Review of Studies on Joint Recovery of Macroalgae and Marine Debris by Hydrothermal Liquefaction
Abstract
:1. Introduction
2. Experience of Hydrothermal Liquefaction of Macroalgae
2.1. Using Macroalgae for Biofuel Production in a One-Step Process
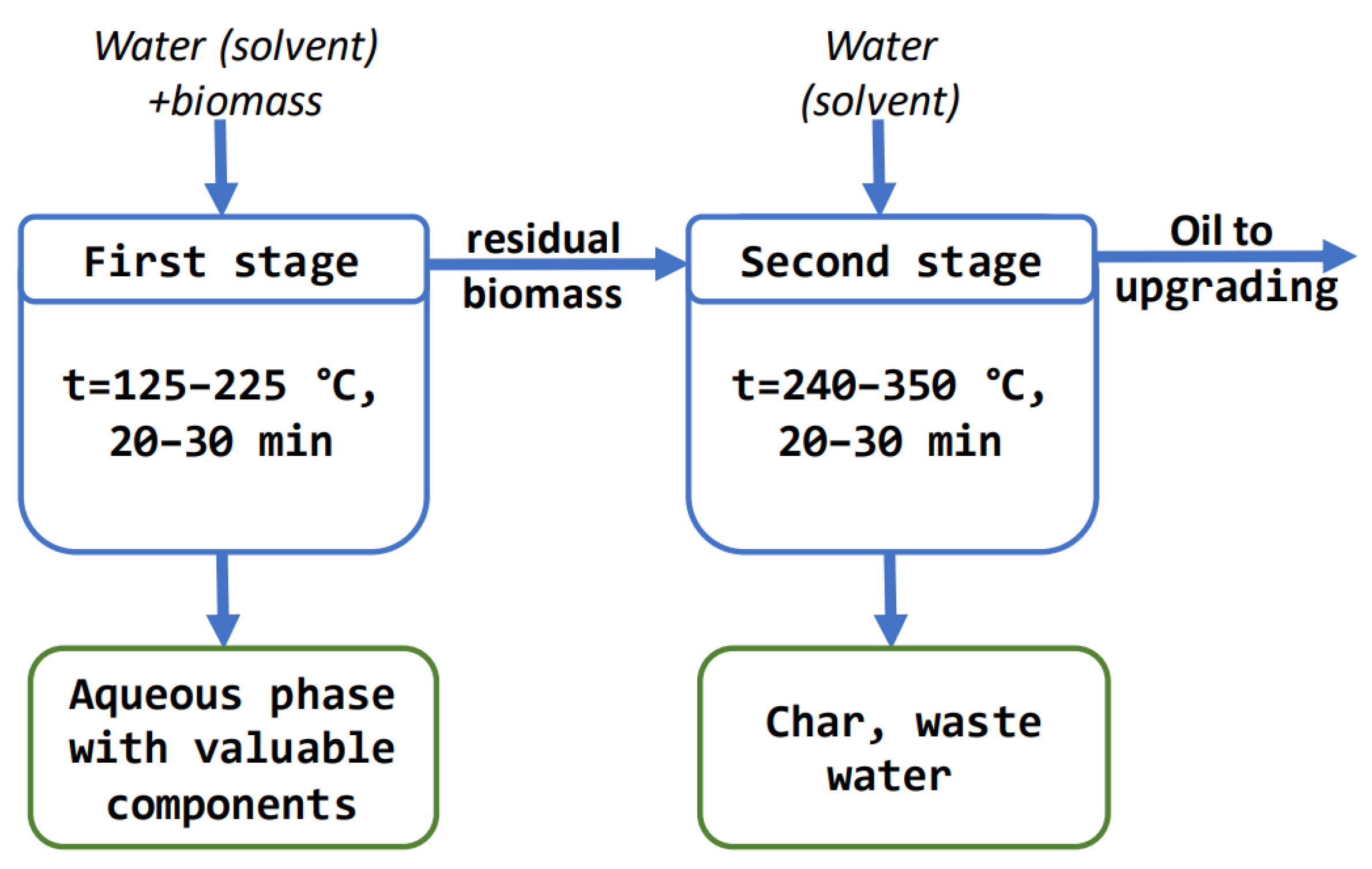
2.2. Application of a Two-Stage Hydrothermal Liquefaction Process for Algae Conversion
- first stage: 125–225 °C, 0.5–30 min
- second stage: 350 °C, 60 min
- decrease in the amount of nitrogen and oxygen amount in oil phase;
- possibility of valuable component preliminary extraction;
- increase of biomass utilization rate.
- if the goal is to extract valuable components from the aqueous phase after the first stage;
- in the case of using pure, monoculture biomass;
- if it is vitally important to reduce the amount of nitrogen in bio-oil.
3. Prospects for Joint Processing of Macroalgae Biomass and Marine Debris
3.1. Hydrothermal Liquefaction of Polymers and Their Mixtures
- PET behaved very different from others, since upon addition of a catalyst the formed insoluble terephthalic acid transformed into a soluble salt, increasing the COD of the aqueous phase [46].
- PVC was the only polymer that exhibited carbonization reactions rather than liquefaction reactions. The addition of alkali did not lead to significant differences in the yields of oil and char; however, a large difference was noted in the volumes of the gas phase. Treatment of PVC in the presence of alkali gives an increased gas yield due to the release of Cl2 amount. Solid residues of PVC were strongly dechlorinated, which indicates that this fraction can be further used as a carbon source [46].
- In general, each type of synthetic polymer exhibits its own depolymerization characteristics at HTL, which creates opportunities and challenges for future applications for net and mixed streams. Short exposure time of subcritical HTL was not efficient for polyolefins and polystyrene, however it could be useful for polymers, which contain heteroatoms.
3.2. Joint Hydrothermal Liquefaction of Polymers and Biomass
4. Discussion
- Maximum oil yield during the HTL process could be achieved by using alcohols as co-solvents, since the presence of alcohols leads to an esterification reaction with carboxylic acids [31];
- As catalytic systems, it is worth paying attention to heterogeneous catalysis with the use of mineral and metal-containing catalysts, which provide an increase in the bio-oil yield and “soften” the conditions of the process.
- The presence of polymers, typical representatives of marine waste (TeraPak, high- and low-pressure polyethylene, polypropylene, and nylon) do not provide a significant synergistic effect, and with a significant proportion of polymers in the macroalgae biomass (more than 10% by weight in terms of dry matter), an increase in the yield of non-target solid fraction and a decrease in the yield of biofuel should be expected;
- Optimum process temperatures, providing the maximum yield of liquid fraction, for most polymers are in the range of 350–400 °C, while for macroalgae the optimum temperature is in the range of 260–290 °C.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joniver, C.F.H.; Photiades, A.; Moore, P.J.; Winters, A.L.; Woolmer, A.; Adams, J.M.M. The global problem of nuisance macroalgal blooms and pathways to its use in the circular economy. Algal Res. 2021, 58, 102407. [Google Scholar] [CrossRef]
- Ingle, K.; Vitkin, E.; Robin, A.; Yakhini, Z.; Mishori, D.; Golberg, A. Macroalgae Biorefinery from Kappaphycus alvarezii: Conversion Modeling and Performance Prediction for India and Philippines as Examples. Bioenerg. Res. 2018, 11, 22–32. [Google Scholar] [CrossRef]
- Stepanova, E.M.; Lugovaya, E.A. Macro- and microelements in some species of marine life from the Sea of Okhotsk. Foods Raw Mater. 2021, 9, 302–309. [Google Scholar] [CrossRef]
- Tabakaev, A.V.; Tabakaeva, O.V.; Piekoszewski, W.; Kalenik, T.K.; Poznyakovsky, V.M. Antioxidant properties of edible sea weed from the Northern Coast of the Sea of Japan. Foods Raw Mater. 2021, 9, 262–270. [Google Scholar] [CrossRef]
- Del Río, P.G.; Domínguez, E.; Domínguez, V.D.; Romaní, A.; Domingues, L.; Garrote, G. Third generation bioethanol from invasive macroalgae Sargassum muticum using autohydrolysis pretreatment as first step of a biorefinery. Renew. Energy 2019, 141, 728–735. [Google Scholar] [CrossRef] [Green Version]
- Tedesco, S.; Stokes, J. Valorisation to biogas of macroalgal waste streams: A circular approach to bioproducts and bioenergy in Ireland. Chem. Pap. 2017, 71, 721–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tedesco, S.; Daniels, S. Optimisation of biogas generation from brown seaweed residues: Compositional and geographical parameters affecting the viability of a biorefinery concept. Appl. Energy 2018, 228, 712–723. [Google Scholar] [CrossRef]
- Torres, M.D.; Kraan, S.; Domínguez, H. Seaweed biorefinery. Rev. Environ. Sci. Biotechnol. 2019, 18, 1–54. [Google Scholar] [CrossRef]
- Álvarez-Viñas, M.; Flórez-Fernández, N.; Torres, M.D.; Domínguez, H. Successful Approaches for a Red Seaweed Biorefinery. Mar. Drugs 2019, 17, 620. [Google Scholar] [CrossRef] [Green Version]
- Flórez-Fernández, N.; Domínguez, H.; Torres, M.D. Advances in the biorefinery of Sargassum muticum: Valorisation of the alginate fractions. Ind. Crops Prod. 2019, 138, 111483. [Google Scholar] [CrossRef]
- European Parliament; Council of the European Union. Directive (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018 on the Promotion of the Use of Energy from Renewable Sources: PE/48/2018/REV/1. Off. J. Eur. Union 2018, 328, 1–20. [Google Scholar]
- Kargbo, H.; Harris, J.S.; Phan, N.A. Dropin-fuel production from biomass Critical review on technoeconomic feasibility and sustainability Renewable-and-Sustainable-Energy-Reviews. Renew. Sustain. Energy Rev. 2021, 135, 110168. [Google Scholar] [CrossRef]
- Marulanda, V.A.; Botero Gutierrez, C.D.; Cardone Alzate, C.A. Thermochemical Biological Biochemical, and Hybrid Conversion Methods of Bio-Derived Molecules into Renewable Fuels. In Advanced Bioprocessing for Alternative Fuels, Biobased Chemicals, and Bioproducts; Hosseini, M., Ed.; Elsevier: Duxford, UK, 2019; pp. 59–81. [Google Scholar] [CrossRef]
- Huang, S.; Liu, T.; Peng, B.; Geng, A. Enhanced ethanol production from industrial lignocellulose hydrolysates by a hydrolysate-cofermenting Saccharomyces cerevisiae strain. Bioproc. Biosyst. Eng. 2019, 42, 883–896. [Google Scholar] [CrossRef]
- Rech, F.R.; Fontana, R.C.; Rosa, C.A.; Camassola, M.; Ayub, M.A.Z.; Dillon, A.J. Fermentation of hexoses and pentoses from sugarcane bagasse hydrolysates into ethanol by Spathaspora hagerdaliae. Bioproc. Biosyst. Eng. 2019, 42, 83–92. [Google Scholar] [CrossRef]
- Zhu, L.; Li, P.; Sun, T.; Kong, M.; Li, X.; Ali, S.; Liu, W.; Fan, S.; Qiao, J.; Li, S.; et al. Overexpression of SFA1 in engineered Saccharomyces cerevisiae to increase xylose utilization and ethanol production from different lignocellulose hydrolysates. Bioresour. Technol. 2020, 313, 123724. [Google Scholar] [CrossRef] [PubMed]
- Rajak, C.; Jacob, S.; Kim, B.S. A holistic zero waste biorefinery approach for macroalgal biomass utilization: A review. Sci. Total Environ. 2020, 716, 137067. [Google Scholar] [CrossRef] [PubMed]
- Brex´o, R.P.; Sant’Ana, A.S. Impact and significance of microbial contamination during fermentation for bioethanol production. Renew. Sustain. Energ. Rev. 2017, 73, 423–434. [Google Scholar] [CrossRef]
- Winjobi, O.; Shonnard, D.R.; Bar-Ziv, E.; Zhou, W. Techno-economic assessment of the effect of torrefaction on fast pyrolysis of pine. Biofuels Bioprod. Biorefin. 2016, 10, 117–128. [Google Scholar] [CrossRef]
- Brigljevic, B.; Liu, J.; Lim, H. Comprehensive feasibility assessment of a polygeneration process integrating fast pyrolysis of S. japonica and the Rankine cycle. Appl. Energy 2019, 254, 113704. [Google Scholar] [CrossRef]
- Brilman, D.W.F.; Drabik, N.; Wądrzyk, M. Hydrothermal co-liquefaction of microalgae, wood, and sugar beet pulp. Biomass Convers. Biorefin. 2017, 7, 445–454. [Google Scholar] [CrossRef] [Green Version]
- Ciuffi, B.; Loppi, M.; Rizzo, A.M.; Chiaramonti, D.; Rosi, L. Towards a better understanding of the HTL process of lignin-rich feedstock. Sci. Rep. 2021, 11, 15504. [Google Scholar] [CrossRef] [PubMed]
- Ellersdorfer, M. Hydrothermal co-liquefaction of chlorella vulgaris with food processing residues, green waste and sewage sludge. Biomass Bioenergy 2020, 142, 105796. [Google Scholar] [CrossRef]
- Yang, J.; Quan, H.Q.S.; Corscadden, K.; Niu, H.; Lin, J.; Astatkie, T. Advanced models for the prediction of product yield in hydrothermal liquefaction via a mixture design of biomass model components coupled with process variables. Appl. Energy 2019, 233, 906–915. [Google Scholar] [CrossRef]
- Koley, S.; Khadase, M.S.; Mathimani, T.; Raheman, H.; Mallick, N. Catalytic and non-catalytic hydrothermal processing of Scenedesmus obliquus biomass for bio-crude production—A sustainable energy perspective. Energy Convers. Manag. 2018, 163, 111–121. [Google Scholar] [CrossRef]
- Han, Y.; Hoekman, K.; Jena, U.; Das, P. Use of Co-Solvents in Hydrothermal Liquefaction (HTL) of Microalgae. Energies 2020, 13, 124. [Google Scholar] [CrossRef] [Green Version]
- Nazari, L.; Yuan, Z.; Ray, M.B.; Xu, C. Co-conversion of waste activated sludge and sawdust through hydrothermal liquefaction: Optimization of reaction parameters using response surface methodology. Appl. Energy 2017, 203, 1–10. [Google Scholar] [CrossRef]
- Arun, J.; Gopinath, K.P.; Selvam, P.; Rajan, S.; Malolan, R.; Srinivaasan, P. Hydrothermal liquefaction and pyrolysis of Amphiroa fragilissima biomass: Comparative study on oxygen content and storage stability parameters of bio-oil. Bioresour. Technol. 2020, 11, 100465. [Google Scholar] [CrossRef]
- Greene, J.M.; Gulden, J.; Wood, G.; Huesemann, M.; Quinn, J.C. Techno-economic analysis and global warming potential of a novel offshore macroalgae biorefinery. Algal Res. 2020, 51, 102032. [Google Scholar] [CrossRef]
- Matayeva, A.; Basile, F.; Cavani, F.; Bianchi, D.; Chiaberge, S. Development of Upgraded Bio-Oil via Liquefaction and Pyrolysis. Studies in Surface Science and Catalysis. In Horizons in Sustainable Industrial Chemistry and Catalysis Studies in Surface Science and Catalysis; Albonetti, S., Perathoner, S., Quadrelli, E.A., Eds.; Elsevier: Oxford, UK, 2019; Volume 178, pp. 231–256. [Google Scholar] [CrossRef]
- Basar, I.A.; Liu, H.; Carrere, H.; Trably, E.; Eskicioglu, C. A review on key design and operational parameters to optimize and develop hydrothermal liquefaction of biomass for biorefinery applications. Green Chem. 2021, 23, 1404. [Google Scholar] [CrossRef]
- Ou, L.; Thilakaratne, R.; Brown, R.C.; Wright, M.M. Techno-economic analysis of transportation fuels from defatted microalgae via hydrothermal liquefaction and hydroprocessing. Biomass Bioenergy 2015, 72, 45–54. [Google Scholar] [CrossRef]
- Aierzhati, A.; Watson, J.; Si, B.; Stablein, M.; Wang, T.; Zhang, Y. Development of a mobile, pilot scale hydrothermal liquefaction reactor: Food waste conversion product analysis and techno-economic assessment. Energy Convers. Manag. 2021, 10, 100076. [Google Scholar] [CrossRef]
- Yang, C.; Wu, J.; Deng, Z.; Zhang, B.; Cui, C.; Ding, Y. A comparison of energy consumption in hydrothermal liquefaction and pyrolysis of microalgae. Trends Renew. Energy 2017, 3, 76–85. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Liang, J.; Zhu, M.; Zhao, Y.; Zhang, B. Microplastics in seawater and zooplankton from the Yellow Sea. Environ. Pollut. 2018, 242, 585–595. [Google Scholar] [CrossRef]
- Zhang, K.; Shi, H.; Peng, J.; Wang, Y.; Wu, X.X.C.; Lam, P.K.S. Microplastic pollution in China’s inland water systems: A review of findings, methods, characteristics, effects, and management. Sci. Total Environ. 2018, 630, 1641–1653. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Vethaak, A.D.; Lavorante, B.R.B.O.; Lundebye, A.-K.; Guilhermino, L. Marine microplastic debris: An emerging issue for food security, food safety and human health. Mar. Pollut. Bull. 2018, 133, 336–348. [Google Scholar] [CrossRef]
- Rhinane, H.; Houssa, R.; Loulad, S. The seafloor marine debris on the north and the central part of the moroccan atlantic waters from tangier (35° n) to sidi ifni (29° n): Composition, abundance, spatial distribution, sources and movement. ISPRS 2019, 42, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Nurhayati, E.; Tangahu, B.V.; Mahardini, I.R.; Berlianto, M.; Yuliawati, A.A. Marine Debris Monitoring in the Coastal Area of the District of Banyuwangi, Indonesia: Characterization of the Debris Type and composition. In IOP Conference Series: Earth and Environmental Science, Proceedings of the Sustainability and Resilience of Coastal Management, Surabaya, Indonesia, 30 November 2020; IOP Publishing: Bristol, UK, 2021; Volume 799, p. 012039. [Google Scholar] [CrossRef]
- Iñiguez, M.E.; Conesa, J.A.; Fullana, A. Hydrothermal carbonization (HTC) of marine plastic debris. Fuel 2019, 257, 116033. [Google Scholar] [CrossRef]
- Tang, Z.; Fiorilli, S.; Heeres, H.; Pescarmona, P. Multifunctional Heterogeneous Catalysts for the Selective Conversion of Glycerol into Methyl Lactate. ACS Sustain. Chem. Eng. 2018, 6, 10923–10933. [Google Scholar] [CrossRef] [Green Version]
- Helmer Pedersen, T.; Conti, F. Improving the circular economy via hydrothermal processing of high-density waste plastics. Waste Manag. 2017, 68, 24–31. [Google Scholar] [CrossRef]
- Jin, K.; Vozka, P.; Kilaz, G.; Chen, W.-T.; Wang, N.-H.L. Conversion of polyethylene waste into clean fuels and waxes via hydrothermal processing (HTP). Fuel 2020, 273, 117726. [Google Scholar] [CrossRef]
- Jin, K.; Vozka, P.; Gentilcore, C.; Kilaz, G.; Wang, N.-H.L. Low-pressure hydrothermal processing of mixed polyolefin wastes into clean fuels. Fuel 2021, 294, 120505. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Liu, Z.; Su, J.; Fang, C.; Xu, D.; Song, W.; Wangb, S. Influences of operating parameters on liquefaction performances of Tetra Pak in sub-/supercritical water. J. Environ. Manag. 2019, 237, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Passos, J.S.; Glasius, M.; Biller, P. Screening of common synthetic polymers for depolymerization by subcritical hydrothermal liquefaction. Process Saf. Environ. Prot. 2020, 139, 371–379. [Google Scholar] [CrossRef]
- Seshasayee, M.S.; Savage, P.E. Oil from plastic via hydrothermal liquefaction: Production and characterization. Appl. Energy 2020, 278, 115673. [Google Scholar] [CrossRef]
- Ciuffi, B.; Rosi, L.; Miliotti, E.; Lotti, G.; Rizzo, A.M.; Chiaramonti, D. Batch Hydrothermal liquefaction of end-of-life plastic and oil characterization. E3S Web Conf. 2021, 238, 08004. [Google Scholar] [CrossRef]
- Hongthong, S.; Leese, H.S.; Allen, M.J.; Chuck, C.J. Assessing the Conversion of Various Nylon Polymers in the Hydrothermal Liquefaction of Macroalgae. Environments 2021, 8, 34. [Google Scholar] [CrossRef]
- Gu, J.S.; Martinez-Fernandez, N.; Pang, X.; Fu, S.C. Recent development of hydrothermal liquefaction for algal biorefinery. Renew. Sust. Energ. Rev. 2020, 121, 109707. [Google Scholar] [CrossRef]
- Raikova, S.; Knowles, T.D.J.; Allen, M.J.; Christopher, J.; Chuck, C.J. Co-liquefaction of macroalgae with common marine plastic Pollutants. ACS Sustain. Chem. Eng. 2019, 7, 6769–6781. [Google Scholar] [CrossRef] [Green Version]
- Takolander, A.; Cabeza, M.; Leskinen, E. Climate change can cause complex responses in Baltic Sea macroalgae: A systematic review. J. Sea Res. 2017, 123, 16–29. [Google Scholar] [CrossRef] [Green Version]
- Andreeva, A.; Budenkova, E.; Babich, O.; Sukhikh, S.; Dolganyuk, V.; Michaud, P.; Ivanova, S. Influence of Carbohydrate Additives on the Growth Rate of Microalgae Biomass with an Increased Carbohydrate Content. Mar. Drugs 2021, 19, 381. [Google Scholar] [CrossRef]
- Andreeva, A.; Budenkova, E.; Babich, O.; Sukhikh, S.; Ulrikh, E.; Ivanova, S.; Prosekov, A.; Dolganyuk, V. Purification, and Study of the Amino Acid Composition of Microalgae Proteins. Molecules 2021, 26, 2767. [Google Scholar] [CrossRef]
- Balina, K.; Romagnoli, F.; Pastare, L.; Blumberga, D. Use of macroalgae for bioenergy production in Latvia: Review of potential availability of marine coastline species. Energy Procedia 2017, 113, 403–410. [Google Scholar] [CrossRef]
- Jatmiko, T.H.; Prasetyo, D.J.; Hernawan, C.D.; Khasanah, M.Y. Nutritional Evaluation of Ulva sp. from Sepanjang Coast, Gunungkidul, Indonesia. In IOP Conference Series: Earth and Environmental Science, Proceedings of the 2nd International Conference on Natural Products and Bioresource Science—2018, Tangerang, Indonesia, 1–2 November 2018; IOP Publishing: Bristol, UK, 2019; Volume 251, p. 012011. [Google Scholar] [CrossRef]
- Schultz-Jensen, N.; Thygesen, A.; Leipold, F.; Thomsen, S.T.; Roslander, C.; Lilholt, H.; Bjerre, A.B. Pretreatment of the macroalgae Chaetomorpha linum for the production of bioethanol–Comparison of five pretreatment technologies. Bioresour. Technol. 2013, 140, 36–42. [Google Scholar] [CrossRef]
- Abomohra, E.-F.; El-Naggar, A.; Hamed, A.; Ali, B.A. Potential of macroalgae for biodiesel production: Screening and evaluation studies. J. Biosci. Bioeng. 2018, 125, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Neveux, N.; Yuen, A.; Jazrawi, C.; Magnusson, M.; Haynes, B.; Masters, A.; Montoya, A.; Paul, N.; Maschmeyer, T.; de Nys, R. Biocrude yield and productivity from the hydrothermal liquefaction of marine and freshwater green macroalgae. Bioresour. Technol. 2014, 155, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Neveux, N.; Magnusson, M.; Maschmeyer, T.; Nys, R.; Paul, N.A. Comparing the potential production and value of high-energy liquid fuels and protein from marine and freshwater macroalgae. GCB Bioenergy 2015, 7, 673–689. [Google Scholar] [CrossRef] [Green Version]
- Biswas, B.; Fernandes, A.C.; Kumar, J.; Muraleedharan, U.D.; Bhaskar, T. Valorization of Sargassum tenerrimum: Value addition using hydrothermal liquefaction. Fuel 2018, 222, 394–401. [Google Scholar] [CrossRef]
- Elliott, D.C.; Hart, T.R.; Neuenschwander, G.G.; Rotness, L.J.; Roesijadi, G.; Zacher, A.H.; Magnuson, J.K. Hydrothermal Processing of Macroalgal Feedstocks in Continuous-Flow Reactors. ACS Sustain. Chem. Eng. 2014, 2, 207–215. [Google Scholar] [CrossRef]
- Fernandes, A.C.; Biswas, B.; Kumar, J.; Bhaskar, T.; Muraleedharan, U.D. Valorization of the red macroalga Gracilaria corticata by hydrothermal liquefaction: Product yield improvement by optimization of process parameters. Bioresour. Technol. 2021, 15. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, S.; Cao, B.; Hu, Y.; Abomohra, E.-F.; Wang, Q.; Qian, L.; Liu, L.; Liu, X.; He, Z.; et al. Optimization of hydrothermal co-liquefaction of seaweeds with lignocellulosic biomass: Merging 2nd and 3rd generation feedstocks for enhanced bio-oil production. Energy 2019, 173, 413–422. [Google Scholar] [CrossRef]
- He, Y.T.; Liang, X.; Jazrawi, C.; Montoya, A.; Yuen, A.K.; Cole, A.J.; Neveux, N.; Paul, N.A.; Nys, R.D.; Maschmeyer, T.; et al. Continuous hydrothermal liquefaction of macroalgae in the presence of organic co-solvents. Algal Res.-Biomass Biofuels Bioprod. 2016, 17, 185–195. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, S.; Qian, L.; Barati, B.; Gong, X.; Abomohra, A.E.F.; Wang, X.; Esakkimuthu, S.; Hu, Y.; Liu, L. Effect of cosolvent and addition of catalyst (HZSM-5) on hydrothermal liquefaction of macroalgae. Int. J. Energy Res. 2019, 43, 8841–8851. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, C.; Jiang, J.; Yang, Z.; Feng, W.; Li, L.; Guo, Y.; Hu, J. Catalytic hydrothermal liquefaction of Gracilaria corticata macroalgae: Effects of process parameter on bio-oil up-gradation. Bioresour. Technol. 2021, 319, 124163. [Google Scholar] [CrossRef] [PubMed]
- Biswas, B.; Kumar, A.; Fernandes, A.C.; Saini, K.; Negi, S.; Muraleedharan, U.D.; Bhaskar, T. Solid base catalytic hydrothermal liquefaction of macroalgae: Effects of process parameter on product yield and characterization. Bioresour. Technol. 2020, 307, 123232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chen, H.; He, Z. Catalytic hydrothermal liquefaction of Spirulina platensis: Focusing on aqueous phase characterization. Int. J. Energy Res. 2019, 43, 7135–7145. [Google Scholar] [CrossRef]
- Yan, L.; Wang, Y.; Li, J.; Zhang, Y.; Ma, L.; Fu, F.; Chen, B.; Liu, H. Hydrothermal liquefaction of Ulva prolifera macroalgae and the influence of base catalysts on products. Bioresour. Technol. 2019, 292, 121286. [Google Scholar] [CrossRef]
- Li, D.; Chen, L.; Xu, D.; Zhang, X.; Ye, N.; Chen, F.; Chen, S. Preparation and characteristics of bio-oil from the marine brown alga Sargassum patens C. Agardh. Bioresour. Technol. 2012, 104, 737–742. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Le, T.M.; Nguyen, H.V. Iron-catalyzed fast hydrothermal liquefaction of Cladophora socialis macroalgae into high quality fuel precursor. Bioresour. Technol. 2021, 337, 125445. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Geng, J.; Zhang, D.; Ning, X. Hydrothermal liquefaction of macroalgae: Influence of zeolites based catalyst on products. J. Energy Inst. 2020, 93, 581–590. [Google Scholar] [CrossRef]
- Kandasamy, S.; Zhang, B.; He, Z.; Chen, H.; Feng, H.; Wang, Q.; Wang, B.; Ashokkumar, V.; Siva, S.; Bhuvanendran, N.; et al. Effect of low-temperature catalytic hydrothermal liquefaction of Spirulina platensis. Energy 2020, 190, 116236. [Google Scholar] [CrossRef]
- Kandasamy, S.; Zhang, B.; He, Z.; Chen, H.; Feng, H.; Wang, Q.; Wang, B.; Bhuvanendran, N.; Esakkimuthu, S.; Ashokkumar, V.; et al. Hydrothermal liquefaction of microalgae using Fe3O4 nanostructures as efficient catalyst for the production of bio-oil: Optimization of reaction parameters by response surface methodology. Biomass Bioenergy 2019, 131, 105417. [Google Scholar] [CrossRef]
- Suganya, T.; Varman, M.; Masjuki, H.H.; Renganathan, S. Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: A biorefinery approach. Renew. Sustain. Energ. Rev. 2016, 55, 909–941. [Google Scholar] [CrossRef]
- Chen, H.; Zhong, Q. Thermal and UV stability of β-carotene dissolved in peppermint oil microemulsified by sunflower lecithin and Tween 20 blend. Food Chem. 2015, 174, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, W.; Hilten, R.; Jena, U.; Das, K.C.; Kastner, J.R. Effect of low temperature hydrothermal liquefaction on catalytic hydrodenitrogenation of algae biocrude and model macromolecules. Algal Res. 2016, 13, 53–68. [Google Scholar] [CrossRef] [Green Version]
- Hockstad, R.; Obeid, W.; Hatcher, P.G.; Savage, P.E. The Use of Hydrothermal Carbonization to Recycle Nutrients in Algal Biofuel Production. Environ. Prog. Sustain. Energy 2013, 32, 5235–5243. [Google Scholar]
- Jazrawi, C.; Biller, P.; He, Y.; Montoya, A.; Ross, A.B.; Maschmeyer, T.; Haynes, B.S. Two-stage hydrothermal liquefaction of a high-protein microalga. Algal Res. 2015, 8, 15–22. [Google Scholar] [CrossRef] [Green Version]
- European Comission. Sustainable Agriculture, Forestry and Fisheries in the Bioeconomy. A Challenge for Europe; Publications Office of the European Union: Brussels, Belgium, 2015. [Google Scholar] [CrossRef]
- Cesario, M.T.; da Fonseca, M.M.R.; Marques, M.M.; de Almeida, M.C.M. Marine algal carbohydrates as carbon sources for the production of biochemicals and biomaterials. Biotechnol. Adv. 2018, 36, 798–817. [Google Scholar] [CrossRef]
- Miao, C.; Chakraborty, M.; Chen, S. Impact of reaction conditions on the simultaneous production of polysaccharides and bio-oil from heterotrophically grown Chlorella sorokiniana by a unique sequential hydrothermal liquefaction process. Bioresour. Technol. 2012, 110, 617–627. [Google Scholar] [CrossRef]
- Chakraborty, M.; Miao, C.; McDonald, A.; Chen, S. Concomitant extraction of bio-oil and value added polysaccharides from Chlorella sorokiniana using a unique sequential hydrothermal extraction technology. Fuel 2012, 95, 63–70. [Google Scholar] [CrossRef]
- Prapaiwatcharapan, K.; Sunphorka, S.; Kuchonthara, P.; Kangvansaichol, K.; Hinchiranan, N. Single-and two-step hydrothermal liquefaction of microalgae in a semi-continuous reactor: Effect of the operating parameters. Bioresour. Technol. 2015, 191, 426–432. [Google Scholar] [CrossRef]
- Selvaratnam, T.; Reddy, H.; Muppaneni, T.; Holguin, F.; Nirmalakhandan, N.; Lammers, P.J.; Deng, S. Optimizing energy yields from nutrient recycling using sequential hydrothermal liquefaction with Galdieria sulphuraria. Algal Res. 2015, 12, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhao, S.; Cheng, X.; Qian, L.; Barati, B.; Gong, X.; Cao, B.; Yuan, C. Study on two-step hydrothermal liquefaction of macroalgae for improving bio-oil. Bioresour. Technol. 2021, 319, 124176. [Google Scholar] [CrossRef] [PubMed]
- Hongthong, S.; Raikova, S.; Leese, H.S.; Chuck, C.J. Co-processing of common plastics with pistachio hulls via hydrothermal liquefaction. Waste Manag. 2020, 102, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Arun, J.; Gopinath, K.P.; Rajan, P.S.S.; Marudai, J.M.; Vargees, F. Co-liquefaction of Prosopis juliflora with polyolefin waste for production of high grade liquid hydrocarbons. Bioresour. Technol. 2019, 274, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Seshasayee, M.S.; Savage, P.E. Synergistic interactions during hydrothermal liquefaction of plastics and biomolecules. Chem. Eng. J. 2021, 417, 129268. [Google Scholar] [CrossRef]
- Chen, W.T.; Jin, K.; Wang, L.N.H. Use of Supercritical Water for the Liquefaction of Polypropylene into Oil. ACS Sustain. Chem. Eng. 2019, 7, 3749–3758. [Google Scholar] [CrossRef]

| Species | Derbesia | Ulva | Cladophora | Polysiphonia |
|---|---|---|---|---|
| Value in Mass % d.m. | ||||
| Ash | 34.7 | 30.7–31.4 | 17.8–25.5 | 15.1–32.32 |
| Moisture | 6.4 | 7.2 | 5.7–6.7 | - |
| Lipid | 10.4 | 8.3–12.5 | 4.6–5.3 | 0.33–2.78 |
| Protein | 21.6 | 14.27–16.35 | 17.8–26.8 | 11.7–34.45 |
| Carbohydrates | 26.9 | 28.65–43.6 | 44.4–45.4 | - |
| C | 29.2 | 27.7 | 30.9–37.5 | - |
| H | 4.8 | 5.5 | 5.0–5.9 | - |
| O | 27.4 | 41.1 | 32.9–34.9 | - |
| N | 4.5 | 3.5 | 5.2–6.5 | - |
| S | 2.8 | 5.0 | 1.8–2.3 | - |
| HHV (MJ/kg) | 12.4 | 11.7 | 12.7–16.4 | 15.8 |
| Algae Type | Conditions | Fuel Yield Efficiency | Reference |
|---|---|---|---|
| HTL water as solvent, without catalyst | |||
| Sargassum tenerrimum | Temperature 260–300 °C, 15 min | Maximum 16.3% | [61] |
| Amphiroa fragilissima | Temperature 280 °C, pressure 16–24 MPa | Maximum fuel yield 28.9% | [28] |
| Saccharina spp. | 364 °C, 40 min | Maximum fuel yield was 27.7% | [62] |
| HTL different solvents, with or without catalyst | |||
| Gracilaria corticata | Temperature 260, 280, 300 °C, solvent: water, ethanol, methanol, acetone, a mixture of ethanol and water | Maximum yield 16.6% (with acetone), 5.25 wt% (water) | [63] |
| Enteromorpha clathrata; mixture with rice husk | Optimal conditions: temperature 300 °C, 50% ethanol as a co-solvent, 45 min | Maximum oil yield 71.7% for the mixture 1:1 algae:husk. For Enteromorpha cl. maximum yield 26.0% | [64] |
| Oedogonium intermedium | Temperature 350 °C, 3 min, solvents: 10% N-heptane and 10% toluene, 10% anisole | Maximum fuel yield was 24.0% with water, 20% with N-heptane, 21% with toluene and 28% with anisole | [65] |
| Enteromorpha clathrata | Temperature 250–350 °C, pressure 5–45 MPa, co-solvent ethanol 0–100%, 15–75 min, catalysts HZSM-5 (10–30%) | Maximum oil yield 46.75% with a conversion rate of 95.5% with 300 °C, 45 min, 75% ethanol; with HZSM-5 oil yield decreased to 44.5% | [66] |
| Gracilaria corticata | Temperature 260–300 °C with iron-nickel catalysts at a temperature of 260–300 °C using water, methyl, and ethyl alcohol as solvents | Maximum fuel yield was 56.2% when using Ga/NiFe-LDO/AC catalyst at 280 °C | [67] |
| Sargassum tenerrimum | Temperature 260, 280, and 300 °C, pressure 4.5–12 MPa, catalysts: CaO, applied on CeO2, Al2O3 and ZrO2 in doses of 5 to 25 wt%. Contact time 15 min. Solvent: water, ethanol, and a mixture of water: ethanol | The fuel yield in the non-catalytic process was 3.3 wt%, 23.3 wt%, and 32.0 wt% when using water, ethanol, and their mixture, respectively. In the catalytic process, the maximum yield when using CaO/ZrO2 as a catalyst was 25.2 wt% and 33.0 wt% when using ethanol and a mixture of ethanol and water, respectively | [68] |
| Homogeneous acid/alkaline catalysts with water as solvent | |||
| Spirulina platensis | 300 °C, 35 min, 0.34% catalysts CH3COOH | Maximum fuel yield 29.7% without catalyst, with CH3COOH 28% | [69] |
| Ulva prolifera | Temperature 270, 290, and 310 °C) and reaction time (10, 20, and 30 min), catalysts: KOH, NaOH and Na2CO3 | Maximum yield was 12.0 wt% at 290 °C without catalysis. In the catalytic reaction, the maximum yield was 26.7 wt% when using KOH | [70] |
| Sargassum patens | Temperature 340 °C, time 15 min, catalyst: 5% Na2CO3 | Maximum yield was without catalyst 32%, with Na2CO3 yield was 28% | [71] |
| Cladophora sociali | Temperature 350 °C, catalysts: KOH, K2CO3, H3PO4, HCOOH, zeolites, Ni-Re, Ru/C, Fe | Maximum yield was 36.2% in the presence of Fe | [72] |
| Heterogeneous catalysts with water as solvent | |||
| Ulva prolifera | Temperature 260–300 °C, time 15–45 min, pressure 4.3–7 MPa, catalyst: zeolites | The maximum bio-oil yield was 16.6 wt% without catalyst and 29.3 wt% with catalyst | [73] |
| Spirulina platensis | Temperature 320 °C, 37 min, catalysts 0.15% Fe3O4 | HHV rose from 23.5% to 30.98 MJ/kg and fuel yield rose from 24.5% to 32.3% with Fe3O4 | [74] |
| Spirulina platensis | Temperature 250 °C, 30 min, catalysts 0.4% CeO2 | Fuel yield without catalyst 16%, with CeO2—26% (+62.5%) | [75] |
| Polymers | Conditions | Fuel Yield Efficiency | HHV of the Oil | References |
|---|---|---|---|---|
| High- and low-pressure polyethylene | Temperature 400–450 °C, 0.5 and 4 h. Raw material dose 1:1.75 | The maximum yield (97 wt%) of a mixture of paraffinic and α-olefin waxes was obtained at 425 °C and 30–40 min. The maximum fuel yield (87 wt%) was obtained at 425 °C, 2.5 h or 450 °C, 45 min. | 42.7–42.9 MJ/kg | [43] |
| Polypropylene and polyethylene | Pressure 0.25, 1.55, 3.75, 10.25, and 23 MPa. Polymer: water ratio 1:1.75; 200:1; 7.3:1; 2.3:1, time 45–60 min, temperature 450 °C | The minimum coal yield and the optimum degree of destruction were achieved at a water content of 5% and 1.55 MPa. The light ends of the fuel, obtained from a mixture of PP:PE in a 1:1 ratio, meet all the requirements for high-quality pure gasoline, and the heavy ends meet the requirements for ultra-low sulfur diesel fuel. | 42.6–42.7 MJ/kg for oil from polyethylene and 42.2–42.4 MJ/kg for oil from polypropylene | [44] |
| Tetra Pak | Temperature 300–420 °C, pressure 16–24 MPa, time 5–60 min, and polymer concentration 5–40%. | The maximum fuel yield of 35.55% was achieved at 360 °C, 22 MPa, 30 min and a feed concentration of 20 wt%. The maximum fuel calorific value of 48.747 MJ/kg and the maximum energy extraction efficiency of 46.49% were observed at 420 °C, 20 MPa, 30 min and a feed concentration of 20 wt% | The maximum fuel HHV 48.747 MJ/kg and the maximum energy extraction efficiency of 46.49% were observed at 420 °C, 20 MPa, 30 min and a feed concentration of 20 wt%. | [45] |
| 12 polymers: polyacrylonit-rylbutadiene-styrene (ABS), bisphenol-A epoxy resin, high pressure polyethylene, low pressure polyethylene, polyamide 6 polyamide 66, polyethylene terephthalate, polycarbonate, polypropylene, polystyrene and polyurethane foam, polyvinyl chloride. | Temperature 350 °C, exposure time 14 min and 1:17 (polymer:water ratio), catalyst KOH 17.2 g/L | LDPE, HDPE, PP, and PS showed a solid yield of more than 90%, the formation of oil did not exceed 5%. The catalyst allowed increasing the destruction of ABS, epoxy resin, polyamides, PET, polycarbonate, and polyurethane foam to achieve an average solid fraction yield of 0–25%, while without a catalyst this value was 50–86%. | n/a | [46] |
| Polybutylene terephthalate, polycarbonate, polyethylene terephthalate, polylactate, polymethyl methacrylate, polyoxymethylene, styrene butadiene (SBD), polyvinyl acetate (PVA) | Temperature 400 °C, 25 MPa, 15 min, raw material dose 1:10 | The synthetic crude oil yield ranged from 0% (for PET, PBT, polylactate) and almost 100% for PC. SBD and PVA also demonstrated high biofuel yield up to 80% | n/a | [42] |
| Polypropylene, polystyrene, polycarbonate, and polyethylene terephthalate. | Time: 0.5 h, 1 h, temperature 350–450 °C, pressure 16.52 and 25 MPa | The maximum yield was from 16 wt% for PET and up to 86 wt% for polystyrene. Depolymerization of plastics to oil was fastest under supercritical conditions (T > 400 °C). | 30–46 MJ/kg for oil from polypropylene; 36–46 MJ/kg for oil from polystyrene; 28–30 MJ/kg for oil from polycarbonate; 28–29 MJ/kg for oil from PET | [47] |
| Polymer mixture: cellulose, PET, nylon, PVA, polyethylene, polypropylene | Temperature 340 °C, 5 h, raw material dose 1:10, catalyst 2% NaOH (for PET hydrolysis) | Comparison of the results of the two tests showed that the addition of NaOH led to a decrease in the percentage of solid residue (from 75.1% to 65.5%) and an increase in the percentage of water-soluble organic substances (from 6.2% to 16%), the fuel yield remained practically the same (7.7% and 7.4%, respectively). | n/a | [48] |
| A mixture of pistachio shells, polyethylene, PET, polypropylene, nylon-6 | The weight ratios of biomass and plastic are 100:0, 90:10, and 80:20. Temperature 350 °C, time 15, and 60 min. | Polyolefins led to a decrease in bio-oil yield from 34% to 20%, PET and nylon provided a slight increase in oil yield to 36.1 and 34.6%, respectively. | 34 MJ/kg for oil from pistachio shells; shells with 10–20% of PET 33–35 MJ/kg; shells with 10–20% of PE 37–38 MJ/kg; shells with 10–20% of PP 32–33 MJ/kg; shells with 10–20% of nylon 32.5–33 MJ/kg. | [88] |
| Biomass of Prosopis juliflora shrub and polyolefins | Biomass: Polymers Ratio: 0:1, 1:0, 1:1, 2:1, 3:1, 4:1, and 5:1, temperature 340–440 °C, catalyst: bentonite clay activated with hydrochloric acid | The maximum bio-oil yield was about 61.23 wt% at 420 °C for a 3:1 mixture with the addition of 3 wt% catalyst with a holding time of 60 min. | 45.2 MJ/kg for oil from Prosopis juliflora; 44.18 MJ/kg for oil from polyolefins; 46.0 MJ/kg for oil from blend 3:1 | [89] |
| Cellulose, starch, soy protein isolate, lignin, stearic acid, glucose and various polymers: polypropylene, polystyrene, polycarbonate, and polyethylene terephthalate | Time: 0.5 h, temperature 300, 350, 400, 425 °C, pressure 25 MPa | Increase in oil yield by two (45%), with joint processing with polystyrene, decrease in the decomposition temperature of polyolefins (up to 300 °C). | 27.9–32.1 MJ/kg for oil from biomolecules blend; 36.4–38.5 MJ/kg for oil from polymers blend; 30.9–34.0 MJ/kg for oil from 9 components blend (polymers and biomolecules) | [90] |
| A mixture of macroalgae and plastic marine debris (polyethylene, polypropylene, nylon) | Temperature 340 °C, pressure 16.5 MPa, time 12 and 27 min, plastic dose 0, 10%, 25%, 50% of the mixture mass | The biofuel yield did not exceed 15%, but with the addition of plastics, a significant increase was noted (from 10% to 15%), the calorific value of the fuel increased from 33.5 to 39.0 MJ/kg | With the addition of plastics, a significant increase was noted (from 10% to 15%), HHV of the fuel increased from 33.5 to 39.0 MJ/kg | [91] |
| A mixture of Fucus serratus macroalgae and nylon | 350 °C during 10 min, 4 grades of nylon: nylon 6, nylon 6/6, nylon 12, and nylon 6/12 in a mixture of 5, 20, and 50 wt% nylon in relation to biomass | 100% of nylon 6 and 6/6 degrades under HTL conditions while forming cyclopentanone. Nylon 6/12 and nylon 12 were less reactive, only traces of monomer were observed | HHV of oil from algae 33 MJ/kg, HHV of oil from algae+ nylon 28–35 MJ/kg | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulikova, Y.; Sukhikh, S.; Ivanova, S.; Babich, O.; Sliusar, N. Review of Studies on Joint Recovery of Macroalgae and Marine Debris by Hydrothermal Liquefaction. Appl. Sci. 2022, 12, 569. https://doi.org/10.3390/app12020569
Kulikova Y, Sukhikh S, Ivanova S, Babich O, Sliusar N. Review of Studies on Joint Recovery of Macroalgae and Marine Debris by Hydrothermal Liquefaction. Applied Sciences. 2022; 12(2):569. https://doi.org/10.3390/app12020569
Chicago/Turabian StyleKulikova, Yuliya, Stanislav Sukhikh, Svetlana Ivanova, Olga Babich, and Natalia Sliusar. 2022. "Review of Studies on Joint Recovery of Macroalgae and Marine Debris by Hydrothermal Liquefaction" Applied Sciences 12, no. 2: 569. https://doi.org/10.3390/app12020569
APA StyleKulikova, Y., Sukhikh, S., Ivanova, S., Babich, O., & Sliusar, N. (2022). Review of Studies on Joint Recovery of Macroalgae and Marine Debris by Hydrothermal Liquefaction. Applied Sciences, 12(2), 569. https://doi.org/10.3390/app12020569








