DLP Light-Curing 3D Printing Combustible Lighting Shell and Performance Study
Abstract
1. Introduction
2. Experimental Section
2.1. Reagents and Materials
2.2. Preparation of Combustible Lighting Shell
- (1)
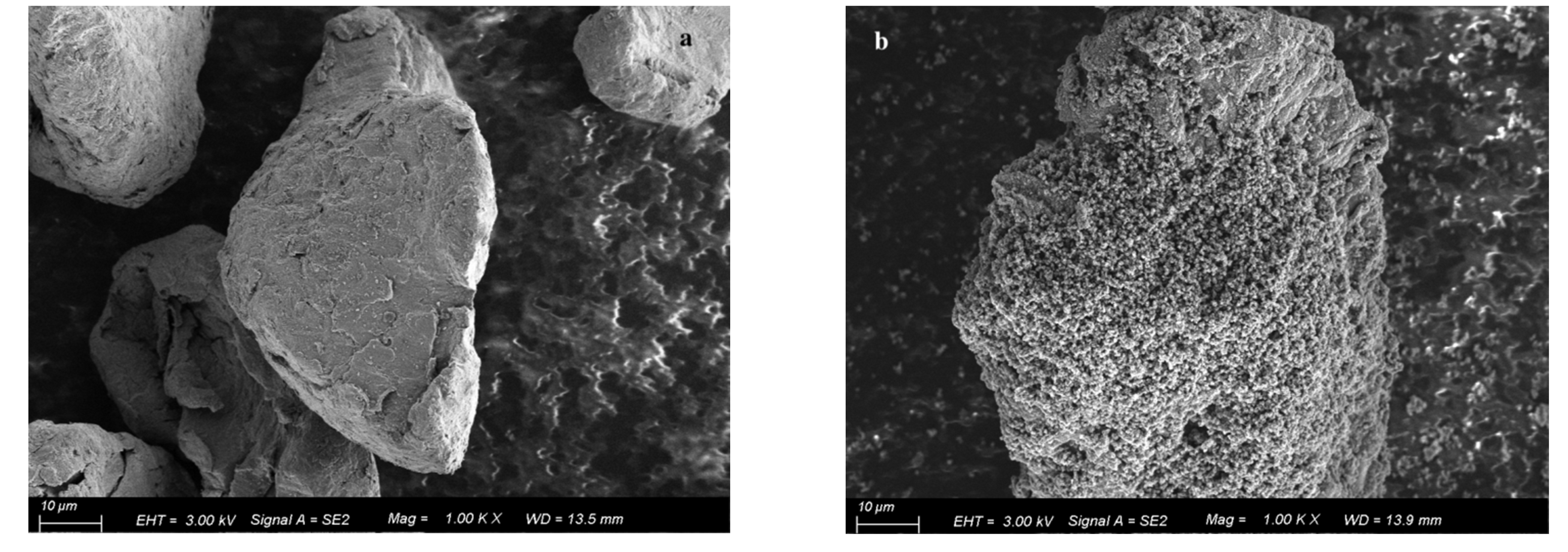
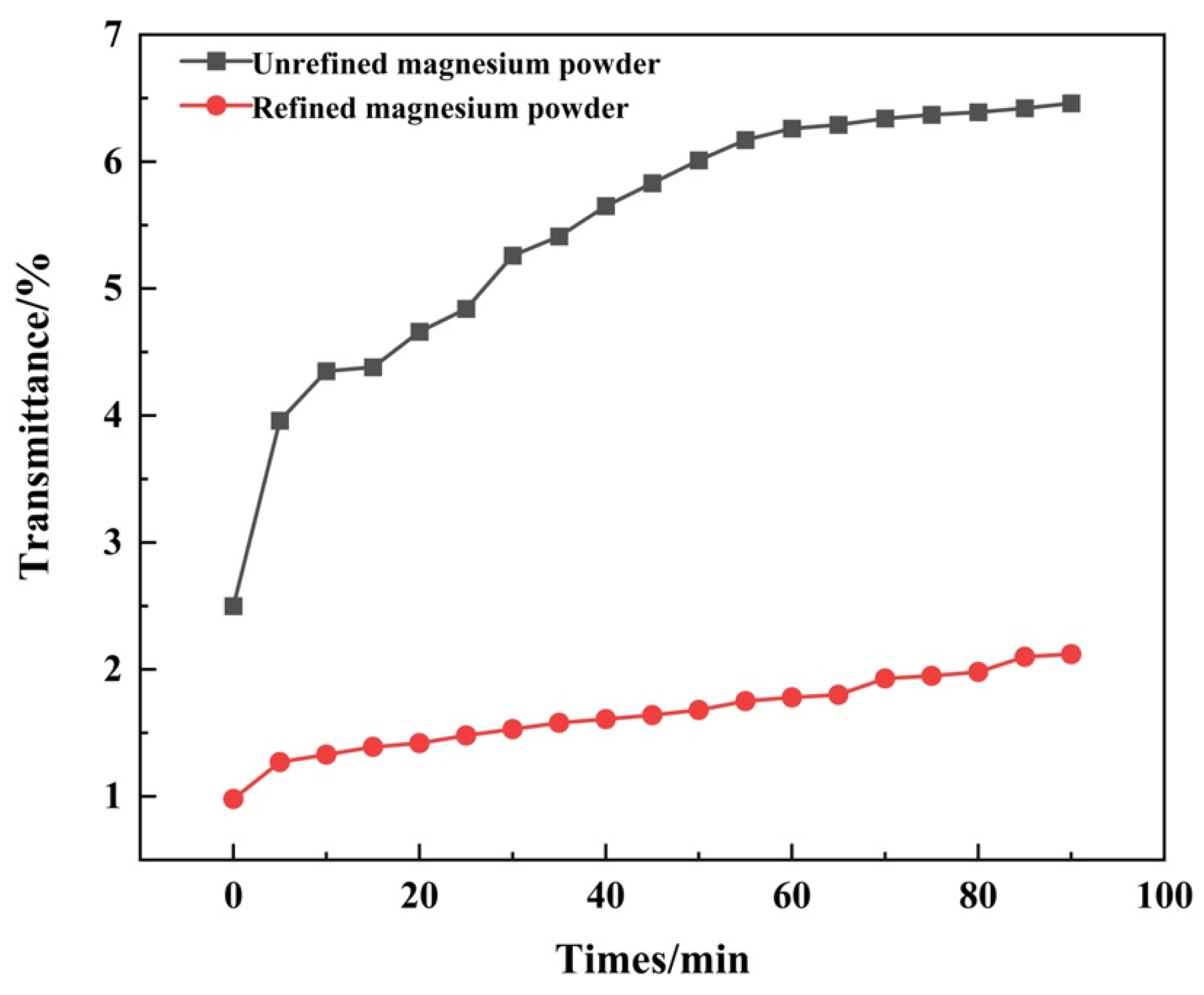
- Solid filler pretreatment: sodium nitrate and magnesium powder were used as the solid-phase filler. Magnesium powder was acid-etched and passed through a 500-mesh sieve, and sodium nitrate was ground and refined through a 500-mesh sieve.
- (2)
- Preparation of resin slurry: resin slurry includes prepolymer EA, PUA; dilution monomer HDDA, HPA; photoinitiator TPO; additives (leveling agent, defoamer, adhesion promoter). The formula (mass fraction) is 11.42% EA, 45.74% PUA, 30.48% HDDA, 7.62% HPA, 3.81% TPO, 0.96% additives. The slurry configuration process is shown in Figure 1. The prepared resin slurry was printed into a pillar for testing of the flame color and combustion spectrum of the burning resin.
- (3)
- Combustible lighting shell printing: The combustible lighting shell is a resin slurry combined with a solid filler and then printed using the DP-002 3D printer of Creative 3D Technology Co., Ltd. (Shanghai, China). The solid filler was added to the resin slurry and stirred evenly for 1 h using a magnetic stirrer, and the stirred slurry was placed in the 3D printer tank; the model was printed for the hollow cylindrical tube shell. The size was ∅ 17 mm × 15 mm, the wall thickness was 3 mm, and the bottom thickness was 3.4 mm. Print parameters were set for a first layer exposure time of 45 s, a first layer exposure layer of 10 layers, brightness 80%, and the rest of the layers had exposure time of 20 s. The finished print is shown in Figure 2, with a mass of 4.805 g and a density of 1.912.15 g/cm3. At the same time, we prepared a traditional low-carbon steel illuminating candle shell as a structural comparison, as shown in Figure 2c.
2.3. Preparation of Illuminant
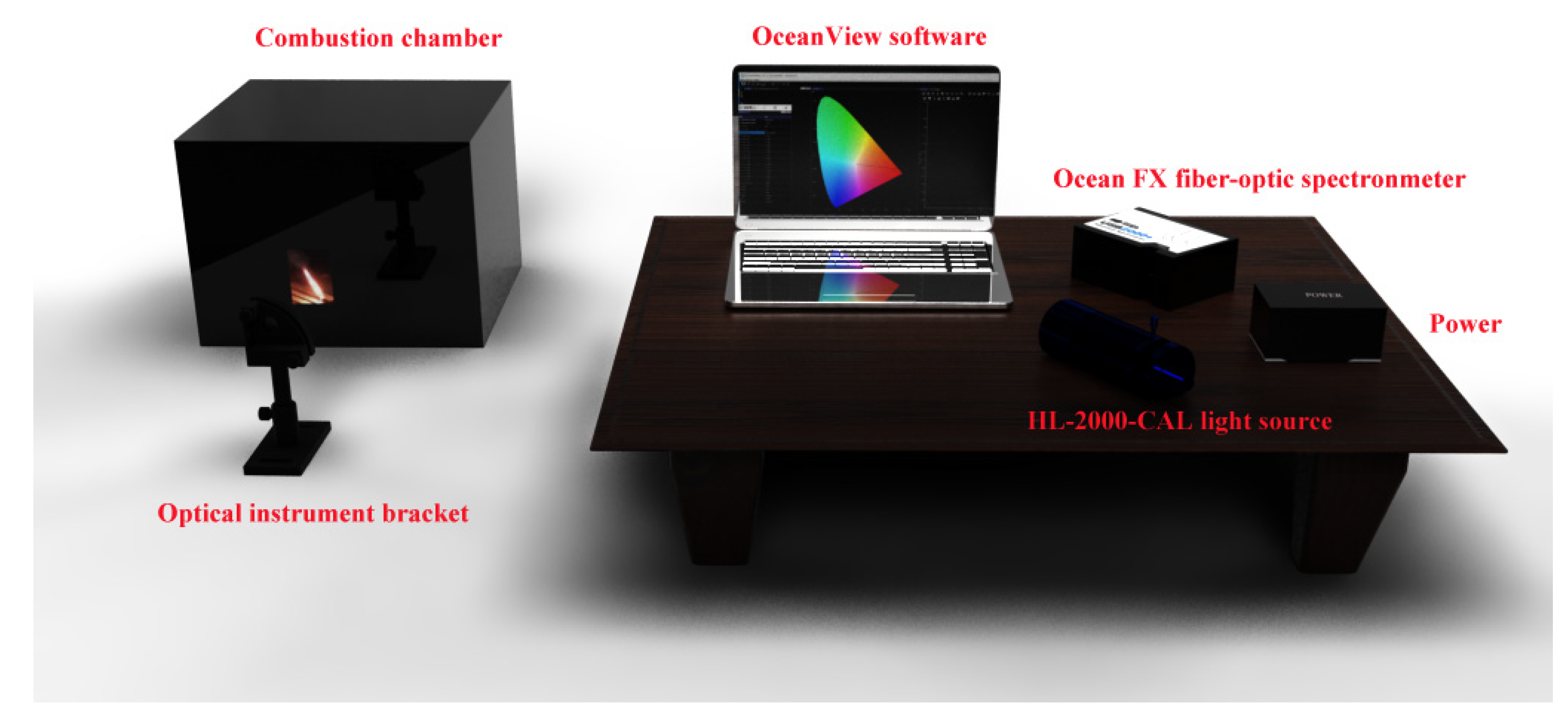
2.4. Material Characterization
3. Results and Discussion
3.1. Performance of Modified Magnesium Powder
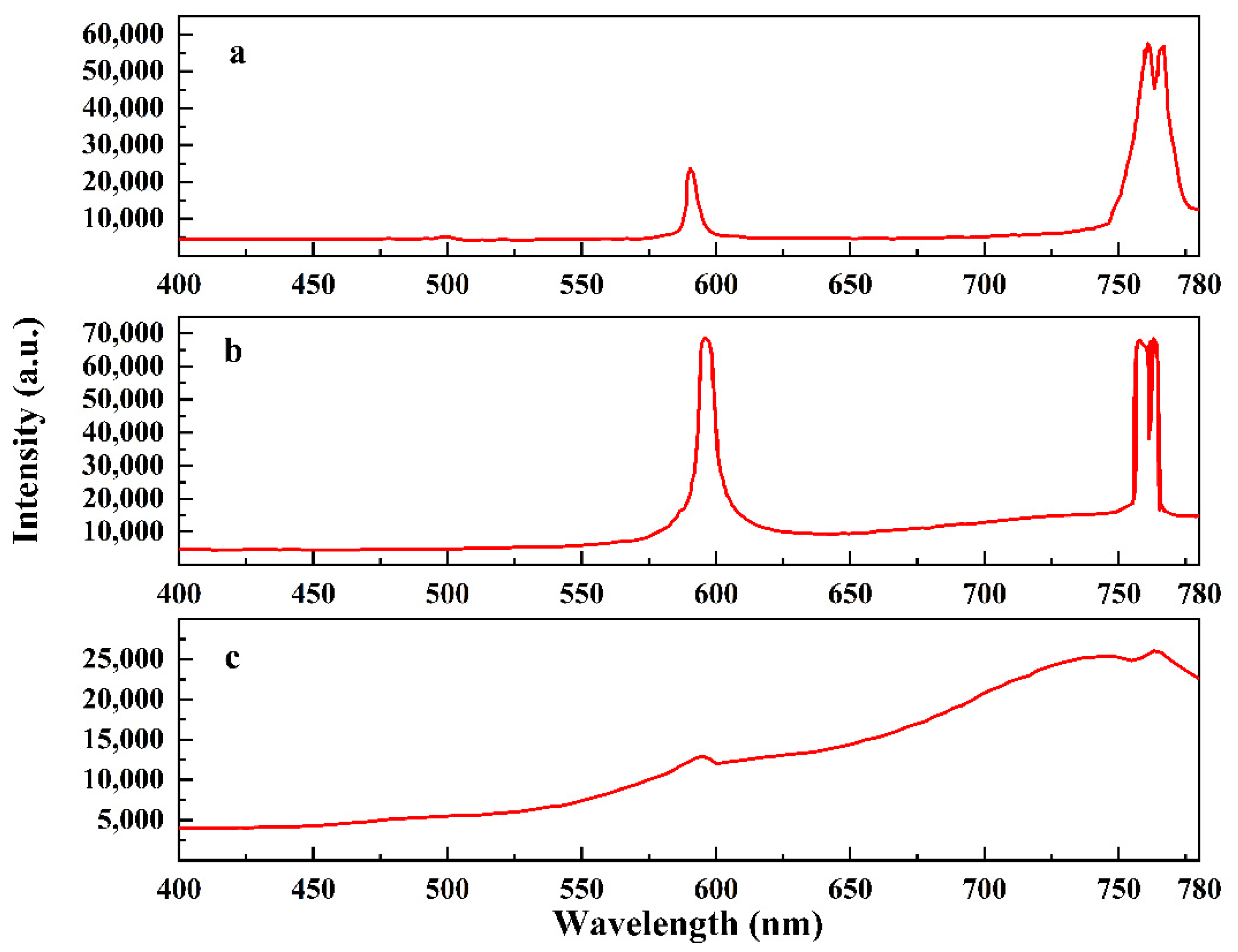
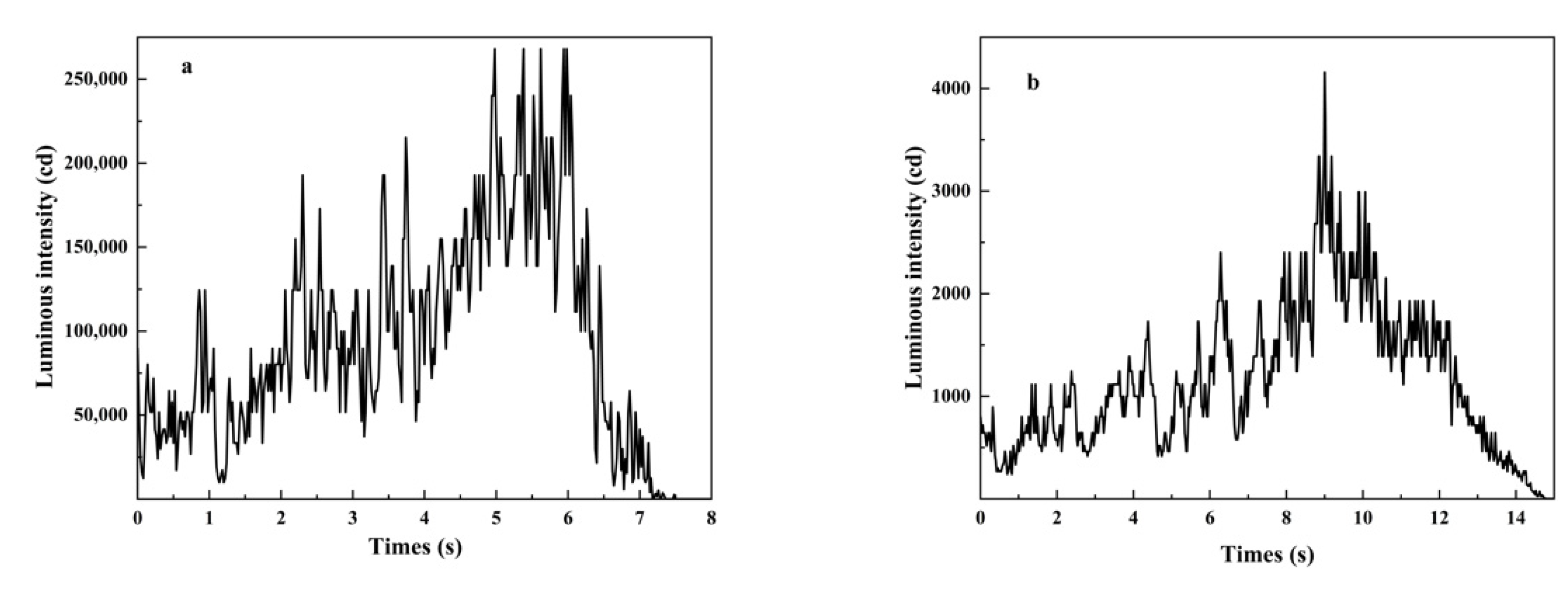
3.2. Luminous Performance
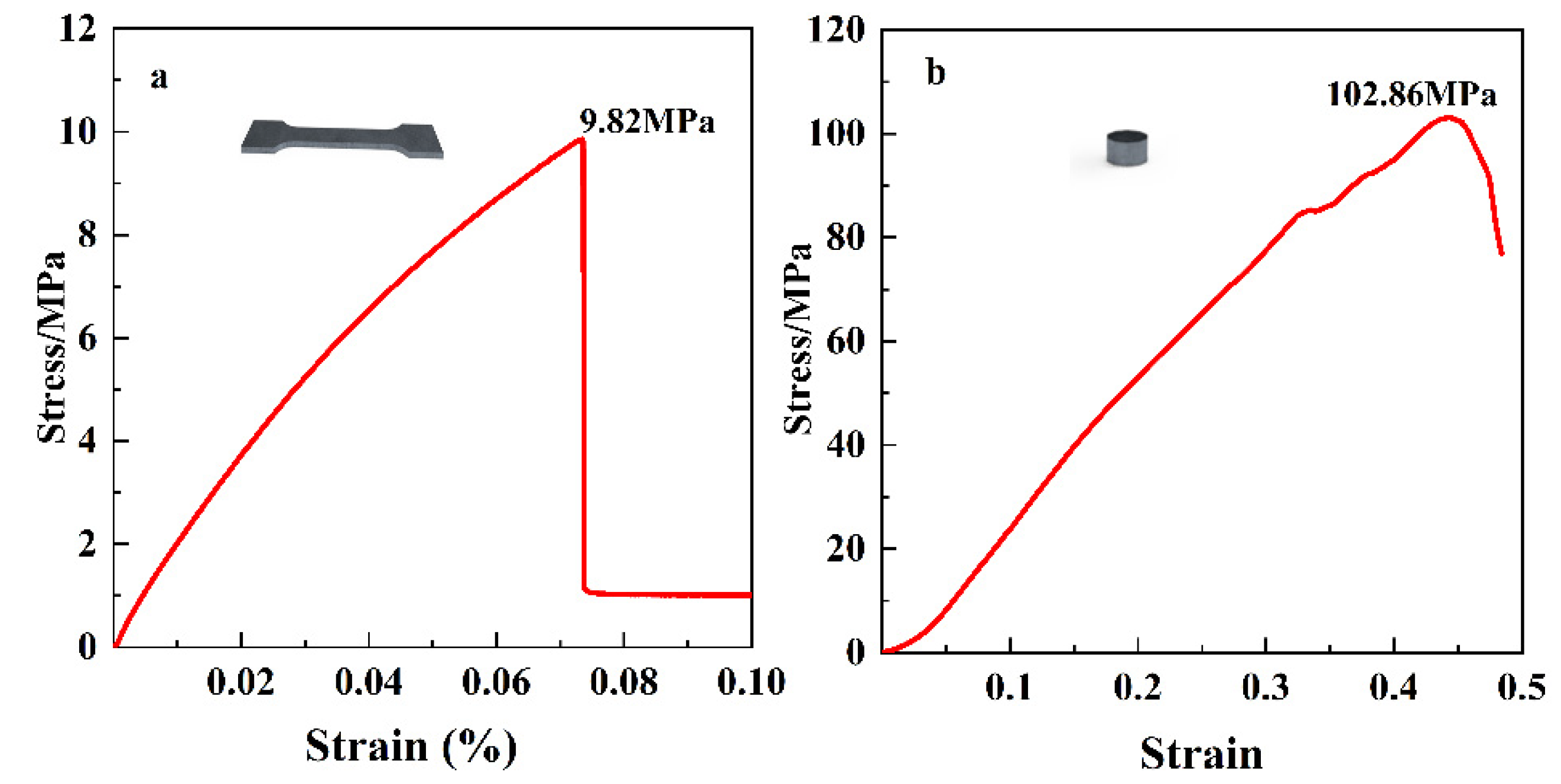
3.3. Mechanical Properties of Combustible Lighting Shell Specimens
3.4. Compatibility of Combustible Lighting Shell
3.5. Moisture Absorption Test of Combustible Lighting Shell
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Back, S.; Son, Y.; Lim, S.; Myung, I. Storage life estimation of magnesium flare material for 81 mm illuminating projectile. J. Korea Inst. Mil. Sci. Technol. 2015, 18, 267–274. [Google Scholar] [CrossRef][Green Version]
- Berger, B. Military Pyrotechnics. CHIMIA 2004, 58, 363. [Google Scholar] [CrossRef]
- Lu, E. Artillery Flare Design; National Defense Industry Press: Beijing, China, 1978. [Google Scholar]
- Li, D.; Ding, Y.; Wang, Y. Improvement of lighting candle payload. J. Ordnance Equip. Eng. 2008, 29, 3. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Y.; Yan, X. Measurement and Calculation of Combustion Performance of Combustible Cartridge Under High Pressure. J. Ballistics. 2017, 29, 54–57. [Google Scholar]
- Li, Y.; Guo, D.; Zhao, C.; Zhou, W.; Xu, F. Characterization of Combustible Cartridge Cases Enhanced by Novel Energetic Fibers. Chin. J. Energetic Mater. 2009, 17, 5. [Google Scholar] [CrossRef]
- Putra, N.E.; Leeflang, M.A.; Minneboo, M.; Taheri, P.; Fratila-Apachitei, L.E.; Mol, J.M.C.; Zhou, J.; Zadpoor, A.A. Extrusion-based 3D printed biodegradable porous iron. Acta Biomaterialia. 2021, 121, 741–756. [Google Scholar] [CrossRef] [PubMed]
- Kollamaram, G.; Croker, D.M.; Walker, G.M.; Goyanes, A.; Basit, A.W.; Gaisford, S. Low temperature fused deposition modeling (FDM) 3D printing of thermolabile drugs. Int. J. Pharm. 2018, 545, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Tian, L.; Peng, H.; Zhou, Y.; Lu, L. Constrained stacking in DLP 3D printing. Comput. Graph. 2021, 95, 60–68. [Google Scholar] [CrossRef]
- Vadodaria, S.; Mills, T. Jetting-based 3D printing of edible materials. Food Hydrocoll. 2020, 106, 105857. [Google Scholar] [CrossRef]
- Karakurt, I.; Lin, L. 3D printing technologies: Techniques, materials, and post-processing. Curr. Opin. Chem. Eng. 2020, 28, 134–143. [Google Scholar] [CrossRef]
- Li, M.; Yang, W.; Xu, M.; Hu, R.; Zheng, L. Study of photocurable energetic resin based propellants fabricated by 3D printing. Mater. Design. 2021, 207, 109891. [Google Scholar] [CrossRef]
- Yang, W.; Hu, R.; Zheng, L.; Yan, G.; Yan, W. Fabrication and investigation of 3D-printed gun propellants. Mater. Design. 2020, 192, 108761. [Google Scholar] [CrossRef]
- Li, M.; Gao, Y.; Hu, R.; Wang, Q.; Yang, W. Thermal study of APNIMMO/CL-20 based propellants fabricated by 3D printing. Thermochim. Acta. 2021, 706, 179072. [Google Scholar] [CrossRef]
- Zhou, M.; Nan, F.; He, W.; Wang, M. Design and Preparation of Propellant 3D Printer Based on Extrusion Deposition Technology. Chin. J. Energetic Mater. 2021, 29, 6. [Google Scholar] [CrossRef]
- Dunju, W.; Changping, G.; Ruihao, W.; Baohui, Z.; Bing, G.; Fude, N. Additive manufacturing and combustion performance of CL-20 composites. J. Mater. Sci. 2020, 55, 2836–2845. [Google Scholar] [CrossRef]
- Quan, H.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X. Photo-curing 3D printing technique and its challenges. Bioact. Mater. 2020, 5, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, Z.; Pei, Z.; Qiu, J.; Wang, S. Current progress on the 3D printing of thermosets. Adv. Compos. Hybrid Mater. 2020, 3, 462–472. [Google Scholar] [CrossRef]
- Chen, Y.; Ba, S.; Ren, H. Additive Manufacturing of a Special-Shaped Energetic Grain and Its Performance. Micromachines 2021, 12, 1509. [Google Scholar] [CrossRef]
- Meyerriecks, W.; Kosanke, K.L. Color values and spectra of the principal emitters in colored flames. J. Pyrotech. 2003, 18, 710–731. [Google Scholar]
- Ba, S.; Yang, Y.; Shen, H.; Wang, S.; Sun, W. Delay Property of Energetic Grain via Digital Light Processing Photocurable 3D Printing. Chin. J. Energetic Mate. 2022, 30, 363–369. [Google Scholar] [CrossRef]





| Number | Shell Quality/g | Quality of Shell after Hygroscoping/g | Shell Added Mass/g | Rate of Weight Change/% |
|---|---|---|---|---|
| 1 | 4.805 | 4.8282 | 0.0232 | 0.48 |
| 2 | 4.811 | 4.8293 | 0.0183 | 0.38 |
| 3 | 4.797 | 4.8195 | 0.0225 | 0.47 |
| 4 | 4.807 | 4.8268 | 0.0198 | 0.41 |
| 5 | 4.815 | 4.8378 | 0.0228 | 0.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Ba, S.; Zhang, B.; Li, L. DLP Light-Curing 3D Printing Combustible Lighting Shell and Performance Study. Appl. Sci. 2022, 12, 10401. https://doi.org/10.3390/app122010401
Wang H, Ba S, Zhang B, Li L. DLP Light-Curing 3D Printing Combustible Lighting Shell and Performance Study. Applied Sciences. 2022; 12(20):10401. https://doi.org/10.3390/app122010401
Chicago/Turabian StyleWang, Haibo, Shuhong Ba, Bo Zhang, and Linpeng Li. 2022. "DLP Light-Curing 3D Printing Combustible Lighting Shell and Performance Study" Applied Sciences 12, no. 20: 10401. https://doi.org/10.3390/app122010401
APA StyleWang, H., Ba, S., Zhang, B., & Li, L. (2022). DLP Light-Curing 3D Printing Combustible Lighting Shell and Performance Study. Applied Sciences, 12(20), 10401. https://doi.org/10.3390/app122010401






