On the Issues of NOx as Greenhouse Gases: An Ongoing Discussion…
Abstract
1. Introduction
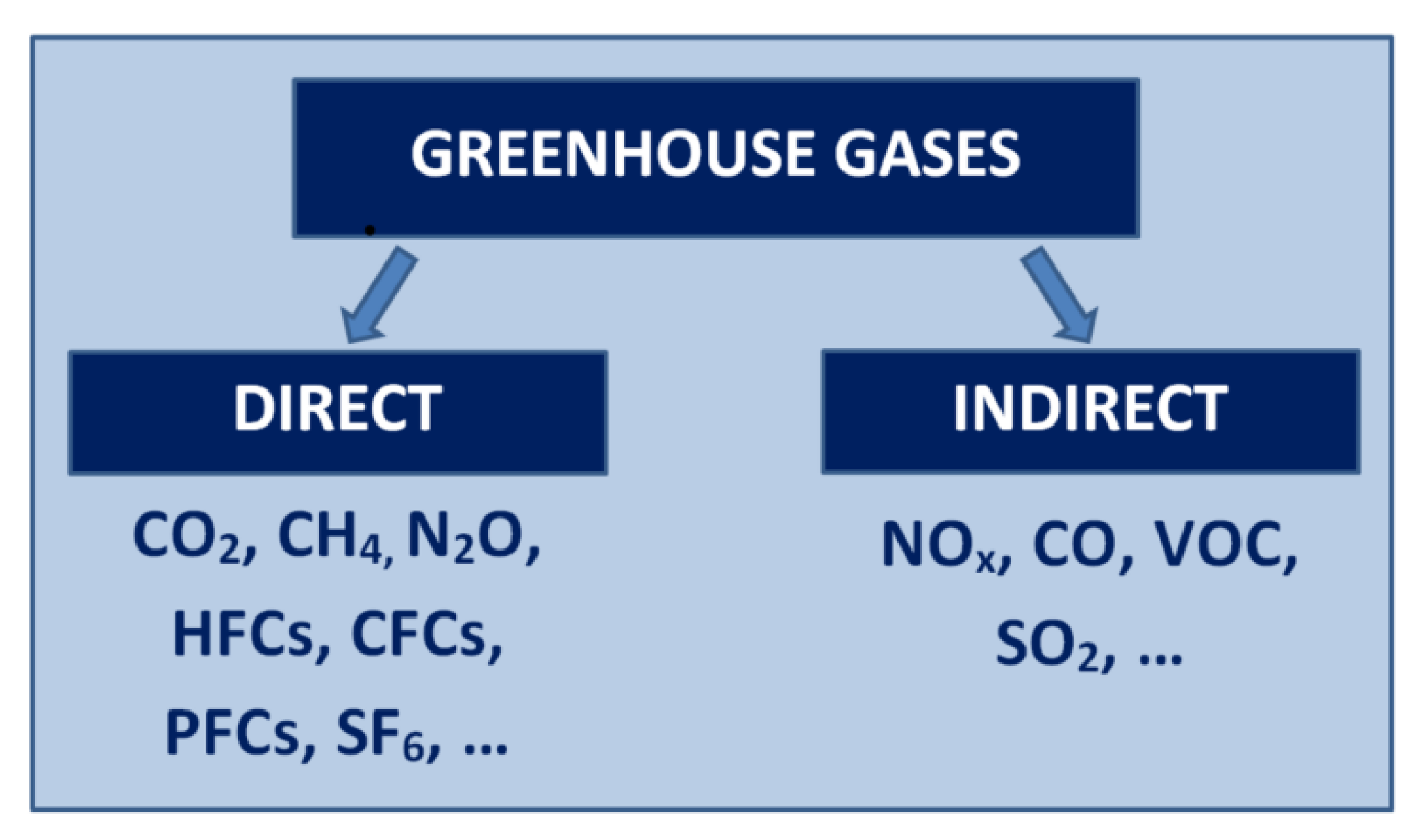
1.1. NOx Environmental Impact
- NOx are GHGs and influence global warming;
- NOx are not GHGs and do not influence the GHE;
- NOx are indirect GHGs and influence global warming;
- NOx are indirect GHGs and influence global cooling.
1.2. Methodology
1.3. NOx: The Matter of Definition
2. The Role of NOx in the Greenhouse Effect
3. Global Warming Potential
4. Dualistic Nature of NOx Impact on the Greenhouse Effect
4.1. Warming Nature
4.2. Cooling Nature
4.3. Summary
5. Simplified Evaluation of NOx (Surface Sources) Impact on GHE
6. NOx in Waste Incineration and Co-Incineration Plants
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| AGWP | Absolute global warming potential |
| C | Total emission limit values for co-firing fuels with waste |
| Cwaste | Emission limit values for waste incineration plants and co-firing |
| FBC | Fluidized-bed combustor |
| GHE | Greenhouse effect anthropogenic) sources |
| GHG | Greenhouse gas |
| GWP | Global warming potential |
| hν | Energy of photon (photochemical reactions) as the multiplication of Planck constant, h and photon’s frequency, ν |
| MSW | Municipal solid waste |
| pptv | Parts-per-trillion (volumetric) |
| RDF | Refuse-derived fuel |
| SCR | Selective catalytic reduction |
| SNCR | Selective non-catalytic reduction |
| STP | Standard temperature and pressure |
| Vproc | Waste gas volume resulting from the plant process including the combustion of the authorized fuels normally used in the plant (waste excluded) |
| Vwaste | Waste gas volume resulting from the incineration of waste only determined from the waste with the lowest calorific value specified in the permit |
| WtE | Waste-to-energy |
References
- Khodakarami, J.; Ghobadi, P. Urban pollution and solar radiation impacts. Renew. Sustain. Energy Rev. 2016, 57, 965–976. [Google Scholar] [CrossRef]
- Munawer, M.E. Human health and environmental impacts of coal combustion and post-combustion wastes. J. Sustain. Min. 2018, 17, 87–96. [Google Scholar] [CrossRef]
- Bhatraju, P.; Crawford, J.; Hall, M.; Lang, J.D., Jr. Inhaled nitric oxide: Current clinical concepts. Nitric Oxide 2015, 50, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, A.; Miller, C.C.; McMullin, B.; Ghahary, A. Potential application of gaseous nitric oxide as a topical antimicrobial agent. Nitric Oxide 2006, 14, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Butterbach-Bahl, K.; Nemitz, E.; Zaehle, S.; Billen, G.; Boeckx, P.; Erisman, J.; Garnier, J.; Upstill-Goddard, R.; Kreuzer, M.; Oenema, O. Nitrogen as a Threat to the European Greenhouse Balance in: The European Nitrogen Assessment; Sutton, M.A., Howard, C.M., Erisman, J.W., Billen, G., Bleeker, A., Grennfelt, P., van Grinsven, H., Grizzetti, B., Eds.; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Pinder, R.W.; Bettez, N.D.; Bonan, G.B.; Greaver, T.L.; Wieder, W.R.; Schlesinger, W.H.; Davidson, E.A. Impacts of human alteration of the nitrogen cycle in the US on radiative forcing. Biogeochemistry 2013, 114, 25–40. [Google Scholar] [CrossRef]
- Lawrence, M.G.; Crutzen, P.J. Influence of NOx emissions from ships on tropospheric photochemistry and climate. Nature 1999, 402, 167–170. [Google Scholar] [CrossRef]
- Stratmann, G.; Ziereis, H.; Stock, P.; Brenninkmeijer, C.A.M.; Zahn, A.; Rauthe-Schöch, A.; Velthoven, P.V.; Schlager, H.; Volz-Thomas, A. NO and NOy in the upper troposphere: Nine years of CARIBIC measurements onboard a passenger aircraft. Atmos. Environ. 2016, 133, 93–111. [Google Scholar] [CrossRef]
- Gradoń, B. Rola podtlenku azotu w modelowaniu emisji NO z procesów spalania paliw gazowych w piecach wysokotemperaturowych (The role of the nitrous oxide in modelling of the NO emission from combustion processes of gaseous fuels in hightemperature furnaces). Zesz. Nauk. Politech. Śląskiej Hut. 2003, 67. (In Polish) [Google Scholar]
- Wasiuk, D.K.; Khan, M.A.H.; Shallcross, D.E.; Lowenberg, M.H. The impact of global aviation NOx emissions on tropospheric composition changes from 2005 to 2011. Atmos. Res. 2016, 178, 73–83. [Google Scholar] [CrossRef]
- Liu, C.; Shi, J.-W.; Gao, C.; Niu, C. Manganese oxide-based catalysts for low-temperature selective catalytic reduction of NOx with NH3: A review. Appl. Catal. A Gen. 2016, 522, 54–69. [Google Scholar] [CrossRef]
- Hernández Rodríguez, M.J.; Pulido Melián, E.; González Díaz, O.; Araña, J.; Macías, M.; González Orive, A.; Doña Rodríguez, J.M. Comparison of supported TiO2 catalysts in the photocatalytic degradation of NOx. J. Mol. Catal. A Chem. 2016, 413, 56–66. [Google Scholar] [CrossRef]
- Ramalingam, S.; Rajendran, S.; Ganesan, P. Performance improvement and exhaust emissions reduction in biodiesel operated diesel engine through the use of operating parameters and catalytic converter: A review. Renew. Sustain. Energy Rev. 2018, 81, 3215–3222. [Google Scholar] [CrossRef]
- Mat Yasin, M.H.; Mamat, R.; Najafi, G.; Ali, O.M.; Yusop, A.F.; Ali, M.H. Potentials of palm oil as new feedstock oil for a global alternative fuel: A review. Renew. Sustain. Energy Rev. 2017, 79, 1034–1049. [Google Scholar] [CrossRef]
- Manan, Z.A.; Mohd Nawi, W.N.R.; Wan Alwi, S.R.; Klemeš, J.J. Advances in Process Integration research for CO2 emission reduction—A review. J. Clean. Prod. 2017, 167, 1–13. [Google Scholar] [CrossRef]
- Sun, M.; Wang, Y.; Shi, L.; Klemeš, J.J. Uncovering energy use, carbon emissions and environmental burdens of pulp and paper industry: A systematic review and meta-analysis. Renew. Sustain. Energy Rev. 2018, 92, 823–833. [Google Scholar] [CrossRef]
- Tahir, S.N.A.; Rafique, M.; Alaamer, A.S. Biomass fuel burning and its implications: Deforestation and greenhouse gases emissions in Pakistan. Environ. Pollut. 2010, 158, 2490–2495. [Google Scholar] [CrossRef] [PubMed]
- Abu-Madi, M.; Rayyan, M.m.A. Estimation of main greenhouse gases emission from household energy consumption in the West Bank, Palestine. Environ. Pollut. 2013, 179, 250–257. [Google Scholar] [CrossRef]
- Choi, J.; Ahn, M.; Kwak, S.; Lee, J.G.; Yoon, Y. Flame structure and NOx emission characteristics in a single hydrogen combustor. Int. J. Hydrog. Energy 2022, 47, 29542–29553. [Google Scholar] [CrossRef]
- Beccaria, M.; Piparo, M.; Zou, Y.; Stefanuto, P.-H.; Purcaro, G.; Mendes Siqueira, A.L.; Maniquet, A.; Giusti, P.; Focant, J.-F. Analysis of mixed plastic pyrolysis oil by comprehensive two-dimensional gas chromatography coupled with low- and high-resolution time-of-flight mass spectrometry with the support of soft ionization. Talanta 2023, 252, 123799. [Google Scholar] [CrossRef] [PubMed]
- Santhanam, K.S.V.; Ahamed, N.N.N. Greenhouse Gas Sensors Fabricated with New Materials for Climatic Usage: A Review. ChemEngineering 2018, 2, 38. [Google Scholar] [CrossRef]
- Meng, D.; Zhan, W.; Guo, Y.; Guo, Y.; Wang, Y.; Wang, L.; Lu, G. A highly effective catalyst of Sm-Mn mixed oxide for the selective catalytic reduction of NOx with ammonia: Effect of the calcination temperature. J. Mol. Catal. A Chem. 2016, 420, 272–281. [Google Scholar] [CrossRef]
- Kampfl, G.; Kristóf, K.; Algaidi, A.A.; Bayoumi Hamuda, H.E.A.F.; Heltai, G. Study of NOx and CO2 production of cultivated soil in closed microcosm experimental system. Microchem. J. 2007, 85, 31–38. [Google Scholar] [CrossRef]
- Anpo, M.; Kim, T.-H.; Matsuoka, M. The design of Ti-, V-, Cr-oxide single-site catalysts within zeolite frameworks and their photocatalytic reactivity for the decomposition of undesirable molecules—The role of their excited states and reaction mechanisms. Catal. Today 2009, 142, 114–124. [Google Scholar] [CrossRef]
- Abas, N.; Kalair, A.; Khan, N.; Kalair, A.R. Review of GHG emissions in Pakistan compared to SAARC countries. Renew. Sustain. Energy Rev. 2017, 80, 990–1016. [Google Scholar] [CrossRef]
- Imanaka, N.; Masui, T. Advances in direct NOx decomposition catalysts. Appl. Catal. A Gen. 2012, 431–432, 1–8. [Google Scholar] [CrossRef]
- Normann, F.; Andersson, K.; Leckner, B.; Johnsson, F. Emission control of nitrogen oxides in the oxy-fuel process. Prog. Energy Combust. Sci. 2009, 35, 385–397. [Google Scholar] [CrossRef]
- Tomeczek, J.; Gradoń, B. The role of N2O and NNH in the formation of NO via HCN in hydrocarbon flames. Combust. Flame 2003, 133, 311–322. [Google Scholar] [CrossRef]
- Amponsah, N.Y.; Wang, J.; Zhao, L. A review of life cycle greenhouse gas (GHG) emissions of commonly used ex-situ soil treatment technologies. J. Clean. Prod. 2018, 186, 514–525. [Google Scholar] [CrossRef]
- Xia, L.; Ti, C.; Li, B.; Xia, Y.; Yan, X. Greenhouse gas emissions and reactive nitrogen releases during the life-cycles of staple food production in China and their mitigation potential. Sci. Total Environ. 2016, 556, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, D.; Choo, S.; Pham, H.T. Estimation of the Non-Greenhouse Gas Emissions Inventory from Ships in the Port of Incheon. Sustainability 2020, 12, 8231. [Google Scholar] [CrossRef]
- Mitchell, J.F. The “greenhouse” effect and climate change. Rev. Geophys. 1989, 27, 115–139. [Google Scholar] [CrossRef]
- Fuglestvedt, J.S.; Shine, K.P.; Berntsen, T.; Cook, J.; Lee, D.S.; Stenke, A.; Skeie, R.B.; Velders, G.J.M.; Waitz, I.A. Transport impacts on atmosphere and climate: Metrics. Atmos. Environ. 2010, 44, 4648–4677. [Google Scholar] [CrossRef]
- El-Shehawy, R.; Gorokhova, E.; Fernandez-Pinas, F.; del Campo, F.F. Global warming and hepatotoxin production by cyanobacteria: What can we learn from experiments? Water Res. 2012, 46, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- 2018. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C10102439&Units=SI&Mask=80 (accessed on 28 May 2018).
- Ehhalt, D.; Prather, M.; Dentener, F.; Derwent, R.; Dlugokencky, E.J.; Holland, E.; Isaksen, I.; Katima, J.; Kirchhoff, V.; Matson, P.; et al. Atmospheric Chemistry and Greenhouse Gases; Pacific Northwest National Laboratory (PNNL): Richland, WA, USA, 2001. [Google Scholar]
- Lasek, J.; Yu, Y.H.; Wu, J.C.S. Removal of NOx by photocatalytic processes. J. Photochem. Photobiol. C Photochem. Rev. 2013, 14, 29–52. [Google Scholar] [CrossRef]
- Lammel, G.; Graßl, H. Greenhouse effect of NOx. Environ. Sci. Pollut. Res. 1995, 2, 40–45. [Google Scholar] [CrossRef]
- Skowron, A.; Lee, D.S.; De León, R.R. Variation of radiative forcings and global warming potentials from regional aviation NOx emissions. Atmos. Environ. 2015, 104, 69–78. [Google Scholar] [CrossRef]
- Mahashabde, A.; Wolfe, P.; Ashok, A.; Dorbian, C.; He, Q.; Fan, A.; Lukachko, S.; Mozdzanowska, A.; Wollersheim, C.; Barrett, S.R.H.; et al. Assessing the environmental impacts of aircraft noise and emissions. Prog. Aerosp. Sci. 2011, 47, 15–52. [Google Scholar] [CrossRef]
- Macintosh, A.; Wallace, L. International aviation emissions to 2025: Can emissions be stabilised without restricting demand? Energy Policy 2009, 37, 264–273. [Google Scholar] [CrossRef]
- Eickenscheidt, N.; Brumme, R. NOx and N2O fluxes in a nitrogen-enriched European spruce forest soil under experimental long-term reduction of nitrogen depositions. Atmos. Environ. 2012, 60, 51–58. [Google Scholar] [CrossRef]
- Johnson, C.; Henshaw, J.; Mclnnes, G. Impact of aircraft and surface emissions of nitrogen oxides on tropospheric ozone and global warming. Nature 1992, 355, 69. [Google Scholar] [CrossRef]
- Wuebbles, D.J.; Grant, K.E.; Connell, P.S.; Penner, J.E. The role of atmospheric chemistry in climate change. Japca 1989, 39, 22–28. [Google Scholar] [CrossRef]
- Fuglestvedt, J.S.; Isaksen, I.S.A.; Wang, W.-C. Estimates of indirect global warming potentials for CH4, CO and NOx. Clim. Chang. 1996, 34, 405–437. [Google Scholar] [CrossRef]
- Adouani, N.; Limousy, L.; Lendormi, T.; Sire, O. N2O and NO emissions during wastewater denitrification step: Influence of temperature on the biological process. Comptes Rendus Chim. 2015, 18, 15–22. [Google Scholar] [CrossRef]
- Skowron, A.; Lee, D.S.; De León, R.R.; Lim, L.L.; Owen, B. Greater fuel efficiency is potentially preferable to reducing NOx emissions for aviation’s climate impacts. Nat. Commun. 2021, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Banasiewicz, A.; Janicka, A.; Michalak, A.; Włostowski, R. Photocatalysis as a method for reduction of ambient NOx in deep underground mines. Measurement 2022, 200, 111453. [Google Scholar] [CrossRef]
- Miller, C.J.; Prashanth, P.; Allroggen, F.; Grobler, C.; Sabnis, J.S.; Speth, R.L.; Barrett, S.R.H. An environmental cost basis for regulating aviation NOx emissions. Environ. Res. Commun. 2022, 4, 055002. [Google Scholar] [CrossRef]
- Johnson, C.E.; Derwent, R.G. Relative radiative forcing consequences of global emissions of hydrocarbons, carbon monoxide and NOx from human activities estimated with a zonally-averaged two-dimensional model. Clim. Chang. 1996, 34, 439–462. [Google Scholar] [CrossRef]
- Zhang, R.; Tie, X.; Bond, D.W. Impacts of anthropogenic and natural NOx sources over the U.S. on tropospheric chemistry. Proc. Natl. Acad. Sci. USA 2003, 100, 1505–1509. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Biagioni, R.N.; Alarcón-Herrera, M.T.; Rivas-Lucero, B.A. An overview of nitrate sources and operating processes in arid and semiarid aquifer systems. Sci. Total Environ. 2018, 624, 1513–1522. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130122. [Google Scholar] [CrossRef]
- Wild, O.; Prather, M.J.; Akimoto, H. Indirect long-term global radiative cooling from NOx emissions. Geophys. Res. Lett. 2001, 28, 1719–1722. [Google Scholar] [CrossRef]
- Kimochi, Y.; Inamori, Y.; Mizuochi, M.; Xu, K.-Q.; Matsumura, M. Nitrogen removal and N2O emission in a full-scale domestic wastewater treatment plant with intermittent aeration. J. Ferment. Bioeng. 1998, 86, 202–206. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, J.; Xie, H.; Li, S.; Zhang, T.; Wang, J. Identifying sources of nitrous oxide emission in anoxic/aerobic sequencing batch reactors (A/O SBRs) acclimated in different aeration rates. Enzym. Microb. Technol. 2011, 49, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Negoro, M.; Shioji, N.; Miyamoto, K.; Micira, Y. Growth of microalgae in high CO2 gas and effects of SOx and NOx. Appl. Biochem. Biotechnol. 1991, 28, 877. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xu, G.; Rong, J.; Chen, H.; He, C.; Giordano, M.; Wang, Q. The acclimation of Chlorella to high-level nitrite for potential application in biological NOx removal from industrial flue gases. J. Plant Physiol. 2016, 195, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Scott, J.T. Throwing fuel on the fire: Synergistic effects of excessive nitrogen inputs and global warming on harmful algal blooms. Environ. Sci. Technol. 2010, 44, 7756–7758. [Google Scholar] [CrossRef]
- Wagner, C.; Adrian, R. Cyanobacteria dominance: Quantifying the effects of climate change. Limnol. Oceanogr. 2009, 54, 2460–2468. [Google Scholar] [CrossRef]
- Kumar, K.; Dasgupta, C.N.; Nayak, B.; Lindblad, P.; Das, D. Development of suitable photobioreactors for CO2 sequestration addressing global warming using green algae and cyanobacteria. Bioresour. Technol. 2011, 102, 4945–4953. [Google Scholar] [CrossRef]
- Wang, C.; Yu, X.; Lv, H.; Yang, J. Nitrogen and phosphorus removal from municipal wastewater by the green alga Chlorella sp. J. Environ. Biol. 2013, 34, 421. [Google Scholar]
- Yen, H.-W.; Ho, S.-H.; Chen, C.-Y.; Chang, J.-S. CO2, NOx and SOx removal from flue gas via microalgae cultivation: A critical review. Biotechnol. J. 2015, 10, 829–839. [Google Scholar] [CrossRef]
- Kyprianidis, K.G.; Dahlquist, E. On the trade-off between aviation NOx and energy efficiency. Appl. Energy 2017, 185, 1506–1516. [Google Scholar] [CrossRef]
- Fan, W.; Sun, Y.; Zhu, T.; Wen, Y. Emissions of HC, CO, NOx, CO2, and SO2 from civil aviation in China in 2010. Atmos. Environ. 2012, 56, 52–57. [Google Scholar] [CrossRef]
- Kurniawan, J.S.; Khardi, S. Comparison of methodologies estimating emissions of aircraft pollutants, environmental impact assessment around airports. Environ. Impact Assess. Rev. 2011, 31, 240–252. [Google Scholar] [CrossRef]
- Vet, R.; Artz, R.S.; Carou, S.; Shaw, M.; Ro, C.-U.; Aas, W.; Baker, A.; Bowersox, V.C.; Dentener, F.; Galy-Lacaux, C.; et al. A global assessment of precipitation chemistry and deposition of sulfur, nitrogen, sea salt, base cations, organic acids, acidity and pH, and phosphorus. Atmos. Environ. 2014, 93, 3–100. [Google Scholar] [CrossRef]
- 2022. Available online: https://www.epa.gov/ghgemissions/global-greenhouse-gas-emissions-data#Trends (accessed on 2 September 2022).
- Battye, W.; Aneja, V.P.; Schlesinger, W.H. Is nitrogen the next carbon? Earth’s Future 2017, 5, 894–904. [Google Scholar] [CrossRef]
- Lee, C.T.; Lim, J.S.; Fan, Y.V.; Liu, X.; Fujiwara, T.; Klemeš, J.J. Enabling low-carbon emissions for sustainable development in Asia and beyond. J. Clean. Prod. 2018, 176, 726–735. [Google Scholar] [CrossRef]
- Gonzalez-Salazar, M.A.; Kirsten, T.; Prchlik, L. Review of the operational flexibility and emissions of gas- and coal-fired power plants in a future with growing renewables. Renew. Sustain. Energy Rev. 2018, 82, 1497–1513. [Google Scholar] [CrossRef]
- Jaeglé, L.; Steinberger, L.; Martin, R.V.; Chance, K. Global partitioning of NOx sources using satellite observations: Relative roles of fossil fuel combustion, biomass burning and soil emissions. Faraday Discuss. 2005, 130, 407–423. [Google Scholar] [CrossRef]
- Tong, D.; Zhang, Q.; Davis, S.J.; Liu, F.; Zheng, B.; Geng, G.; Xue, T.; Li, M.; Hong, C.; Lu, Z. Targeted emission reductions from global super-polluting power plant units. Nat. Sustain. 2018, 1, 59. [Google Scholar] [CrossRef]
- Miyazaki, K.; Bowman, K.; Sekiya, T.; Takigawa, M.; Neu, J.L.; Sudo, K.; Osterman, G.; Eskes, H. Global tropospheric ozone responses to reduced NOx emissions linked to the COVID-19 worldwide lockdowns. Sci. Adv. 2021, 7, eabf7460. [Google Scholar] [CrossRef]
- Doumbia, T.; Granier, C.; Elguindi, N.; Bouarar, I.; Darras, S.; Brasseur, G.; Gaubert, B.; Liu, Y.; Shi, X.; Stavrakou, T. Changes in global air pollutant emissions during the COVID-19 pandemic: A dataset for atmospheric modeling. Earth Syst. Sci. Data 2021, 13, 4191–4206. [Google Scholar] [CrossRef]
- 2017. Available online: http://cdiac.ornl.gov/ftp/ndp030/global.1751_2014.ems (accessed on 31 August 2017).
- Miyazaki, K.; Eskes, H.; Sudo, K. Global NOx emission estimates derived from an assimilation of OMI tropospheric NO2 columns. Atmos. Chem. Phys. 2012, 12, 2263–2288. [Google Scholar] [CrossRef]
- Lamsal, L.; Martin, R.; Padmanabhan, A.; van Donkelaar, A.; Zhang, Q.; Sioris, C.; Chance, K.; Kurosu, T.; Newchurch, M. Application of satellite observations for timely updates to global anthropogenic NOx emission inventories. Geophys. Res. Lett. 2011, 38, 1–5. [Google Scholar] [CrossRef]
- Miyazaki, K.; Eskes, H.; Sudo, K.; Boersma, K.F.; Bowman, K.; Kanaya, Y. Decadal changes in global surface NOx emissions from multi-constituent satellite data assimilation. Atmos. Chem. Phys. 2017, 17, 807. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Vassilev, V.S. Advantages and disadvantages of composition and properties of biomass in comparison with coal: An overview. Fuel 2015, 158, 330–350. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the chemical composition of biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Van Caneghem, J.; Brems, A.; Lievens, P.; Block, C.; Billen, P.; Vermeulen, I.; Dewil, R.; Baeyens, J.; Vandecasteele, C. Fluidized bed waste incinerators: Design, operational and environmental issues. Prog. Energy Combust. Sci. 2012, 38, 551–582. [Google Scholar] [CrossRef]
- Sajid, M.; Raheem, A.; Ullah, N.; Asim, M.; Ur Rehman, M.S.; Ali, N. Gasification of municipal solid waste: Progress, challenges, and prospects. Renew. Sustain. Energy Rev. 2022, 168, 112815. [Google Scholar] [CrossRef]
- Yang, X.; Liao, Y.; Wang, Y.; Chen, X.; Ma, X. Research of coupling technologies on NOx reduction in a municipal solid waste incinerator. Fuel 2022, 314, 122769. [Google Scholar] [CrossRef]
- Leckner, B.; Lind, F. Combustion of municipal solid waste in fluidized bed or on grate—A comparison. Waste Manag. 2020, 109, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Anufriev, I.S. Review of water/steam addition in liquid-fuel combustion systems for NOx reduction: Waste-to-energy trends. Renew. Sustain. Energy Rev. 2021, 138, 110665. [Google Scholar] [CrossRef]
- Skalska, K.; Miller, J.S.; Ledakowicz, S. Trends in NOx abatement: A review. Sci. Total Environ. 2010, 408, 3976–3989. [Google Scholar] [CrossRef]
- Makarichi, L.; Jutidamrongphan, W.; Techato, K.-a. The evolution of waste-to-energy incineration: A review. Renew. Sustain. Energy Rev. 2018, 91, 812–821. [Google Scholar] [CrossRef]

| Case | Assumptions |
|---|---|
| Coal combustion (100%) | LHV = 22 MJ/kg, new installation, continuous measurement (daily average values) |
| Biomass combustion (100%) | LHV = 18 MJ/kg, new installation, continuous measurement (daily average values) |
| RDF combustion (100%) | LHV = 15 MJ/kg, new installation, continuous measurement (daily average values) |
| Coal/RDF co-firing (10% by energy) | LHV = 22 MJ/kg (coal) and LHV = 15 MJ/kg (RDF), RDF share of 10% (by energy), new installation, continuous measurement (daily average values), installation capacity (thermal power in fuel) of 1.5 MW, 15 MW, 105 MW, and 305 MW |
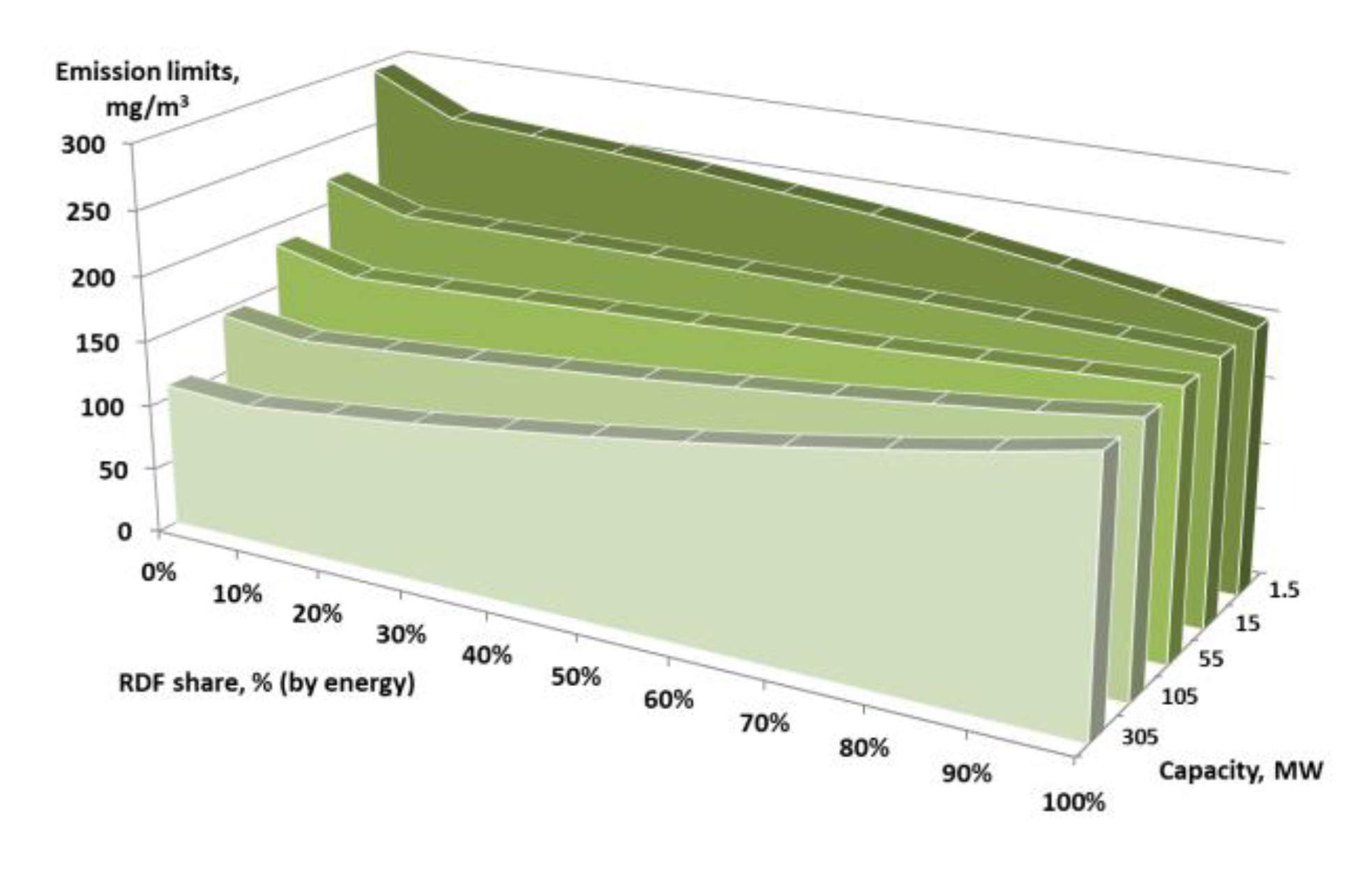
| Biomass/RDF co-firing | LHV = 22 MJ/kg (coal) and LHV = 15 MJ/kg (RDF), RDF share of 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, and 90% (by energy), new installation, continuous measurement (daily average values), installation capacity (thermal power in fuel) of 1.5 MW, 15 MW, 105 MW, and 305 MW |
| Warming | Cooling | Cross Impact |
|---|---|---|
| Air | ||
| In the short-term, NOx emissions contribute to warming by enhancing tropospheric O3 concentrations (on a daily time scale), which are recognized as GHG [6,32]. | NOx enhances OH production. CH4 (GHG) is oxidized in the presence of OH [6,39]. NOx can lead to decreases in O3 concentration on a decadal time scale because it causes an increase in OH radical concentration, which decreases CH4 concentration, which decreases NO2 formation, which decreases O3 formation. [6,39]. The formation of fine particles called aerosols. Aerosols are powerful cooling agents, both directly by scattering or absorbing light, and indirectly by affecting the cloud formation, their lifetime, and brightness [6,7]. | NOx leads to O3 decreasing (on a decadal time scale) or increasing (on a daily time scale) [6]. |
| Soil and vegetation aboveground | ||
| Nitrogen is a substrate for N2O production by nitrifying and denitrifying bacteria in soils. Thus, the deposition of nitrogen (Nr) onto ecosystems can increase N2O emissions and decrease the uptake of atmospheric CH4 by soil microorganisms. Soil microbes that consume CH4 often preferentially consume ammonium (NH4+), leading to reduced CH4 consumption rates in the presence of abundant NH4+ [6]. Inhibition of photosynthesis and a reduction of atmospheric CO2 sequestration by the plant biomass due to an increase of O3 concentration in the atmosphere (impacted by NOx). Reduction of aboveground C storage and reduction of belowground C assimilation and allocation [5,6] In some cases, the excess of N leads to the enhanced mortality of plants due to nutrient imbalances or acidification [6]. | In some cases, inputs of Nr from atmospheric deposition enhance plant growth rates because of the fundamental constraint of N availability on plant productivity and CO2 uptake into plant biomass. N additions to soil typically increase C capture and storage [6]. Foliar N may also increase the albedo of the canopy, enhancing the reflectivity of the Earth’s surface, and hence contributing to cooling [6]. | Warming and cooling effects are possible. The effect of N on net C flux (both above and below ground pools) differs among ecosystems [5,6]. |
| Water | ||
| Nitrogen is a substrate for N2O production by nitrifying and denitrifying bacteria in water bodies [6]. Denitrification occurring in water can emits N2O [46]. Nitrous oxide (N2O) can be emitted from wastewater treatment processes [46,55,56]. Both SO2 and NO inhibited algal growth at a high level of CO2 [57,58]. | N- water can accelerate to grow algae growth. Nevertheless, the harmful (toxic, food-web altering, hypoxia-generating) algal blooms (HABs) have been linked to human nutrient (phosphorus (P) and nitrogen (N)) over enrichment [59] The serious problem is cyanobacterial bloom formation. Decreasing P and N loads can counteract the direct positive effect of warming temperatures on bloom proliferation [34,60]. Some algae species can sequestrate the CO2 from the flue gas including SOX and NO [61]. In the case of some species (green alga Chlorella sp.), the presence of NOx can enhance algae growth [62] | NOx and SOx might be beneficial to the growth of microalgae as they can provide additional nutrients. However, this is true only when the culture pH is stably controlled and the NOx/SOx concentrations should be lower than the inhibitory level [63]. |
| CO2 Emission Data from [12,76] | NOx Emission | Data from | Calculated as GtCO2/Year | Calculated as GtNOx/Year | GWP100 = 10 | Contribution of NOx in the GHE Compared to CO2 Emissions from Fossil Fuels Usage and Industrial Processes, % | Contribution of NOx in the GHE Compared to the Emission of all GHGs, % | |
|---|---|---|---|---|---|---|---|---|
| Year | GtC/Year | GtN/Year | GtCO2/Year | GtNOx/Year | NOx-eq.CO2, GtCO2/Year | % (Calculated from Equation (9)) | %(Total), (Calculated from Equation (10)) | |
| 2000 | 6.733 | 0.0256 | [72] | 24.7 | 0.084 | 0.8 | 3.3 | 2.2 |
| 2005 | 8.042 | 0.0454 | [77] | 29.5 | 0.149 | 1.5 | 4.8 | 3.2 |
| 2006 | 8.336 | 0.0191 | [78] | 30.6 | 0.063 | 0.6 | 2.0 | 1.3 |
| 2009 | 8.697 | 0.0209 | [78] | 31.9 | 0.069 | 0.7 | 2.1 | 1.4 |
| 2014 | 9.855 | 0.0475 | [79] | 36.1 | 0.156 | 1.6 | 4.1 | 2.7 |
| Case | Emission Standard (Executed) | ||||
|---|---|---|---|---|---|
| 1.5 MW | 15 MW | 55 MW | 105 MW | 305 MW | |
| Coal combustion (100%) | 400 (req.) (base 267) | 300 (req.) (base 200) | 220 (req.) (base 200) | 146 (req.) (base 133) | 110 (req.) (base 100) |
| Biomass combustion (100%) | 294 (req.) (base 267) | 220 (req.) (base 200) | 184 (req.) (base 167) | 146 (req.) (base 133) | 110 (req.) (base 100) |
| RDF combustion (100%) | 200 | 200 | 200 | 200 | 200 |
| Biomass/RDF (10%) co-firing | 264 | 201 | 169 | 138 | 107 |
| Coal/RDF (10%) co-firing | 265 | 202 | 202 | 138 | 106 |
| RDF Share (% by Energy) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Capacity | 0% | 10% | 20% | 30% | 40% | 50% | 60% | 70% | 80% | 90% | 100% |
| 1.5 MW | 294 (req.) 267 (base) | 264 | 260 | 256 | 251 | 245 | 239 | 231 | 222 | 212 | 200 |
| 15 MW | 220 (req.) 200 (base) | 200.8 | 201.5 | 202.0 | 202.5 | 202.6 | 202.7 | 202.5 | 202.0 | 201.2 | 200 |
| 55 MW | 184 (req.) 167 (base) | 169 | 172 | 175 | 178 | 181 | 185 | 188 | 192 | 196 | 200 |
| 105 MW | 146 (req.) 133 (base) | 138 | 143 | 148 | 154 | 160 | 167 | 174 | 182 | 190 | 200 |
| 305 MW | 110 (req.) 100 (base) | 107 | 114 | 121 | 130 | 139 | 149 | 160 | 172 | 185 | 200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lasek, J.A.; Lajnert, R. On the Issues of NOx as Greenhouse Gases: An Ongoing Discussion…. Appl. Sci. 2022, 12, 10429. https://doi.org/10.3390/app122010429
Lasek JA, Lajnert R. On the Issues of NOx as Greenhouse Gases: An Ongoing Discussion…. Applied Sciences. 2022; 12(20):10429. https://doi.org/10.3390/app122010429
Chicago/Turabian StyleLasek, Janusz Andrzej, and Radosław Lajnert. 2022. "On the Issues of NOx as Greenhouse Gases: An Ongoing Discussion…" Applied Sciences 12, no. 20: 10429. https://doi.org/10.3390/app122010429
APA StyleLasek, J. A., & Lajnert, R. (2022). On the Issues of NOx as Greenhouse Gases: An Ongoing Discussion…. Applied Sciences, 12(20), 10429. https://doi.org/10.3390/app122010429







