Abstract
Ye’elimite is a dominant phase in calcium sulfoaluminate cement, which is a promising alternative type of cementitious binder. Ca3.8Na0.2Al5.6Fe0.2Si0.2SO16 (abbreviated as ss-C4A3$) is a kind of typical doped solid-solution ye’elimite. In this study, the formation process of ss-C4A3$ was investigated. Clinkers of ss-C4A3$ were sintered at various temperatures for different holding times. X-ray diffraction tests and Rietveld quantitative phase analysis were conducted to determine the phase compositions of the clinkers. Meanwhile, the formation process of ss-C4A3$ was analyzed by kinetic theory. The results show that solid reactions between intermediate phases (calcium aluminate phases) and anhydrite mainly resulted in the formation of ss-C4A3$. In the conditions of 1150–1250 °C, ss-C4A3$ tended to be formed and stable until 4 h. However, when the sintering temperature was 1300 °C, the ss-C4A3$ decreased to generate calcium aluminate phases after 2 h. Compared to other kinetic models, the three-dimensional diffusion model mostly conformed with the formation process of ss-C4A3$, and the fitting results obtained by the Jander model exhibited the highest correlation coefficients. The activation energy of ss-C4A3$ formation equaled 285.6 kJ/mol, which was smaller than that of stoichiometric ye’elimite.
1. Introduction
As the production process of calcium sulfoaluminate (CSA) cement produces fewer CO2 emissions and consumes less energy compared to Portland cement, CSA cement is treated as a type of alternative and low-carbon binder [1,2]. Due to characteristics of early strength and tailored expansion, CSA cement has also been widely used as a rapid repairing material since the 1970s and it has recently been promoted for application in numerous domains, such as soft soil stabilization and 3D printing [3,4,5]. Ye’elimite is a dominant phase in CSA cement and generally accounts for over 50 wt.% in CSA clinkers [6,7]. Sintering conditions of CSA clinkers are determined by the forming conditions of ye’elimite; meanwhile, the main hydration products of CSA cement result from the reactions between ye’elimite and gypsum [8,9,10,11,12].
The chemical formula of stoichiometric ye’elimite is Ca4Al6SO16, which can be abbreviated as st-C4A3$ (hereafter, nomenclatures are used—C: CaO, $: SO3, A: Al2O3, S: SiO2). The crystallographic structure of st-C4A3$ has been identified to be orthorhombic at room temperature, with a space group of Pcc2. Meanwhile, the formation and hydration mechanisms of st-C4A3$ have also been intensively investigated by a number of studies [13,14,15]. On the other hand, certain types of doping ions (such as iron ions and sodium ions et al.) enter into the lattices of ye’elimite in the manufacturing process of CSA clinkers, which leads to the formation of doped/solid-solution ye’elimite [16,17,18]. Specifically, when solid waste is utilized as raw materials, various types of solid-solution ye’elimite tend to be formed more easily [19]. A series of recent studies focused on different types of doped ye’elimite, including strontium-bearing ye’elimite, barium-bearing ye’elimite, and iron-bearing ye’elimite.
The consensus obtained by studies on doped ye’elimite is that dopants result in a crystallographic system of ye’elimite, partly or totally transforming from an orthorhombic system to a cubic system [16,17,20]. Particularly, Ca3.8Na0.2Al5.6Fe0.2Si0.2SO16 (abbreviated as ss-C4A3$), a typical kind of doped solid-solution ye’elimite, has attracted special attention, as the crystallographic system of ss-C4A3$ has been proved to be purely cubic at room temperature [21]. Comparing it to st-C4A3$, ss-C4A3$ also exhibited obviously distinct hydration characters [22]. Additionally, elemental compositions of ss-C4A3$ approached sintered ye’elimite phases in CSA clinkers due to the existence of iron ions and silicon ions in raw materials [16].
In this study, the formation process of ss-C4A3$ was investigated. Clinkers of ss-C4A3$ were sintered at various temperatures for different holding times. X-ray diffraction (XRD) tests and Rietveld quantitative phase analysis (RQPA) were conducted to determine the phase compositions of the clinkers. According to the results of the RQPA, different models of solid-state reactions were employed to fit the isothermal formation process of ss-C4A3$. By comparing the obtained correlation coefficients, the formation mechanism of ss-C4A3$ was confirmed. Lastly, the value of activation energy for ss-C4A3$ was acquired by the Arrhenius equation.
2. Materials and Methods
2.1. Raw Materials and Sintering Conditions for Clinkers
According to the elemental molar ratios of st-C4A3$ and ss-C4A3$, analytical reagents of CaCO3, Na2CO3, Al2O3, Fe2O3, SiO2, and CaSO4 were weighed and mixed by a planetary ball mill for homogeneousness to prepare raw materials (purities of all analytical reagents were more than 99.99 wt.%). Afterwards, well-mixed raw materials were compressed into cylindroid samples (Φ5.0 cm × H1.0 cm) and placed in a high-temperature furnace for sintering. As for st-C4A3$, through a heating rate of 10 °C/min, the raw materials were sintered at 1300 °C for 240 min. With regard to ss-C4A3$, the raw materials were respectively sintered at 1150 °C, 1200 °C, 1250 °C, and 1300 °C (10 °C/min heating rate) for various holding times (0 min, 15 min, 30 min, 60 min, 120 min, 180 min, and 240 min).
2.2. Test Methods
After sintering, synthesized clinkers were finely ground to pass a sieve of 45 μm for X-ray diffraction (XRD) testing. A Bruker diffractometer with CuKα1,2 radiation (without a monochromator, λ1 = 0.15406 nm, λ2 = 0.15444 nm) was employed at 40 kV and 40 mA. For quantitative phase analysis, the range of data collection was 5–80° (in this paper, all angles refer to a 2θ value) with a step size of 0.02°, and the total measurement time for each sample was approximately 30 min. When the lattice parameters needed to be refined, the range of data collection was expanded to be 5–120°, and the total measurement time for each sample was increased to approximately 120 min.
XRD patterns obtained by direct tests were firstly analyzed qualitatively in Bruker Evolution software to determine the phases in the clinkers. Then, Topas 4.2 software was employed to perform RQPA for dealing with the obtained XRD patterns. Table 1 exhibits the COD codes for all the phases involved in the RQPA.

Table 1.
COD codes and references of phases involved in the RQPA.
2.3. Kinetic Theory
To characterize the process of chemical reactions for a certain type of product, reaction degree α is calculated according to Equation (1).
In Equation (1), t is the reaction time at the isothermal stage and m0 is the initial mass percentage of the product for the isothermal stage. The mass percentage of the product at holding time t is written as mt, and m∞ represents the (theoretical) final mass percentage of the product for the isothermal stage.
In isothermal conditions, the temperature is constant. Based on the kinetic reaction equation, the reaction degree α can be related to holding time t, as shown in Equation (2).
For Equation (2), g(α) and f(α) are the integral and differential forms of the kinetic models, respectively. The models used for the solid-state reactions in this study are exhibited in Table 2.

Table 2.
Kinetic models for solid-state reactions [28].
Based on the results of linear fitting for Equation (2), the slopes of the kinetic equation k for various temperatures were obtained. Furthermore, the values of the parameters in the Arrhenius Equation, expressed in the logarithmic form and exhibited in Equation (3), were confirmed.
lnk = lnA − Ea/RT
In Equation (3), A is the preexponential factor, k is the slope of the kinetic equation, Ea represents activation energy, and R is the gas constant (8.314 J/(mol·K)).
3. Results
3.1. Crystallographic Distinctions between st-C4A3$ and ss-C4A3$
To distinguish the crystallographic distinctions between orthorhombic and cubic ye’elimite, XRD patterns of st-C4A3$ (sintered at 1300 °C for 240 min) and ss-C4A3$ (sintered at 1250 °C for 240 min) were compared, as shown in Figure 1. As can be seen, except for some low-intensity peaks for monocalcium aluminate (CaAl2O4) and calcium oxide (CaO), all peaks belonged to orthorhombic and cubic ye’elimite. Meanwhile, the patterns for st-C4A3$ and ss-C4A3$ tended to be similar, as the major peaks of both patterns overlap at 23.7°, 33.7°, 41.6°, (angles refer to 2θ in this paper) and so on. However, it should also be noted that, compared to ss-C4A3$, the pattern of st-C4A3$ exhibited some additional characteristic peaks (such as the peaks at 18.1°, 20.6°, 35.8°, and 37.3°), which can be observed in the zoomed-in images of Figure 1. The additional characteristic peaks were used to distinguish the st-C4A3$ from the ss-C4A3$.
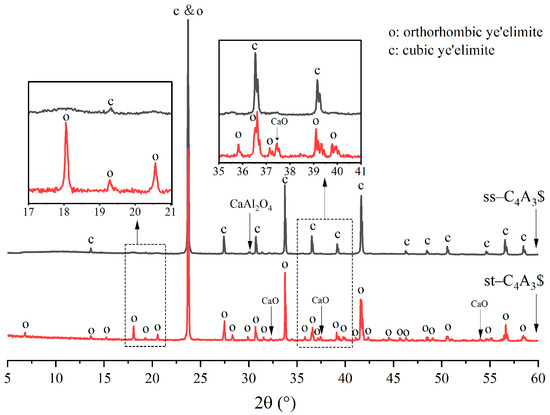
Figure 1.
XRD patterns of solid-solution ye’elimite (sintered at 1250 °C for 4 h) and stoichiometric ye’elimite (sintered at 1300 °C for 4 h).
To further confirm the lattice parameters of synthesized st-C4A3$ and ss-C4A3$, the Rietveld method was employed to refine the patterns in Figure 1. The fitting result of ss-C4A3$ can be seen in Figure 2, and the obtained values of the lattice parameters are exhibited in Table 3. According to the results of the Rietveld refinement, the crystallographic characters of synthesized st-C4A3$ and ss-C4A3$ in this paper were consistent with those of previous studies.
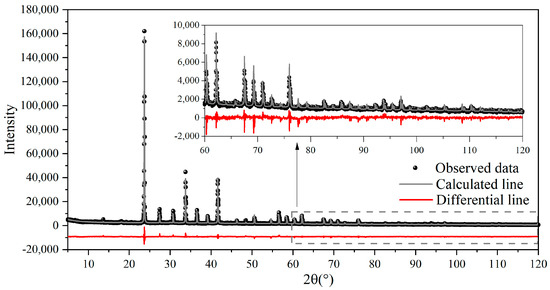
Figure 2.
Rietveld plot for XRD pattern of ss-C4A3$ (sintered at 1250 °C for 4 h).

Table 3.
Lattice parameters of synthesized solid-solution and stoichiometric ye’elimite.
3.2. Formation Process of ss-C4A3$
The formation process of st-C4A3$ can be divided into three stages, including the stage for the decomposition of raw materials (mainly referring to the decomposition of CaCO3, as shown in Equation (4)), the stage for the formation of intermediate phases (mainly referring to the formation of CA, as shown in Equation (5)), and the formation of ye’elimite (mainly referring to the reaction between CA and C$, as shown in Equation (6)). To determine the involved solid reactions in the formation process of ss-C4A3$, the XRD patterns of clinkers sintered at various temperatures for different holding times were compared, as shown in Figure 3.
CaCO3 → CaO + CO2
CaO + Al2O3 → CaAl2O4
3CaAl2O4 + CaSO4 → Ca4Al6SO16
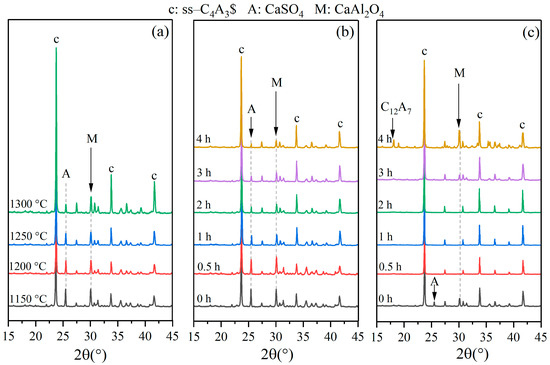
Figure 3.
XRD patterns of clinkers for ss-C4A3$, (a) sintered at various temperatures without holding times, (b) sintered in conditions of different holding times at 1150 °C, (c) sintered at 1300 °C for different holding times.
Comparing the XRD patterns for st-C4A3$ and ss-C4A3$ in Section 3.1, it was observed that the additional characteristic peaks of st-C4A3$ were not in any of the clinkers of ss-C4A3$ in Figure 3, which means that the formation process of ss-C4A3$ did not involve the formation of st-C4A3$. Clinkers sintered at various temperatures without holding times are exhibited in Figure 3a. It can be seen that certain amounts of ss-C4A3$ were formed at 1150 °C, but CaAl2O4 and CaSO4 can obviously still be detected. With sintering temperatures increasing from 1150 to 1300 °C, the intensity of the peaks of ss-C4A3$ steadily increased; meanwhile, the intensity of the peaks of CaAl2O4 and CaSO4 decreased. Similar variational tendencies for the peaks of ss-C4A3$, CaAl2O4, and CaSO4 can also be observed in the clinkers sintered at 1150 °C for different holding times (see Figure 3b). However, for clinkers sintered at 1300 °C (see Figure 3c), CaSO4 could not be detected after a 0.5 h holding time, and peaks of CaAl2O4 reappeared after sintering for over 3 h. To further determine the contents of the phases in clinkers sintered in different conditions, Rietveld quantitative phase analysis was conducted to identify the XRD patterns. Taking the analysis of the clinkers sintered at 1150 °C for 60 min as an example, the selected range and fitting results of the Rietveld quantitative phase analysis are shown in Figure 4.
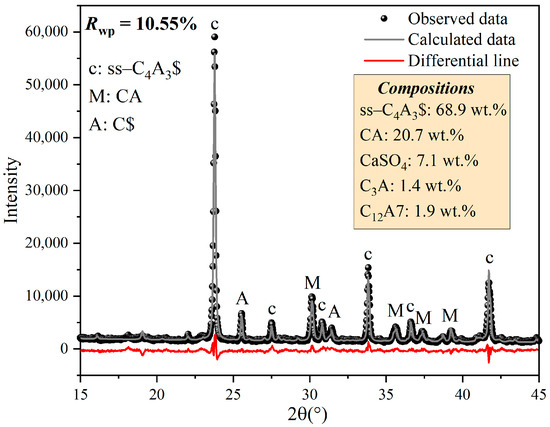
Figure 4.
Selected range and fitting results of the Rietveld quantitative phase analysis of clinkers sintered at 1150 °C for 60 min.
Figure 5 exhibits the contents of the phases in clinkers sintered at different temperatures as a function of holding time, of which three stages were identified. The first stage was the forming stage, which included 1150 °C (within 240 min), 1200 °C (within 240 min), 1250 °C (the first 120 min), and 1300 °C (the first 30 min). In the forming stage, the contents of ss-C4A3$ steadily increased with increasing holding time; meanwhile, the contents of calcium aluminate phases (in most instances, CA accounted for the overwhelming majority) and anhydrite continually decreased. The variation tendency illustrates that ss-C4A3$ was formed through the reaction between the calcium aluminate phases and anhydrite in the forming stage, which was similar to the reaction in Equation (6). After the forming stage, the stable stage was observed at 1250 °C (in the range of 120-240 min) and 1300 °C (in the range of 30–120 min). In the stable stage, the contents of ss-C4A3$ remained stable and generally remained over 90 wt.% in the clinkers; meanwhile, the calcium aluminate phases and anhydrite accounted for the absolute minority (less than 10 wt.%). It should also be noted that the contents of ss-C4A3$ tended to decrease and generate calcium aluminate phases when the clinkers were sintered over 120 min at 1300 °C, which can be identified as the decomposing stage.
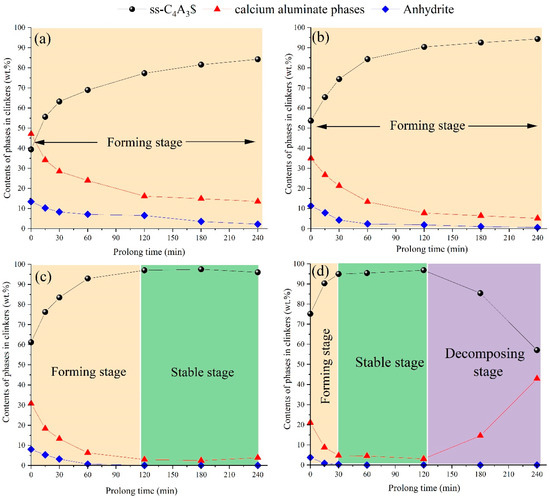
Figure 5.
Contents of phases in clinkers sintered at different temperatures as a function of holding time: (a) 1150 °C, (b) 1200 °C, (c) 1250 °C, (d) 1300 °C.
3.3. Kinetic Analysis for the Formation Process of ss-C4A3$
The reaction degrees α of ss-C4A3$ in the forming stage were calculated according to the contents of the phases in the clinkers obtained by the RQPA. Afterwards, the linear relationship between the reaction degrees and the holding times were fitted by Equation (2) (the integral form of kinetic models), and the correlation coefficients (R2) were compared to determine the potential optimal models.
According to the comparison in Table 4, all diffusion models exhibited obviously higher correlation coefficients than other types of models. It should be also noted that the correlation coefficients of the D3 (Jander) model and the D4 (Ginstling–Brounstein) model were very close and higher than the results yielded from the D1 and D2 models. The results illustrate that the formation reactions of ss-C4A3$ take place in a process of three-dimensional diffusion, which accords with st-C4A3$ and several types of solid-solution ye’elimite [29,30]. Considering the D3 model exhibited the most acceptable fitting results, the values for k in Equation (2) were determined by the D3 model, and the fitting results can be seen in Figure 6a. Afterwards, the values of Ea were obtained through a linear fitting for Equation (3) (Arrhenius equation), and they are shown in Figure 6b. According to the results of the Arrhenius fitting, the Ea of ss-C4A3$ equaled 285.6 kJ/mol in this study.

Table 4.
Correlation coefficients (R2) fitted by different reaction models.
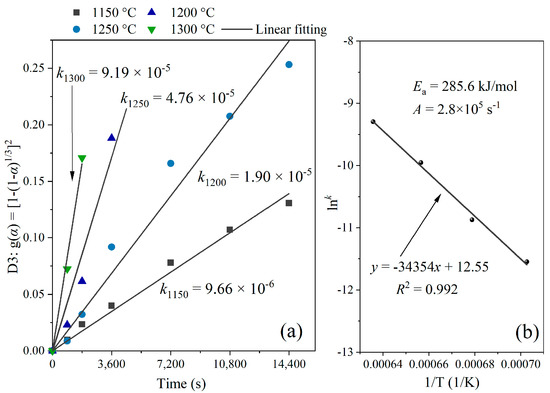
Figure 6.
Plot of kinetic analysis for ss-C4A3$, (a) Jander fitting of ss-C4A3$ formation at different temperatures, (b) Arrhenius fitting for the formation of ss-C4A3$.
3.4. Influence of Sintering Temperature on Microstructural Morphologies of ss-C4A3$
Microstructural morphologies of four types of clinkers containing over 90 wt.% of ss-C4A3$ are compared in Figure 7. As shown in Figure 7a, the morphology features of ss-C4A3$ sintered at 1200 °C for 240 min were identified as approximately 1–2 μm polyhedral granules with clear boundaries. For ss-C4A3$ sintered at 1250 °C for 240 min (shown in Figure 7b), the majority of granules still exhibited clear boundaries, but a few granules tended to fuse and combine with adjacent granules (shown by the arrow). When prolonged times extended to 240 min at 1250 °C (see Figure 7c), fusion among the granules of ss-C4A3$ was more evident (shown in the circles). As for ss-C4A3$ sintered at 1300 °C for 120 min (see Figure 7d), partial granules fused to be larger than 2 μm; meanwhile, the boundaries of the granules tended to be more vague.
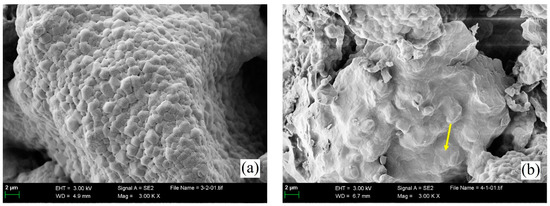
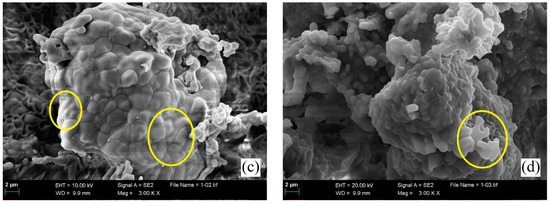
Figure 7.
Microstructural morphologies of ss-C4A3$ sintered at various temperatures for different times (a) 1200 °C for 240 min, (b) 1250 °C for 120 min, (c) 1250 °C for 240 min, (d) 1300 °C for 120 min.
4. Discussion
Values of Ea for different types of ye’elimite were compared and are shown in Table 5. It can be seen that the Ea of ss-C4A3$ was slightly smaller than that of st-C4A3$ and obviously smaller than that of strontium/barium-bearing ye’elimite. From the point of view of physical significance, the value of Ea reflects the barrier needed to be overcome for a reaction [28]. This indicates that the ss-C4A3$ tended to be more easily formed than the st-C4A3$ and strontium/barium-bearing ye’elimite in the same sintering conditions.

Table 5.
Values of activation energy (Ea) for different types of ye’elimite formation.
Less barrier for forming ss-C4A3$ can be mainly attributed to the effect of different types on the formation process of ye’elimite. For strontium/barium-bearing ye’elimite, strontic/baric ions introduce strontic/baric sulfates or strontic/baric aluminates as distinct intermediate phases, which results in different solid reactions for forming strontium/barium-bearing ye’elimite compared to st-C4A3$ [29,30]. It should also be noted that strontic/baric sulfates are more stable than calcium sulfate at high temperatures, and thus, higher barriers need to be overcome for forming strontium/barium-bearing ye’elimite compared to st-C4A3$ [29]. However, for st-C4A3$, the formation process of ss-C4A3$ mainly resulted from the solid reactions between the calcium aluminate phases and anhydrite, which is in accordance with the st-C4A3$. Meanwhile, considering the mineralizing effect caused by the substitution of Fe3+ ions with Al3+ in the crystal structure of ye’elimite, ss-C4A3$ tended to be more easily formed than st-C4A3$ [31].
5. Conclusions
Based on the Rietveld quantitative phase analysis for clinkers sintered at various temperatures for different holding times, the formation process of ss-C4A3$ was investigated through the lens of kinetic theory. The following conclusions can be made:
- (1)
- XRD patterns of st-C4A3$ and ss-C4A3$ tended to be similar, but st-C4A3$ was distinguished from ss-C4A3$ by some additional characteristic peaks. The formation process of ss-C4A3$ mainly resulted from solid reactions between the intermediate phases (calcium aluminate phases) and anhydrite, which did not involve the formation of st-C4A3$. In the conditions of 1150–1250 °C, ss-C4A3$ tended to be formed and stable for 4 h. However, when the sintering temperature increased to 1300 °C, ss-C4A3$ decomposed to generate calcium aluminate phases after 2 h.
- (2)
- Compared to other kinetic models, the three-dimensional diffusion model mostly conformed with the formation process of ss-C4A3$, and the fitting results obtained by the Jander model exhibited the highest correlation coefficients. The activation energy of ss-C4A3$ formation equaled 285.6 kJ/mol, which was lower than that of stoichiometric and strontium/barium-bearing ye’elimite.
- (3)
- The morphology features of ss-C4A3$ sintered at 1200 °C for 240 min were identified as approximately 1–2 μm polyhedral granules with clear boundaries; higher sintering temperatures or longer holding times would lead to granules fusing together.
Author Contributions
J.Z.: Conceptualization, funding acquisition, writing—original draft; J.H.: data curation, investigation, visualization; C.Y.: data curation, investigation; C.C.: project administration, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Fundamental Research Funds for the Central Universities (No. 3132020167).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Glasser, F.; Zhang, L. High-performance cement matrices based on calcium sulfoaluminate-belite compositions. Cem. Concr. Res. 2001, 31, 1881–1886. [Google Scholar] [CrossRef]
- Zhou, Q.; Milestone, N.; Hayes, M. An alternative to Portland Cement for waste encapsulation—The calcium sulfoaluminate cement system. J. Hazard. Mater. 2006, 136, 120–129. [Google Scholar] [CrossRef]
- Meng, K.; Cui, C.; Liang, Z.; Li, H.; Pei, H. A new approach for longitudinal vibration of a large-diameter floating pipe pile in visco-elastic soil considering the three-dimensional wave effects. Comput. Geotech. 2020, 128, 103840. [Google Scholar] [CrossRef]
- Khalil, N.; Aouad, G.; El Cheikh, K.; Rémond, S. Use of calcium sulfoaluminate cements for setting control of 3D-printing mortars. Constr. Build. Mater. 2017, 157, 382–391. [Google Scholar] [CrossRef]
- Cui, C.; Meng, K.; Xu, C.; Liang, Z.; Li, H.; Pei, H. Analytical solution for longitudinal vibration of a floating pile in saturated porous media based on a fictitious saturated soil pile model. Comput. Geotech. 2020, 131, 103942. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Zajac, M.; Ben Haha, M. CSA raw mix design: Effect on clinker formation and reactivity. Mater. Struc. 2015, 12, 3895–3911. [Google Scholar] [CrossRef]
- Alvarez-Pinazo, G.; Cuesta, A.; Garcia-Mate, M.; Santacruz, I.; Losilla, E.R.; Torre, A.G.; Leon-Reina, L.; Aranda, M.A.G. Rietveld quantitative phase analysis of Yeelimite-containing cements. Cem. Concr. Res. 2012, 42, 960–971. [Google Scholar] [CrossRef] [Green Version]
- Juenger, M.C.G.; Winnefeld, F.; Provis, J.L.; Ideker, J.H. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 12, 1232–1243. [Google Scholar] [CrossRef]
- Shi, C.; Fernandez, A.; Palomo, A. New cements for the 21st century: The pursuit of an alternative to Portland cement. Cem. Concr. Res. 2011, 7, 750–763. [Google Scholar] [CrossRef]
- Hargis, C.W.; Telesca, A.; Monteiro, P.J.M. Calcium sulfoaluminate (Ye’elimite) hydration in the presence of gypsum, calcite, and vaterite. Cem. Concr. Res. 2014, 65, 15–20. [Google Scholar] [CrossRef]
- Winnefeld, F.; Martin, L.H.J.; Muller, C.J.B. Lothenbach, using gypsum to control hydration kinetics of CSA cements. Constr. Build. Mater. 2017, 155, 154–163. [Google Scholar] [CrossRef]
- Bizzozero, J.; Gosselin, C.; Scrivener, K.L. Expansion mechanisms in calcium aluminate and sulfoaluminate systems with calcium sulfate. Cem. Concr. Res. 2014, 56, 190–202. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Zajac, M.; Ben Haha, M.; Scrivener, K.L. Factors influencing the hydration kinetics of Ye’elimite; effect of mayenite. Cem. Concr. Res. 2018, 116, 113–119. [Google Scholar] [CrossRef]
- El, Y.; El, Y.; Smith, A. Examination of ye’elimite formation mechanisms. J. Eur. Ceram. Soc. 2019, 39, 5086–5095. [Google Scholar]
- Cuesta, A.; De la Torre, A.G.; Losilla, E.R.; Peterson, V.K.; Rejmak, P.; Ayuela, A.; Frontera, C.; Aranda, M.A.G. Structure, Atomistic Simulations, and Phase Transition of Stoichiometric Yeelimite. Chem. Mater. 2013, 25, 1680–1687. [Google Scholar] [CrossRef] [Green Version]
- Zea-Garcia, J.; Santacruz, I.; Aranda, M.; De, G. Alite-belite-ye’elimite cements: Effect of dopants on the clinker phase com-position and properties. Cem. Concr. Res. 2019, 115, 192–202. [Google Scholar] [CrossRef]
- Li, C.; Wu, M.; Yao, W. Effect of coupled B/Na and B/Ba doping on hydraulic properties of belite-ye’elimite-ferrite cement. Constr. Build. Mater. 2019, 208, 23–35. [Google Scholar] [CrossRef]
- Andaç, O.; Glasser, F.P. Polymorphism of calcium sulphoaluminate (Ca4Al6O16·SO3) and its solid solutions. Adv. Cem. Res. 1994, 6, 57–60. [Google Scholar] [CrossRef]
- Chang, J.; Cheng, X.; Liu, F.; Lu, L.; Teng, B. Influence of fluorite on the Ba-bearing sulphoaluminate cement. Cem. Concr. Res. 2001, 31, 213–216. [Google Scholar] [CrossRef]
- Zhao, J.; Chang, J. Crystallographic Analysis of Sr-Bearing Ye’elimite. J. Inorg. Organomet. Polym. Mater. 2017, 27, 1694–1702. [Google Scholar] [CrossRef]
- Cuesta, A.; de la Torre, Á.G.; Losilla, E.; Santacruz, I.; Aranda, M. Pseudocubic crystal structure and phase transition in doped ye’elimite. Cryst. Growth Des. 2014, 14, 5158–5163. [Google Scholar] [CrossRef] [Green Version]
- Cuesta, A.; Pinazo, G.; Sanfélix, S.; Peral, I.; Aranda, M.; De la Torre, A. Hydration mechanisms of two polymorphs of synthetic Ye’elimite. Cem. Concr. Res. 2014, 63, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Lazic, B.; Krüger, H.; Kahlenberg, V.; Konzett, J.; Kaindl, R. Incommensurate structure of Ca2Al2O5 at high temperatures—Structure investigation and Raman spectroscopy. ACS Catal. 2010, 64, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.C.H.; Zussman, J. The crystal structure of anhydrite (CaSO4). Acta Crystallogr. 1963, 16, 767–769. [Google Scholar] [CrossRef] [Green Version]
- Boysen, H.; Lerch, M.; Stys, A.; Senyshyn, A. Structure and oxygen mobility in mayenite (Ca12Al14O33): A high-temperature neutron powder diffraction study. Acta Crystallogr. Sect. B Struct. Sci. 2007, 63, 675–682. [Google Scholar] [CrossRef]
- Mondal, P.; Jeffery, J.W. The crystal structure of tricalcium aluminate, Ca3Al2O6. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1975, 31, 689–697. [Google Scholar] [CrossRef]
- Verbraeken, M.; Suard, E.; Irvine, J. Structural and electrical properties of calcium and strontium hydrides. J. Chem. Educ. 2009, 19, 2766–2770. [Google Scholar] [CrossRef]
- Laidler, K.J. The development of the Arrhenius equation. J. Chem. Educ. 1984, 61, 494. [Google Scholar] [CrossRef]
- Bao, X.; Zhao, P.; Liang, C.; Li, Q.; Wang, S.; Cheng, X. Phase formation mechanism and kinetics in solid-state synthesis of Ba-doped Ye′elimite: The effect of Ba-doping concentration on C4−xBxA3$ systems. Ceramics-Silikáty 2020, 64, 338–347. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Chang, J. Kinetic Analysis for Formation Process of Sr-Bearing Ye’elimite. J. Inorg. Organomet. Polym. Mater. 2017, 27, 1861–1869. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Scholten, T.; Scrivener, K.; Ben Haha, M.; Wolter, A. Formation, composition and stability of Ye’elimite and iron-bearing solid solutions. Cem. Concr. Res. 2020, 131, 106009. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).