Fundamental Investigations on the High-Temperature Corrosion of Spinel-Forming Alumina Castables by Steel Slags
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Spinel-Forming Alumina Castablesand Characterization Methods
2.2. Preparation of Synthetic Steel Slags
2.3. Thermodynamic Simulation of the Effect of Slag Chemistry on the Corrosion Behavior Ofspinel-Forming Alumina Castables
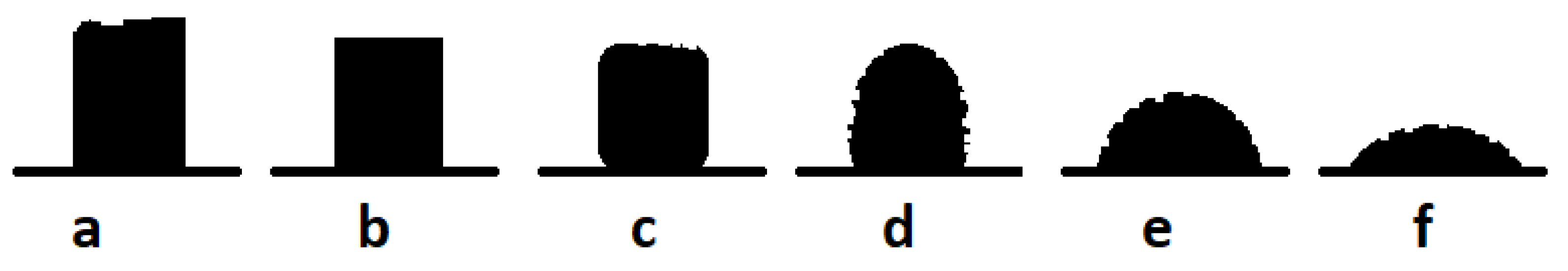
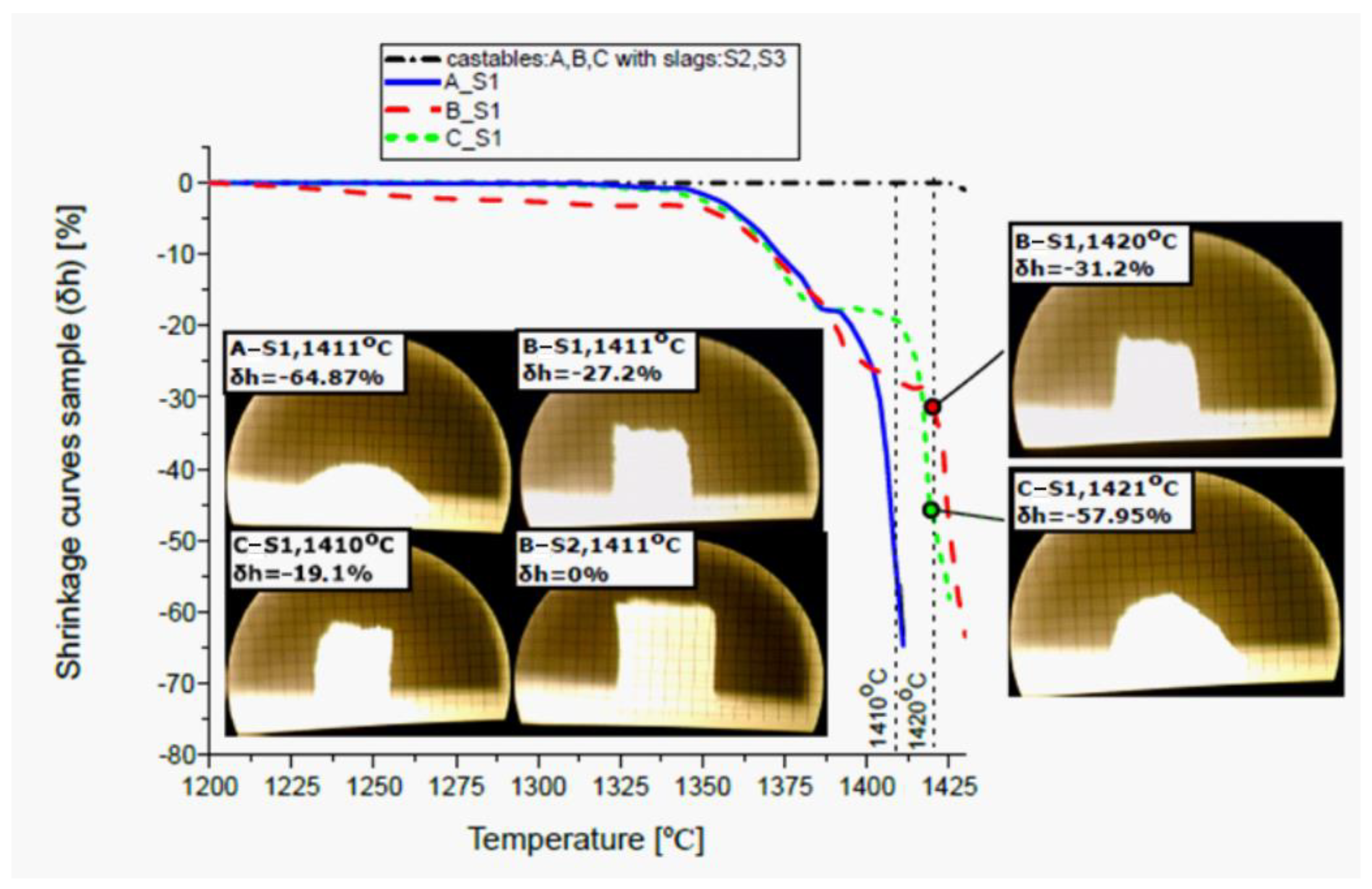
2.4. Laboratory-Scale Investigations of the Mixtures Containing Alumina-Spinel Castablesand Synthetic Slags Using the Heating Microscopy Thermal Analysis (HMTA) and X-Ray Diffraction (XRD)
- —change of sample linear dimensions,
- —sample height at room temperature,
- h(T)—sample height at temperature T.
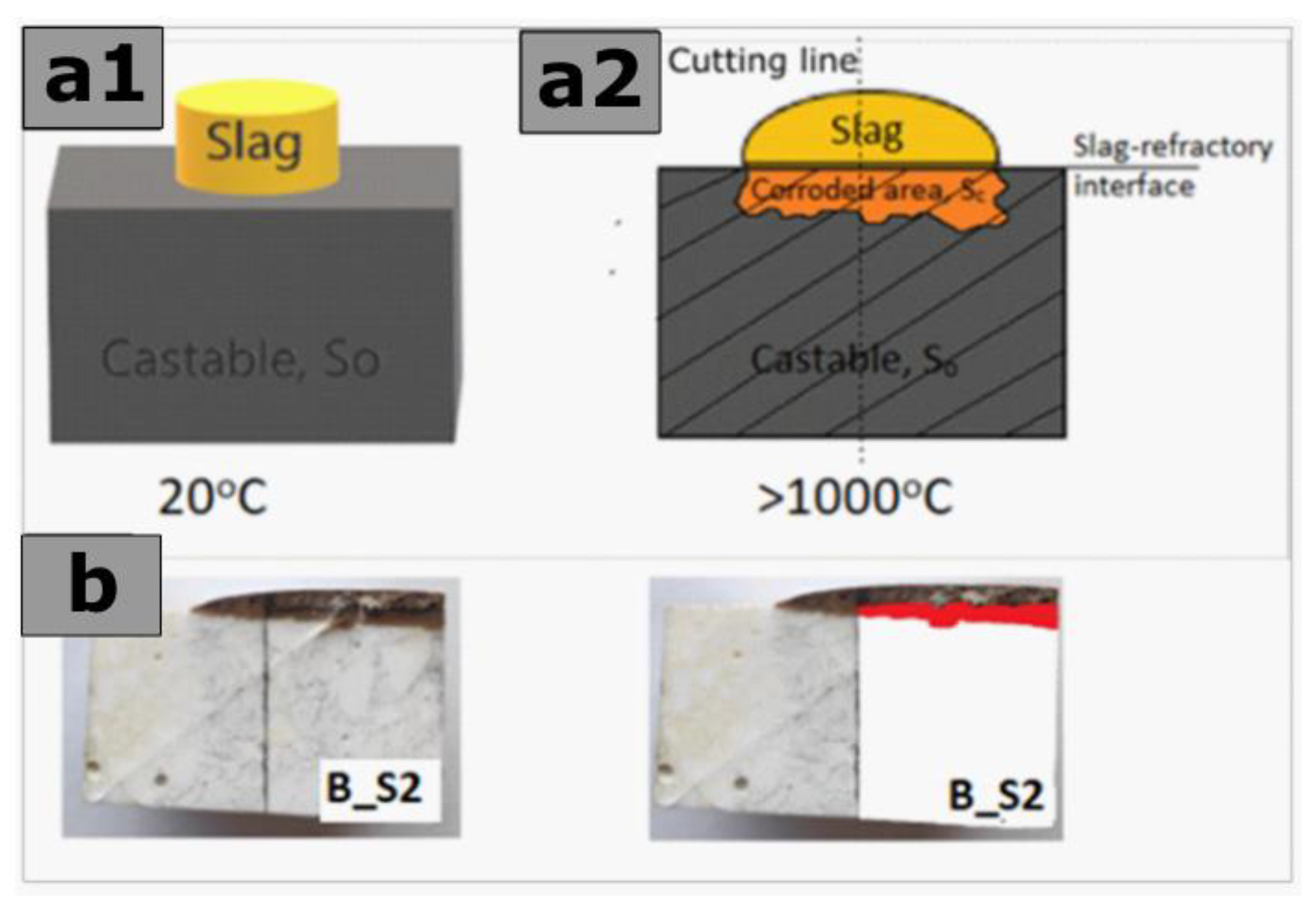
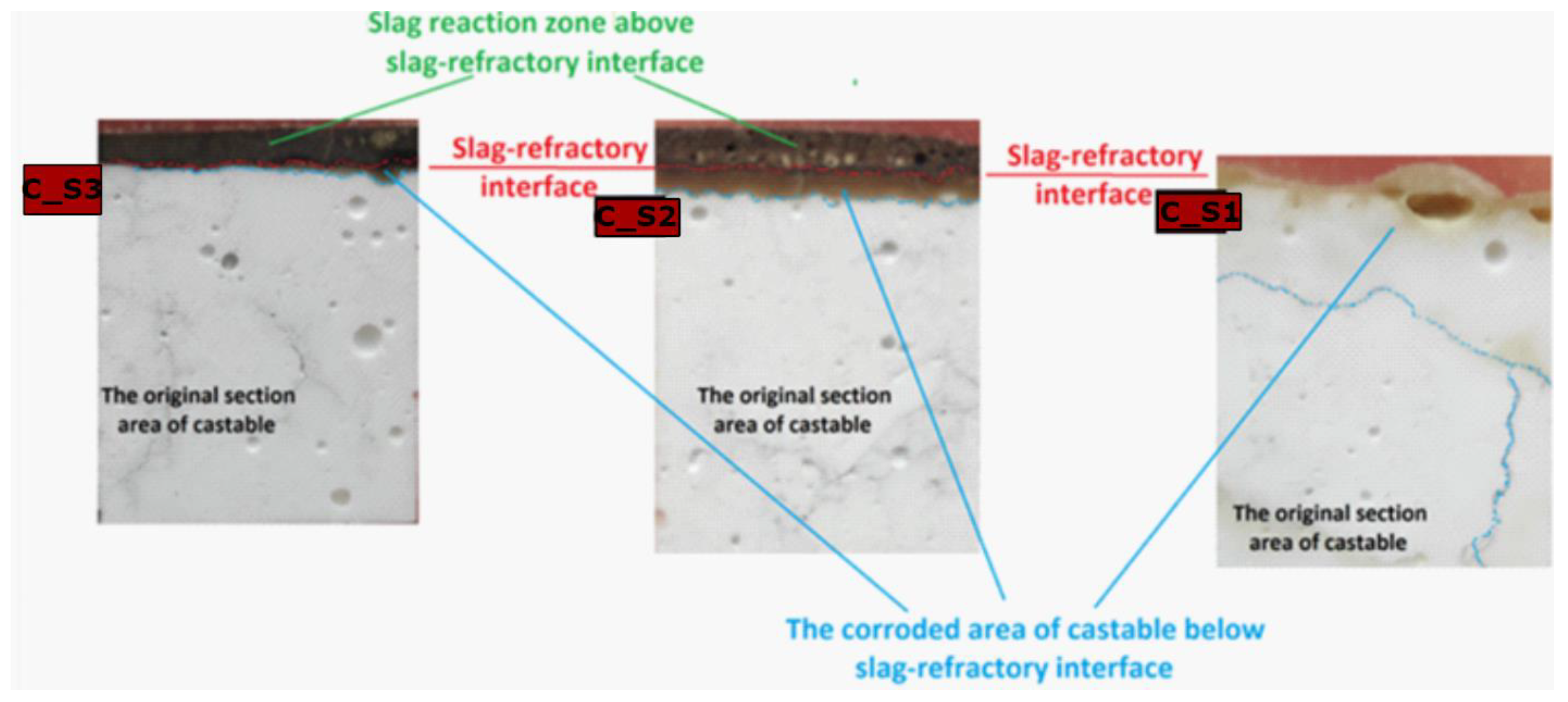
2.5. Laboratory-Scale Static Corrosion Test by the Coating Method
- IC—the corrosion index,
- SC—the corroded area of castable below the slag-refractory interface,
- SO—the original section area of castable.
3. Results
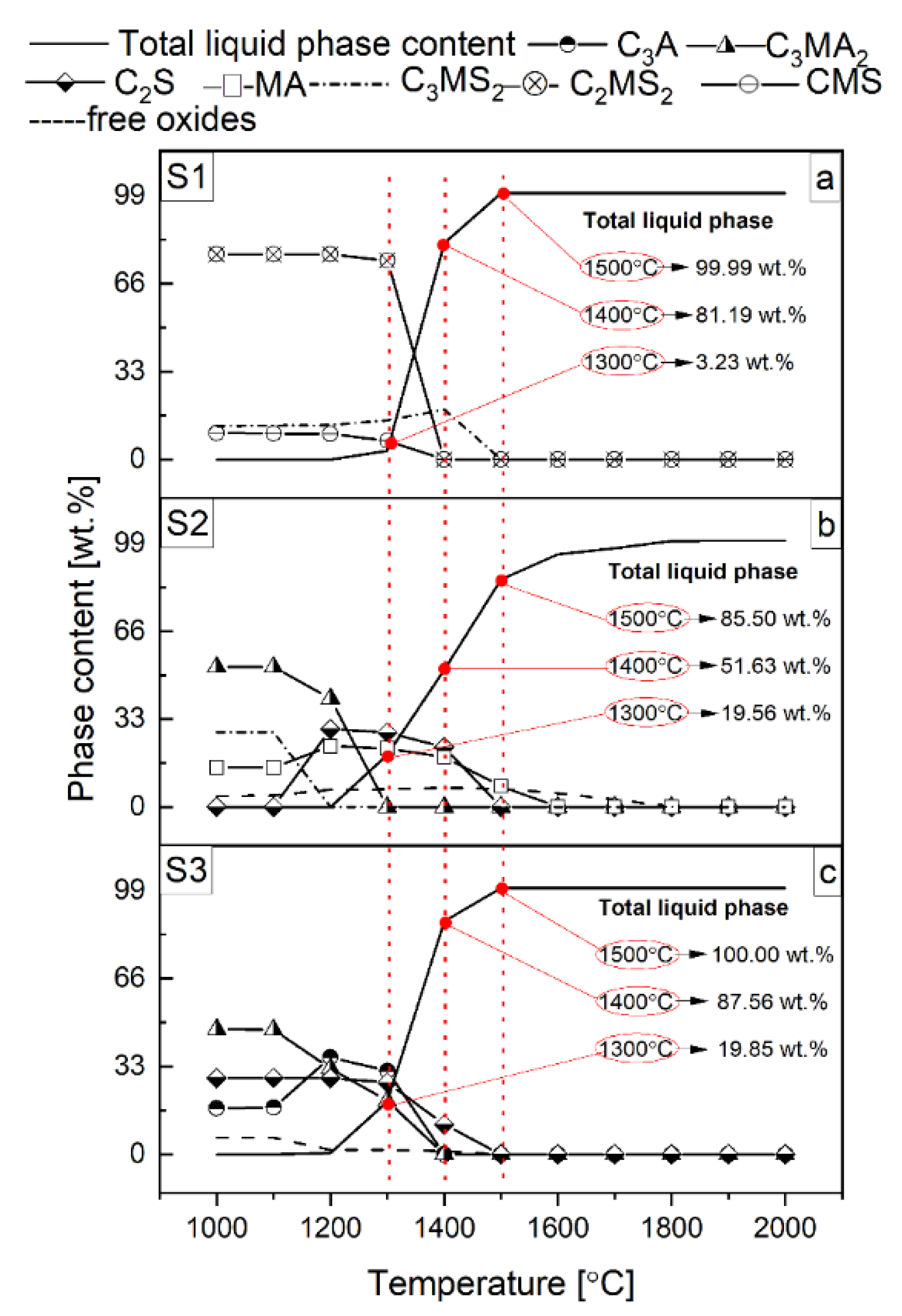
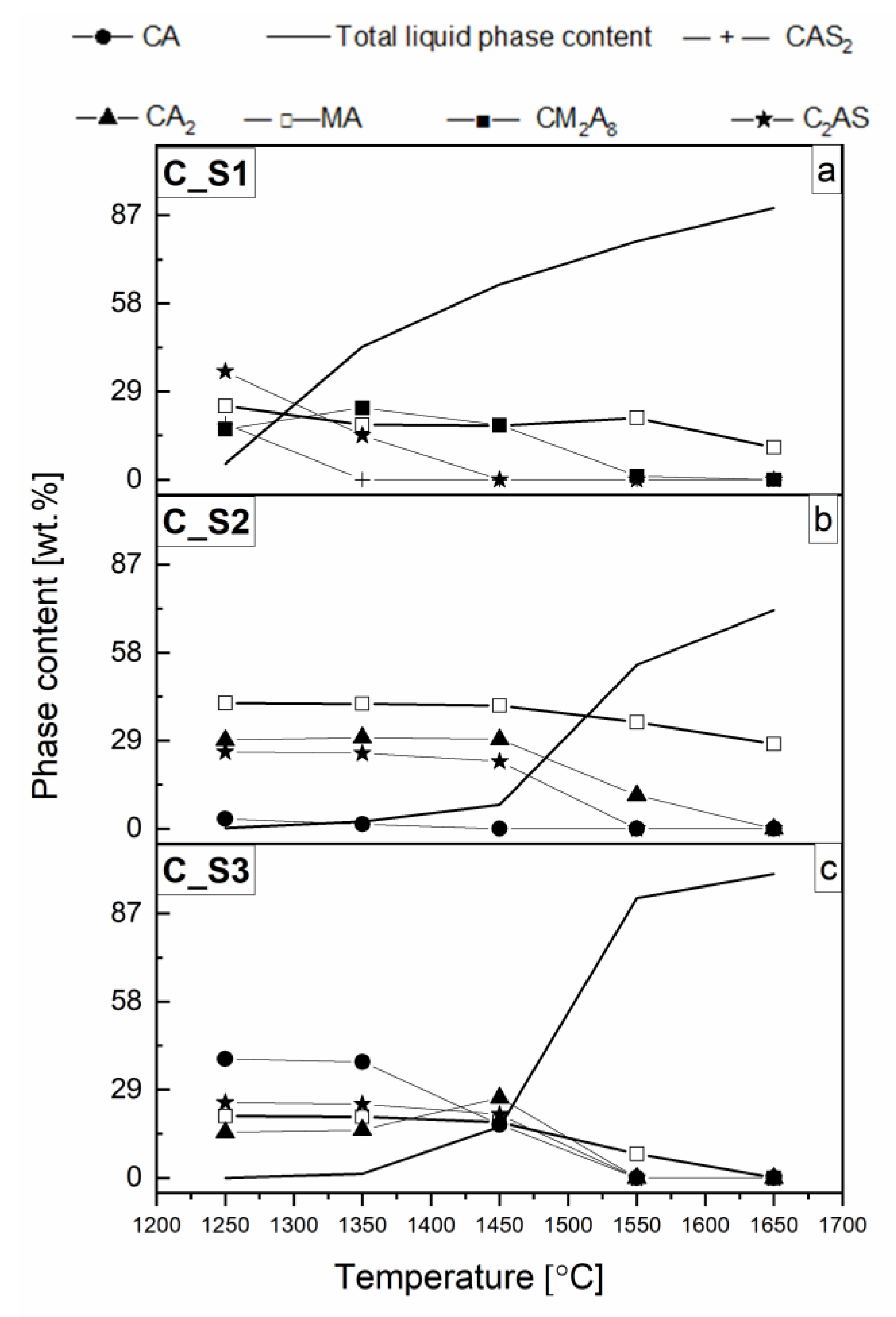
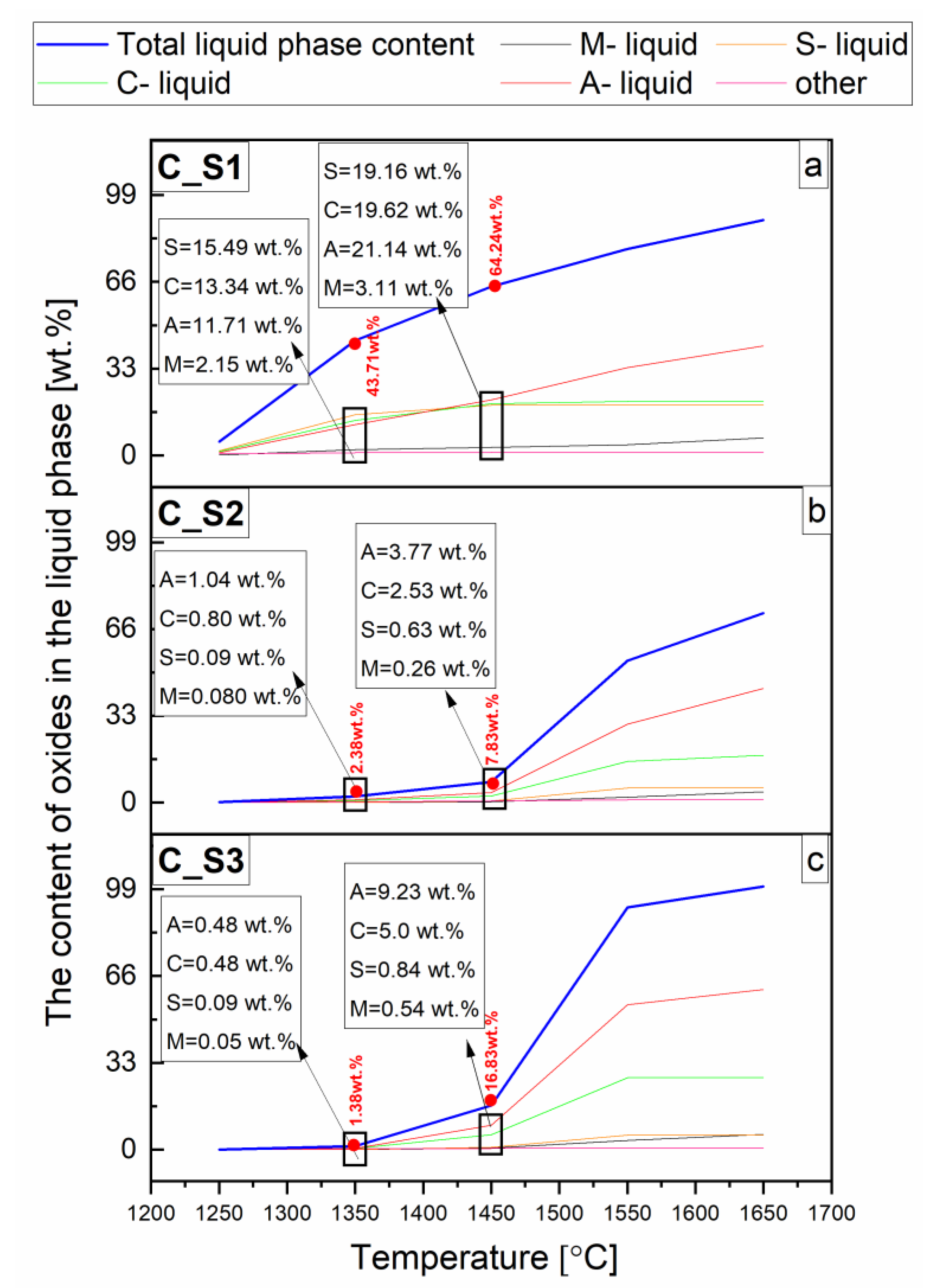
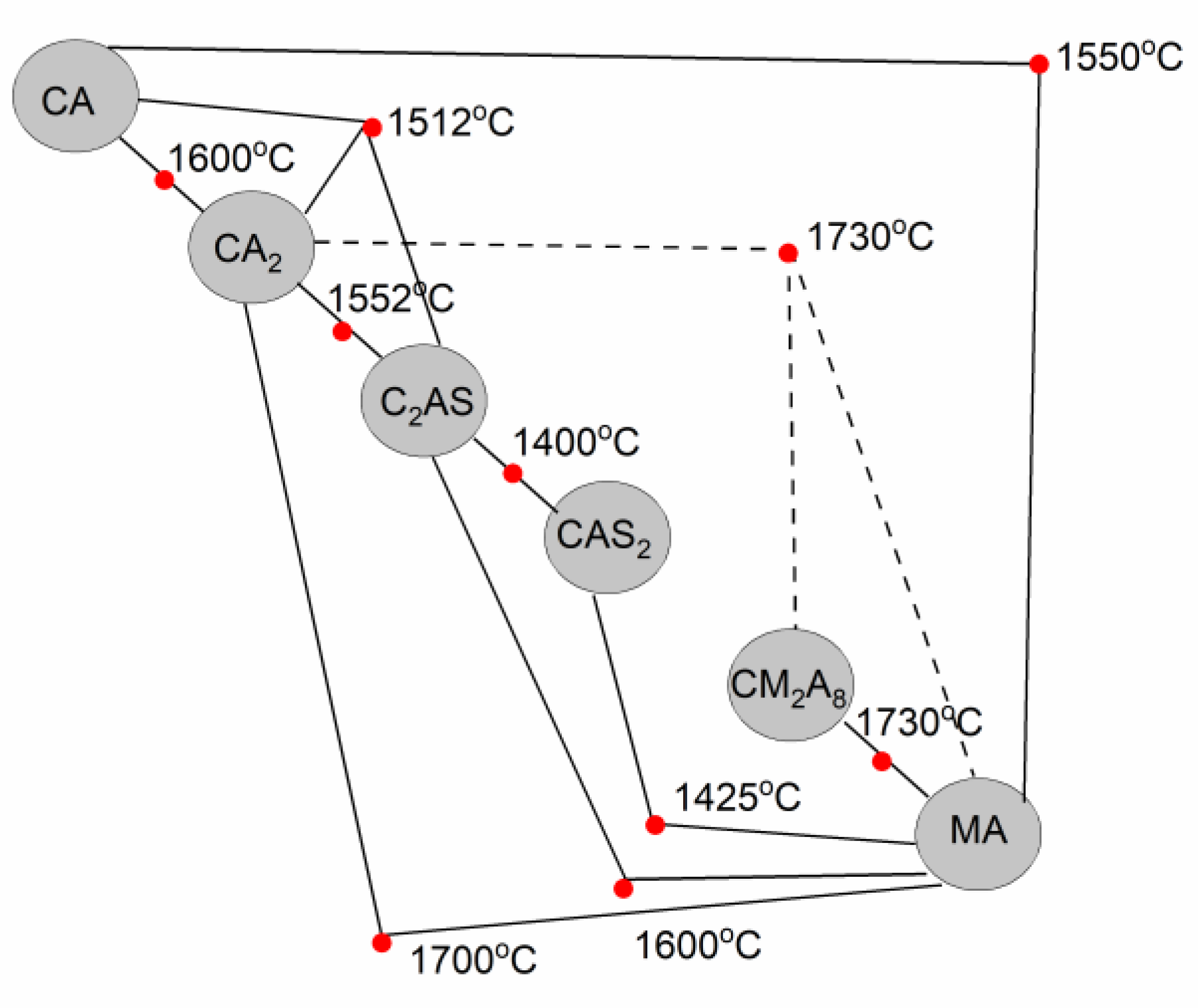
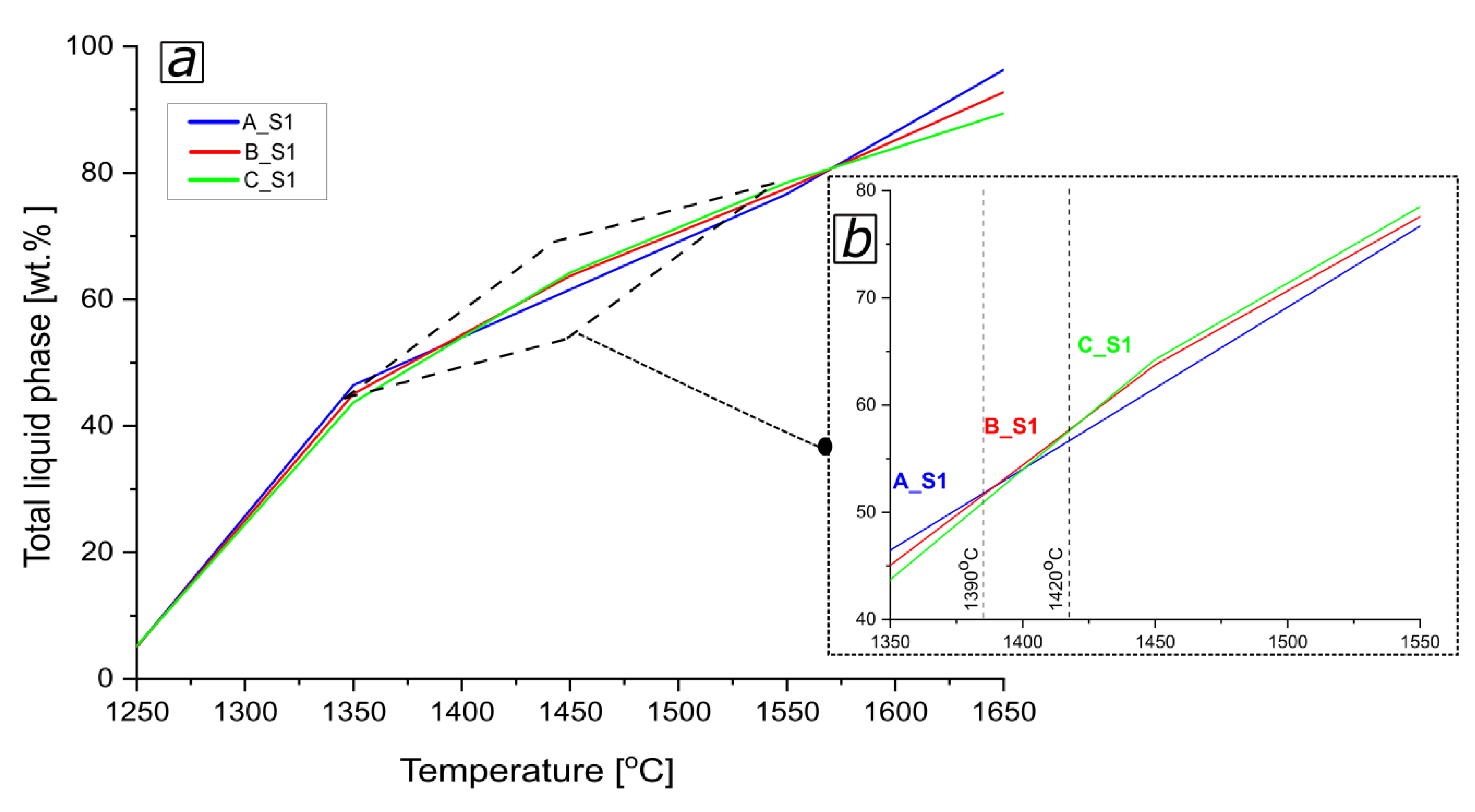
3.1. Thermodynamic Analysis of Reactive Mixtures Containing Components of Spinel-Forming Alumina Castables and Steel Slags and Thermal Analysis with Microscopic Heating (HMTA)
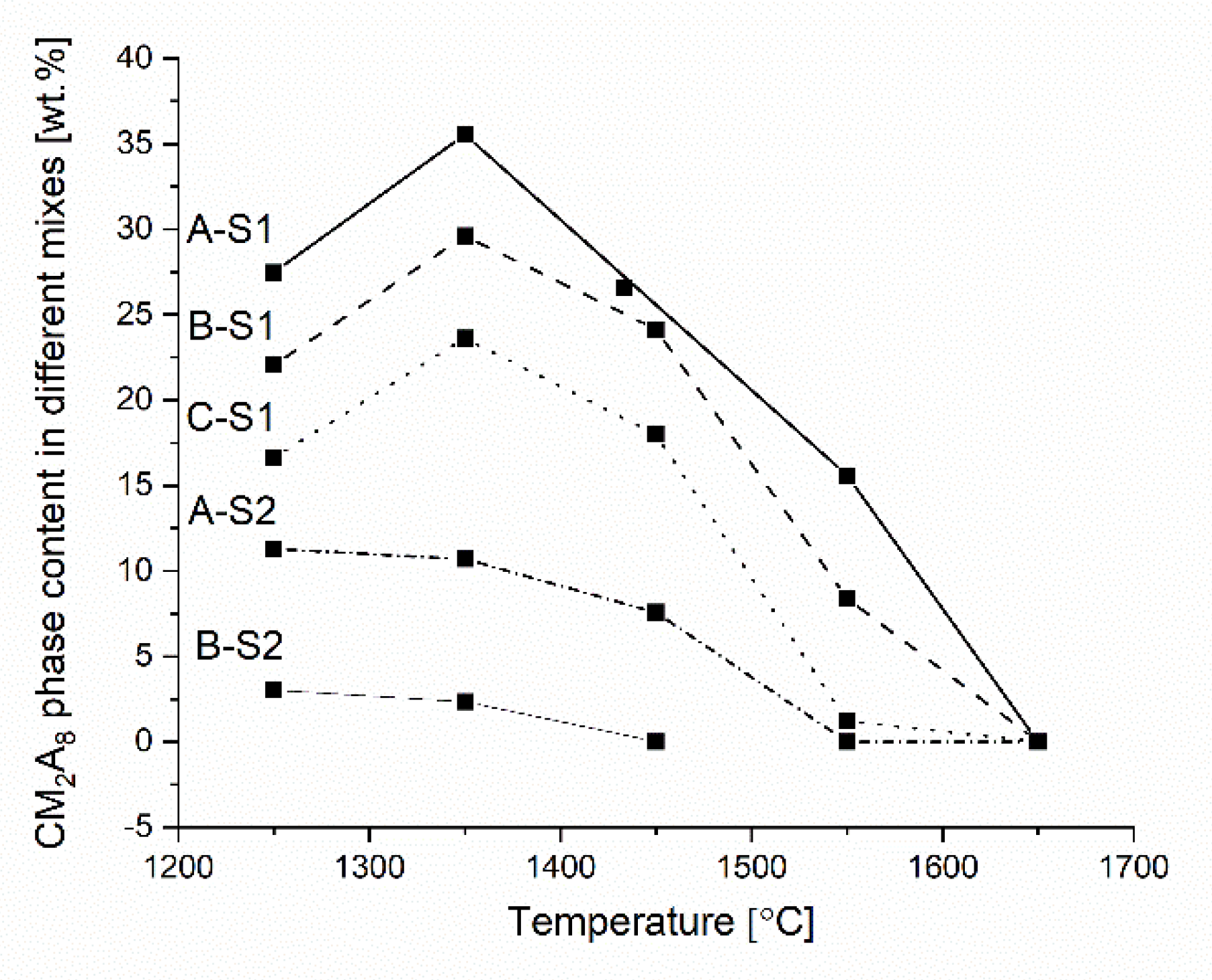
3.2. An Overview of Reaction Characteristics of Cement-Free Corundum-Spinel Castables
3.3. X-Ray Investigations of High-Temperature Relations within the Mixtures Containing Spinel-Forming Alumina Castables and Synthetic Slags
3.4. Corrosion Index
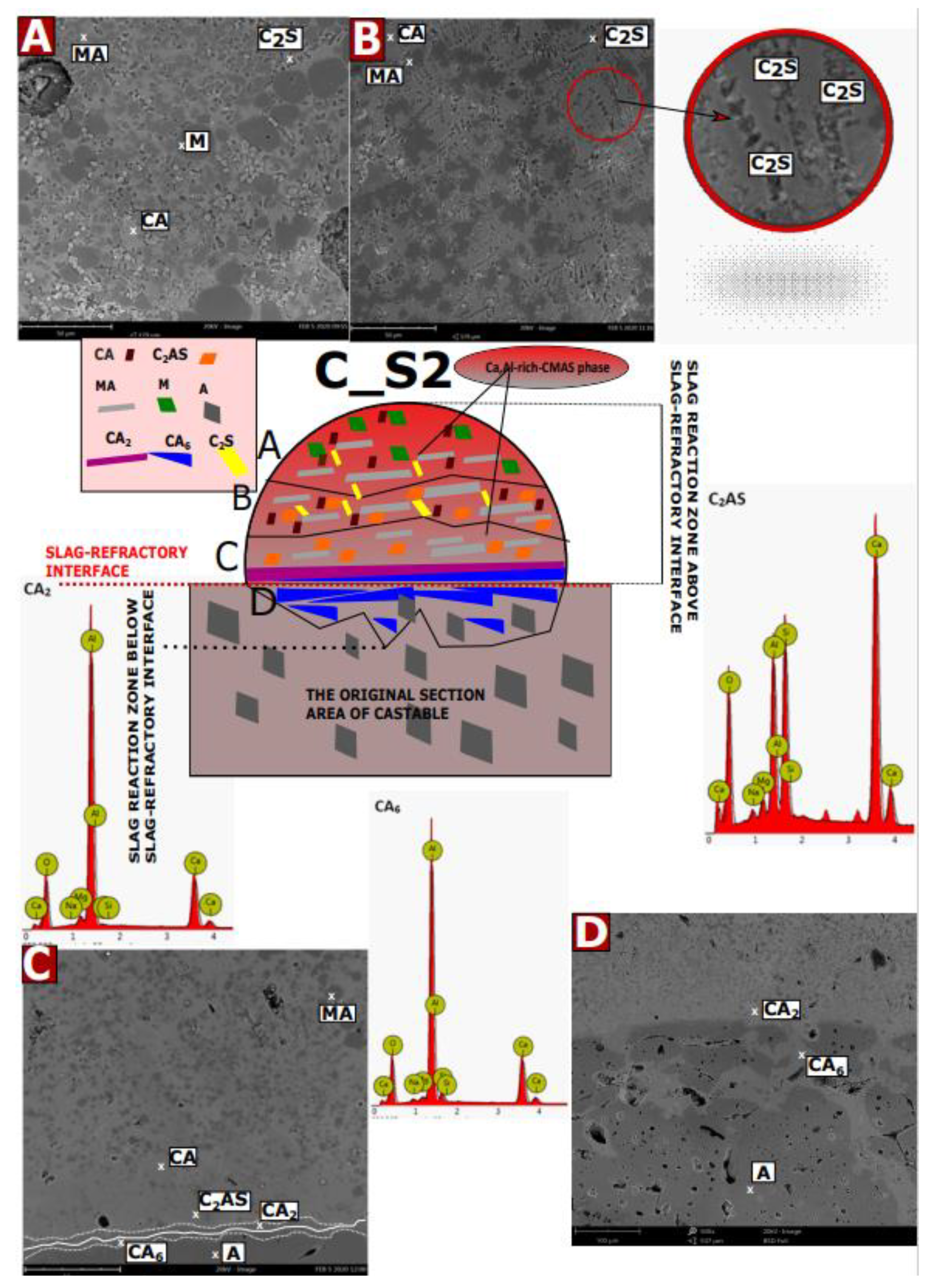
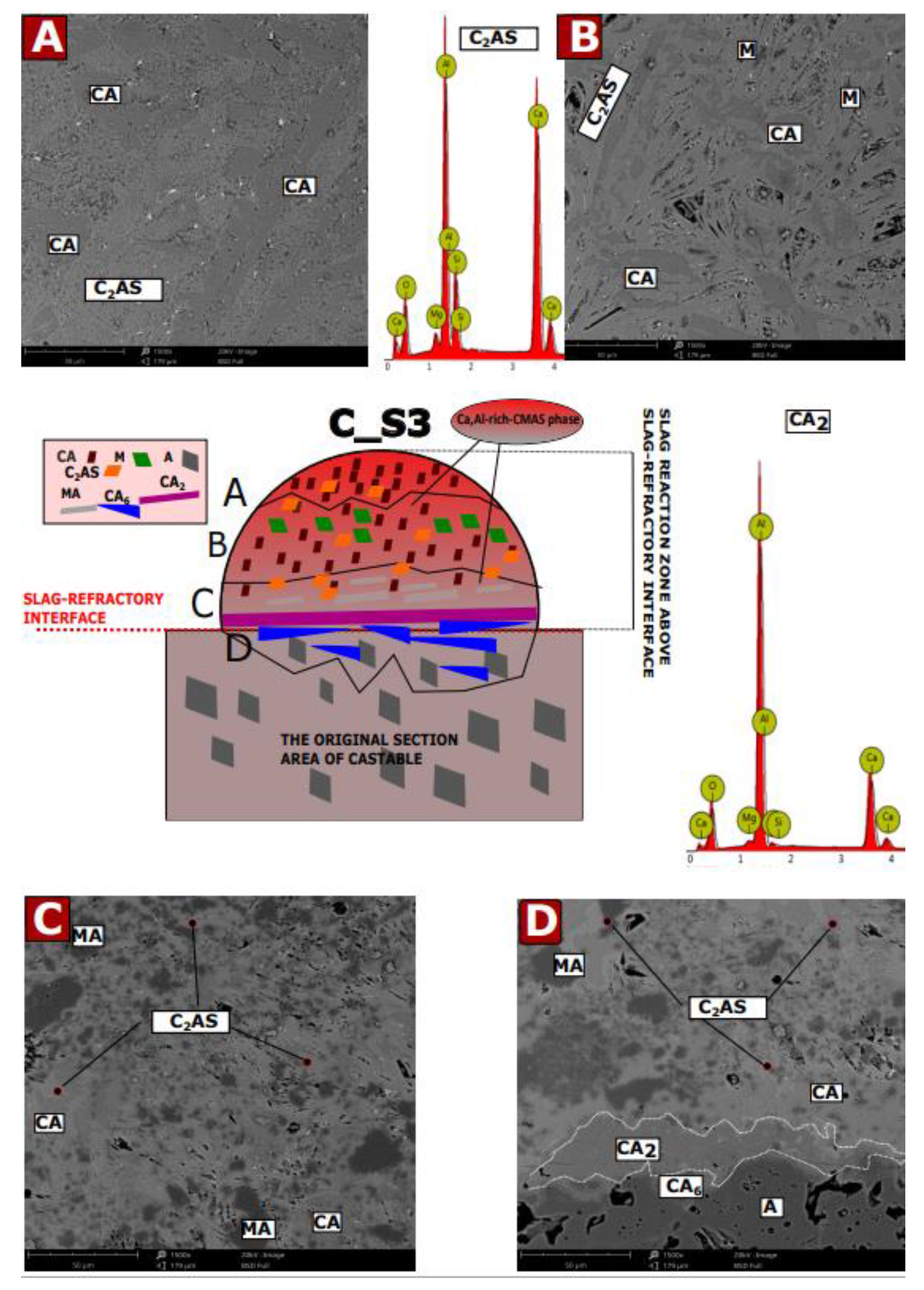
3.5. SEM-EDS Studies of Corroded Castables
4. Summary and Conclusions
- -
- As the content of SiO2-rich phases in the slag increases, the tendency for accelerated liquid phase formation in a narrow temperature range also increases.
- -
- The macroscopic image is influenced by the composition and quantity of the liquid phase.
- -
- CAS2, formed as a result of corrosion of the slag with a lower C/S content, reduces the temperature of the liquid phase formation and increases the degradation of the material
- -
- The lower the C/S mass ratio in the slag, the greater the area of castable that will be degraded.
- -
- It was found that with an increase in the MgO content in the material, the IC decreases (with increasing MgO content in castable, the castable’s resistance to slag increases), while with the decrease in the mass ratio C/S in the slag, the IC increases, i.e., the aggressive character of the slag increases.
- -
- Insights on in situ secondary MgAl2O4 formation mechanism and its correlation with the corrosion resistance study were conducted of spinel-containing refractory castables.
- -
- The Ca2+ ions that most deeply diffuse into the material form a continuous zone of CA2 and CA6, which prevents further degradation of the material. As the C/S ratio decreases, this zone is deeper in the material.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Nakabo, K.; Nishida, S.; Kitamura, M. Technologies of alumina-magnesia refractories for steel ladles part II, castable properties under restrained heating. In Proceedings of the 72° Congresso Anual da ABM, Sao Paulo, Brazil, 2–8 October 2017; Volume 72, pp. 291–301. [Google Scholar]
- Nieformowanewyrobyogniotrwałe—Część 1: Wprowadzenieiklasyfikacja, PN-EN 1402-1:2005. Available online: https://sklep.pkn.pl/pn-en-1402-1-2005p.html (accessed on 2 September 2021).
- Warmuz, K.; Madej, D. Effect of the particle size on the reactivity of MgO-Al2O3hydratingmixtures: A long-term kinetic investigation of hydrotalcite synthesis. Appl. Clay Sci. 2021, 211, 106196. [Google Scholar] [CrossRef]
- Lee, W.E.; Vieira, W.; Zhang, S.; Ahari, K.G.; Sarpoolaky, H.; Parr, C. Castable refractory concretes. Int. Mater. Rev. 2001, 46, 145–167. [Google Scholar] [CrossRef]
- Zawrah, M.F.M.; Khalil, N.M. Effect of mullite formation on properties of refractory castables. Ceram. Int. 2001, 27, 689–694. [Google Scholar] [CrossRef]
- Myhre, B. Strength development of bauxite-based ultralow-cement castables. Am. Ceram. Soc. Bull. 1994, 73, 68–73. [Google Scholar]
- Aïtcin, P.C. Supplementary cementitious materials and blended cements. In Science and Technology of Concrete Admixtures; Woodhead Publishing: Cambridge, UK, 2016; pp. 53–73. [Google Scholar] [CrossRef]
- Firoozjaei, E.A.; Saidi, A.; Monshi, A.; Koshy, P. The Effect of Microsilica and Refractory Cement Content on the Properties of Andalusite Based Low Cement Castables Used in Aluminum Casthouse. Cerâmica 2010, 56, 411–421. [Google Scholar] [CrossRef]
- Majchrowicz, I.; Witel, J.; Barański, J.; Cholewa, M. Monolitycznemateriałyogniotrwałe do zastosowań w odlewnictwie. XII Konferencjaodlewnicza Technical; 2010. Available online: http://docplayer.pl/14339381-Monolityczne-materialy-ogniotrwale-do-zastosowan-w-odlewnictwie.html (accessed on 15 September 2021).
- Souza, T.M.; Braulio, M.A.L.; Luz, A.P.; Bonadia, P.; Pandolfelli, V.C. Systemic analysis of MgO hydration effects on alumina–magnesia refractory castables. Caram. Int. 2012, 38, 3969–3976. [Google Scholar] [CrossRef]
- Chan, C.F.; Ko, Y.C. Effect of CaO content on the hot strength of alumina–spinel castables in the temperature range of 1000 °C to 1500 °C. J. Am. Ceram. Soc. 2005, 81, 2957–2960. [Google Scholar] [CrossRef]
- Chen, S.; Cheng, M.; Lin, S.; Ko, Y. Thermal characteristics of Al2O3–MgO and Al2O3–spinel castables for steel ladles. Ceram. Int. 2002, 28, 811–817. [Google Scholar] [CrossRef] [Green Version]
- Diaz, L.A.; Torrecillas, R.; de Aza, A.; Pena, P.; de Aza, S. Alumina-rich refraktory concretes with added spinel, periclase and dolomite: A comparative study of their microstructural evolution with temperature. J. Eur. Ceram. 2005, 25, 1499–1506. [Google Scholar] [CrossRef]
- Nanba, M.; Kaneshige, T.; Hamazaki, Y.; Nishio, H.; Ebizawa, I. Thermal characteristics of castables for teeming ladles. Taikabutsu Overseas 1996, 16, 17–21. [Google Scholar]
- Sako, E.Y.; Braulio, M.A.L.; Milanez, D.H.; Brant, P.O.; Pandolfelli, V.C. Microsilica role in the CA6 formation in cement-bonded spinelrefractorycastables. J. Mater. Process. Technol. 2009, 209, 5552–5557. [Google Scholar] [CrossRef]
- Braulio, M.A.L.; Rigaud, M.; Buhr, A.; Parr, C.; Pandolfelli, V.C. Spinel-containing alumina-based refractory castables. Ceram. Int. 2011, 37, 1705–1724. [Google Scholar] [CrossRef]
- Sumimura, H.; Yamamura, T.; Kubota, Y.; Kaneshige, T. Study on slag penetration of alumina-spinel castable. In Proceedings of the UNITECR’99, Berlin, Germany, 6–9 September 1999; pp. 97–101. [Google Scholar]
- Fujii, K.; Furusato, I.; Takita, I. Composition of spinel clinker for teeming ladle casting materials. Taikabutsu Overseas 1999, 12, 4–9. [Google Scholar]
- Kriechbaum, G.W.; Wohrmeyer, C.; Routschka, G. Neue Spinell-Rohstoffe fur FeuerfestauskleidungenimStahlbereich. In Proceedings of the 35th International Colloquium on Refractories, Aachen, Germany, 26–27 February 1992. [Google Scholar]
- Mori, J. Structure change of alumina castable by addition of magnesia or spinel. Taikabutsu Overseas 1995, 15, 20–23. [Google Scholar]
- Nagasoe, A.; Tsurumoto, S.I.; Kitamura, A. Refractory characteristics of spinels with various MgO contents. Taikabutsu Overseas 1991, 11, 20–28. [Google Scholar]
- Mori, J.; Yoshimura, M.; Oguchi, Y.; Kawakami, T.; Ohishi, I. Effect of slag composition on wear of alumina-spinel castable for steel ladle. Taikabutsu Overseas 1992, 12, 40–45. [Google Scholar]
- Naigai, B.; Matsumoto, O.; Isobe, T.; Nishiumi, Y. Wear mechanism of castasble for steel ladle by slag. Taikabutsu Overseas 1992, 12, 15–20. [Google Scholar]
- Sato, Y.; Joguchi, H.; Hiroki, N. Test results of alumina-spinel castable for steel ladle. Taikabutsu Overseas 1992, 12, 10–14. [Google Scholar]
- Yamamura, T.; Kubota, Y.; Kaneshige, T.; Nanba, M. Effect of spinel clinker composition on properties of alumina-spinel castable. Taikabutsu Overseas 1994, 13, 39–45. [Google Scholar]
- Holleyn, F.; Krause, O.; Rossdeutscher, J.; Brochen, E.; Dannert, C.; Odziomek, M. Matrix Design for Improved Spinel Formation in High Alumina Refractory Monolithics that is Adjusted to the Service Conditions. In Proceedings of the UNITECR, Santiago, Chile, 26–29 September 2017; pp. 465–468. [Google Scholar]
- Zhang, S.; Lee, W.E. Spinel-containing refractories. In Refractories Handbook; Schacht, C.A., Monticello, M.D., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 81–139. [Google Scholar]
- Yamamura, T.; Hamazaki, Y.; Kaneshige, T.; Toyoda, T.; Nishi, M.; Kato, H. Alumina-spinel castable refractories for steel teeming ladle. Taikabutsu Overseas 1992, 12, 21–27. [Google Scholar]
- Salomao, R.; Bittencourt, L.R.M.; Pandolfelli, V.C. A novel approach for magnesia hydration assessment in refractory castables. Ceram. Int. 2007, 33, 803–810. [Google Scholar] [CrossRef]
- Yilmaz, S. Corrosion of high alumina spinel castables by steel ladle slag. Ironmak. Steelmak. 2006, 33, 151–156. [Google Scholar] [CrossRef]
- Qiu, W.; Ruan, G.; Ouyang, J.; Zhao, Y. Effect of Spinel Kinds on Properties of Alumina-Spinel Castables. Int. J. Appl. Ceram. Technol. 2014, 11, 993–1000. [Google Scholar] [CrossRef]
- Diaz, L.A.; Torrecillas, R.; de Aza, A.H.; Pena, P. Effect of spinel content on slag attack resistance of high alumina refractory castables. J. Eur. Ceram. Soc. 2007, 27, 4623–4631. [Google Scholar] [CrossRef]
- Pogorzałek, J.; Różański, P. Steelmaking Slags Utilization. Prace IMŻ 1, 2010. Available online: https://fbc.pionier.net.pl/details/nnlt3T5 (accessed on 6 July 2021).
- Guo, M.; Parada, S.; Smets, S.; Jones, P.T.; van Dyck, J.; Blanpain, B.; Wollants, P. Laboratory study of the interaction mechanisms between magnesia-chromite refractories and Al2O3-rich VOD slags. In Proceedings of the VII International Conference on Molten Slags Fluxes and Salts, Institute of Mining and Metallurgy, Cape Town, South Africa, 25–28 January 2004. [Google Scholar]
- Rose, L.; Reichel, J.; Germershausen, T. Innovations in Refining Process Technique Combined Blowing Vacuum Converter with CO2; SMS Siemag AG: Düsseldorf, Germany, 2013. [Google Scholar]
- Jonczy, I.; Lata, L. Characteristic of chemical composition of converter and blast furnace slags. Górnictwo I Geol. 2013, 8, 51–61. [Google Scholar]
- Belmonte, D.; Ottonello, G.; VetuschiZuccolini, M.; Attene, M. The system MgO-Al2O3-SiO2 under pressure: A computational study of melting relations and phase diagrams. Chem. Geol. 2017, 461, 54–64. [Google Scholar] [CrossRef]
- Prince, A.T. Phase Equilibrium Relationships in a Portion of the System MgO-A12O3-2CaO·SiO2. J. Am. Ceram. Soc. 1951, 34, 44–51. [Google Scholar] [CrossRef]
- Prince, A.T. Liquidus Relationships on 10% MgO Plane of the System Lime-Magnesia-Alumina-Silica. J. Am. Ceram. Soc. 1954, 37, 402–408. [Google Scholar] [CrossRef]
- Shin, J.W. The experimental and theoretical determination of the thermodynamic properties of melts and glasses from the system CaO-MgO-Al2O3-SiO2. Aachen Techn. Hochsch. Diss. 2005. Available online: http://publications.rwth-aachen.de/record/59938/files/59938.pdf (accessed on 6 June 2021).
- Lee, W.E.; Zhang, S. Direct and indirect slag corrosion of oxide and oxide-c refractories. In Proceedings of the VII International Conference on Molten Slags Fluxes and Salts, Cape Town, South Africa, 25–28 January 2004; pp. 309–319. [Google Scholar]
- Chen, L.; Li, S.; Jones, P.T.; Guo, M.; Blanpain, B.; Malfliet, A. Identification of magnesia–chromite refractory degradation mechanisms of secondary copper smelter linings. J. Eur. Ceram. Soc. 2016, 36, 2119–2132. [Google Scholar] [CrossRef]
- Mosner, P.; Vorokhta, M.; Koudelka, L. Application of heating microscopy to the study of thermal behaviour of ZnO–P2O5–WO3 glasses. J. Therm. Anal. Calorim. 2013, 112, 659–664. [Google Scholar] [CrossRef]
- Rankin, G.A.; Wright, F.E. The ternary system CaO-Al2O3-SiO2. Am. J. Sci. 1915, 39, 1–79. [Google Scholar] [CrossRef]
- Shu, Q.; Wang, Y.; Li, J.; Liu, Y.; Li, P.; Chou, K. Effect of Na2O on Dissolution Rate of Alumina in CaO–Al2O3–MgO–SiO2 Slag. ISIJ Int. 2015, 55, 2297–2303. [Google Scholar] [CrossRef] [Green Version]
- Rao, M.R. Liquidus Relations in the Quaternary Subsystem CaA12O4-CaAl4O7-Ca2Al2SiO7-MgAl2O4. J. Am. Ceram. Soc. 1968, 51, 50–54. [Google Scholar] [CrossRef]
- De Aza, A.H.; Iglesias, J.E.; Pena, P.; de Aza, S. Ternary System Al2O3–MgO–CaO: Part II, Phase Relationships in the Subsystem Al2O3–MgAl2O4–CaAl4O7. J. Am. Ceram. Soc. 2000, 83, 919–927. [Google Scholar] [CrossRef]
- Sako, E.Y.; Braulio, M.A.L.; Zinngrebe, E.; van der Laan, S.R.; Pandolfelli, V.C. In-Depth Microstructural Evolution Analyses of Cement-Bonded Spinel Refractory Castables: Novel Insights Regarding Spinel and CA6 Formation. J. Am. Ceram. Soc. 2012, 95, 1732–1740. [Google Scholar] [CrossRef]
- Wang, Z.; Sohn, I. Effect of the Al2O3/SiO2 mass ratio on the crystallization behavior of CaO-SiO2-MgO-Al2O3 slags using confocal laser scanning microscopy. Ceram. Int. 2018, 44, 19268–19277. [Google Scholar] [CrossRef]
- Allu, A.R.; Balaji, S.; Tulyaganov, D.U.; Mather, G.C.; Margit, F.; Pascual, M.J.; Siegel, R.; Milius, W.; Senker, J.; Agarkov, D.A.; et al. Understanding the Formation of CaAl2Si2O8 in Melilite Based Glass-Ceramics: Combined Diffraction and Spectroscopic Studies. J. Am. Chem. Soc. 2017, 29, 6233–6243. [Google Scholar] [CrossRef]
- Martinez, A.G.T.; Luz, A.P.; Braulio, M.A.L.; Sako, E.Y.; Pandolfelli, V.C. Revisiting CA6 formation in cement-bonded alumina-spinel refractory castables. J. Eur. Ceram. 2017, 37, 5023–5034. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, K.H.; Kang, S.J.L. Formation of CaO6Al2O3 and MgAl2O4 During Dissolution of Al2O3 into CaMgSiO4 Melt. J. Mater. Sci. 1992, 11, 315–316. [Google Scholar]
- Geiger, C.A.; Kleppa, O.J.; Mysen, B.O.; Lattimer, J.M.; Grossman, L. Enthalpies of formation of CaAl4O7 and CaAl12O19 (hibonite) by high temperature, alkali borate solution calorimetry. Geochim. Cosmochim. Acta 1988, 52, 1729–1736. [Google Scholar] [CrossRef]
- Mercury, J.M.R.; de Aza, A.H.; Pena, P. Synthesis of CaAl2O4 from powders: Particle size effect. J. Eur. Ceram. 2005, 25, 3269–3279. [Google Scholar] [CrossRef]
- Johnson, W.C.; Stein, D.F. Additive and Impurity Distributions at Grain Boundaries in Sintered Alumina. J. Am. Ceram. Soc. 1975, 58, 485–489. [Google Scholar] [CrossRef]


| Sample | SiO2 | CaO | MgO | Al2O3 | TiO2 | Fe2O3 | MnO | CaF2 | C/S | M/A | C/A | C/M |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | Mass Ratio | |||||||||||
| A_S1 | 19.16 | 20.65 | 7.47 | 51.33 | 0.15 | 0.70 | 0.30 | 0.25 | 1.08 | 0.15 | 0.40 | 2.76 |
| A_S2 | 5.57 | 17.91 | 9.71 | 65.66 | 0.15 | 0.65 | 0.10 | 0.25 | 3.21 | 0.15 | 0.27 | 1.84 |
| A_S3 | 5.48 | 27.36 | 3.79 | 62.83 | 0.15 | 0.05 | 0.10 | 0.25 | 5.00 | 0.06 | 0.44 | 7.22 |
| B_S1 | 19.16 | 20.65 | 8.47 | 50.33 | 0.15 | 0.70 | 0.30 | 0.25 | 1.08 | 0.17 | 0.41 | 2.44 |
| B_S2 | 5.57 | 17.91 | 10.71 | 64.66 | 0.15 | 0.65 | 0.10 | 0.25 | 3.21 | 0.17 | 0.28 | 1.67 |
| B_S3 | 5.48 | 27.36 | 4.79 | 61.83 | 0.15 | 0.05 | 0.10 | 0.25 | 5.00 | 0.08 | 0.44 | 5.71 |
| C_S1 | 19.16 | 20.65 | 9.47 | 49.33 | 0.15 | 0.70 | 0.30 | 0.25 | 1.08 | 0.19 | 0.42 | 2.18 |
| C_S2 | 5.57 | 17.91 | 11.71 | 63.66 | 0.15 | 0.65 | 0.10 | 0.25 | 3.21 | 0.18 | 0.28 | 1.53 |
| C_S3 | 5.48 | 27.36 | 5.79 | 60.83 | 0.15 | 0.05 | 0.10 | 0.25 | 5.00 | 0.10 | 0.45 | 4.73 |
| Reactive Mixtures | Temperature (°C) |
|---|---|
| A_S1 | 1086 |
| B_S1 | |
| C_S1 | |
| A_S2 | 1221 |
| B_S2 | 1226 |
| C_S2 | 1203 |
| A_S3 | 1230 |
| B_S3 | 1231 |
| C_S3 |
| Samples | ||||
|---|---|---|---|---|
| A_S1 | B_S1 | C_S1 | A,B,C_S2 and A,B,C_S3 | |
| Temperature of Characteristic Points [°C] | ||||
| Deformation point | 1350 | 1320 | 1340 | out of measuring range |
| Softening point | 1365 | 1370 | 1365 | |
| Sphere point | 1377 | 1392 | 1392 | |
| Half sphere point | 1408 | 1420 | 1421 | |
| Total melting point/Flow | 1411 | 1431 | 1425 | |
| Sample | Phases | |||||
|---|---|---|---|---|---|---|
| A | MA | C2AS | CA | CA2 | CAS2 | |
| A | 80.9 | 19.1 | - | - | - | - |
| B | 74.8 | 25.2 | - | - | - | - |
| C | 62.1 | 37.9 | - | - | - | - |
| A_S3 | 15.6 | 14.2 | 21.4 | 37.2 | 11.0 | - |
| B_S3 | 11.6 | 18.0 | 21.5 | 40.2 | 7.9 | - |
| C_S3 | 7.9 | 23.1 | 21.5 | 41.0 | 6.1 | - |
| A_S2 | 16.6 | 33.2 | 19.0 | 11.2 | 20.1 | - |
| B_S2 | 14.8 | 38.8 | 18.2 | 15.1 | 12.7 | - |
| C_S2 | 11.9 | 43.5 | 18.5 | 14.1 | 12.1 | - |
| A_S1 | 11.7 | 33.8 | 36.5 | - | - | 18.0 |
| B_S1 | 8.3 | 39.3 | 34.6 | - | - | 17.8 |
| C_S1 | 5.3 | 41.4 | 35.5 | - | - | 17.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramult, J.; Wiśniewska-Tobiasz, K.; Prorok, R.; Madej, D. Fundamental Investigations on the High-Temperature Corrosion of Spinel-Forming Alumina Castables by Steel Slags. Appl. Sci. 2022, 12, 704. https://doi.org/10.3390/app12020704
Ramult J, Wiśniewska-Tobiasz K, Prorok R, Madej D. Fundamental Investigations on the High-Temperature Corrosion of Spinel-Forming Alumina Castables by Steel Slags. Applied Sciences. 2022; 12(2):704. https://doi.org/10.3390/app12020704
Chicago/Turabian StyleRamult, Jakub, Klaudia Wiśniewska-Tobiasz, Ryszard Prorok, and Dominika Madej. 2022. "Fundamental Investigations on the High-Temperature Corrosion of Spinel-Forming Alumina Castables by Steel Slags" Applied Sciences 12, no. 2: 704. https://doi.org/10.3390/app12020704
APA StyleRamult, J., Wiśniewska-Tobiasz, K., Prorok, R., & Madej, D. (2022). Fundamental Investigations on the High-Temperature Corrosion of Spinel-Forming Alumina Castables by Steel Slags. Applied Sciences, 12(2), 704. https://doi.org/10.3390/app12020704







