Enzymatic Synthesis of Galacto-Oligosaccharides from Concentrated Sweet Whey Permeate and Its Application in a Dairy Product
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
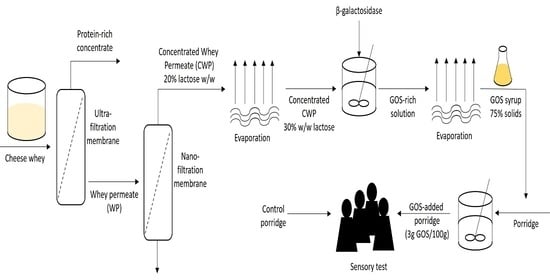
2.2. Concentrated Whey Permeate Preparation
2.3. GOS Production Kinetics
2.4. Carbohydrate Quantification
2.5. Analytical Methods
2.6. GOS Application in a Dairy Product
3. Results
3.1. Concentrated Whey Permeate
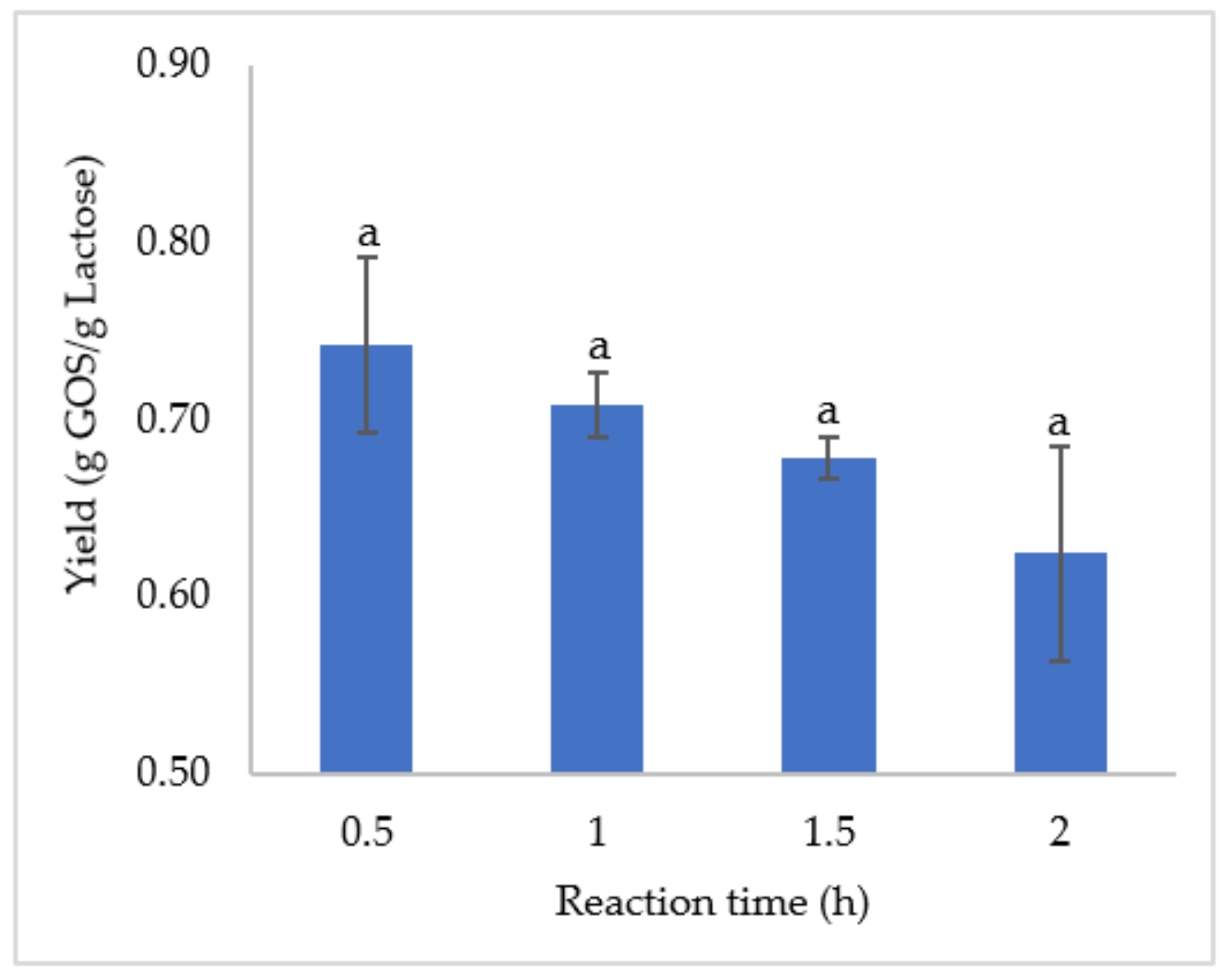
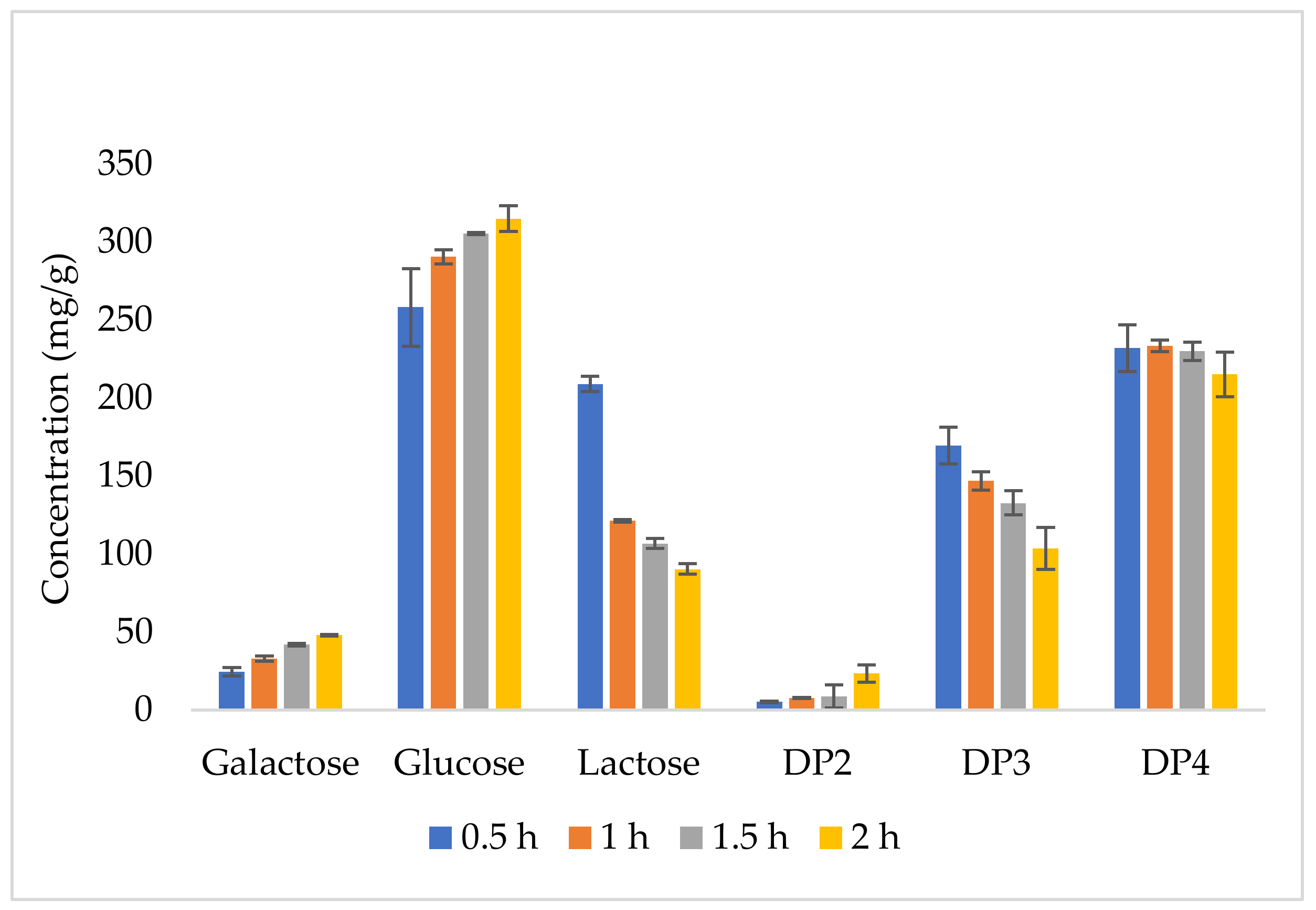
3.2. GOS Production Kinetics
3.3. GOS Application in a Dairy Product
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopes, A.C.A.; Eda, S.H.; Andrade, R.P.; Amorim, J.C.; Duarte, W.F. New Alcoholic Fermented Beverages—Potentials and Challenges. In Fermented Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Cambridge, UK, 2019; Volume 5, pp. 577–603. ISBN 9780128152713. [Google Scholar]
- Lu, L.; Xiao, M. Recent Progress on Galactooligosaccharides Synthesis by Microbial β-Galactosidase. In Functional Carbohydrates; Chen, J., Zhu, Y., Liu, S., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 147–182. ISBN 9781315371061. [Google Scholar]
- Román, A.; Wang, J.; Csanádi, J.; Hodúr, C.; Vatai, G. Experimental Investigation of the Sweet Whey Concentration by Nanofiltration. Food Bioprocess Technol. 2011, 4, 702–709. [Google Scholar] [CrossRef]
- Cuartas-Uribe, B.; Alcaina-Miranda, M.I.; Soriano-Costa, E.; Mendoza-Roca, J.A.; Iborra-Clar, M.I.; Lora-García, J. A Study of the Separation of Lactose from Whey Ultrafiltration Permeate Using Nanofiltration. Desalination 2009, 241, 244–255. [Google Scholar] [CrossRef]
- Eskandarloo, H.; Abbaspourrad, A. Production of Galacto-Oligosaccharides from Whey Permeate Using β-Galactosidase Immobilized on Functionalized Glass Beads. Food Chem. 2018, 251, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Sen, D.; Sarkar, A.; Bhattacharyya, S.; Bhattacharjee, C. A Comparative Study on the Production of Galacto-Oligosaccharide from Whey Permeate in Recycle Membrane Reactor and in Enzymatic Batch Reactor. Ind. Eng. Chem. Res. 2011, 50, 806–816. [Google Scholar] [CrossRef]
- Lamsal, B.P. Production, Health Aspects and Potential Food Uses of Dairy Prebiotic Galactooligosaccharides. J. Sci. Food Agric. 2012, 92, 2020–2028. [Google Scholar] [CrossRef]
- Boons, G.J. Synthetic Oligosaccharides: Recent Advances. Drug Discov. Today 1996, 1, 331–342. [Google Scholar] [CrossRef]
- Osman, A. Synthesis of Prebiotic Galacto-Oligosaccharides: Science and Technology. In Probiotics, Prebiotics, and Synbiotics: Bioactive Foods in Health Promotion; Watson, R., Preedy, V., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 135–154. ISBN 978-0-12-802189-7. [Google Scholar]
- Padilla, B.; Ruiz-Matute, A.I.; Belloch, C.; Cardelle-Cobas, A.; Corzo, N.; Manzanares, P. Evaluation of Oligosaccharide Synthesis from Lactose and Lactulose Using β-Galactosidases from Kluyveromyces Isolated from Artisanal Cheeses. J. Agric. Food Chem. 2012, 60, 5134–5141. [Google Scholar] [CrossRef]
- Carević, M.; Bezbradica, D.; Banjanac, K.; Milivojević, A.; Fanuel, M.; Rogniaux, H.; Ropartz, D.; Veličković, D. Structural Elucidation of Enzymatically Synthesized Galacto-Oligosaccharides Using Ion-Mobility Spectrometry-Tandem Mass Spectrometry. J. Agric. Food Chem. 2016, 64, 3609–3615. [Google Scholar] [CrossRef]
- Urrutia, P.; Bernal, C.; Wilson, L.; Illanes, A. Improvement of Chitosan Derivatization for the Immobilization of Bacillus Circulans β-Galactosidase and Its Further Application in Galacto-Oligosaccharide Synthesis. J. Agric. Food Chem. 2014, 62, 10126–10135. [Google Scholar] [CrossRef]
- Tzortzis, G.; Goulas, A.K.; Gibson, G.R. Synthesis of Prebiotic Galactooligosaccharides Using Whole Cells of a Novel Strain, Bifidobacterium Bifidum NCIMB 41171. Appl. Microbiol. Biotechnol. 2005, 68, 412–416. [Google Scholar] [CrossRef]
- Torres, D.P.M.; Gonçalves, M.D.P.F.; Teixeira, J.A.; Rodrigues, L.R. Galacto-Oligosaccharides: Production, Properties, Applications, and Significance as Prebiotics. Compr. Rev. Food Sci. Food Saf. 2010, 9, 438–454. [Google Scholar] [CrossRef]
- Otieno, D.O. Synthesis of β-Galactooligosaccharides from Lactose Using Microbial β-Galactosidases. Compr. Rev. Food Sci. Food Saf. 2010, 9, 471–482. [Google Scholar] [CrossRef]
- Depeint, F.; Tzortzis, G.; Vulevic, J.; I’Anson, K.; Gibson, G.R. Prebiotic Evaluation of a Novel Galactooligosaccharide Mixture Produced by the Enzymatic Activity of Bifidobacterium Bifidum NCIMB 41171, in Healthy Humans: A Randomized, Double-Blind, Crossover, Placebo-Controlled Intervention Study. Am. J. Clin. Nutr. 2008, 87, 785–791. [Google Scholar] [CrossRef]
- Liu, Y.; Gibson, G.R.; Walton, G.E. An in Vitro Approach to Study Effects of Prebiotics and Probiotics on the Faecal Microbiota and Selected Immune Parameters Relevant to the Elderly. PLoS ONE 2016, 11, e0162604. [Google Scholar] [CrossRef] [PubMed]
- Nath, A.; Haktanirlar, G.; Varga, Á.; Molnár, M.A.; Albert, K.; Galambos, I.; Koris, A.; Vatai, G. Biological Activities of Lactose-Derived Prebiotics and Symbiotic with Probiotics on Gastrointestinal System. Medicina 2018, 54, 18. [Google Scholar] [CrossRef] [PubMed]
- Oozeer, R.; Van Limpt, K.; Ludwig, T.; Ben Amor, K.; Martin, R.; Wind, R.D.; Boehm, G.; Knol, J. Intestinal Microbiology in Early Life: Specific Prebiotics Can Have Similar Functionalities as Human-Milk Oligosaccharides. Am. J. Clin. Nutr. 2013, 98, 561S–571S. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, V.; Tomar, S.K.; Singh, R.R.B.; Singh, A.K.; Ali, B. Galactooligosaccharides: Novel Components of Designer Foods. J. Food Sci. 2011, 76, R103–R111. [Google Scholar] [CrossRef]
- Wang, Y. Prebiotics: Present and Future in Food Science and Technology. Food Res. Int. 2009, 42, 8–12. [Google Scholar] [CrossRef]
- Fischer, C.; Kleinschmidt, T. Synthesis of Galactooligosaccharides in Milk and Whey: A Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 678–697. [Google Scholar] [CrossRef]
- Ebeling, M.E. The Dumas Method for Nitrogen in Feeds. J. AOAC Int. 1968, 51, 766–770. [Google Scholar] [CrossRef]
- Maubois, J.L.; Lorient, D. Dairy Proteins and Soy Proteins in Infant Foods Nitrogen-to-Protein Conversion Factors. Dairy Sci. Technol. 2016, 96, 15. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 14th ed.; Association of Analytical Chemists: Washington, DC, USA, 1985. [Google Scholar]
- Mukhopadhyay, R.; Talukdar, D.; Chatterjee, B.P.; Guha, A.K. Whey Processing with Chitosan and Isolation of Lactose. Process Biochem. 2003, 39, 381–385. [Google Scholar] [CrossRef]
- Piggott, J.R.; Simpson, S.J.; Williams, S.A.R. Sensory Analysis. Int. J. Food Sci. Technol. 1998, 33, 7–18. [Google Scholar] [CrossRef]
- Splechtna, B.; Nguyen, T.H.; Steinböck, M.; Kulbe, K.D.; Lorenz, W.; Haltrich, D. Production of Prebiotic Galacto-Oligosaccharides from Lactose Using β-Galactosidases from Lactobacillus Reuteri. J. Agric. Food Chem. 2006, 54, 4999–5006. [Google Scholar] [CrossRef] [PubMed]
- Van Leusen, E.; Torringa, E.; Groenink, P.; Kortleve, P.; Geene, R.; Schoterman, M.; Klarenbeek, B. Industrial Applications of Galactooligosaccharides. In Food Oligosaccharides: Production, Analysis and Bioactivity; Moreno, F.J., Sanz, M.L., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2014; pp. 470–491. ISBN 978-1-118-42649-4. [Google Scholar]
- Kaur, N.; Sharma, P.; Jaimni, S.; Kehinde, B.A.; Kaur, S. Recent Developments in Purification Techniques and Industrial Applications for Whey Valorization: A Review. Chem. Eng. Commun. 2019, 207, 123–138. [Google Scholar] [CrossRef]
- Hackenhaar, C.R.; Spolidoro, L.S.; Flores, E.E.E.; Klein, M.P.; Hertz, P.F. Batch Synthesis of Galactooligosaccharides from Co-Products of Milk Processing Using Immobilized β-Galactosidase from Bacillus Circulans. Biocatal. Agric. Biotechnol. 2021, 36, 102136. [Google Scholar] [CrossRef]
- Illanes, A.; Vera, C.; Wilson, L. Enzymatic Production of Galacto-Oligosaccharides. In Lactose-Derived Prebiotics; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 111–189. ISBN 9780128027240. [Google Scholar]
- Yañez-Ñeco, C.V.; Cervantes, F.V.; Amaya-Delgado, L.; Ballesteros, A.O.; Plou, F.J.; Arrizon, J. Synthesis of β(1 → 3) and β(1 → 6) Galactooligosaccharides from Lactose and Whey Using a Recombinant β-Galactosidase from Pantoea Anthophila. Electron. J. Biotechnol. 2021, 49, 14–21. [Google Scholar] [CrossRef]
- Slominski, B.A. Hydrolysis of Galactooligosaccharides by Commercial Preparations of α-Galactosidase and β-Fruetofuranosidase: Potential for Use as Dietary Additives. J. Sci. Food Agric. 1994, 65, 323–330. [Google Scholar] [CrossRef]
- Zhao, R.; Duan, F.; Yang, J.; Xiao, M.; Lu, L. Integrated Utilization of Dairy Whey in Probiotic β-Galactosidase Production and Enzymatic Synthesis of Galacto-Oligosaccharides. Catalysts 2021, 11, 658. [Google Scholar] [CrossRef]
- Yañez-Ñeco, C.V.; Rodriguez-Colinas, B.; Amaya-Delgado, L.; Ballesteros, A.O.; Gschaedler, A.; Plou, F.J.; Arrizon, J. Galactooligosaccharide Production from Pantoea Anthophila Strains Isolated from “Tejuino”, a Mexican Traditional Fermented Beverage. Catalysts 2017, 7, 242. [Google Scholar] [CrossRef]
- Füreder, V.; Rodriguez-Colinas, B.; Cervantes, F.V.; Fernandez-Arrojo, L.; Poveda, A.; Jimenez-Barbero, J.; Ballesteros, A.O.; Plou, F.J. Selective Synthesis of Galactooligosaccharides Containing β(1→3) Linkages with β-Galactosidase from Bifidobacterium Bifidum (Saphera). J. Agric. Food Chem. 2020, 68, 4930–4938. [Google Scholar] [CrossRef]
- Böger, M.; van Leeuwen, S.S.; van Bueren, A.L.; Dijkhuizen, L. Structural Identity of Galactooligosaccharide Molecules Selectively Utilized by Single Cultures of Probiotic Bacterial Strains. J. Agric. Food Chem. 2019, 67, 13969–13977. [Google Scholar] [CrossRef]
- Kittibunchakul, S.; Maischberger, T.; Domig, K.; Kneifel, W.; Nguyen, H.; Haltrich, D.; Nguyen, T. Fermentability of a Novel Galacto-Oligosaccharide Mixture by Lactobacillus Spp. and Bifidobacterium Spp. Molecules 2018, 23, 3352. [Google Scholar] [CrossRef]
- Sako, T.; Matsumoto, K.; Tanaka, R. Recent Progress on Research and Applications of Non-Digestible Galacto-Oligosaccharides. Int. Dairy J. 1999, 9, 69–80. [Google Scholar] [CrossRef]


| Porridge | ||
|---|---|---|
| Ingredients (%) | Control | GOS-Added |
| Water | 65.1 | 58.6 |
| Banana puree | 14.4 | 14.4 |
| Milk | 11.4 | 11.4 |
| Rice flour | 6.7 | 6.7 |
| Starch | 2.1 | 2.1 |
| Whey protein (WPC 80) | 0.3 | 0.3 |
| GOS syrup | 6.5 | |
| Total | 100 | 100 |
| Component, % wb | CWP | E-CWP |
|---|---|---|
| pH | 6.1 | 6.1 |
| Soluble solids | 25 | 37 |
| Protein | 0.4 | 0.6 |
| Lactose | 20.6 | 30.5 |
| Ash | 1.2 | 1.8 |
| Potassium | 0.28 | 0.41 |
| Sodium | 0.08 | 0.12 |
| Calcium | 0.15 | 0.22 |
| Phosphorous | 0.03 | 0.04 |
| Others | 2.2 | 3.26 |
| Fat | 0 | 0 |
| Water | 75 | 63 |
| Component | % db |
|---|---|
| GOS * | 40.7 |
| Lactose | 20.9 |
| Glucose | 25.8 |
| Galactose | 2.5 |
| Protein | 1.9 |
| Ash | 3.7 |
| Fat | 0.1 |
| Others | 4.4 |
| Color | |||||
|---|---|---|---|---|---|
| pH | % SS * | L | a | b | |
| Control | 5.97 | 10.7 | 63.05 | 6.29 | 15.31 |
| GOS-added | 5.87 | 16 | 66.55 | 5.12 | 16.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orrego, D.; Klotz-Ceberio, B. Enzymatic Synthesis of Galacto-Oligosaccharides from Concentrated Sweet Whey Permeate and Its Application in a Dairy Product. Appl. Sci. 2022, 12, 10229. https://doi.org/10.3390/app122010229
Orrego D, Klotz-Ceberio B. Enzymatic Synthesis of Galacto-Oligosaccharides from Concentrated Sweet Whey Permeate and Its Application in a Dairy Product. Applied Sciences. 2022; 12(20):10229. https://doi.org/10.3390/app122010229
Chicago/Turabian StyleOrrego, David, and Bernadette Klotz-Ceberio. 2022. "Enzymatic Synthesis of Galacto-Oligosaccharides from Concentrated Sweet Whey Permeate and Its Application in a Dairy Product" Applied Sciences 12, no. 20: 10229. https://doi.org/10.3390/app122010229
APA StyleOrrego, D., & Klotz-Ceberio, B. (2022). Enzymatic Synthesis of Galacto-Oligosaccharides from Concentrated Sweet Whey Permeate and Its Application in a Dairy Product. Applied Sciences, 12(20), 10229. https://doi.org/10.3390/app122010229