Abstract
Dexmedetomidine (DEX) used for sedation was reported to have organ-protecting effects in ischemia–reperfusion injury model animals. However, no testicular cell-protecting effect was observed with DEX treatment. The effects of DEX on a normal testis in vivo have not been reported. Therefore, DEX was administered to mice for 14 days to investigate the reproductive toxicology of DEX on the testis and the localization of DEX-responsive receptors. The testes, pituitary glands, and serum were examined and analyzed using real-time PCR, immunofluorescence staining, and liquid chromatography–mass spectrometry. In the testis, α2A-adrenergic receptors were observed in the cytoplasm of Leydig cells, while imidazoline receptors were observed in germ cells and Leydig cell cytoplasm. The levels of luteinizing hormone and follicle-stimulating hormone mRNA in the pituitary gland significantly temporarily decreased. Serum DEX could not be detected 26 h after DEX administration. DEX administration did not affect serum testosterone levels, some testicular mRNA related to spermatogenesis, and oxidative stress factors. Therefore, although DEX receptors are present in the testis, DEX is metabolized relatively quickly, and DEX administration has no damaging effects on the testis. This study is the first in vivo report about the effects of DEX administration on the testis.
1. Introduction
Dexmedetomidine (DEX) is used for intraoperative anesthesia and sedation in intensive care. It is an α2-adrenergic receptor agonist that also acts on imidazoline receptors. There are three α2-adrenergic receptor subtypes, and DEX mainly reacts with α2A-adrenergic receptors [1]. Normally, α2-adrenergic receptors bind to the noradrenaline released by itself and suppress the further release of noradrenaline. Many α2-adrenergic receptors are present in the locus coeruleus of the pons, and when DEX binds to them, noradrenaline release is suppressed. The site of the central nervous system responsible for excitement and arousal is also suppressed, making it sedated. DEX causes less respiratory depression than other sedative drugs and is approved for use in children in Japan. It also has a relatively short half-life of about 2 h and is easy to use [1]. Recently, it was reported that it also has a protective effect on various organs [2].
The administration of DEX to a myocardial ischemia–reperfusion mouse model activated endothelial nitric oxide synthase in vascular endothelial cells, resulting in coronary artery dilation, decreased IL-1β/IL-6/TNF-α mRNA expression in myocardial cells, and increased antioxidant enzyme levels (SOD, CAT, GPx). DEX was reported to have cardioplegic, anti-inflammatory, and antioxidant effects [3]. In addition, Hyunyoung et al. reported that the administration of DEX to a hepatic ischemia–reperfusion rat model has antiapoptotic and anti-inflammatory effects that are due to an increased Bcl-2 mRNA expression and decreased IL-6 mRNA expression in hepatocytes [4]. Zhixiang et al. reported that its administration to a spinal ischemia–reperfusion rabbit model has anti-inflammatory and antiapoptotic effects that are due to suppressed TNF-α/IL-1β and reduced number of caspase 3-positive neurons [5]. Therefore, DEX is considered to have a protective effect on various organs.
However, there is a report that the organ-protective effect of DEX on the testis did not improve the pathological condition in the reperfusion model. Tuglu et al. studied the testicular condition 6 h after a single intraperitoneal administration of DEX 60 min before torsion in rats. Compared to the torsion group, in the DEX + torsion group, the total oxidative ability (TOS) increased at 25 μg/kg of administration of DEX, the total antioxidant ability (TAS) increased, and the oxidative stress index (OSI) decreased at 50 µg/kg administration. It was reported that the testicular tissue was unchanged in the DEX + torsion group compared to the torsion group [6,7]. Jing et al. ligated the rabbit spermatic cord for 4 h, administered 50 µg/kg single intraperitoneally to DEX 1 h before reperfusion and removed the testes four weeks later. No significant differences in testis volume, spermatogenic cell count, MDA concentration, and SOD activity between the ligated group and DEX + ligated group were reported [8]. Therefore, there are no reports on the improvement in pathological conditions in testis reperfusion model animals by DEX administration, and it is possible that they do not have an organ-protective effect.
In an in vitro study where DEX (0.015–1.5 μM) was exposed to Leydig cells isolated from 2-week-old rats, Wang et al. reported that 3β-HSD/p450 17A1/p450c11 mRNA expression and enzyme levels decreased, androgen synthesis was suppressed, and the number of reactive oxygen species (ROS)-induced apoptotic Leydig cells increased [9]. The studies in which DEX was treated alone are only in vitro studies, and there are no reports of in vivo studies. The in vivo administration of DEX alone may adversely affect the testis. In addition, since DEX reacts with cultured Leydig cells [9], Leydig cells might have a site of action for DEX. However, the localization of α2A-adrenergic and imidazoline receptors in the testis is unknown.
Therefore, to understand the reproductive toxicity of DEX, we studied whether normal mice that received DEX alone at varying concentrations and durations would have testicular dysfunction. Furthermore, we studied the localization of α2A-adrenergic and imidazoline receptors in the testis, which are the sites of action of DEX.
2. Materials and Methods
2.1. Animals
Male A/J mice (n = 71, 7–8 weeks old) were purchased from SLC (Shizuoka, Japan). These were maintained at the Animal Department of Support Center for Medical Re-search and Education of Tokai University for one week. In previous studies, the A/J mice were used as a pathological model for autoimmune orchitis [10,11,12]. In the present study, the A/J mice were used to obtain baseline data for future studies on autoimmune orchitis. The mice were kept on a 12 h light/12 h dark schedule (lights on from 07:00 to 19:00) and allowed food and water ad libitum. All animal experiments were performed in accordance with institutional guidelines and were approved by the Institutional Animal Care and Use Committee (permit nos. 212001 at Tokai University). All efforts were made to minimize the number of animals used and animal suffering.
2.2. Experimental Design
The mice were divided into four groups. The normal group (n = 17) was managed in the same environment as other mice without any treatment. The sham group (n = 17) received a dose of saline with dissolved DEX intraperitoneally corresponding to the treatment group.
The 10 µg/kg group (n = 17) received DEX (Nipro, Osaka, Japan) at a concentration of 10 µg/kg intraperitoneally for 14 days. The 40 µg/kg group (n = 20) received DEX at 40 µg/kg intraperitoneally for 14 days. The method for determining the DEX administration concentration this time was if the total antioxidant capacity (TOS) increased by 25 µg/kg and if the total antioxidant status (TAS) increased by 50 µg/kg from the previous report.
The administration was performed every day at 7:00 am for 14 days, and the mice were euthanized at 9:00 (n = 4 per group) and 13:00 (n = 5 in the 40 µg/kg group; n = 4 in other groups) on day 14 and at 9:00 (n = 5 in the 40 µg/kg group; n = 4 in other groups) and 13:00 (n = 6 in the 40 µg/kg group; n = 5 in other groups) on day 15. Since the half-life of DEX is short, and it is used as a continuous administration in clinical practice, it is continuously administered every few days depending on the condition in the intensive care unit. Data were collected in a short period after DEX administration for 14 days (Figure S1). The mice fell asleep about 15 min after DEX administration and were sedated for about 2 h thereafter.
Weights were measured before the mice were sacrificed. After being in deep general anesthesia, their heart blood sample, testis, and pituitary gland were collected. The testes were weighed and stored by each method. The serum was separated at 3000 rpm 4 °C for 15 min; 200 µL of mouse serum was dispensed, and blood testosterone concentration was outsourced from SRL, Inc. and measured by ECLIA (electro chemiluminescence immunoassay).
2.3. Histochemistry
The mouse testes were fixed in Bouin’s fluid for three days and dehydrated in 70% alcohol. The testes were wrapped in paraffin, cut into 2.25 µm thick slices, and placed on a slide glass. The slices were stained using hematoxylin and eosin and were observed using a light microscope.
2.4. Immunofluorescence
2.4.1. 3β HSD (3 Beta-hydroxysteroid dehydrogenase), α2A-Adrenergic Receptor, and Imidazoline Receptor
The testes were cut into 5 µm thick slices using a cryostat (Leica CM3050 S; Leica Biosystems, Wetzlar, Germany), and the testis sections were placed on a slide glass. The sections were fixed with acetone for 5 min. After washing with PBS, these sections were blocked using an avidin/biotin blocking kit (Vector Laboratories, CA, USA) for 15 min and blocked using a DAKO Protein Block Serum-Free for 20 min. The testis sections were incubated with goat antimouse 3β HSD antibodies (Santa cruz biotechnology, TX, USA: sc-30820), rabbit antimouse α2A-adrenergic receptor antibodies (Sigma-Aldrich, Darmstadt, Germany: A271), and rabbit antimouse imidazoline receptor antibodies (US biological, MA, USA: Q80TM9) at 4 °C overnight. As the secondary antibody, the sections were incubated with biotin donkey antirabbit antibodies (Vector Laboratories: BA-4001) for 60 min at room temperature (25 °C). As the third antibody, the sections were incubated with Streptavidin 594 (Molecular Probes, MA, USA: S11227) for 60 min at room temperature. As the fourth antibody, the sections were incubated with rabbit antigoat Alexa 488 antibodies (Thermo Fisher Scientific, MA, USA: A21222) for 60 min. The nuclei were counterstained with DAPI. Immunofluorescent images were taken using a Zeiss LSM 700 Microscope (Carl Zeiss Micro Imaging, Thüringen, Germany).
2.4.2. PNA (Peanut Agglutinin) and Imidazoline Receptor
The testes were cut into 5 µm thick slices using a cryostat, and the testis sections were placed on a slide glass. These sections were fixed with 4% paraformaldehyde for 10 min. After washing with PBS, these sections were blocked using an avidin/biotin blocking kit (Vector Laboratories) for 15 min and a DAKO Protein Block Serum-Free for 20 min. The testis sections were incubated with rabbit antimouse imidazoline receptor antibodies at 4 °C overnight. As the secondary antibody, the sections were incubated with Lectin PNA Alexa Fluor 594 (Thermo Fisher Scientific: L32459) for 30 min at room temperature. As the third antibody, the sections were incubated with biotin donkey antirabbit antibodies for 60 min at room temperature. As the fourth antibody, the sections were incubated with Streptavidin 488 (Molecular Probes: S11223) for 60 min at room temperature. The nuclei were counterstained with DAPI. Immunofluorescent images were taken using a Zeiss LSM 700 Microscope (Carl Zeiss Micro Imaging).
2.5. Liquid Chromatography–Mass Spectrometry (LC/MS)
Each sample was analyzed for DEX. Chemical standards with a purity of >99% for DEX analytes were obtained (Toronto Research Chemicals, TO, Canada: D299000). DEX-d4 (Toronto Research Chemicals: D299006) was used as the internal standard and recovery surrogate. Then, 20 µL of the mouse serum was dispensed, 1 µL of the prepared D4-DEX stock was added thereto, and 300 µL of acetonitrile was added. This was vortexed for 3 min, centrifuged (15,000 rpm, 4 °C, 10 min), and dried using a Thermo Savant SPD-Series SpeedVac Concentrator (Thermo Fisher Scientific). These were redissolved in 1 mL of 50% acetonitrile, vortexed for 10 min, and centrifuged for 3 min. The supernatant was passed through a filter, and 50 µL was used for LC/MS. LCMS-8050 (SHIMAZU, Kyoto, Japan) was used as the LC/MS system. The column was an L-column 2 ODS (Chemicals Evaluation and Research Institute, Tokyo, Japan; 50 mm × 2.0 mm). The mobile phase used was 0.1% acetic acid, 0.05% formic acid, and 95% acetonitrile. The flow rate was 0.30 mL/min, and the column temperature was 40 °C. The values were calculated from a calibration curve (Figure S2).
2.6. Western Blotting of α2A-Adrenergic Receptors and Imidazoline Receptors
The testis tissues were homogenized in a radioimmunoprecipitation assay lysis buffer. After homogenization, the protein concentration was measured using a bicinchoninic acid (BCA) protein assay kit (Thermo Fisher Scientific). An equal amount of protein (10 µg) per lane was run on a polyacrylamide gel and was transferred onto polyvinylidene difluoride membranes. Membranes containing the transferred proteins were blocked with 5% skim milk in TBS containing 0.1% Tween 20 solution (TBST) for 1 h at room temperature. The membranes were incubated with primary antibodies of rabbit antimouse α2A-adrenergic receptor antibody (Sigma-Aldrich: A271) and rabbit antimouse imidazoline receptor antibody (US biological: Q80TM9) at 4 °C overnight. The membrane was incubated with antirabbit IgG-HRP antibody (GE healthcare, IL, USA: NA9340V) as the secondary antibody after rinsing with TBST for 1 h. To confirm the equal loading of the samples, rabbit anti-GAPDH polyclonal antibodies (Sigma-Aldrich: G9545) were used as an internal control. Chemiluminescence (Millipore, Darmstadt, Germany: Immobilon Western HRP Substrate peroxide solution/Luminol Reagent) was used to expose the protein bands on the membrane using Fusion (Vilber Bio Imaging, Paris, France).
2.7. Real-Time PCR
The total RNA was isolated from each group’s testes and pituitary glands using TRIzol RNA extraction, and this was reverse-transcribed into cDNA using a high-capacity cDNA reverse-transcription kit according to the manufacturer’s instructions. cDNA quantification was performed using a cobas Z 480 analyzer (Roche-diagnostics, Basel, switzerland). The sequences of cholesterol side-chain cleavage cytochrome P450 (P450scc), 3β-hydroxysteroid dehydrogenase (3βHSD), luteinizing hormone receptor (LHR), follicle-stimulating hormone receptor (FSHR), luteinizing hormone (LH), follicle-stimulating hormone (FSH), imidazoline receptor, α2A-adrenergic receptor, caspase 3, catalase (CAT), glutathione peroxidase (GPx), superoxide dismutase 2 (SOD2), adrenoleukodystrophy protein from subfamily D, member 1 (ABCD1), ABCD3, glucose-regulated protein, 78kDa (GRP78), 8-oxoguanine DNA glycosylase 1 (OGG1), Ki67, topoisomerase 2-alpha (Top2a), synaptonemal complex protein 3 (Sycp3), MutL homolog 1 (Mlh1), acrosin (Acr), transition protein (Tnp1), inhibin-A (InhA), and SHBG (sex-hormone-binding globulin) were obtained from FASMAC (Table S1). The data were analyzed using thermal cycler dice real-time system software (Takara-Bio, Shiga, Japan), and the comparative Ct method (2ΔΔCt) was used to quantify the gene expression levels. Real-time RT-PCR data were standardized to the internal control GAPDH.
2.8. Statistical Analysis
Data are expressed as mean ± standard deviation (SD). IBM SPSS statistical software was used to analyze the data. Specifically, the data were analyzed using one-way analysis of variance, multiple comparisons tests (Tukey’s honest significant difference and Games–Howell tests), and the Kruskal–Wallis test based on the results of the Shapiro–Wilk test. Differences were considered statistically significant at p < 0.05. It was judged that there was no significant difference between the results of real-time PCR unless the values were doubled or more than half, even if there was a statistically significant difference.
3. Results
3.1. Localization and Expression of α2A-Adrenergic Receptors and Imidazoline Receptors in Normal Mice
α2A-adrenergic receptor and imidazoline receptor mRNA were expressed in the testes of normal mice (Figure 1A). In immunofluorescent staining, 3βHSD specifically stains Leydig cells in the testicular interstitium [13], while PNA specifically stains acrosomes [14]. α2A-adrenergic receptors were observed in Leydig cell and vascular endothelial cell cytoplasm of normal mice (Figure 1B1), while imidazoline receptors were observed in Leydig cells (Figure 1B2) and germ cells in normal mice (Figure 1B3).
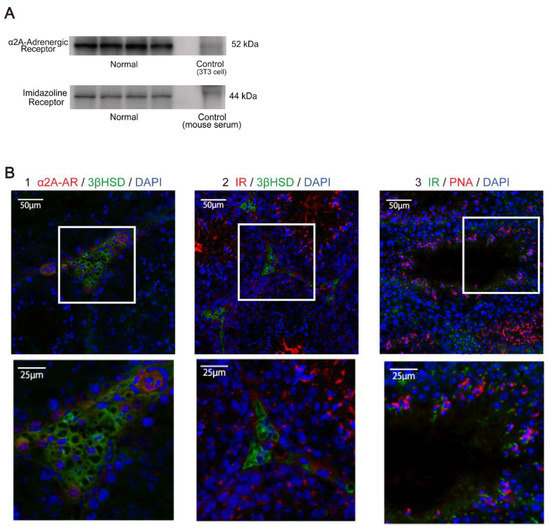
Figure 1.
Localization and expression of α2A-adrenergic and imidazoline receptors in normal mouse. (A) α2A-adrenergic and imidazoline receptors were measured by Western blot in the testis of the normal group. (B) Localization sites of α2A-adrenergic receptor (α2A-AR) and imidazoline receptor (IR) were observed in the testis of the normal group by immunofluorescence analysis. (1) α2A-adrenergic receptors were stained with Alexa594, while 3βHSD (3 beta-hydroxysteroid dehydrogenase) was stained with Alexa488. α2A-adrenergic receptors were expressed around the nucleus of Leydig cells and in vascular endothelial cells. α2A-adrenergic receptor, 3βHSD, and DAPI are shown in red, green, and blue, respectively. (2) IRs were stained with Alexa594, while 3βHSD was stained with Alexa488. IRs were expressed around the nucleus of Leydig cells. IR, 3βHSD, and DAPI are shown in red, green, and blue, respectively. (3) IRs were stained with Alexa488, while PNA was stained with Alexa594. Imidazoline receptors were expressed in sperm cells and the like in fine tubes. Imidazoline receptors, PNA, and DAPI are shown in green, red, and blue, respectively.
3.2. Effects of DEX Administration on Testicular Morphology and Receptors
DEX treatment started from day 0 at 7:00 am to day 14. The sampling points from mice were 9:00 on day 14 (D14-9), 13:00 on day 14 (D14-13), 9:00 on day 15 (D15-9), and 13:00 on day 15 (D15-13). The testis weight/body weight ratio in the 10 µg/kg and 40 µg/kg groups was not significantly different compared to that in the normal and sham group at all sampling points (Figure 2A). Moreover, the testes in all groups showed no histological changes (Figure 2B). The α2A-adrenergic receptor and imidazoline receptor mRNA expression levels were not significantly different from those of the normal group at all sampling points (Figure 2C). Therefore, the receptors on which DEX acts and testicular histology were not affected by DEX administration. Albeit statistically significant, the differences in the imidazoline receptor mRNA expression levels in the following comparisons were rejected because they did not meet our criteria for significance: 10 µg/kg compared to sham on D14-9 (p = 0.018); 10 µg/kg compared to sham (p = 0.002) and 40 µg/kg compared to sham (p = 0.02) at D14-13; and 10 µg/kg compared to normal (p = 0.035) and 40 µg/kg compared to normal (p = 0.03) at D15-13.
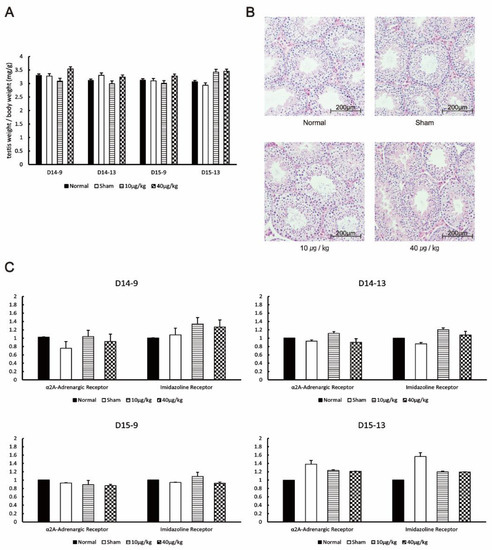
Figure 2.
Effects of DEX administration on testicular morphology and receptors. (A) The testis weight/body weight ratio of the mice was compared at each testis collection point. (B) HE staining of mouse testis collected by D15-13. (C) The expression levels of α2A-adrenergic and imidazoline receptors at each testis collection period were measured by RT-PCR. The values in the graph are presented as means ± SDs. The sampling points after 14 days of continuous DEX administration were 9:00 on day 14 (D14-9), 13:00 on day 14 (D14-13), 9:00 on day 15 (D15-9), and 13:00 on day 15 (D15-13).
3.3. Blood Concentration of DEX in DEX-Administered Mice
On average, DEX was administered at 0.69 nmol/mL/mouse/day in the 10 µg/kg group and 2.77 nmol/mL/mouse/day in the 40 µg/kg group. The average blood concentration at D14-9 (2 h after DEX administration) was 0.036 fmol/mL in the 10 µg/kg group and 0.948 fmol/mL in the 40 µg/kg group (Table 1). At D14-13 (4 h after D14-9), DEX was not detected in the 10 µg/kg group, while it had an average of 0.082 fmol/mL in the 40 µg/kg group. The blood concentration at D15-9 (26 h after 14 days of continuous administration) and D15-13 (30 h after 14 days of continuous administration) was not detected (Table 1). Hence, it was found that DEX was not detected in the blood 6 h after administration in the 10 µg/kg group and 26 h after administration in the 40 µg/kg group.

Table 1.
Blood concentration of DEX in experimental mice.
3.4. Effects of DEX Administration on the Pituitary Gland of Mice
At D14-9, the mRNA expression levels of LH and FSH in the DEX-administered group were significantly lower than those in the normal and sham group as follows: LH, 10 µg/kg compared to normal (p = 0.028), 40 µg/kg compared to normal (p = 0.041), 10 µg/kg compared to sham (p = 0.007), and 40 µg/kg compared to sham (p = 0.01); FSH, 10 µg/kg compared to normal (p = 0.027), 40 µg/kg compared to normal (p = 0.027), 10 µg/kg compared to sham (p = 0.018), and 40 µg/kg compared to sham (p = 0.018). After D14-13, LH and FSH mRNA expression was not significantly different from that in the normal and sham group (Figure 3A). α2A-adrenergic receptor mRNA and imidazoline receptor mRNA were expressed in the pituitary gland. At D14-9, the mRNA expression of α2A-adrenergic receptors was significantly increased in the DEX-administered group as compared with that in the normal and sham group (α2A-adrenergic receptor: 10 µg/kg compared to normal, p = 0.009; 10 µg/kg compared to sham, p = 0.001). However, no changes were observed in imidazoline receptors. After D14-13, there was no significant difference in the mRNA expression levels of α2A-adrenergic receptors and imidazoline receptors (Figure 3B). Hence, it was found that the mRNA expression of LH and FSH decreased, while the mRNA expression of α2A-adrenergic receptors increased 2 h after DEX administration. The expression of LH, FSH, and α2A-adrenergic receptors returned to the same level as in the normal group 6 h after DEX administration. The following factors were statistically significant but were rejected because they did not meet our criteria for significance: LH at D15-13 (10 µg/kg compared to normal, p = 0.03) and imidazoline receptor at D15-13 (10 µg/kg compared to normal, p = 0.003; 40 µg/kg compared to normal, p = 0.02).
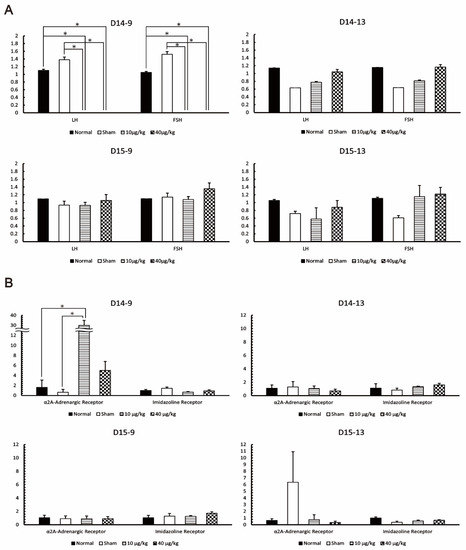
Figure 3.
Effect of DEX administration on mouse pituitary gland. (A) The expression levels of luteinizing hormone (LH) and Follicle-Stimulating Hormone (FSH) at each pituitary gland collection point were measured by RT-PCR. (B) The expression levels of α2A-adrenergic and imidazoline receptors at each pituitary gland collection period were measured by RT-PCR. The values in the graph are presented as means ± SDs. * The difference was significant compared with the other group according to the statistical method of this study. The sampling points after 14 days of continuous DEX administration were 9:00 on day 14 (D14-9), 13:00 on day 14 (D14-13), 9:00 on day 15 (D15-9), and 13:00 on day 15 (D15-13).
3.5. Effects of DEX Administration to Androgens
Testicular cells in the interstitium were examined using the primers of P450scc, 3βHSD, and LHR (markers of Leydig cells). There was no significant difference in the mRNA expression levels of P450scc, 3βHSD, and LHR at all sampling points (Figure 4A). Serum testosterone levels were not significantly different among all groups at D15-13 (Figure 4B). Therefore, DEX administration had no effect on testosterone synthesis pathway enzymes in Leydig cells, and the testosterone concentration was also unaffected.
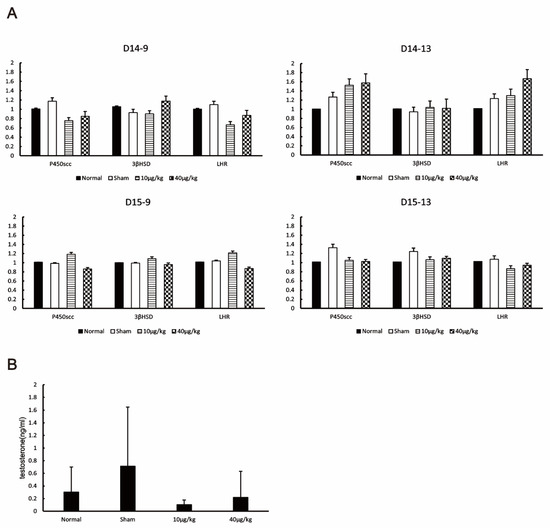
Figure 4.
Effects of DEX administration on androgens. (A) The expression levels of cholesterol side-chain cleavage cytochrome P450 (P450scc), 3 beta-hydroxysteroid dehydrogenase (3βHSD), and luteinizing hormone receptor (LHR) at each testis collection point were measured by RT-PCR. The values are presented as means ± SDs. (B) The testosterone concentration in the blood of mice from each group collected by D15-13 was measured by ECLIA. The values in the graph are presented as means ± SDs. Testosterone concentrations were not significantly different among the four groups based on the Kruskal–Wallis test (p = 0.429). The sampling points after 14 days of continuous DEX administration from mice were 9:00 on day 14 (D14-9), 13:00 on day 14 (D14-13), 9:00 on day 15 (D15-9), and 13:00 on day 15 (D15-13).
3.6. Effects of DEX Administration on Germ Cells and Sertoli Cells
Testicular cells in the seminiferous tubules were examined with the primers of Ki67 (markers for Mitosis), Top2 (markers for Meiosis), Sycp3, Mlh1 (markers for pachytene spermatocyte), Acr, Tnp1 (markers for round spermatid), InhA, and SHBG (markers for Sertoli cells). There was no significant difference in all markers at all sampling points compared to the normal group and sham group (Figure 5). Therefore, DEX administration did not affect the spermatogenic process. The following factors were statistically significant but were rejected because they did not meet our criteria for significance: Ki67 at D14-9 (40 µg/kg compared to normal, p = 0.003), Top2a at D14-13 (10 µg/kg compared to normal, p = 0.033; 40 µg/kg compared to normal, p = 0.016), Sycp3 at D15-9 (10 µg/kg compared to normal, p = 0.026), Sycp3 at D15-13 (10 µg/kg compared to sham, p = 0.004), Mlh1 at D14-13 (10 µg/kg compared to normal, p = 0.042), Mlh1 at D14-13 (10 µg/kg compared to sham, p = 0.006), Mlh1 at D15-13 (10 µg/kg compared to sham, p = 0.003; 40 µg/kg compared to sham, p = 0.001), Acr at D14-9 (10 µg/kg compared to normal, p = 0.002; 10 µg/kg compared to sham, p = 0.005), Acr at D15-13 (10 µg/kg compared to normal, p = 0.005; 40 µg/kg compared to normal, p = 0.001), Tnp1 at D14-9 (10 µg/kg compared to normal, p = 0.04; 40 µg/kg compared to normal, p = 0.029), Tnp1 at D14-13 (40 µg/kg compared to normal, p = 0.034), inhA at D14-9 (10 µg/kg compared to normal, p = 0.003; 40 µg/kg compared to normal, p = 0.008; 10 µg/kg compared to sham, p = 0.004; 40 µg/kg compared to sham, p = 0.012), inhA at D14-13 (40 µg/kg compared to normal, p = 0.04), SHBG at D15-9 (40 µg/kg compared to normal, p = 0.013; 40 µg/kg compared to sham, p = 0.002), and FSHR at D15-13 (40 µg/kg compared to sham, p = 0.019).
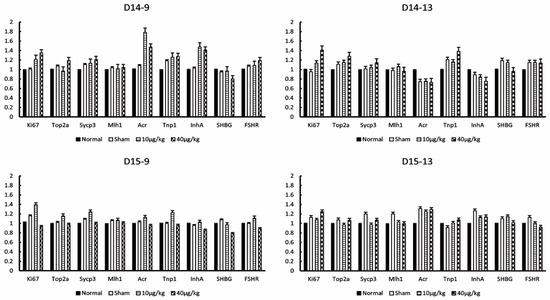
Figure 5.
Effects of DEX administration on germ cells and Sertoli cells. The expression levels of Ki67, TOP2a (topoisomerase 2-alpha), Sycp3 (synaptonemal complex protein 3), Mlh1 (MutL homolog 1), Acr (acrosin), Tnp1 (transition protein 1), InhA (Inhibin-A), SHBG (sex-hormone-binding globulin), and FSHR (follicle-stimulating hormone receptor) at each testis collection point were measured by RT-PCR. The values of graph are presented as the means ± SDs. The sampling points after 14 days continuous DEX administration from mice were 9:00 on day 14 (D14-9), 13:00 on day 14 (D14-13), 9:00 on day 15 (D15-9), and 13:00 on day 15 (D15-13).
3.7. Effects of DEX Administration on Oxidant Stress Factors in the Mouse Testes
There was no significant difference in the expression of antioxidant enzymes CAT, GPx, SOD2, apoptotic factor Caspase3, and oxidizing substances ABCD1, ABCD3, GRP78, and OGG1 at all sampling points (Figure 6). Therefore, DEX administration had no effect on apoptosis or oxidative stress in the testis. The following factors were statistically significant but were rejected because they did not meet our criteria for significance: CAT at D15-13 (40 µg/kg compared to sham, p = 0.003), GPx at D14-13 (10 µg/kg compared to normal, p = 0.016; 40 µg/kg compared to normal, p = 0.001; 10 µg/kg compared to sham, p = 0.027; 40 µg/kg compared to sham, p = 0.002), GPx at D15-9 (10 µg/kg compared to normal, p = 0.036; 40 µg/kg compared to normal, p = 0.001; 40 µg/kg compared to sham, p = 0.026), SOD2 at D14-9 (10 µg/kg compared to normal, p = 0.001; 40 µg/kg compared to normal, p = 0.001; 10 µg/kg compared to sham, p = 0.001; 40 µg/kg compared to sham, p = 0.001), SOD2 at D15-9 (10 µg/kg compared to sham, p = 0.008), SOD2 at D15-13 (40 µg/kg compared to normal, p = 0.01), Caspase3 D14-9 (10 µg/kg compared to normal, p = 0.029; 10 µg/kg compared to Sham, p = 0.014), Caspase3 at D14-13 (10 µg/kg compared to normal, p = 0.028; 40 µg/kg compared to normal, p = 0.01; 40 µg/kg compared to sham, p = 0.021), ABCD1 at D15-13 (10 µg/kg compared to normal, p = 0.007; 40 µg/kg compared to normal, p = 0.002), ABCD3 at D15-9 (40 µg/kg compared to normal, p = 0.017; 40 µg/kg compared to sham, p = 0.017), GRP78 at D14-9 (40 µg/kg compared to normal, p = 0.044), OGG1 at D14-9 (10 µg/kg compared to normal, p = 0.002), OGG1 at D15-13 (10 µg/kg compared to normal, p = 0.037; 40 µg/kg compared to sham, p = 0.001).
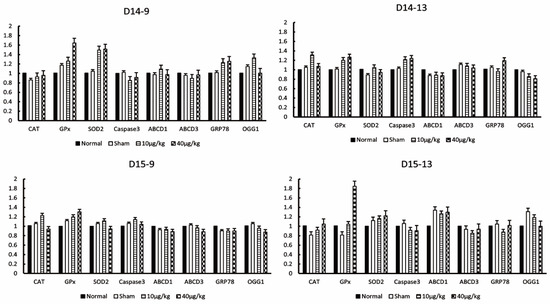
Figure 6.
Effects of DEX administration on oxidant stress factors in the mouse testes. The expression levels of catalase (CAT), glutathione peroxidase (GPx), superoxide dismutase 2 (SOD2), caspase3, adrenoleukodystrophy protein from subfamily D, member 1 (ABCD1), ABCD, member 3 (ABCD3), glucose-regulated protein, 78kDa (GRP78), and 8-oxoguanine DNA glycosylase 1 (OGG1) at each testis collection point were measured by RT-PCR. The values in the graph are presented as means ± SDs. The sampling points after 14 days of continuous DEX administration were 9:00 on day 14 (D14-9), 13:00 on day 14 (D14-13), 9:00 on day 15 (D15-9), and 13:00 on day 15 (D15-13).
4. Discussion
To investigate the reproductive toxicity of DEX, 10 µg/kg and 40 µg/kg of DEX alone were administered intraperitoneally to normal mice daily for two weeks. No changes in mRNA expression were observed in cells within the interstitium or seminiferous tubules of the testis. In the pituitary gland, the expression of LH and FSH mRNAs was significantly decreased 2 h after DEX administration, and the expression of α2A-adrenergic receptor mRNA was significantly increased, but it rapidly recovered thereafter. Furthermore, in the testis, the site of action of DEX was localized to the cytoplasm of Leydig cells and vascular endothelial cells for adrenergic receptors and to the cytoplasm of Leydig cells and germ cells for IRs.
In general, α2A-adrenergic receptors are present in the cell membrane, and the sympathetic neurotransmitter noradrenaline responds to the receptors by inhibiting the release of noradrenaline and exerting sedation, hypnosis, and sympatholytic effects [15,16,17,18,19,20]. α2A adrenergic receptors are localized in pancreatic β-cells, platelets, and vascular smooth muscle cells and induces various physiological responses, such as insulin release suppression, platelet aggregation activation, and vasoconstriction [15]. Brum et al. reported that α2A-adrenergic receptors are localized in the cell membranes of nerve cell bodies, axons, and dendrites in the superior cervical ganglion of mice based on immunofluorescent staining [17]. They also reported that pretreatment with DEX suppresses the increase in cytosolic calcium flux after potassium administration [17]. Nasser et al. reported that α2A-adrenergic receptors are localized in the cytoplasm of nerve fibers and neuronal cell bodies in guinea pig neurons and in the cytoplasm of glial cells and neuronal cell bodies in mice and rats [18]. In Madin–Darby canine kidney cells (a cell line derived from canine renal tubular epithelial cells), α2A adrenergic receptors were reported to be localized in the cell membrane [21]. It was reported that α2A-adrenergic receptors are localized in ciliary smooth muscle cells and in the cytoplasm [22]. Thus, α2A-adrenergic receptors are localized in the cell membrane and cytoplasm.
IRs were reported to be present on cell membranes [23] and are involved in the regulation of several functions, such as blood pressure and heart rate reduction, sodium excretion from the kidneys, and catecholamine release from adrenal chromaffin cells [24,25,26]. In addition, IR mRNA expression was also reported in rat tissues, such as the brain, liver, testis, and skeletal muscle [27]. IRs are expressed in the brainstem of rats, and are localized in the facial nucleus, inferior olive (dorsal nucleus), lateral reticular nucleus, axons, dendrites, and astrocytes based on immunohistochemical staining, which appears to be stained around the nucleus [28]. In a report investigating the localization of IRs in HEK293 cells transplanted with pEGFPC1-IRAS plasmid, they were found to be punctate in the cytoplasm [29]. Therefore, imidazoline receptors are localized both in the plasma membrane and cytoplasm.
In the testis, it is present in the tunica albuginea of rats, and noradrenaline reacts with α2A-adrenergic receptors to cause contraction of the tunica albuginea of the testis. It was reported to move the sperm from the seminiferous tubules to the epididymis [30]. DEX administration to Leydig cells isolated from rats inhibited testosterone synthase activities, such as 3βHSD [9]. α2A-adrenergic receptors and imidazoline receptors are present in the cytoplasm of testicular cells and are thought to be functional. DEX is a stable formulation of dexmedetomidine hydrochloride in saline between pH 4.5 and 7.0. Dexmedetomidine hydrochloride changes to fat-soluble DEX depending on blood pH [31]. Therefore, it is thought that it also reacts with receptors present in the cytoplasm. This study is the first to examine the localization of α2A-adrenergic receptors and imidazoline receptors in the testis.
In this study, a decrease in pituitary LH and FSH mRNA expression after DEX administration was observed. Gonadotropin-releasing hormone (GnRH), which is secreted from the medial preoptic area of the hypothalamus and arcuate nucleus, stimulated the secretion of LH and FSH from the pituitary gland.
Hunger stress increased the release of renal corticotropin-releasing hormone (CRH) produced in the paraventricular nucleus via α2-adrenergic receptors of the hypothalamus and suppressed GnRH [32].
The administration of noradrenaline to the paraventricular nucleus of normal rats reduced LH [33]. Furthermore, the administration of α2-adrenergic receptor agonists inhibited GnRH and LH in fasting stress-induced LH suppression experiments [34]. These findings suggest that noradrenergic neurons entering the paraventricular nucleus suppress GnRH and LH via CRH [32]. It was reported that the administration of clonidine, an α2A-adrenergic receptor agonist similar to DEX, to the paraventricular nucleus of the hypothalamus in rats significantly decreased plasma LH concentrations [33,35,36]. Therefore, it is thought that LH and FSH mRNA expression also decreased because GnRH was suppressed by noradrenaline and CRH stimulation. GnRH and CRH should also be investigated in the future.
The number of α2-adrenergic receptors is known to be inversely related to noradrenaline levels, as adrenergic receptors are downregulated throughout the brain in the presence of high agonist concentrations [37,38]. However, in adrenalectomized rats, α2-adrenergic receptors are decreased in the paraventricular nucleus but increased in the supraoptic nucleus [39]. The short-term and long-term administration of cortisol to tree shrews also decreased the hypothalamic α2-adrenergic receptor expression in the short term and increased it in the long term [40]. Thus, α2-adrenergic receptor expression is regulated by adrenal hormones and noradrenaline. Since the expression of α2-adrenergic receptors differs depending on each part of the brain, the treatment period, and the treatment concentration, it was found that in the case of the pituitary gland in this study, administration of 10 μg/kg DEX increased the mRNA expression of α2-adrenergic receptors. The absence of changes in α2-adrenergic receptor mRNA following the administration of 40 μg/kg DEX compared to that of 10 μg/kg DEX may be due to the presence of high concentrations of the agonist [37,38]. The half-life of DEX in the blood is only 2.36–2.91 h. In mice, the blood concentration of DEX decreased to about 0.1 fmol/mL in 6 h in the 40 μ group. Since DEX has such a short half-life, the expression of α2-adrenergic receptor was transiently increased, while the expression of LH and FSH was decreased, but it recovered quickly.
In a study of Leydig cells (0.05 × 10⁶ cells/well) isolated from rats, DEX administration (approximately 3.6–360 µg/L) inhibited testosterone synthase activity in a dose-dependent manner [9]. It also increased cells undergoing apoptosis [9]. However, in this study, administration of DEX (40 μg/kg) to normal mice had no effect on blood testosterone levels. It also had no effect on the expression of P450scc, 3βHSD, LHR, and Caspase3 mRNAs. There is a controversy about the effects of testosterone levels after glyphosate administration. The administration of glyphosate to cultured TM3 cells (0.1 mM) and rat primary Leydig cells (1 ppm) decreased the testosterone levels [41,42]. The administration of glyphosate (2.5–500 mg/kg) to rats did not alter the testosterone levels [43,44]. Dai et al. also administered 500 mg/kg of glyphosate to rats, but no significant difference was found; however, there were reduced testosterone levels by more than half [44]. Thus, in vitro and in vivo drug administration have a large difference in effective concentration. Therefore, increasing the dose of DEX in this study may also affect the testosterone levels.
In the ischemia–reperfusion model, ROS overproduction after ischemia–reperfusion causes cytokine release from macrophages and neutrophil infiltration, etc.; this ultimately causes cell damage because of apoptosis and inflammation [3]. It was reported that the administration of DEX to ischemia–reperfusion models of various organs has antiapoptotic, anti-inflammatory, and antioxidant effects, and it improves the pathology [3,4,5,45,46,47,48]. DEX is believed to have organ-protective effects. However, when administered to a rat testicular torsion model, TOS increased at 25 μg/kg administration, TAS increased at 50 μg/kg administration, and OSI decreased. The testicular pathology did not improve [6,7]. In myocardial and spinal cord ischemia–reperfusion, the administration of DEX at 20 µg/kg or 5 µg/kg/30 min was reported to have organ-protective effects, including antiapoptotic and anti-inflammatory effects [3,5]. In this study, when DEX was administered to normal mice at 10 and 40 μg/kg, no changes were observed in the expression of intracellular mRNAs in the testicular stroma (P450scc/3βHSD/LHR) and seminiferous tubules (Ki67, Top2, Sycp3, Mlh1, Acr, Tnp1, InhA, and SHBG). Furthermore, there were no changes in antioxidant enzymes CAT, GPx, and SOD2 and oxidative factors ABCD1, ABCD3, GRP78, and OGG1. Caspase3, an apoptotic factor, also showed no changes. Therefore, DEX does not appear to have a male reproductive toxicity in mice. However, differences in chemical sensitivity between mice and rats must be considered [49].
5. Conclusions
In this study, α2A-adrenergic receptors, which are the site of action of DEX, are localized in the cytoplasm of Leydig cells, while imidazoline receptors are localized in the cytoplasm and germ cells of Leydig cells, and these may have pharmacological effects. In addition, LH, FSH, and α2A-adrenergic receptor mRNA expression were changed by various hormone actions caused by DEX administration. It was considered that a low blood concentration of DEX caused rapid recovery. Furthermore, the administration of DEX to mice did not adversely affect various factors in the testis, including the testosterone levels. There was a large difference in the effective concentration between in vitro and in vivo drug administration. The reproductive toxicity of DEX was expected; a clear effect could not be confirmed. In this study, increasing the DEX dose further may cause testicular dysfunction, but clinically using more than this dose is unlikely. Therefore, the DEX concentration used in this study is unlikely to exhibit male reproductive toxicity.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/app122010409/s1, Figure S1: Experimental schedule of DEX administration to mice. DEX treatment started at 7:00 am of Day 0 to Day 14. The collection time of the testes, blood, and pituitary glands from the mice were 9:00 on Day 14 (D14-9), 13:00 on Day 14 (D14-13), 9:00 on Day 15 (D15-9), and 13:00 on Day 15 (D15-13). Figure S2: Calibration curve used in liquid chromatography–mass spectrometry. Table S1: Nucleotide sequences of primers used in real-time PCR.
Author Contributions
Conceptualization, H.N., H.T., K.S. (Kou Sakabe) and T.S.; formal analysis and writing—original draft preparation, review, and editing, H.N., H.T., N.Q., D.K., S.T., S.H., K.S. (Kou Sakabe) and T.S.; data curation and investigation, H.N., H.T., D.K. and K.S. (Kosuke Shirose); funding acquisition, and supervision, K.S. (Kou Sakabe) and T.S.; project administration, H.N., H.T., K.S. (Kou Sakabe) and T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Tokai University on Animal Research (Approval No. 201084).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are openly available on request from the corresponding author.
Acknowledgments
The authors would like to thank Kaori Suyama, Yuko Furuya, and Mika Izumitani (Tokai University School of Medicine, Kanagawa, Japan) for excellent advisory and secretarial support. We are grateful to Masatoshi Ito, Ayumi Sasaki, Shunji Amano, Ting Wang, Hideyuki Matsuzawa, Yuka Kitamura, Yoshinori Okada, and Yoko Kameyama (Medical Science College Office, Tokai University) for excellent technical support.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results. The Department of Environmental Preventive Medicine (Yamada Bee Company, Inc.) is an endowment department supported by a grant from the Yamada Bee Company, Inc.
References
- Afonso, J.; Reis, F. Dexmedetomidine: Current Role in Anesthesia and Intensive Care. Rev. Bras. Anestesiol. 2012, 62, 118–133. [Google Scholar] [CrossRef]
- Bao, N.; Tang, B. Organ-Protective Effects and the Underlying Mechanism of Dexmedetomidine. Mediat. Inflamm. 2020, 2020, 6136105. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jiang, C.; Jiang, J.; Qiu, L. Dexmedetomidine protects mice against myocardium ischaemic/reperfusion injury by activating an AMPK/PI3K/Akt/eNOS pathway. Clin. Exp. Pharmacol. Physiol. 2017, 44, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Kim, T.Y.; Kim, S.Y.; Ro, S.J.; Koh, S.R.; Ryu, S.; Ko, J.S.; Jeong, M.A. The Protective Effects of Dexmedetomidine Preconditioning on Hepatic Ischemia/Reperfusion Injury in Rats. Transplant. Proc. 2021, 53, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhao, T.; Lv, S.; Gao, Y.; Masters, J.; Weng, H. Dexmedetomidine attenuates spinal cord ischemia–reperfusion injury through both anti-inflammation and anti-apoptosis mechanisms in rabbits. J. Transl. Med. 2018, 16, 209. [Google Scholar] [CrossRef] [PubMed]
- Tuglu, D.; Yuvanc, E.; Ozan, T.; Bal, F.; Yilmaz, E.; Atasoy, P.; Kisa, U.; Batislam, E. Protective effects of udenafil citrate, piracetam and dexmedetomidine treatment on testicular torsion/detorsion-induced ischaemia/reperfusion injury in rats. Andrologia 2016, 48, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Tuglu, D.; Yuvanc, E.; Yılmaz, E.; Gencay, I.Y.; Atasoy, P.; Kisa, U.; Batislam, E. The antioxidant effect of dexmedetomidine on testicular ischemia-reperfusion injury. Acta Cir. Bras. 2015, 30, 414–421. [Google Scholar] [CrossRef]
- Xiao, J.; Wan, W.; Zhang, Y.; Ma, J.; Yan, L.; Luo, Y.; Tang, J. Administration of Dexmedetomidine Does Not Produce Long-Term Protective Effect on Testicular Damage Post Testicular Ischemia-Reperfusion Injury. Drug Des. Dev. Ther. 2021, 15, 315–321. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Ni, C.; Fang, Y.; Wu, K.; Zheng, W.; Li, X.; Lin, H.; Fan, L.; Ge, R.-S. Effects of dexmedetomidine on the steroidogenesis of rat immature Leydig cells. Steroids 2019, 149, 108423. [Google Scholar] [CrossRef]
- Terayama, H.; Hirai, S.; Naito, M.; Qu, N.; Katagiri, C.; Nagahori, K.; Hayashi, S.; Sasaki, H.; Moriya, S.; Hiramoto, M.; et al. Specific autoantigens identified by sera obtained from mice that are immunized with testicular germ cells alone. Sci. Rep. 2016, 6, 35599. [Google Scholar] [CrossRef]
- Terayama, H.; Itoh, M.; Naito, M.; Hirai, S.; Qu, N.; Kuerban, M.; Musha, M. Experimental model of autoimmune orchitis with abdominal placement of donor’s testes, epididymides, and vasa deferentia in recipient mice. J. Reprod. Immunol. 2011, 90, 195–201. [Google Scholar] [CrossRef]
- Naito, M.; Terayama, H.; Hirai, S.; Qu, N.; Lustig, L.; Itoh, M. Experimental autoimmune orchitis as a model of immunological male infertility. Med. Mol. Morphol. 2012, 45, 185–189. [Google Scholar] [CrossRef]
- Wang, Q.; Dong, J.; Lu, W.; He, H.; Sun, X.; Zhang, K.; Song, Q.; Jiang, Y.; Wang, Y.; Li, C.; et al. Phenylethanol glycosides from Cistanche tubulosa improved reproductive dysfunction by regulating testicular steroids through CYP450-3β-HSD pathway. J. Ethnopharmacol. 2020, 251, 112500. [Google Scholar] [CrossRef]
- Wakayama, T.; Nakata, H.; Kumchantuek, T.; Gewaily, M.; Iseki, S. Identification of 5-bromo-2′-deoxyuridine-labeled cells during mouse spermatogenesis by heat-induced antigen retrieval in lectin staining and immunohistochemistry. J. Histochem. Cytochem. 2015, 63, 190–205. [Google Scholar] [CrossRef]
- MacMillan, L.B.; Lakhlani, P.; Lovinger, D.; Limbird, L.E. Alpha 2-adrenergic receptor subtypes: Subtle mutation of the alpha 2A-adrenergic receptor in vivo by gene targeting strategies reveals the role of this subtype in multiple physiological settings. Recent Prog. Horm. Res. 1998, 53, 25–42. [Google Scholar]
- Sun, Y.; Wang, W.; Zhou, C.; Ma, X.; Bai, W.; Zhang, J.; Yang, Q.; Wang, K.; Jia, J.; Liu, G.; et al. Changes in TRPV1 expression in the POA of ovariectomized rats regulated by NE-dependent α2-ADR may be involved in hot flashes. Ann. Anat. 2020, 232, 151565. [Google Scholar] [CrossRef]
- Brum, P.C.; Hurt, C.M.; Shcherbakova, O.G.; Kobilka, B.; Angelotti, T. Differential targeting and function of α2A and α2C adrenergic receptor subtypes in cultured sympathetic neurons. Neuropharmacology 2006, 51, 397–413. [Google Scholar] [CrossRef]
- Nasser, Y.; Ho, W.; Sharkey, K.A. Distribution of adrenergic receptors in the enteric nervous system of the guinea pig, mouse, and rat. J. Comp. Neurol. 2006, 495, 529–553. [Google Scholar] [CrossRef]
- Kable, J.W.; Murrin, L.C.; Bylund, D.B. In vivo gene modification elucidates subtype-specific functions of alpha(2)-adrenergic receptors. J. Pharmacol. Exp. Ther. 2000, 293, 1–7. [Google Scholar]
- Cai, J.; Li, J.; Mao, Y.; Bai, X.; Xu, L.; Wang, H. Immunohistochemical Localization of α2-Adrenergic Receptors in the Neonatal Rat Cochlea and the Vestibular Labyrinth. J. Mol. Neurosci. 2013, 51, 1010–1020. [Google Scholar] [CrossRef]
- Keefer, J.; Limbird, L. The alpha 2A-adrenergic receptor is targeted directly to the basolateral membrane domain of Madin-Darby canine kidney cells independent of coupling to pertussis toxin-sensitive GTP-binding proteins. J. Biol. Chem. 1993, 268, 11340–11347. [Google Scholar] [CrossRef]
- Ooi, Y.H.; Oh, D.-J.; Rhee, D.J. Analysis of α2-adrenergic Receptors and Effect of Brimonidine on Matrix Metalloproteinases and Their Inhibitors in Human Ciliary Body. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4237–4243. [Google Scholar] [CrossRef] [PubMed]
- Ernsberger, P. The I1-imidazoline receptor and its cellular signaling pathways. Ann. N. Y. Acad. Sci. 1999, 881, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Van Zwieten, P.A. Central imidazoline (I1) receptors as targets of centrally acting antihypertensives: Moxonidine and Rilmenidine. J. Hypertens. 1997, 15, 117–125. [Google Scholar] [CrossRef]
- Maltsev, A.V.; Evdokimovskii, E.V.; Kokoz, Y.M. Disturbance of I1-imidazoline receptor signal transduction in cardiomyocytes of Spontaneously Hypertensive Rats. Arch. Biochem. Biophys. 2019, 671, 62–68. [Google Scholar] [CrossRef]
- Bousquet, P.; Hudson, A.; García-Sevilla, J.A.; Li, J.-X. Imidazoline Receptor System: The Past, the Present, and the Future. Pharmacol. Rev. 2020, 72, 50–79. [Google Scholar] [CrossRef]
- Piletz, J.E.; Jones, J.C.; Zhu, H.; Bishara, O.; Ernsberger, P. Imidazoline receptor antisera-selected cDNA clone and mRNA distribution. Ann. N. Y. Acad. Sci. 1999, 881, 1–7. [Google Scholar] [CrossRef]
- Nagakura, Y.; Ide, R.; Saiki, C.; Hashizume, N.S.; Imai, T. Expression of nischarin, an imidazoline 1 receptor candidate protein, in the ventrolateral medulla of newborn rats. Neurosci. Lett. 2021, 761, 136113. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Y.; Shan, Y.; Yao, Z.; Liu, X.; Su, R.; Sun, Q.; Cong, Y.; Li, J. Generation and Characterization of Novel Human IRAS Monoclonal Antibodies. J. Biomed. Biotechnol. 2009, 2009, 973754. [Google Scholar] [CrossRef]
- Da Silva Júnior, E.D.; de Souza, B.P.; Rodrigues, J.Q.D.; Caricati-Neto, A.; Jurkiewicz, A.; Jurkiewicz, N.H. Effects of clonidine in the isolated rat testicular capsule. Eur. J. Pharmacol. 2014, 726, 16–26. [Google Scholar] [CrossRef]
- Pharmaceutical Interview Form. Dexmedetomidine. Available online: https://www.pfizermedicalinformation.jp/ja-jp/system/files/content_files/pcd02if.pdf?pmidf (accessed on 7 October 2022).
- Tsukamura, H. Neuroendocrine Mechanisms Regulating Pulsatile Luteinizing Hormone Secretion. J. Reprod. Dev. 1995, 41, j103–j111. [Google Scholar] [CrossRef]
- Tsukamura, H.; Nagatani, S.; Cagampang, F.R.; Kawakami, S.; Maeda, K. Corticotropin-releasing hormone mediates suppression of pulsatile luteinizing hormone secretion induced by activation of alpha-adrenergic receptors in the paraventricular nucleus in female rats. Endocrinology 1994, 134, 1460–1466. [Google Scholar] [CrossRef]
- Cagampang, F.R.; Ohkura, S.; Tsukamura, H.; Coen, C.W.; Ota, K.; Maeda, K. Alpha 2-adrenergic receptors are involved in the suppression of luteinizing hormone release during acute fasting in the ovariectomized estradiol-primed rats. Neuroendocrinology 1992, 56, 724–728. [Google Scholar] [CrossRef]
- Briski, K.P.; Shakya, M. Mu Opioid Receptor Regulation of Gonadotropin-Releasing Hormone-Luteinizing Hormone Axis during Short-Term Food Deprivation: Role of Alpha1-Adrenoreceptor Signaling. Neuro Endocrinol. Lett. 2018, 39, 363–370. [Google Scholar]
- Brann, D.W.; Mahesh, V.B. Detailed Examination of the Mechanism and Site of Action of Progesterone and Corticosteroids in the Regulation of Gonadotropin Secretion: Hypothalamic Gonadotropin-Releasing Hormone and Catecholamine Involvement. Biol. Reprod. 1991, 44, 1005–1015. [Google Scholar] [CrossRef][Green Version]
- Collins, S.; Caron, M.G.; Lefkowitz, R.J. Regulation of adrenergic receptor responsiveness through modulation of receptor gene expression. Annu. Rev. Physiol. 1991, 53, 497–508. [Google Scholar] [CrossRef]
- Flügge, G.; van Kampen, M.; Meyer, H.; Fuchs, E. Alpha2A and alpha2C-adrenoceptor regulation in the brain: Alpha2A changes persist after chronic stress. Eur. J. Neurosci. 2003, 17, 917–928. [Google Scholar] [CrossRef]
- Jhanwar-Uniyal, M.; Leibowitz, S.F. Impact of circulating corticosterone on alpha1- and alpha2-noradrenergic receptors in discrete brain areas. Brain Res. 1986, 368, 404–408. [Google Scholar] [CrossRef]
- Flügge, G. Effects of cortisol on brain alpha2-adrenoceptors: Potential role in stress. Neurosci. Biobehav. Rev. 1999, 23, 949–956. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, J.; Yang, L.; Zhang, H.; Zhang, Y.; Gao, D.; Jiang, H.; Li, Y.; Dong, H.; Ma, T.; et al. Glyphosate exposure attenuates testosterone synthesis via NR1D1 inhibition of StAR expression in mouse Leydig cells. Sci. Total Environ. 2021, 785, 147323. [Google Scholar] [CrossRef]
- Clair, E.; Mesnage, R.; Travert, C.; Séralini, G.É. A glyphosate-based herbicide induces necrosis and apoptosis in mature rat testicular cells in vitro, and testosterone decrease at lower levels. Toxicol. In Vitro 2012, 26, 269–279. [Google Scholar] [CrossRef]
- Johansson, H.K.L.; Schwartz, C.L.; Nielsen, L.N.; Boberg, J.; Vinggaard, A.M.; Bahl, M.I.; Svingen, T. Exposure to a glyphosate-based herbicide formulation, but not glyphosate alone, has only minor effects on adult rat testis. Reprod. Toxicol. 2018, 82, 25–31. [Google Scholar] [CrossRef]
- Dai, P.; Hu, P.; Tang, J.; Li, Y.; Li, C. Effect of glyphosate on reproductive organs in male rat. Acta Histochem. 2016, 118, 519–526. [Google Scholar] [CrossRef]
- Gu, J.; Chen, J.; Xia, P.; Tao, G.; Zhao, H.; Ma, D. Dexmedetomidine attenuates remote lung injury induced by renal ischemia-reperfusion in mice. Acta Anaesthesiol. Scand. 2011, 55, 1272–1278. [Google Scholar] [CrossRef]
- Qiao, H.; Sanders, R.D.; Ma, D.; Wu, X.; Maze, M. Sedation improves early outcome in severely septic Sprague Dawley rats. Crit. Care 2009, 13, R136. [Google Scholar] [CrossRef]
- Taniguchi, T.; Kidani, Y.; Kanakura, H.; Takemoto, Y.; Yamamoto, K. Effects of dexmedetomidine on mortality rate and inflammatory responses to endotoxin-induced shock in rats. Crit. Care Med. 2004, 32, 1322–1326. [Google Scholar] [CrossRef]
- Rajakumaraswamy, N.; Ma, D.; Hossain, M.; Sanders, R.D.; Franks, N.; Maze, M. Neuroprotective interaction produced by xenon and dexmedetomidine on in vitro and in vivo neuronal injury models. Neurosci. Lett. 2006, 409, 128–133. [Google Scholar] [CrossRef]
- Terayama, H.; Qu, N.; Endo, H.; Ito, M.; Tsukamoto, H.; Umemoto, K.; Kawakami, S.; Fujino, Y.; Tatemichi, M.; Sakabe, K. Effect of acetamiprid on the immature murine testes. Int. J. Environ. Health Res. 2018, 28, 683–696. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).