Influence of Effective Microorganisms, Colloidal Nanosilver and Silver Compounds on Water Content in New and Used Engine Oil: A Preliminary Study
Abstract
1. Introduction
2. Oil Contamination with Water
3. Materials and Methodology
3.1. Materials Used in the Research
- Maximum dynamic viscosity of 6600 cP at −30 °C;
- Maximum pumpability temperature of 60,000 cP at the temperature of −35 °C;
- Kinematic viscosity at temperature 100 °C min, 3.8 mm2/s to 9.3–12.5 mm2/s;
- Viscosity HTHS in 150 °C min, 2.9 cP.
3.2. Methodology
- The Aquagent Coulometric solvent (100 mL) was poured into the measuring vessel;
- An ampoule of iodine in the amount of 5 mL was added;
- The parts were twisted together and the device was placed on the stand;
- The “G” generation electrode and the “D” detection electrode were connected;
- It was then possible to start conditioning, i.e., to begin removing the moisture from the measuring system in order for the obtained measurement to correspond to the tested sample;
- It was necessary to fill the glass syringe with test oil and weigh the oil-filled syringe, then the sample itself, to obtain the weight of the test sample;
- After completing the measurement of the water content, the previously measured mass of the tested oil was fed into the device in order to obtain the water content as a percentage.
4. Results and Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| ACE | Automatic Error Compensation |
| ACF | Activated Carbon Fiber |
| DNA | Deoxyribonucleic Acid |
| EM | Effective Microorganisms |
| HTHS | High Temperature High Shear Rate |
| SAE | Society of Automotive Engineers |
| SS | Silver solution |
| CN | Colloidal nanosilver |
References
- Duda, A. Problems of microbial contamination in the distribution and storage of petroleum fuels. Oil-Gas 2002, 58, 160–167. (In Polish) [Google Scholar]
- Janda, K. Microbiological contamination of fuels. Adv. Microbiol. 2005, 44, 157–169. (In Polish) [Google Scholar]
- Du, Y.; Wu, T.; Gong, R. Properties of water-contaminated lubricating oil: Variation with temperature and small water content. Tribol.-Mater. Surf. Interfaces 2017, 11, 1–6. Available online: https://www.tandfonline.com/doi/abs/10.1080/17515831.2017.1279845 (accessed on 12 July 2022). [CrossRef]
- Fass, R.; Ben-Asher, J.; Shavit, A.B. Effects of microbial contamination in storage tanks on the long-term stability of jet fuel. In Proceedings of the 2nd International Conference on Long-Term Storage Stabilities of Liquid Fuels, San Antonio, TX, USA, 29 July–1 August 1986; pp. 253–263. [Google Scholar]
- Vidal-Verdú, À.; Gómez-Martínez, D.; Latorre-Pérez, A.; Peretó, J.; Porcar, M. The car tank lid bacteriome: A reservoir of bacteria with potential in bioremediation of fuel. NPJ Biofilms Microbiomes 2022, 8, 32. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, H.; Yin, X.; Shi, Y.; Zhang, Y.; Tan, L. Surface coating on aluminum substrate with polymeric guanidine derivative to protect jet fuel tanks from microbial contamination. Surf. Coat. Technol. 2021, 422, 127521. [Google Scholar] [CrossRef]
- Gaylarde, C.C.; Bento, F.; Kelley, J. Microbial contamination of stored hydrocarbon fuels and its control. Rev. Microbiol. 1999, 30, 1–10. Available online: https://www.scielo.br/j/rm/a/YTvpcRy9YY8V5WgzHjBTnhP/?format=pdf&lang=en (accessed on 12 July 2022). [CrossRef]
- Turkiewicz, A. The Role of Microorganisms in the Oil and Gas Industry. Ochr. Środowiska 2011, 13, 227–240. [Google Scholar]
- Passman, F.J. Microbial contamination and its control in fuels and fuel systems since 1980—A review. Int. Biodeterior. Biodegrad. 2013, 81, 88–104. Available online: https://www.sciencedirect.com/science/article/pii/S0964830512002120 (accessed on 12 July 2022). [CrossRef]
- Higa, T. Effective microorganisms—Technology of the 21st century. In Proceedings of the Effective Microorganisms in the World, London, UK, 23 July 2005. [Google Scholar]
- Higa, T. A Revolution in Protecting Our Planet; Foundation for Development of the Warsaw University of Life Sciences: Warsaw, Poland, 2003. (In Polish) [Google Scholar]
- Kolasa-Więcek, A. Will effective microorganisms revolutionize the world? Adv. Food Process. Technol. 2010, 1, 66–69. Available online: http://yadda.icm.edu.pl/baztech/element/bwmeta1.element.baztech-article-BPL2-0021-0030 (accessed on 30 August 2022). (In Polish).
- Mayer, J.; Scheid, S.; Widmer, F.; Fließbach, A.; Oberholzer, H.-R. How effective are ‘Effective microorganisms® (EM)’? Results from a field study in temperate climate. Appl. Soil Ecol. 2010, 46, 230–239. [Google Scholar] [CrossRef]
- Rizwan, S.A.; Khan, H.; Bier, T.A.; Adnan, F. Use of Effective Micro-organisms (EM) technology and self-compacting concrete (SCC) technology improved the response of cementitious systems. Constr. Build. Mater. 2017, 152, 642–650. [Google Scholar] [CrossRef]
- Morones-Ramirez, J.R.; Winkler, J.A.; Spina, C.S.; Collins, J.J. Silver enhances antibiotic activity against Gram-negative bacteria. Sci. Transl. Med. 2013, 5, 190ra81. [Google Scholar] [CrossRef]
- Dzikowska, A.; Gościańska, J.; Nowak, I. Synthesis, physicochemical properties and applications of silver nanoparticles in cosmetics. In Cosmetics—Chemicals for the Body; Cursiva Publisher: Kostrzyń, Poland, 2011; pp. 163–182. (In Polish) [Google Scholar]
- Available online: https://www.mydelkoznanosrebrem.pl/nanosrebro.php (accessed on 12 July 2022). (In Polish).
- Aluyor, E.O.; Ori-jesu, M. Biodegradation of mineral oils—A review. Afr. J. Biotechnol. 2009, 8, 915–920. Available online: http://www.academicjournals.org/AJB (accessed on 12 July 2022).
- Feyisayo, V.A. Crude Oil Contaminated Water Treatment: Development of Water Filter from Locally Sourced Materials. Procedia Manuf. 2017, 7, 465–471. Available online: https://www.sciencedirect.com/science/article/pii/S2351978916302025 (accessed on 12 July 2022).
- Demello, J.A.; Carmichael, C.A.; Peacook, E.E. Biodegradation and environmental behavior of biodiesel mixtures in the sea: An initial study. Mar. Pollut. Bull. 2007, 54, 894–904. Available online: https://pubmed.ncbi.nlm.nih.gov/17481669/ (accessed on 12 July 2022). [CrossRef] [PubMed]
- Farah, M.A.; Oliveira, R.C.; Caldas, J.N.; Rajagopal, K. Viscosity of water-in-oil emulsions: Variation with temperature and water volume fraction. J. Pet. Sci. Eng. 2005, 48, 169–184. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0920410505001129 (accessed on 12 July 2022). [CrossRef]
- Niewczas, A.; Wrona, J.; Wrona, R. Contamination of the lubricating oil and their influence on the durability of the internal combustion engine. Buses Technol. Oper. Transp. Syst. 2010, 11, 6. Available online: http://yadda.icm.edu.pl/baztech/element/bwmeta1.element.baztech-article-BWAK-0037-0042 (accessed on 12 July 2022). (In Polish).
- Nowak, P.; Kucharska, K.; Kaminski, M. Ecological and Health Effects of Lubricant Oils Emitted into the Environment. Int. J. Environ. Res. Public Health 2019, 16, 3002. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6720566/ (accessed on 12 July 2022). [CrossRef] [PubMed]
- Available online: https://www.ecol.com.pl/pl/zanieczyszczenie-oleju-woda-i-metody-jej-usuwania-artykul/ (accessed on 12 July 2022). (In Polish).
- Das, N.; Chandran, P. Microbial Degradation of Petroleum Hydrocarbon Contaminants: An overview. Biotechnol. Res. Int. 2011, 2011, 13. Available online: https://pubmed.ncbi.nlm.nih.gov/21350672/ (accessed on 12 July 2022). [CrossRef] [PubMed]
- Lasocki, J.; Karwowska, E. The impact of microorganisms in the environment of diesel oil and biodiesel on the fuel system of vehicles powered by self-ignition engines. Automot. Arch. 2010, 3, 167–183. Available online: http://yadda.icm.edu.pl/baztech/element/bwmeta1.element.baztech-article-BGPK-3062-2091 (accessed on 12 July 2022). (In Polish).
- Robbins, J.A.; Levy, R. A review of the microbiological degradation of fuel. Dir. Microbicides Prot. Mater. 2005, 177–201. Available online: https://link.springer.com/content/pdf/10.1007%2F1-4020-2818-0_11.pdf (accessed on 12 July 2022).
- Zyska, B. Microbiological Decomposition and Corrosion of Technical Materials. Technical Microbiology. conference materials. Lodz University of Technology, Lodz, Polish, 2000. (In Polish). [Google Scholar]
- Available online: https://badania-olejow.pl/zanieczyszczenie-oleju-woda/ (accessed on 12 July 2022). (In Polish).
- Available online: https://dieselship.com/marine-technical-articles/motor-engineering-knowledge/microbial-degradation-of-lubricating-oils-onboard/ (accessed on 12 July 2022).
- Maduka, C.M.; Okpokwasili, G.C. Microbial growth in brake fluids. Int. Biodeterior. Biodegrad. 2016, 114, 31–38. [Google Scholar] [CrossRef]
- Fregolente, P.B.L.; Fregolente, L.V.; Wolf-Maciel, M.R. Water Content in Biodiesel, Diesel, and Biodiesel–Diesel Blends. J. Chem. Eng. Data 2012, 57, 1817–1821. [Google Scholar] [CrossRef]
- Dealtry, S.; Ghizelinib, A.M.; Mendonça-Hagler, L.C.S.; Chaloubd, R.M.; Reinert, F.; de Campos, T.M.P.; Gomes, N.C.M.; Smallag, K. Petroleum contamination and bioaugmentation in bacterial rhizosphere communities from Avicennia schaueriana. Environ. Microbiol. 2018, 49, 757–769. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6175736/ (accessed on 12 July 2022). [CrossRef]
- Kulkarni, S.J. Biological treatment of petroleum wastewater: A review on research and studies. Int. J. Pet. Petrochem. Eng. 2016, 2, 17–21. Available online: https://www.arcjournals.org/ijppe/volume-2-issue-2/4 (accessed on 12 July 2022).
- Kvenvolden, K.A.; Cooper, C.K. Natural seepage of crude oil into the marine environment. Geo-Mar. Lett. 2003, 23, 140–146. Available online: https://link.springer.com/article/10.1007/s00367-003-0135-0 (accessed on 12 July 2022). [CrossRef]
- Sander, J. Water Contamination: Management of Water during the Lubricant Life Cycle; Lubrication Engineers Corp.: Wichita, KS, USA, 2009; Available online: https://docplayer.net/21508315-Water-contamination-management-of-water-during-the-lubricant-life-cycle.html (accessed on 12 July 2022).
- Available online: https://www.ucando.pl/blog/olej-samochodowy-5w30-cos-dla-kierowcow-z-lekka-noga-ceniacych-sobie-ekonomike-jazdy-oraz-jej-wplyw-na-ochrone-srodowiska/ (accessed on 12 July 2022). (In Polish).
- Available online: https://incar.pl/baza-wiedzy/olej-silnikowy-5w30-czym-sie-charakteryzuje,ranking.html (accessed on 12 July 2022). (In Polish).
- Available online: https://www.aptekarzpolski.pl/2015/01/01-2015-technologia-efektywnych-mikroorganizmow/ (accessed on 12 July 2022). (In Polish).
- Schicht, M.L. To understand EM. EMECHO 2008, 8, 3. [Google Scholar]
- Available online: https://emsustains.co.uk/EM-ceramics.html (accessed on 12 July 2022).
- Available online: https://falaem.pl/rurki-ceramiczne-uzdatnianie-wody (accessed on 12 July 2022). (In Polish).
- Available online: https://www.ecoshop.com.pl/mikroorganizmy/3556-greenland-em-melasa-1l-5907738935220.html (accessed on 12 July 2022). (In Polish).
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. Available online: https://www.sciencedirect.com/science/article/pii/S1549963406003467?casa_token=J6KTF9cUdVIAAAAA:xFmuaoDImoQCz6K3_fJyRoRWNKrLKwo8paA-s2seeKTkHO1I33Ah2nXcHHK_TCot3a3SzW526iCO (accessed on 12 July 2022). [CrossRef]
- Available online: https://cen.acs.org/articles/93/web/2015/07/Antimicrobial-Silver-Nanoparticles-Strengthen-Bacterial.html (accessed on 12 July 2022).
- Mirzajani, F.; Ghassempour, A.; Aliahmadi, A.; Esmaeili, M.A. Antibacterial effect of silver nanoparticles on Staphylococcus aureus. Res. Microbiol. 2011, 162, 542–549. [Google Scholar] [CrossRef]
- Available online: https://auraherbals.pl/blog/srebro-koloidalne-jak-wybrac-prawdziwe-i-nie-dac-sie-oszukac/ (accessed on 12 July 2022).
- Available online: https://allegro.pl/oferta/srebro-koloidalne-jonowe-ag-500ml-9929512556 (accessed on 12 July 2022).
- Available online: https://enaturalnie.pl/argentum200-niejonowe-srebro-100-ppm-plyn-tonik-500-ml-aura-herbals_2838.html (accessed on 12 July 2022).
- Available online: https://archive-resources.coleparmer.com/Manual_pdfs/94101-00.pdf (accessed on 12 July 2022).
- Binks, B.P. Particles as surfactants—Similarities and differences. Curr. Opin. Colloid Interface Sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Kapellos, G.E. Microbial strategies for oil biodegradation. In Modeling of Microscale Transport in Biological Processes; Academic Press: Cambridge, MA, USA, 2017; pp. 19–39. [Google Scholar] [CrossRef]
- Valdivia-Rivera, S.; Ayora-Talavera, T.; Lizardi-Jiménez, M.A.; García-Cruz, U.; Cuevas-Bernardino, J.C.; Pacheco, N. Encapsulation of microorganisms for bioremediation: Techniques and carriers. Rev. Environ. Sci. Bio/Technol. 2021, 20, 815–838. [Google Scholar] [CrossRef]
- Geetha, S.J.; Banat, I.M.; Joshi, S.J. Biosurfactants: Production and potential applications in microbial enhanced oil recovery (MEOR). Biocatal. Agric. Biotechnol. 2018, 14, 23–32. [Google Scholar] [CrossRef]
- Krakowski, R. Research on the effect of the effective microorganisms, silver solution and colloidal nanosilver addition on the engine oil acid number (TAN). Combust. Engines 2021, 186, 59–63. Available online: http://www.combustion-engines.eu/Research-on-the-effect-of-the-effective-microorganisms-silver-solution-and-colloidal,140730,0,2.html (accessed on 12 July 2022). [CrossRef]
- Krakowski, R. Research on the effect of the effective microorganisms, silver solution and colloidal nanosilver addition on the engine oil base number (TBN). Combust. Engines 2021, 187, 8–11. Available online: http://www.combustion-engines.eu/Research-on-the-effect-of-the-effective-microorganisms-silver-solution-and-colloidal,140112,0,2.html (accessed on 12 July 2022). [CrossRef]
- Krakowski, R. Research into the effects of the effective microorganisms addition on the engine oil viscosity. J. KONES 2019, 26, 105–112. Available online: https://kones.eu/ep/2019/vol26/no3/105-112_JOK_2019_NO.3_VOL.26_ISSN_1231-4005-KRAKOWSKI.pdf (accessed on 12 July 2022). [CrossRef][Green Version]

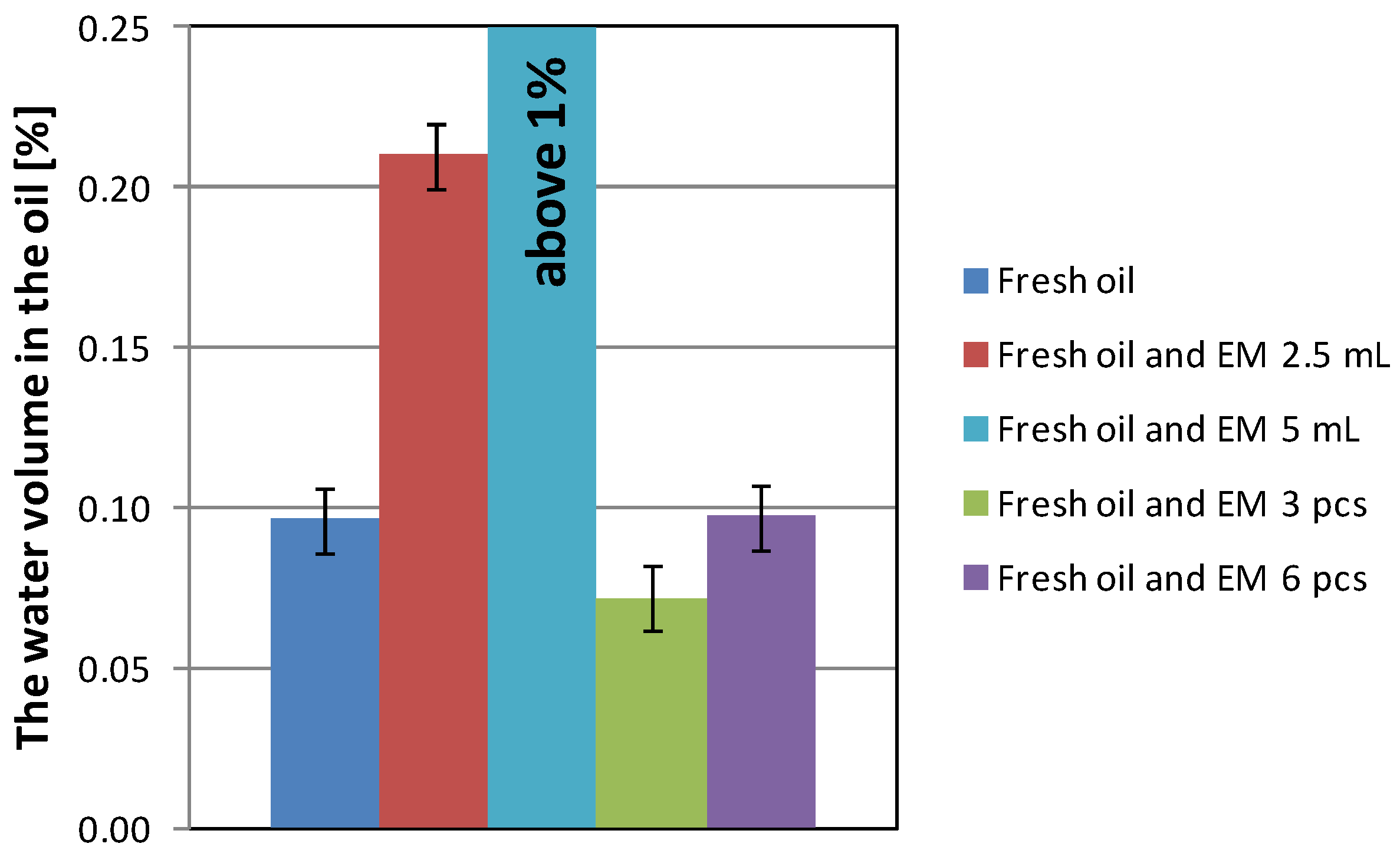
| Parameters | Cou-Lo Aquamax KF Moisture Meter |
|---|---|
| Titration method | Coulometric Karl Fischer titration |
| Endpoint detection | AC polarity |
| Measurement range | 1 µg–10 mg of water, max 200 mg of water |
| Moisture range | 1 ppm–100% water |
| Maximum titration rate | 2.24 mg per minute |
| Maximum electrolysis current | 400 mA |
| Drift Compensation | Controlled automatically |
| Minimum titration time | 0–30 min, user programmable |
| Precision | 10–100 µg ± 3 µg, 100 µg–1 mg ± 3 µg (ppm), over 1 mg ± 0.3% |
| Display format | µg, mg/kg, ppm, % |
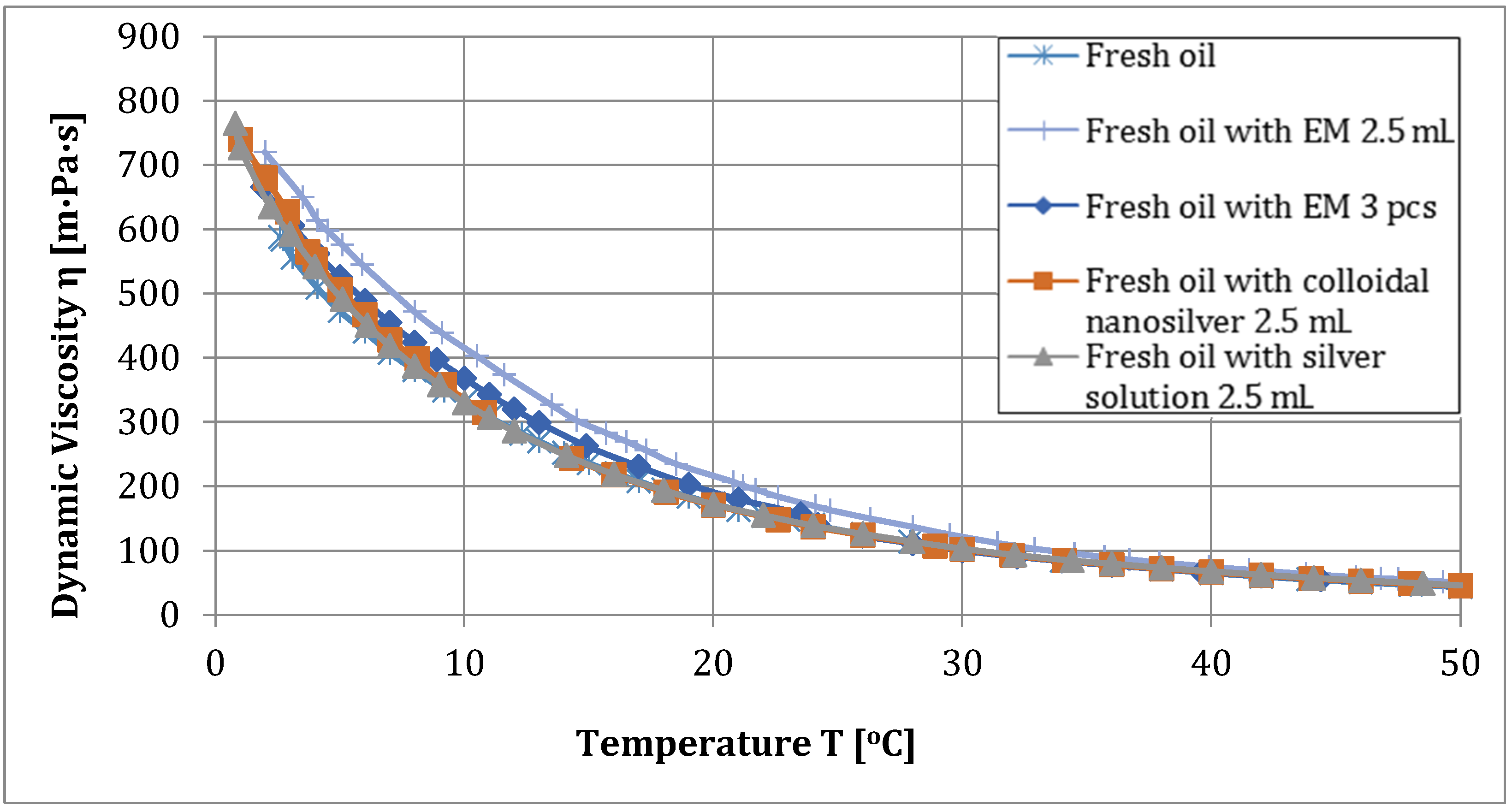
| Dynamic Viscosity in 2 °C | Flash Point | Acid Number | Base Number | Water Content | |
|---|---|---|---|---|---|
| [m∙Pa∙s] | [°C] | [mgKOH/g] | [mgKOH/g] | [%] | |
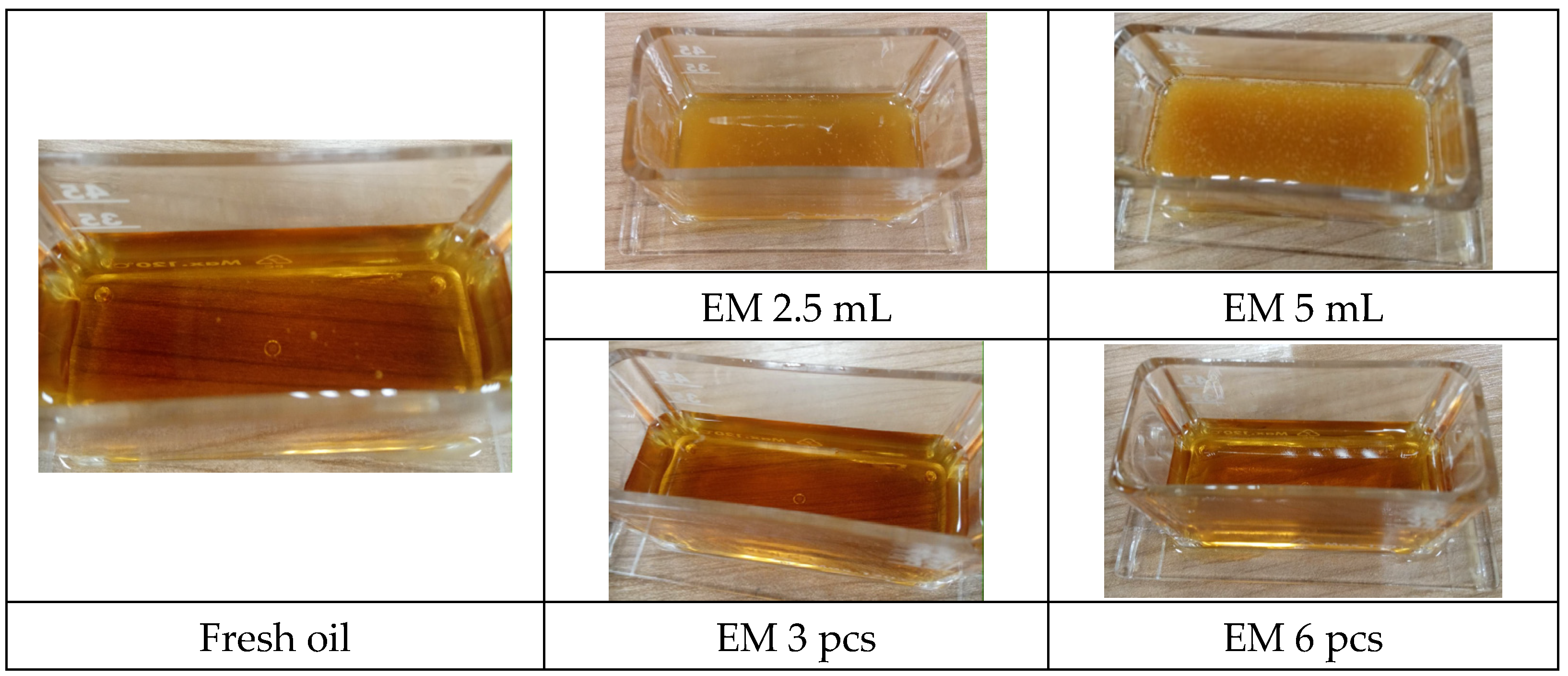
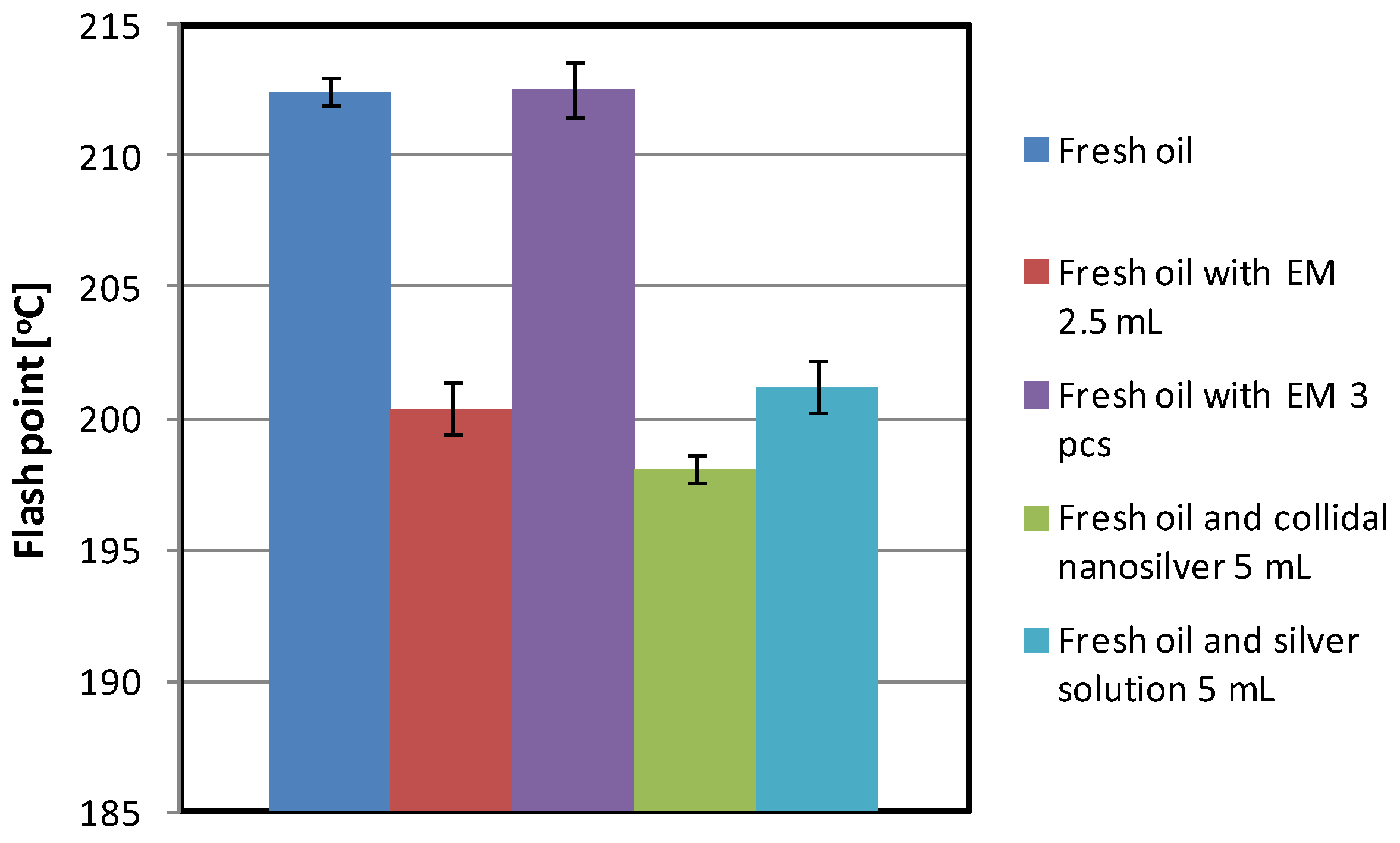
| Fresh oil | 596 | 212.4 | 2.560 | 3.907 | 0.096 |
| Fresh oil and EM 2.5 mL | 720 | 200.4 | 3.021 | 3.606 | 0.210 |
| Fresh oil and EM 5 mL | 713 | 199.6 | 2.497 | 3.444 | above 1 |
| Fresh oil with EM 3 pcs | 667 | 212.5 | 3.113 | 3.962 | 0.072 |
| Fresh oil with EM 6 pcs | 696 | 213.5 | 2.366 | 3.894 | 0.097 |
| Dynamic Viscosity in 2 °C | Flash Point | Acid Number | Base Number | Water Content | |
|---|---|---|---|---|---|
| [m∙Pa∙s] | [°C] | [mgKOH/g] | [mgKOH/g] | [%] | |
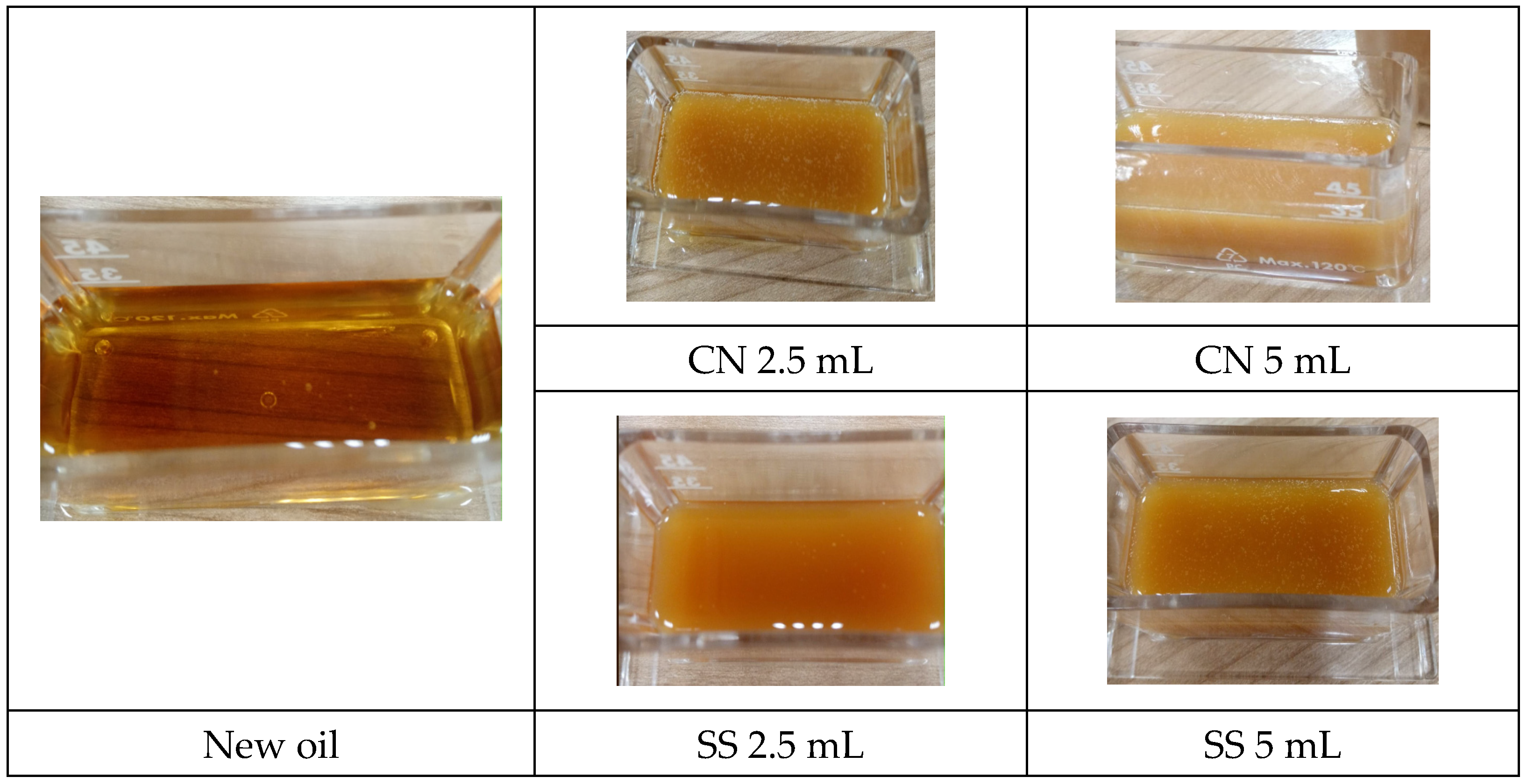
| Fresh oil | 596 | 212.4 | 2560 | 3.907 | 0.096 |
| Fresh oil and colloidal nanosilver 2.5 mL | 686 | 200.4 | 2.668 | 3.604 | above 1 |
| Fresh oil and colloidal nanosilver 5 mL | 742 | 198.4 | 2.659 | 3.751 | above 1 |
| Fresh oil and silver solution 2.5 mL | 698 | 206.9 | 2.682 | 3.479 | above 1 |
| Fresh oil and silver solution 5 mL | 736 | 201.2 | 2.660 | 3.731 | above 1 |
| Dynamic Viscosity in 2 °C | Flash Point | Acid Number | Base Number | Water Content | |
|---|---|---|---|---|---|
| [m∙Pa∙s] | [°C] | [mgKOH/g] | [mgKOH/g] | [%] | |
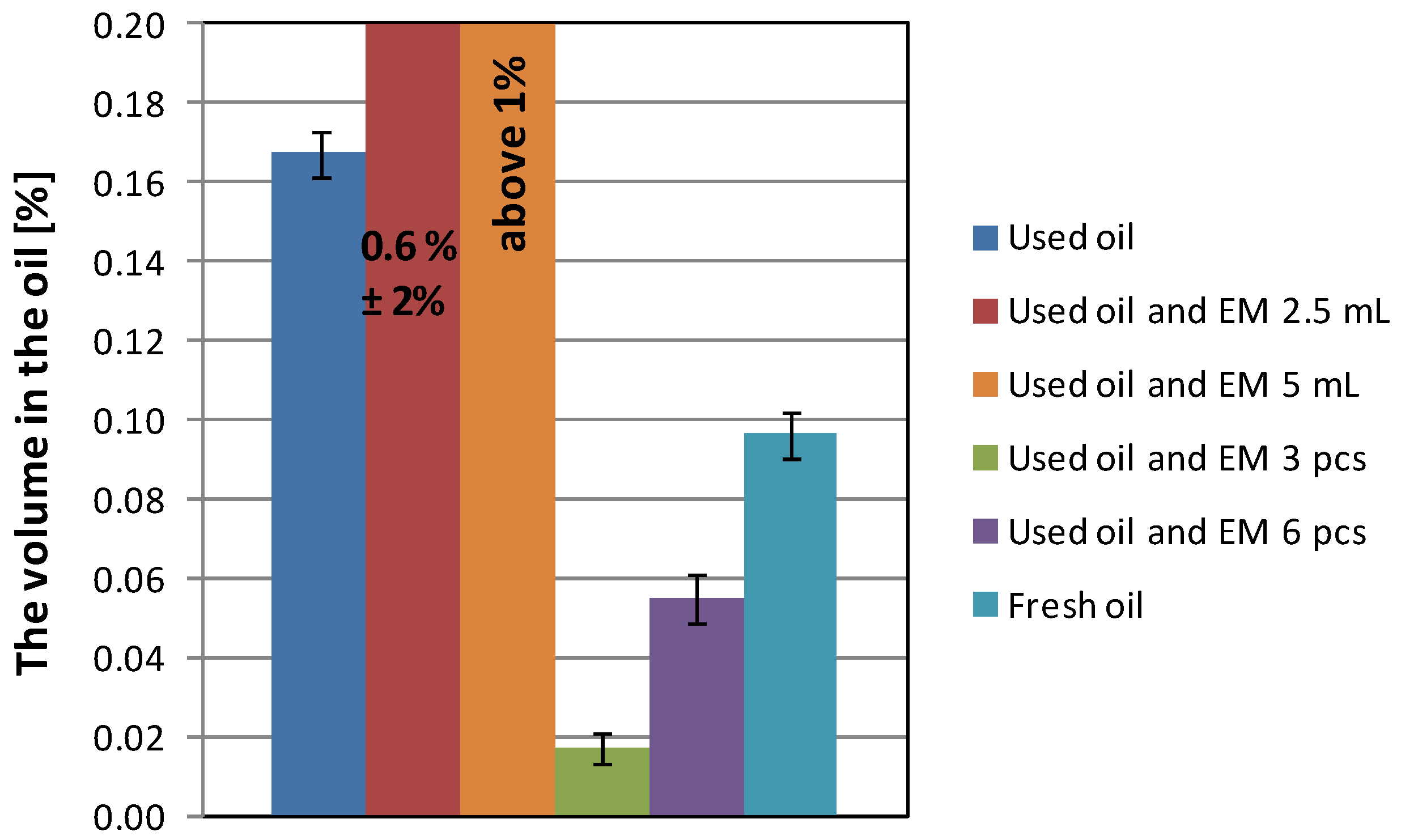
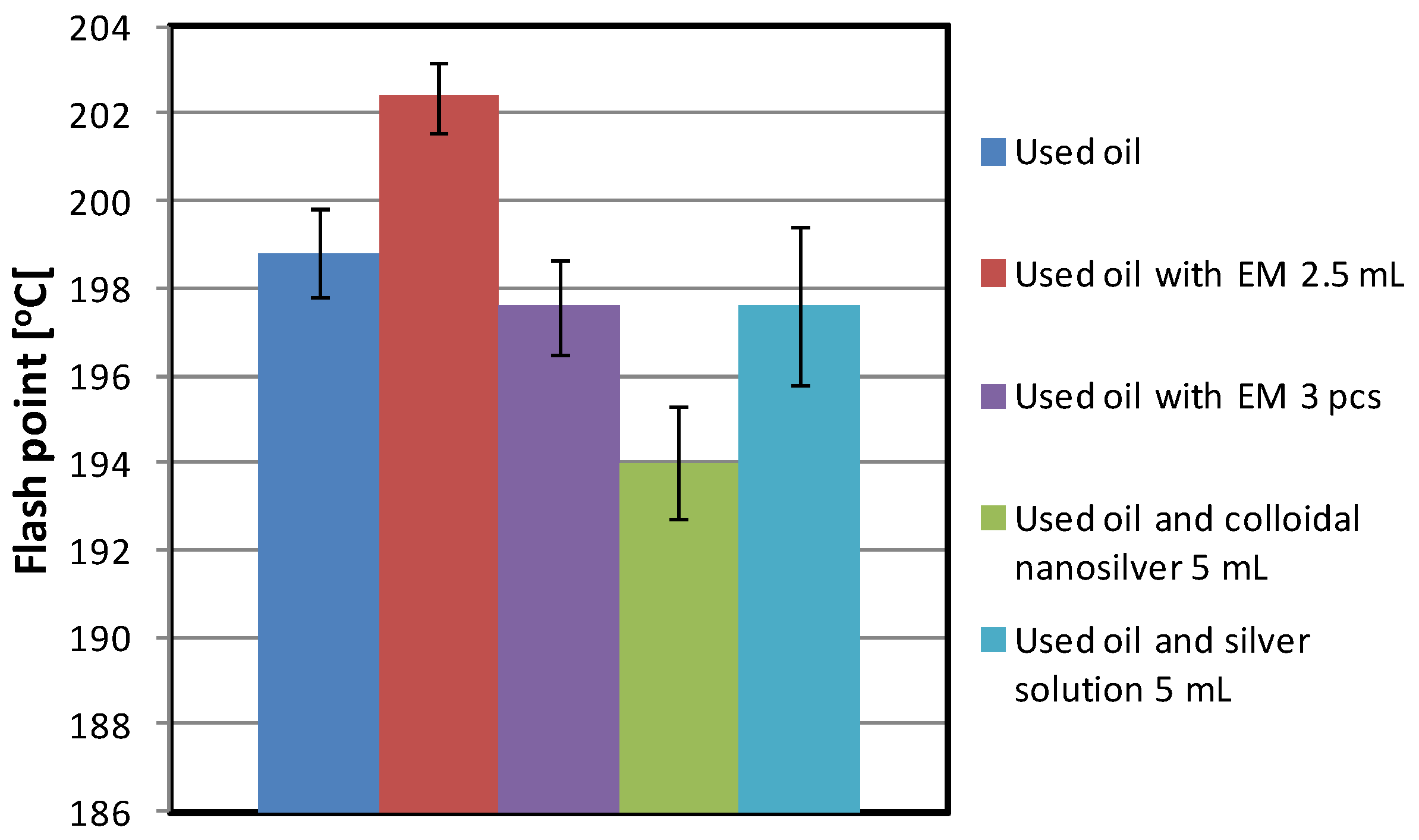
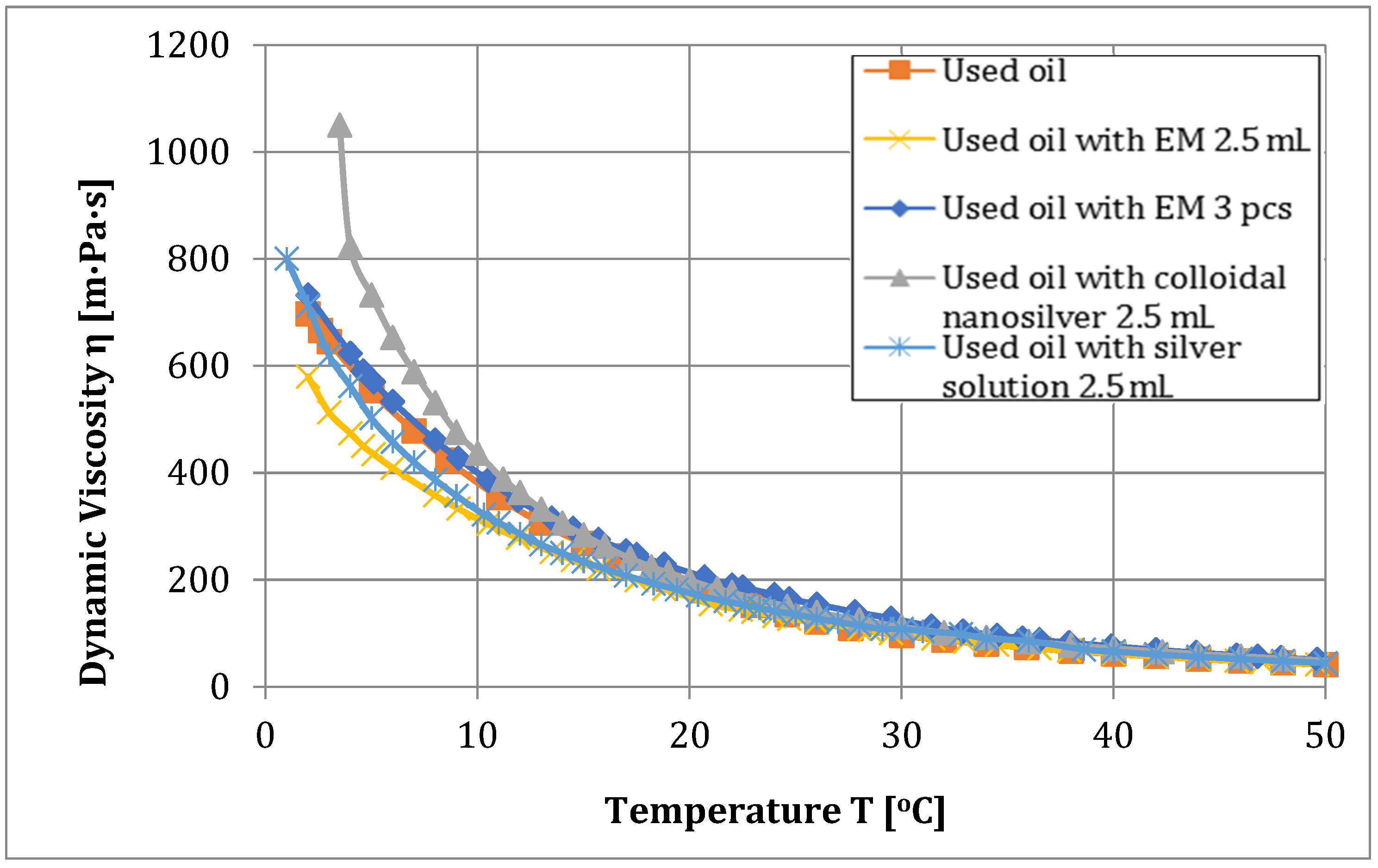
| Used oil | 719 | 198.8 | 4.027 | 1.358 | 0.167 |
| Used oil and EM 2.5 mL | 580 | 202.4 | 4.373 | 0.937 | 0.606 |
| Used oil and EM 5 mL | 758 | 202.1 | 5.411 | 0.844 | above 1 |
| Used oil with EM 3 pcs | 732 | 197.5 | 6.027 | 1.250 | 0.017 |
| Used oil with EM 6 pcs | 652 | 198.1 | 5.499 | 0.749 | 0.054 |
| Dynamic Viscosity in 2 °C | Flash Point | Acid Number | Base Number | Water Content | |
|---|---|---|---|---|---|
| [m∙Pa∙s] | [°C] | [mgKOH/g] | [mgKOH/g] | [%] | |
| Used oil | 719 | 198.8 | 4.027 | 1.358 | 0.167 |
| Used oil and colloidal nanosilver 2.5 mL | 712 | 186.2 | 2.668 | 1.350 | above 1 |
| Used oil and colloidal nanosilver 5 mL | 758 | 194.0 | 2.659 | 1.402 | above 1 |
| Used oil and silver solution 2.5 mL | 796 | 191.6 | 2.682 | 1.037 | above 1 |
| Used oil and silver solution 5 mL | 806 | 197.6 | 2.660 | 1.199 | above 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krakowski, R. Influence of Effective Microorganisms, Colloidal Nanosilver and Silver Compounds on Water Content in New and Used Engine Oil: A Preliminary Study. Appl. Sci. 2022, 12, 10234. https://doi.org/10.3390/app122010234
Krakowski R. Influence of Effective Microorganisms, Colloidal Nanosilver and Silver Compounds on Water Content in New and Used Engine Oil: A Preliminary Study. Applied Sciences. 2022; 12(20):10234. https://doi.org/10.3390/app122010234
Chicago/Turabian StyleKrakowski, Rafał. 2022. "Influence of Effective Microorganisms, Colloidal Nanosilver and Silver Compounds on Water Content in New and Used Engine Oil: A Preliminary Study" Applied Sciences 12, no. 20: 10234. https://doi.org/10.3390/app122010234
APA StyleKrakowski, R. (2022). Influence of Effective Microorganisms, Colloidal Nanosilver and Silver Compounds on Water Content in New and Used Engine Oil: A Preliminary Study. Applied Sciences, 12(20), 10234. https://doi.org/10.3390/app122010234










