Abstract
The production of gamma-decalactone (GDL) by Yarrowia lipolytica is mainly based on the biotransformation of ricinoleic acid, derived from castor oil triglycerides. The main difficulty in this process is the multitude of factors that determine the growth rate of microorganisms, and thus affect the efficiency of lactone synthesis. In order to improve the technological aspects of GDL biosynthesis in batch culture, the influence of three factors was determined: substrate concentration, medium mixing intensity, and its pH, using the Taguchi solid design method (based on orthogonal array design). On the basis of four bioreactor batch cultures, the most favorable culture conditions in terms of GDL synthesis were selected using the statistical Taguchi method. The statistical method of experimental planning has shown that the optimal parameters of lactone biosynthesis are a constant pH at the level of 7, a variable mixing speed in the range of 200–500 rpm, and a substrate concentration at the level of 75 g/L. Using these parameters, about 2.93 ± 0.33 g/L of aroma was obtained. The intensity of mixing turned out to be the most important factor influencing the increase in GDL concentration in the medium.
1. Introduction
Fragrances are the key parameter influencing the acceptance and consumer preferences of food and cosmetic products. Hence, the market for aromas and flavors is constantly evolving. The global Food Flavors market was valued at USD 13.31 billion in 2018 and is expected to reach USD 19.72 billion by 2026, at a CAGR (compound annual growth rate) of 5% [1]. Due to the growing sensitivity of society to ecological problems and the growing trend in the field of a healthy lifestyle [2], de novo synthesis with the use of microorganisms or biotransformations, i.e., synthesis from natural resources, in reactions catalyzed by enzymes, is gaining importance in the production of fragrances [3,4,5].
Among the wide group of compounds synthesized by microorganisms, we can distinguish lactones, including the gamma-decalactone (GDL) C10H18O2, a peach-like aroma compound. Its biotechnological synthesis is based on peroxisomal β-oxidation of ricinoleic acid (R-12-hydroxy-9-octadecanoic acid), the main component of castor oil obtained from Ricinus communis seeds. Ricinoleic acid in the form of ester of coenzyme A is degraded in a four-step β-oxidation, resulting each time in a two-carbon chain-shortening. Finally, from ricinoleyl-CoA, 4-hydroxy-decanoyl-CoA is obtained, which undergoes intramolecular esterification—lactonization (after acidification to pH 2.0 and/or heating) [6,7]. A graphic illustration of the γ-decalactone biosynthesis pathway appears in many publications [6,8,9].
Despite the relatively long history of research on the biosynthesis of GDL (the possibility of producing GDL via yeast Candida tropicalis was discovered in 1963) [10], two issues are still a matter of investigation by the scientific community: the efficiency of the bioprocess [11,12,13,14] as well as the optimization of conditions for the separation of the compound from the biotransformation medium [15,16,17].
Previous studies on the parameters of GDL biosynthesis indicated many factors affecting the biotransformation yield. The selection of appropriate species and strains of microorganisms as well as environmental conditions, including, e.g., temperature, acidity of medium, its composition (including concentration and type of carbon source used), and oxygenation level, seem to have a significant impact. The presence of surfactants, the oxidation–reduction potential of the medium, and the applied technology of biotransformation (step-wise fed-batch cultures, immobilization of cells) could be also crucial for obtaining the maximum lactone concentration [18,19,20,21,22,23,24].
Cultivation in a medium with non-optimized pH reduces access to the carbon substrate and limits cell growth and lactone production [18]. Improper acidity of the medium may affect the intracellular pH of yeast and thus its metabolism. The concentration of GDL also depends on the NAD+/NADH ratio, because NAD+ is a cofactor of 3-hydroxyacyl-CoA dehydrogenase, the activity of which shifts the reaction towards the formation of 3-hydroxy-γ-decalactone. The level of FAD and NAD+ regeneration depends on the proper aeration of the medium [21]. Oxygen plays an important role in the activity of enzymes acyl-CoA oxidase and 3-hydroxyacyl-CoA dehydrogenase, participating in the regeneration of the above-mentioned cofactors. Moreover, it determines the course of the metabolism of hydrophobic substrates by contributing to the production and reconsumption reactions of GDL [25]. In aerobic cultivation processes, the oxygen transfer conditions depend on several factors such as temperature, pressure, composition of the culture medium, air flow rate, medium viscosity, aeration, stirring speed, and configuration of the medium. The studies show that the increase in the oxygen mass transfer rate (kLa) increases the rate of GDL production; however, the final concentration of aroma decreases [26]. Therefore, the mixing rate should also be properly selected to ensure not only the appropriate oxygen concentration in the medium, but also the proper dispersion of hydrophobic substrates [23,27].
The major difficulty in the described process is the great number of various factors determining the growth rate of microorganisms and thus influencing the efficiency of GDL synthesis. Despite the interest in the optimization of biotechnological methods for the biotransformation of castor oil into peach-like fragrance, most experiments have been carried out by means of comprehensive experimental plans. There are only a few scientific reports applying a design of experiment protocols in order to choose the batch culture conditions [21,28]. In one study, it was attempted to optimize the conditions for the biotransformation of castor oil to GDL by yeast Yarrowia lipolytica using the Taguchi robust design method. The approach allowed the researchers to determine the influence of several factors on the efficiency of the process provided in a laboratory-scale bioreactor, with a small number of experiments, based on orthogonal array design [29]. Yarrowia lipolytica, used in the research, is one of the best known “unconventional” yeasts. It is considered non-pathogenic, and the biotechnological processes involving it are generally recognized as safe (GRAS status) by the Food and Drug Administration (FDA, Silver Spring, MD, USA). The unique physiological and biochemical properties of these microorganisms contribute to their wide application in food biotechnology [30,31]. Yarrowia lipolytica is characterized by, among others, high extracellular lipolytic activity, which allows the use of castor oil (a cheap and easily available substrate) in GDL biosynthesis. The yeast produces three extracellularly lipases, named Lip2, Lip7, and Lip8 [32,33].
This article presents the results from batch cultures in which the impacts of medium pH, mixing speed, and castor oil concentration were analyzed based on the statistical Taguchi method. Although this method has already been applied [22], to the best of our knowledge, no study has used the minimal orthogonal array design in the batch bioreactor cultures.
2. Materials and Methods
2.1. Materials
γ-decalactone >98% and γ-undecalactone 98% were purchased from Sigma-Aldrich (Burlington, MA, USA). Diethyl ether, ethyl alcohol 96%, acetone, and ammonia solution were purchased from Avantor Performance Materials (Gliwice, Poland). Tween 20 was purchased from Acros Organics (Belgium, Geel), and castor oil was purchased from Carl Roth (Karlsruhe, Germany). The media ingredients were purchased from BTL Łódź (Lodz, Poland).
2.2. Microorganism and Culture Conditions
In this study, we used Yarrowia lipolytica strain KKP 379 from the Collection of Industrial Microorganisms at the Prof. Wacław Dąbrowski Institute of Agricultural and Food Biotechnology—State Research Institute (Warsaw, Poland). The strain was stored in cryovials containing ceramic beads with a cryoprotective agent at −20 °C (Protect Select, Technical Service Consultants Ltd., Heywood, UK).
Inoculum culture of Y. lipolytica was provided in Erlenmeyer flasks containing 50 mL YPG medium (10 g/L yeast extract, 20 g/L peptone, and 20 g/L glucose) at 27 °C for 24 h on a rotary shaker at the speed of 140 rpm. Biotransformation medium for GDL production was inoculated with the cells in logarithmic growth phase at an initial concentration of OD600 ≈ 0.25.
The composition of the medium for GDL production was as follows: 50 g or 75 g/L of castor oil, 20 g/L of peptone, and 5 g/L of Tween 20. The pH of the medium was corrected with a 25% ammonia solution. Batch cultures were provided in a 4 L operating volume bioreactor Bioflo 3000 (New Brunswick Scientific, Edison, NJ, USA) at 28 °C for about 44–64 h, mixed using the Rushton turbine, and aerated with compressed air at a flow of 100 L/h/L of medium. During culture, the following parameters were monitored: dissolved oxygen (DO2) in the medium, agitation speed, pH, and GDL concentration. Different modes of agitation speed, castor oil concentration (biotransformation substrate), and pH of medium were used in four yeast bioreactor cultures, according to the L4 orthogonal array for trials (Table 1 and Table 2). Each culture in the bioreactor was performed twice.

Table 1.
The L4 orthogonal array for bioreactor cultures.

Table 2.
Levels of factors used in the batch cultures of Y. lipolytica.
2.3. Gamma-Decalactone Extraction and Quantification
In order to determine the content of GDL, a sample of the medium was taken and homogenized for 3 min (approx. 4000 rpm) using an IKA T25 homogenizer (Königswinter, Germany). Next, 1.5 mL samples were collected. In order to maximize the yield of lactonisation of 4-hydroxydecanoic acid, HCl was used in an amount of 10 µL. γ-undecalactone was used as an internal standard and amounted to 20 µL. Lactone was extracted from the biotransformation medium with 1.5 mL diethyl ether. The organic phase was recovered and analyzed with gas chromatography (YL 6100 Young Lin Instrument, Anyang, Gyeonggi-do, Korea) equipped with a capillary column BPX (30 m × 0.25 mm) and a flame ionization detector (280 °C), with nitrogen as a gas vector, at a flow rate of 1.1 mL/min. The split injector temperature was changed as follows: 165 °C to 180 °C at a rate of 3 °C/min and 230 °C at a rate of 5 °C/min. Identification of γ-decalactone and γ-undecalactone was realized on the basis of their retention time (11.5 min and 12.4 min, respectively). All assays were performed in duplicate.
Kinetic parameters of GDL biosynthesis in a batch culture were calculated according to Papanikolaou and Aggelis [34].
2.4. Determination of Biomass Yield
Yeast cells were separated from culture medium by centrifugation (8000 rpm, 10 min, Centrifuge MPW-351R, Warsaw, Poland) and degreased by washing with acetone/ethanol (1:1, v/v) and then with distilled water. Biomass was dried at 105 °C until constant weight. Biomass yield was expressed as cell dry weight in a volume of 1 L of medium (g CDW/L).
2.5. Growth Parameters
For measuring the optical density, 1 mL of culture was centrifuged in an Eppendorf microcentrifuge (5418, ROTH). The supernatant was replaced with 1 mL of distilled water. Samples were diluted (OD between 0.7 and 1.3), and the light scattering property of the solution was measured with a UV-VIS THERMO Scientific Helios spectrophotometer (Waltham, MA, USA) at 600 nm (OD600). Then, the obtained value of the optical density was corrected with the dilution factor. To determine the number of yeast cells, 10 dilutions in sterile 0.85% NaCl were executed. Yeast cells were grown on solid YPD medium at 28 °C for 48 h.
2.6. Hydrophobicity of Cells
The surface hydrophobicity of cells of Y. lipolytica was evaluated using the MATH (Microbial Adhesion to Hydrocarbons) test according to Aguedo et al. [35]. Yeast cells were centrifuged (8000× g, 10 min; Centrifuge MPW-351R) and washed twice with phosphate buffer (0.1 M, pH 7). Then, the cells were resuspended in the buffer to an A600 of 0.70 ± 0.05 (A0). A tube was filled with a 5 mL sample of this suspension and 1 mL decane. The tube was vortexed for 60 s in order to form an emulsion and left for 15 min to allow the separation. The absorbance of the aqueous phase was measured (A). The hydrophobicity of cells was calculated according to the formula:
% adhesion = [(A0 − A)/A0] × 100%
2.7. Statistical Analysis
Four independent bioreactor cultures were carried out in the study, and three input parameters were tested, each at two levels: the concentration of the substrate, the pH regulation of the biotransformation medium, and the intensity of mixing. A tabulated set of a standard orthogonal array was designed (Table 1). The controlled factors used in the study were set at two levels presented in Table 2. After performing all 4 experiments, optimal parameters were determined using the method indicated by Genichi Taguchi [36]. “The larger, the better” method was applied. Higher values of the signal-to-noise ratio (S/N, Eta) identified control factor settings that minimized the effects of the noise factors. The goal was to maximize the response. The results were analyzed using STATISTICA 13.1 software equipped with software supplement DoE—Design of Experiment (TIBCO Software Inc., Palo Alto, CA, USA).
3. Results
3.1. Impact of Concentration of Castor Oil, pH of the Medium, and Agitation Speed on Synthesis of γ-Decalactone in the Batch Culture Carried Out in the Bioreactor
Four bioreactor batch cultures were carried out according to the Taguchi design method (Table 1) aimed at optimizing three parameters of the biotransformation process in terms of GDL synthesis. In each culture, apart from lactone concentration (Figure 1), yeast growth parameters were also monitored, namely optical density, biomass yield, and the number of yeast colonies, as well as their hydrophobicity, by performing the MATH test (Figure 2). These parameters were selected as significant based on literature data and our previous research on GDL synthesis [13,37].
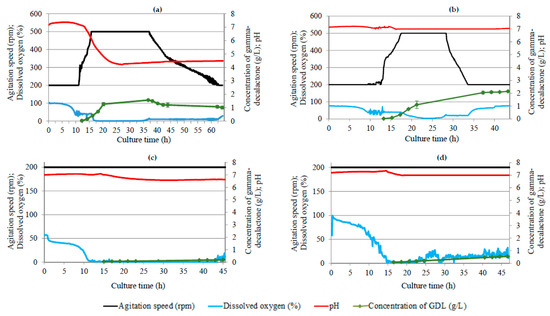
Figure 1.
Changes in dissolved oxygen, agitation speed, and concentration of γ-decalactone in the batch cultures of Y. lipolytica yeast carried out in accordance with L4 orthogonal array. Batch culture parameters: (a) bioreactor culture 1—agitation speed of 200–500 rpm, unregulated pH, castor oil concentration of 75 g/L; (b) bioreactor culture 2—agitation speed of 200–500 rpm, regulated pH, castor oil concentration of 50 g/L; (c) bioreactor culture 3—agitation speed of 200 rpm, regulated pH, castor oil concentration of 50 g/L; (d) bioreactor culture 4—agitation speed of 200 rpm, regulated pH, castor oil concentration of 75 g/L.
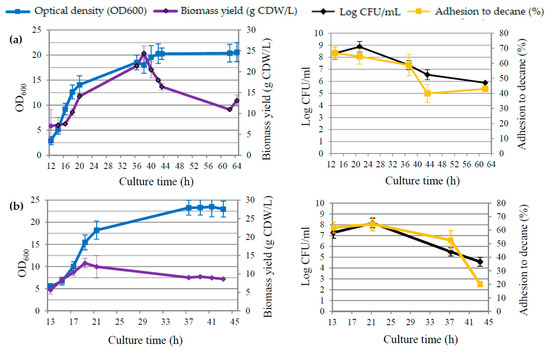
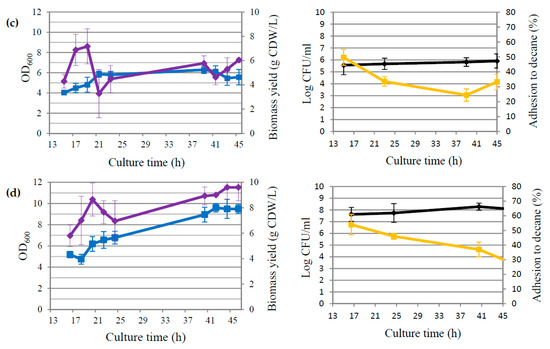
Figure 2.
Growth curves illustrating of either OD600 readings (blue line) and biomass yield (violet line) or viable cell counts (CFU/mL) (black line) and percentage of adhesion to decane (yellow line) for culture 1 (a), culture 2 (b), culture 3 (c), and culture 4 (d).
In the first batch culture (Figure 1a), the substrate (castor oil) concentration was 75 g/L, a variable agitation rate ranging from 200 to 500 rpm was used, and the substrate pH initially at 7 was not corrected throughout the process cycle. The use of such parameters made it possible to obtain a maximum of 1.56 ± 0.04 g/L of GDL in 37 h of biotransformation. On the first day of the biotransformation, the concentration of lactone increased exponentially, reaching the level of 1.26 ± 0.13 g/L already in the 20th hour of the reaction. After reaching the maximum (37 h), the concentration decreased, reaching the value of 1.01 ± 0.11 g/L in the 64th hour of cultivation. Literature data confirm a decrease in GDL concentration in biotransformation reactions conducted by wild-type yeast strain Y. lipolytica [13,23,38]. Therefore, in order to fully capture the kinetics of γ-decalactone biosynthesis by Yarrowia yeast, cultures in bioreactor 1 were performed for 64 h. Observing the maximum concentration of the aroma compound at about 37 h of cultivation, the time of biotransformation reactions in subsequent bioreactors was reduced to two days.
When analyzing the changes in GDL concentration in relation to the process parameters, it can be noticed that only after lowering the pH value below 5 in the medium, the concentration of GDL exceeding 1 g/L was recorded. The maximum concentration of lactone occurred during the period of intensive mixing (500 rpm), with the dissolved oxygen content in the medium at the level of approx. 10%. The parameters of yeast growth in bioreactor 1 (Figure 2a) indicated that on the first day of the experiment, the yeast cells were in the log phase. The OD increased dynamically, reaching the value of 14.06 ± 1.79 in 20 h. During the second day of biotransformation, the cells went into the stationary phase, and since the 40th hour of cultivation, the OD value remained at approx. 20. The biomass yield correlated with the OD values. Within 38 h of culture, a more than threefold increase in cellular biomass was observed, from the level of 7.22 ± 1.87 g CDW/L in the 14th hour of culture to 24.43 ± 1.73 g CDW/L in 38 h. The number of cells in the initial period of the exponential phase, expressed as log CFU/mL, was less than 6 (16 h). After 48 h of culture, the number of colonies increased 100 times.
In the second bioreactor culture (Figure 1b), the acidity of the medium and the concentration of the introduced substrate were modified. The pH was kept constant at 7 throughout the biotransformation process, and the concentration of castor oil was reduced from 75 g/L to 50 g/L. This cultivation variant allowed us to obtain approx. 2.15 ± 0.04 g/L of GDL, which was an approx. 38% higher value in relation to bioreactor 1. The maximum concentration was recorded in 45 h of the reaction, and the cultivation was not carried out any longer. Therefore, it can be assumed that it was possible to obtain a higher concentration of lactone while extending the time of the biotransformation reaction. During this cultivation, the highest concentration of the fragrance compound was obtained, unlike for bioreactor 1, during slow mixing (200 rpm) and at a much higher concentration of dissolved oxygen in the medium, approx. 76%. Both the oxygenation level of the medium and the analyzed yeast growth parameters (Figure 2b) indicated that the highest observed concentration of lactone in the medium was at the final stage of the stationary phase. Comparing the data from bioreactors 1 and 2, it can be seen that on the first day of the experiment, the growth of yeast cells in both cultures was similar—after 21 h in bioreactor 2, the biomass yield was 11.95 ± 3.04 g CDW/L, while in bioreactor 1, it was 14.13 ± 0.04 g CDW/L. The number of live cells was also comparable, at the level of approx. 8–8.2 log CFU/mL. The difference in cell growth became apparent on the second day of biotransformation. In bioreactor 2, cells died faster, so that after 43 h, the living cell population had decreased by almost four log orders—to 4.58 ± 0.65 log CFU/mL. The hydrophobicity of yeast cells correlated with their growth phases. During the first 24 h of culture, the degree of cell adhesion to decane was over 60%, while at the end of the stationary/slow dying phase, it was only about 20.1% (42 h of culture).
The third bioreactor culture (Figure 1c) was carried out by modifying the agitation speed (set at a constant level of 200 rpm) and the pH of the medium (initial pH = 7, not corrected during the cultivation) relative to the second bioreactor. The castor oil concentration was 50 g/L. Under these conditions, the maximum recorded concentration of GDL was 0.19 ± 0.01 g/L (45 h of biotransformation). It was over 8 times lower than in the first culture and over 11 times lower than in the second culture. The concentration of dissolved oxygen in the medium was close to zero since the 12th hour of cultivation. Following the yeast growth parameters (Figure 2c), it is clearly visible that between 15 h and 23 h, there was a relatively small increase in OD compared to the previous bioreactor cultures, from the value of 4.04 ± 0.03 to 5.79 ± 0.94. After 39 h of culture, the optical density was only 6.30 ± 1.06. In this culture, the yield of yeast dry matter did not exceed 7.2 g CDW/L. The slight increase in biomass was accompanied by a small number of colonies. After 15 h of culture, the number of viable cells was 5.56 ± 0.28 log CFU/mL and during the second day of culture, it increased statistically insignificantly to the value of 5.91 ± 0.41 log CFU/mL. Between 15 h and 39 h, the hydrophobicity of Yarrowia cells decreased from 49.7 ± 5.3% to 24.4 ± 4.1%. This may be related to the very low content of dissolved oxygen in the medium [39].
Figure 1d shows the data on the fourth bioreactor setting. This culture used a constant pH of 7 (throughout the cultivation period) and a constant agitation speed of 200 rpm, with a castor oil content of 75 g/L. As a result of the fourth biotransformation, 0.55 ± 0.01 g/L of lactone was obtained in 46 h. Between 16 h and 25 h, the concentration of GDL increased from 0.09 ± 0.01 g/L to 0.20 ± 0.01 g/L, and the level of dissolved oxygen in the medium was 2–3%. On the second day of the reaction, the lactone concentration doubled (0.46 ± 0.02 g/L), and the dissolved oxygen concentration in the medium was about 15–20%, which was more conducive to lactone synthesis. With regard to the first and second biotransformations, the concentrations of lactone were nearly 3 and 4 times lower, respectively. Cells multiplied slightly better than in bioreactor 3, but significantly weaker compared to bioreactors 1 and 2. Optical density (Figure 2d) was correlated to the biomass yield. OD increased from 5.19 ± 0.32 on the first day of biotransformation to 9.60 ± 0.91 on the second day, and the biomass yield increased from 5.80 ± 0.85 g CDW/L to 9.60 ± 0.95 g CDW/L. As in culture 3, extending the reaction time resulted in a decrease in the hydrophobic properties of the cells from 54.2 ± 7.0% to 28.4 ± 4.2%.
Data are presented as the mean ± standard deviation of three replicates.
Comparing the kinetic parameters of all four cultures (Table 3), it can be noticed that the intensity of mixing and aeration of the medium were important in cell proliferation as well as in lactone biosynthesis [40,41,42]. In the first two cultures, in which a variable speed of agitation was used, taking into account the level of dissolved oxygen in the medium, better results of GDL biosynthesis were obtained. The best parameters for both conversion yield of GDL per biomass formed (YL/X) or per carbon substrate (YL/S) and for volumetric rate of GDL production (qLv) or specific rate of GDL production (qL) were obtained in bioreactor 2. The yield of GDL synthesis in terms of carbon substrate in this culture was 0.043 g/g and was twice as high than in culture 1 and more than 10 times higher than in culture 3. Comparing the volumetric rate of GDL production (qLv) g/L/h in individual cultures, it can be seen that between the most efficient and the least efficient biotransformation, the volumetric rate was 12.5 times lower. Similarly, in the case of the specific rate of GDL production efficiency, between the best culture (bioreactor 2) and the worst (bioreactor 3), the specific rate of GDL production differed by 6.5-fold.

Table 3.
Kinetic parameters of γ-decalactone biosynthesis in batch bioreactor cultures of Y. lipolytica, according to the L4 orthogonal array.
3.2. Evaluation of Optimal Conditions for γ-Decalactone Biosynthesis in Bioreactor Culture Means of the Taguchi Statistical Design Method
Performing four experiments in accordance with the assumptions of the Taguchi method of experimental design allowed for the selection of optimal parameters of GDL biosynthesis. The statistical analysis took into account the influence of three parameters, substrate acidity, mixing speed, and substrate concentration (castor oil), on the following initial values: maximum GDL concentration in the medium, dry biomass yield, and yeast cell viability. The results of the statistical analysis are visualized graphically in the form of mean Eta values, marginal means, informing about the input values for which it is forecast to obtain the most desirable value of the selected output parameter. Figure 3 shows the average Eta values obtained on the second day of the biotransformation reaction, on average after 45 h. The data in Figure 3a show that the most important factor influencing the increase in GDL concentration in the medium was the intensity of mixing. Variable mixing speed (200–500 rpm) allowed us to avoid complete oxygen depletion in the substrate, and ensuring adequate oxygenation of the substrate increases the efficiency of biotransformation of castor oil to GDL, because oxygen is a substrate of the β-oxidation pathway. The remaining factors ensuring the maximum concentration of lactone are presented in Table 4. The optimal parameters were revealed during the Taguchi design experiment. Taking into account the obtained cellular biomass yield (Figure 3b), the substrate concentration was the most important factor. Castor oil was the only source of carbon in the media, necessary for the growth of microorganisms and maintenance of vital functions, and was a substrate of the β-oxidation pathway leading to the synthesis of GDL. From the point of view of cell viability (Figure 3c), the optimal conditions were natural acidity at pH 7, no regulation, constant intensity of mixing, and a substrate concentration of 75 g/L.
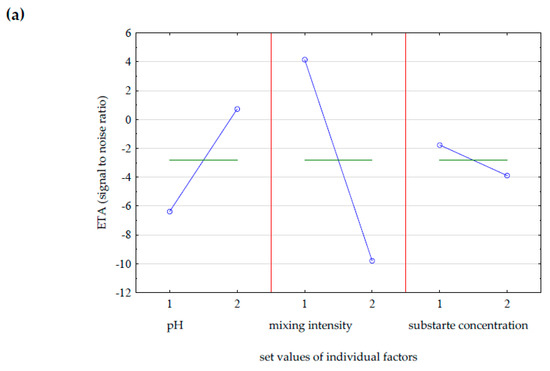
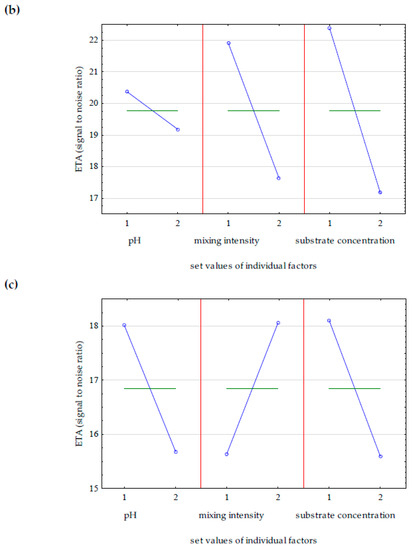
Figure 3.
The influence of individual factors on the Eta value calculated as the larger, the better when the goal was to maximize (a) the concentration of γ-decalactone, (b) the biomass yield, and (c) the number of living cells of Y. lipolytica in batch culture. The values of the controlled factors used in the batch cultures of Y. lipolytica are described in Table 2.

Table 4.
Indication of parameters in S/N group, “the larger, the better”, under which the concentration of γ-decalactone in biotransformation medium at 45 h of Y. lipolytica batch can reach the highest values.
To verify the accuracy of the indicated parameters, a fifth biotransformation reaction was performed. In the bioreactor culture, the parameters presented in Table 4 were used as optimal in obtaining the highest concentration of lactone. The course of this culture along with the obtained GDL concentration is shown in Figure 4. The data analysis confirmed that the statistically selected culture parameters allowed for more efficient biotransformation. The concentration of GDL in this reaction was approx. 36% higher than that of the best bioreactor culture from the first stage (Figure 1b). In this experiment, after 24 h of reaction, over 2 g of lactone/L was obtained, and in 41 h, the concentration of lactone was close to 3 g/L (2.93 ± 0.33 g/L).
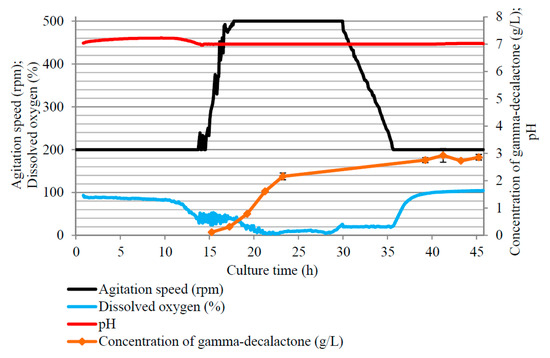
Figure 4.
Changes in dissolved oxygen, agitation speed, and concentration of γ-decalactone in the batch culture of Y. lipolytica yeast carried out in accordance with parameters of Table 4 (agitation speed of 200–500 rpm, regulated pH, castor oil concentration of 75 g/L).
The kinetic parameters for this biotransformation reaction (Table 5) confirm the validity of the assumptions of the Taguchi method. The conversion yield of GDL per biomass formed (YL/X) was higher than the most efficient reaction from the previous stage (Table 3) by approx. 8%. It was similar in the case of the volumetric rate of GDL production (qLv) and the specific rate of GDL production (qL)—these parameters were higher in this reaction by approx. 42% and 13%, respectively. Only the conversion yield of GDL per carbon substrate (YL/S) decreased in this culture.

Table 5.
Kinetic parameters of γ-decalactone biosynthesis in a bioreactor batch culture of Y. lipolytica, according to the parameters in Table 4, determined on the basis of the Taguchi method.
4. Discussion
Three factors with two levels were chosen for the optimization of GDL production by the Taguchi method as a fractional factorial experiment design. The conducted bioreactor cultures, supported by the Taguchi method, confirmed that the selection of appropriate cultivation conditions has a significant impact on the level of GDL biosynthesis. One of the factors determining the concentration of lactone in the medium is the carbon source. Biotransformations leading to the production of GDL described in the literature use castor oil, ricinoleic acid, or methyl ricinoleate [18]. These compounds are sources of carbon in the culture medium, necessary for the growth of microorganisms and maintenance of vital functions, as well as activators for enzymes in the pathway of GDL synthesis. The most widely used is castor oil (as in our research), a cheap, non-toxic, and readily available substrate. Moradi et al. [22] optimized the synthesis of GDL by Y. lipolytica cells using castor oil at the concentration of 10–40 mL/L (v/v). The results of their research indicated that the highest concentration of aroma, at the level of approx. 0.053 g/L, occurred at the dose of castor oil in the range of 10–25 mL/L. The yeast Rhodotorula aurantiaca A19 synthesized within 10 days of the biotransformation of 5.5 g/L, at the oil dose of 20 g/L. The concentration of lactone in the culture of these microorganisms did not increase in proportion to the concentration of the substrate. Higher concentrations of castor oil, in the range of 30–60 g/L, reduced the level of aroma synthesis [20]. Different conclusions result from the studies by Braga and Belo [23]. According to the authors, the use of higher concentrations of hydrophobic substrate, on the order of 60 g/L, in a 25 h cultivation of Yarrowia yeast, enables the synthesis of approx. 5.4 g/L. At lower oil concentrations, a decrease in lactone concentration was observed after some time, until it was completely depleted. A similar relationship was observed in the culture of Y. lipolytica Y-VTP5, where the concentration of 100 g/L made it possible to obtain 1.3 g/L of GDL [43]. In the case of ricinoleic acid, it has been shown that its high concentration may inhibit cell growth and, indirectly, lactone synthesis [22].
As shown by the Taguchi method, the factor that mainly influenced the GDL biosynthesis was the intensity of mixing. Appropriate mixing speed supports the proper oxygenation of the substrate and ensures the dispersion of the hydrophobic substrate (castor oil) in the water phase. The contact surface of fat droplets with the cells of multiplied organisms and the contact surface between the two liquid phases is considered to be a factor determining the degradation of the hydrophobic substrate, and thus the growth of cells and their aroma production [44]. Indications of the Taguchi method, confirmed by bioreactor culture (Figure 4, Table 5), showed that in the analyzed range of three parameters, the highest concentration of lactone was obtained at a variable mixing speed in the range of 200–500 rpm. This is also supported by literature data. Variable stirring speed, ranging from 300 to 600 rpm, used to maintain the proper level of dissolved oxygen in the medium, allowed the fed-batch culture of Y. lipolytica to obtain the highest concentration of 70 mg/L [45]. Mixing at the level of 600 rpm was optimal for productivity and obtaining the maximum concentration of GDL in the study by Gomes et al. [46]. Under these conditions, more than 1 g of lactone per liter of medium was obtained, and the yield was at the level of 87.6 mg/L. The authors report that faster mixing provides a better oxygen transfer to the medium. Similar relationships were shown in the study by Aguedo et al. [40]. The most advantageous in terms of GDL production (110 mg/L) was the mixing speed of 600 rpm (with 0.9 vvm aeration). With 300 rpm slower mixing, the product concentration decreased by nearly 30% (80 mg/L). Increasing the mixing speed is not always associated with increasing the lactone concentration in the medium. However, it may increase its volumetric rate of GDL production. This is evidenced by the team of Braga and Belo [23], who, by increasing the mixing intensity from 400 to 600 rpm, observed a 4.5-fold increase in the aroma productivity (from 16 to 75 mg/L). The different speed of mixing did not affect the differentiation of the emulsification level, but it ensured better dispersion of air bubbles in the medium, increasing the interfacial surface. The emulsification of the medium is extremely important from the point of view of yeast growth (including growth rate). In a non-homogeneous medium, the lag phase can be even 60% longer [47]. The effects of worse homogenization of the substrates, resulting from low mixing values (200 rpm), on the growth of yeast and the duration of the lag phase can be seen in Figure 1 and Table 3. The yield of biomass in these cultures did not exceed 10 g CDW/L, and the lag phase lasted more than 10 h (Figure 1c,d).
The lower efficiency of GDL biosynthesis in the media with agitation at the level of 200 rpm may also be the result of the lower hydrophobicity of yeast cells from these cultures (Figure 2c,d). In biotransformation reactions, there is direct contact of yeast cells with the substrate by adhesion of microorganisms to the surface of larger droplets or by adsorption of smaller droplets on the surface of yeast cells. Lower cell hydrophobicity results in lower levels of intermolecular interactions [35,44].
The third parameter influencing the efficiency of GDL biosynthesis, according to the indicated Taguchi methods, is the pH of the substrate. Acidity influences the activity of enzymes in the β-oxidation pathway. Moreover, in the granulometric tests, it was proved that the size of hydrophobic substrate droplets in the medium also depends on the medium acidity—the droplet size decreases proportionally with the decrease in pH [48]. The optimal pH of lactone biosynthesis depends on the species and type of microorganisms. According to Gomes et al. [28], the yeast Y. lipolytica, despite the different pH of the medium, can regulate the relatively constant intracellular pH. Garcia et al. [21] report that the optimal pH in terms of lactone synthesis by Y. lipolytica W29 is approx. 6.35. Higher acidity of the substrate (pH = 4.45 and lower) combined with a low concentration of dissolved oxygen selectively switches to other pathways in the β-oxidation cycle and affects acyl-CoA hydratase and 3-hydroxy-acyl-CoA dehydrogenase, resulting in 3-hydroxy-gamma-decalactone, dec-2-en-4-olide, and dec-3-en-4-olide. Timoumi et al. [49] also draw attention to the fact that pH influences the morphological changes in Yarrowia lipolytica cells, and thus indirectly their metabolic activity. At pH 7 (used in the cultures described in this article), specifically in batch bioreactors where cells proliferate at their maximum growth rate, mycelia are mainly formed.
5. Conclusions
Research on biotechnological synthesis of γ-decalactone has been conducted for several years and is aimed increasing biosynthesis efficiencies. The influence of many factors (including the biphasic nature of the substrate) on the growth and catalytic activity of microorganisms makes it still difficult to optimize the processes. Complex experimental designs are being used in attempts to determine the optimal conditions for GDL biosynthesis. The Taguchi robust design method used in this study allowed us to determine the influence of three factors (castor oil concentration, pH regulation of growth medium, and mixing intensity) on the lactone yield. A relatively small number of provided experiments can be underlined as an advantage in the proposed approach. Improved GDL recovery was achieved and the facilitated process parameters were tested in bioreactor culture. The concentration of the fragrance compound was 1.4 to 15 times higher than in non-optimized cultures and amounted to 2.93 g/L. The volumetric rate and specific rate of GDL production were higher than in many of the quoted literature examples. This confirms the correctness of comprehensive process optimization. The use of the Taguchi method allowed us to identify the most important factors influencing the analyzed process parameters and prioritize factors having the greatest impact on the lactone concentration obtained in the biotransformation, which was the intensity of mixing. It could also be concluded that the yield of yeast dry matter most strongly depended on the concentration of the lipid substrate in growth medium. The future application of a robust design could consider other factors affecting biotransformation yield, e.g., the intensity of oxygenation and the toxic effect of metabolites. Attempts in the field of fed-batch cultures with immobilized cells should also be considered.
Author Contributions
Conceptualization, J.M.; methodology, J.M. and A.F.; validation, J.M.; formal analysis, J.M.; investigation, J.M. and S.K.; resources, J.M. and D.N.; writing—original draft preparation, J.M.; writing—review and editing, J.M. and A.F.; visualization, J.M., S.K. and A.F.; supervision, J.M. All authors have read and agreed to the published version of the manuscript.
Funding
The study was financially supported by sources of the Ministry of Education and Science within funds of the Institute of Food Sciences of Warsaw University of Life Sciences (WULS) for scientific research.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author (J.M.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- GlobeNewswire: Food Flavors Market to Reach USD 19.72 Billion by 2026. Available online: https://www.globenewswire.com/news-release/2019/10/10/1928250/0/en/Food-Flavors-Market-To-Reach-USD-19-72-Billion-By-2026-Reports-And-Data.html (accessed on 15 August 2022).
- Flavors and Fragrances Market Size, Share & Trends Analysis Report, 2019–2025. Available online: https://www.grandviewresearch.com/industry-analysis/flavors-fragrances-market (accessed on 15 August 2022).
- Braga, A.; Guerreiro, C.; Belo, I. Generation of flavors and fragrances through biotransformation and de novo synthesis. Food Bioprocess Technol. 2018, 11, 2217–2228. [Google Scholar] [CrossRef]
- Sales, A.; Paulino, B.N.; Pastore, G.M.; Bicas, J.L. Biogeneration of aroma compounds. Curr. Opin. Food Sci. 2018, 19, 77–84. [Google Scholar] [CrossRef]
- Paulino, B.N.; Sales, A.; Felipe, L.; Pastore, G.M.; Molina, G.; Bicas, J.L. Recent advances in the microbial and enzymatic production of aroma compounds. Curr. Opin. Food Sci. 2021, 37, 98–106. [Google Scholar] [CrossRef]
- Blin-Perrin, C.; Molle, D.; Duffose, L.; Le-Quere, J.L.; Viel, C.; Mauvais, G.; Feron, G. Metabolism of ricinoleic acid into γ-decalactone: β-oxidation and long chain acyl intermediates of ricinoleic acid in the genus Sporidiobolus sp. FEMS Microbiol. Lett. 2000, 188, 69–74. [Google Scholar] [CrossRef]
- Romero-Guido, C.; Belo, I.; Ta, T.M.N.; Cao-Hoang, L.; Alchihab, M.; Gomes, N.; Thonart, P.; Teixeira, J.A.; Destain, J.; Waché, Y. Biochemistry of lactone formation in yeast and fungi and its utilisation for the production of flavor and fragrance compounds. Appl. Microbiol. Biotechnol. 2011, 89, 535–547. [Google Scholar] [CrossRef]
- AL Mualad, W.N.A.; Bouchedja, D.N.; Selmania, A.; Maadadi, R.; Ikhlef, A.; Kabouche, Z.; Elmechta, L.; Boudjellal, A. Yeast Yarrowia lipolytica as a biofactory for the production of lactone-type aroma gamma-decalactone using castor oil as substrate. Chem. Pap. 2022. [Google Scholar] [CrossRef]
- Małajowicz, J.; Kozłowska, M. Factors affecting the yield in formation of fat-derived fragrance compounds by Yarrowia lipolytica yeast. Appl. Sci. 2021, 11, 9843. [Google Scholar] [CrossRef]
- Okui, S.; Uchiyama, M.; Mizugaki, M. Metabolism of hydroxy fatty acids: 2 Intermediates of the oxidative breakdown of ricinoleic acid by Genus Candida. J. Biochem. 1963, 54, 536–540. [Google Scholar] [CrossRef]
- Guan, S.; Rong, S.; Wang, M.; Cai, B.; Li, Q.; Zhang, S. Enhanced biotransformation productivity of gamma-decalactone from ricinoleic acid based on the expanded vermiculite delivery system. J. Microbiol. Biotechnol. 2019, 29, 1071–1077. [Google Scholar] [CrossRef]
- Rong, S.; Yang, S.; Li, Q.; Cai, B.; Guan, S.; Wang, J.; Zhou, Y.; Chen, Y. Improvement of γ-decalactone production by stimulating the import of ricinoleic acid and suppressing the degradation of γ-decalactone in Saccharomyces cerevisiae. Biocatalal. Biotransfor. 2017, 35, 96–102. [Google Scholar] [CrossRef]
- Małajowicz, J.; Nowak, D.; Fabiszewska, A.; Iuliano, A. Comparison of gamma-decalactone biosynthesis by yeast Yarrowia lipolytica MTLY40-2p and W29 in batch-cultures. Biotechnol. Biotechnol. Equip. 2020, 34, 330–340. [Google Scholar] [CrossRef]
- Narayana, A.V.; Sumalatha, B.; Swamy, A.V.N.; Chary, G.H.V.C. Production of γ-decalactone through fermentation: A review. Int. J. Mod. Agric. 2021, 10, 2189–2192. [Google Scholar]
- Alchihab, M.; Aldric, J.M.; Aguedo, M.; Destain, J.; Wathelet, J.P.; Thonart, P. The use of Macronet resins to recover γ-decalactone produced by Rhodotorula aurantiaca from the culture broth. J. Ind. Microbiol. Biotechnol. 2010, 37, 167–172. [Google Scholar] [CrossRef]
- Kothari, S.D.; Vadgama, R.N.; Bhat, K.H.; Lali, A.M.; Odaneth, A.A. Process optimization for production and purification of γ-decalactone from ricinoleic acid using Yarrowia lipolytica. Biocatal. Agric. Biotechnol. 2022, 39, 1–11. [Google Scholar] [CrossRef]
- Małajowicz, J.; Górska, A.; Bryś, J.; Ostrowska-Ligęza, E.; Wirkowska-Wojdyła, M. Attempt to develop an effective method for the separation of gamma-decalactone from biotransformation medium. Appl. Sci. 2022, 12, 2084. [Google Scholar] [CrossRef]
- Alchihab, M.; Destain, J.; Aguedo, M.; Thonart, P. Production d’arômes de type lactone par des levures. Biotechnol. Agron. Soc. Environ. 2010, 14, 681–691. [Google Scholar]
- Lee, S.L.; Lin, S.J.; Chou, C.C. Growth of and production of γ-decalactone by Sporobolomyces odorus in jar fermentors as affected by pH, aeration and fed-batch technique. J. Ferment. Bioeng. 1995, 80, 195–199. [Google Scholar] [CrossRef]
- Alchihab, M.; Destain, J.; Aguedo, M.; Majad, L.; Ghalfi, H.; Wathelet, J.P.; Thonart, P. Production of γ-decalactone by a psychrophilic and a mesophilic strain of the yeast Rhodotorula aurantiaca. Appl. Biochem. Biotechnol. 2009, 158, 41–50. [Google Scholar] [CrossRef]
- García, E.E.; Belin, J.M.; Waché, Y. Use of a Doehlert factorial design to investigate the effects of pH and aeration on the accumulation of lactones by Yarrowia lipolytica. J. Appl. Microbiol. 2007, 103, 1508–1515. [Google Scholar] [CrossRef]
- Moradi, H.; Asadollahi, M.A.; Nahvi, I. Optimization of gamma-decalactone production by yeast Yarrowia lipolytica using the Taguchi method. J. Microbiol. Biotechnol. Food Sci. 2016, 6, 685–688. [Google Scholar] [CrossRef][Green Version]
- Braga, A.; Belo, I. Production of γ-decalactone by Yarrowia lipolytica: Insights into experimental conditions and operating mode optimization. J. Chem. Technol. Biotechnol. 2015, 90, 559–565. [Google Scholar] [CrossRef]
- Darvishi, F.; Meidani, A.K. Optimization of gamma-decalactone production by Yarrowia lipolytica mutant strain via Response surface methodology. Modares J. Biotechnol. 2021, 12, 13–31. [Google Scholar]
- Pereira de Andrade, D.; Carvalho, B.F.; Schwan, R.F.; Dias, D.R. Production of γ-decalactone by yeast strains under different conditions. Food Technol. Biotechnol. 2017, 55, 225–230. [Google Scholar] [CrossRef]
- Braga, A.; Mesquita, D.P.; Amaral, A.L.; Ferreira, E.C.; Belo, I. Aroma production by Yarrowia lipolytica in airlift and stirred tank bioreactors: Differences in yeast metabolism and morphology. Biochem. Eng. J. 2015, 93, 55–62. [Google Scholar] [CrossRef]
- Puthli, M.S.; Rathod, V.K.; Pandit, A.B. Gaz-liquid mass transfer studies with triple impeller system on a laboratory scale bioreactor. Biochem. Eng. J. 2005, 23, 25–30. [Google Scholar] [CrossRef]
- Gomes, N.; Teixeira, J.A.; Belo, I. Empirical modelling as an experimental approach to optimize lactone production. Catal. Sci. Technol. 2011, 1, 86–92. [Google Scholar] [CrossRef][Green Version]
- Rahul, D.; Pretesh, J. Application of Taguchi-Based Design of Experiments for Industrial Chemical Processes. In Statistical Approaches with Emphasis on Design of Experiments Applied to Chemical Processes; IntechOpen: London, UK, 2018; Chapter 9. [Google Scholar]
- Rong, L.; Liu, S.; Zhu, K.; Kong, J.; Miao, L.; Wang, S.; Xiao, D.; Yu, A. Production of carboxylic acids by metabolically engineered Yarrowia lipolytica: A review. Sheng Wu Gong Cheng Xue Bao 2022, 38, 1360–1372. [Google Scholar] [CrossRef]
- Jach, M.E.; Malm, A. Yarrowia lipolytica as an alternative and valuable source of nutritional and bioactive compounds for humans. Molecules 2022, 27, 2300. [Google Scholar] [CrossRef]
- Fickers, P.; Marty, A.; Nicaud, J.M. The Lipases from Yarrowia lipolytica: Genetics, production, regulation, biochemical characterization and biotechnological applications. Biotechnol. Adv. 2011, 29, 632–664. [Google Scholar] [CrossRef]
- Colacicco, M.; Ciliberti, C.; Agrimi, G.; Biundo, A.; Pisano, I. Towards the physiological understanding of Yarrowia lipolytica growth and lipase production using waste cooking oils. Energies 2022, 15, 5217. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Lipids of oleaginous yeasts. Part II: Technology and potential applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1052–1073. [Google Scholar] [CrossRef]
- Aguedo, M.; Waché, Y.; Coste, F.; Husson, F.; Belin, J.M. Impact of surfactants on the biotransformation of methyl ricinoleate into γ-decalactone by Yarrowia lipolytica. J. Mol. Catal. B Enzym. 2004, 29, 31–36. [Google Scholar] [CrossRef]
- Taguchi, G.; Konishi, S. Orthogonal Arrays and Linear Graphs; American Supplier Institute Inc.: Dearborn, MI, USA, 1987. [Google Scholar]
- Białecka-Florjańczyk, E.; Małajowicz, J.; Dudziak, B. Chemical and biotechnological synthesis of gamma-decalactone. Przem. Chem. 2016, 95, 999–1005. [Google Scholar] [CrossRef]
- Waché, Y.; Aguedo, M.; LeDall, M.T.; Nicaud, J.M.; Belin, J.M. Optimization of Yarrowia lipolytica’s β-oxidation pathway for γ-decalactone production. J. Mol. Catal. B Enzym. 2002, 19–20, 347–351. [Google Scholar] [CrossRef]
- Escamilla-García, E.; O’Riordana, S.; Gomes, N.; Aguedo, A.; Belo, I.; Teixeira, J.A.; Belina, J.M.; Wache, Y. An air-lift biofilm reactor for the production of γ-decalactones by Yarrowia lipolytica. Process Biochem. 2014, 49, 1377–1382. [Google Scholar] [CrossRef]
- Aguedo, M.; Gomes, N.; García, E.E.; Waché, Y.; Mota, M.; Teixeira, J.A.; Belo, I. Decalactone production by Yarrowia lipolytica under increased O2 transfer rates. Biotechnol. Lett. 2005, 27, 1617–1621. [Google Scholar] [CrossRef][Green Version]
- Gomes, N.; Aguedo, M.; Teixeira, J.A.; Belo, I. Oxygen mass transfer in a biphasic medium: Influence on the biotransformation of methyl ricinoleate into γ-decalactone by the yeast Yarrowia lipolytica. Biochem. Eng. J. 2007, 35, 380–386. [Google Scholar] [CrossRef]
- Gomes, N.; Teixeira, J.A.; Belo, I. Oxygen effect in γ-decalactone production through biotransformation of ricinoleic acid. In Proceedings of the 8th World Congress of Chemical Engineering, Montréal, QC, Canada, 23–27 August 2009. [Google Scholar]
- Le Do, T.T.; Vu, N.T.; Phan-Thi, H.; Cao-Hoang, L.; Ta, T.M.N.; Waché, Y.; Nguyen, T.H.T. Traditional fermented sausage ‘Nem chua’ as a source of yeast biocatalysts efficient for the production of the aroma compound γ-decalactone. Int. J. Food Sci. Technol. 2014, 49, 1099–1105. [Google Scholar] [CrossRef]
- Gomes, N.; Waché, Y.; Teixeira, J.A.; Belo, I. Oil-in-water emulsions characterization by laser granulometry and impact on γ-decalactone production in Yarrowia lipolytica. Biotechnol. Lett. 2011, 33, 1601–1606. [Google Scholar] [CrossRef][Green Version]
- Moradi, H.; Asadollahi, M.A.; Nahvi, I. Improved γ-decalactone production from castor oil by fed-batch cultivation of Yarrowia lipolytica. Biocatal. Agric. Biotechnol. 2013, 2, 64–68. [Google Scholar] [CrossRef]
- Gomes, N.; Teixeira, J.A.; Belo, I. The use of methyl ricinoleate in lactone production by Yarrowia lipolytica: Aspects of bioprocess operation that influence the overall performance. Biocatal. Biotransform. 2010, 28, 227–234. [Google Scholar] [CrossRef]
- Waché, Y.; Bergmark, K.; Courthaudon, J.L.; Aguedo, M.; Nicaud, J.M.; Belin, J.M. Medium-size droplets of methyl ricinoleate are reduced by cell-surface activity in the γ-decalactone production by Yarrowia lipolytica. Lett. Appl. Microbiol. 2000, 30, 183–187. [Google Scholar] [CrossRef][Green Version]
- Bouchedja, D.N.; Danthine, S.; Kar, T.; Fickers, P.; Sassi, H.; Boudjellal, A.; Blecker, C.; Delvigne, F. pH level has a strong impact on population dynamics of the yeast Yarrowia lipolytica and oil micro-droplets in multiphasic bioreactor. FEMS Microbiol. Lett. 2018, 365, fny173. [Google Scholar] [CrossRef]
- Timoumi, A.; Cléret, M.; Bideaux, C.; Guillouet, S.E.; Allouche, Y.; Molina-Jouve, C.; Fillaudeau, L.; Gorret, N. Dynamic behavior of Yarrowia lipolytica in response to pH perturbations: Dependence of the stress response on the culture mode. Appl. Microb. Cell Physiol. 2017, 101, 351–366. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).