Reverse Guided Bone Regeneration (R-GBR) Digital Workflow for Atrophic Jaws Rehabilitation
Abstract
Featured Application
Abstract
1. Introduction
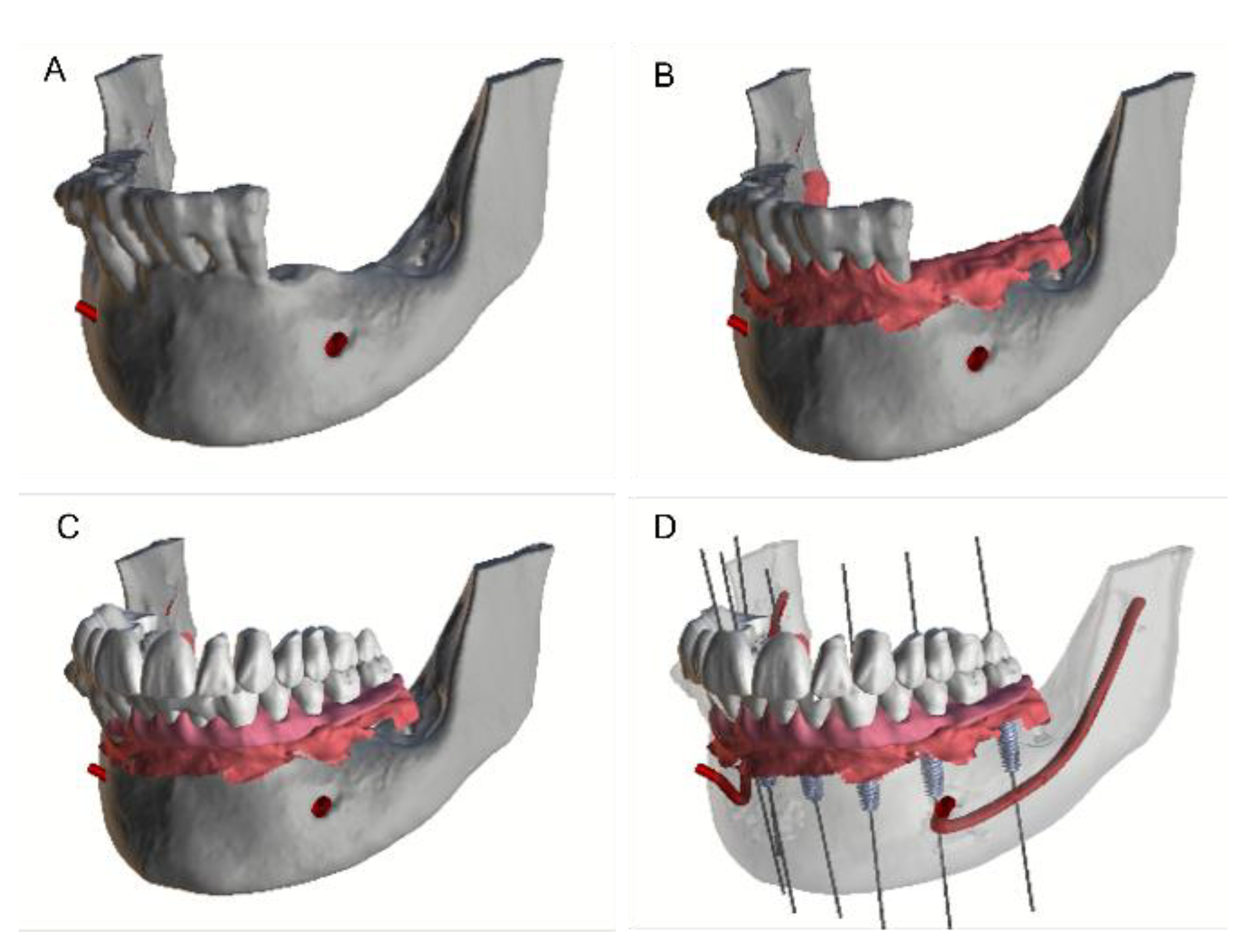
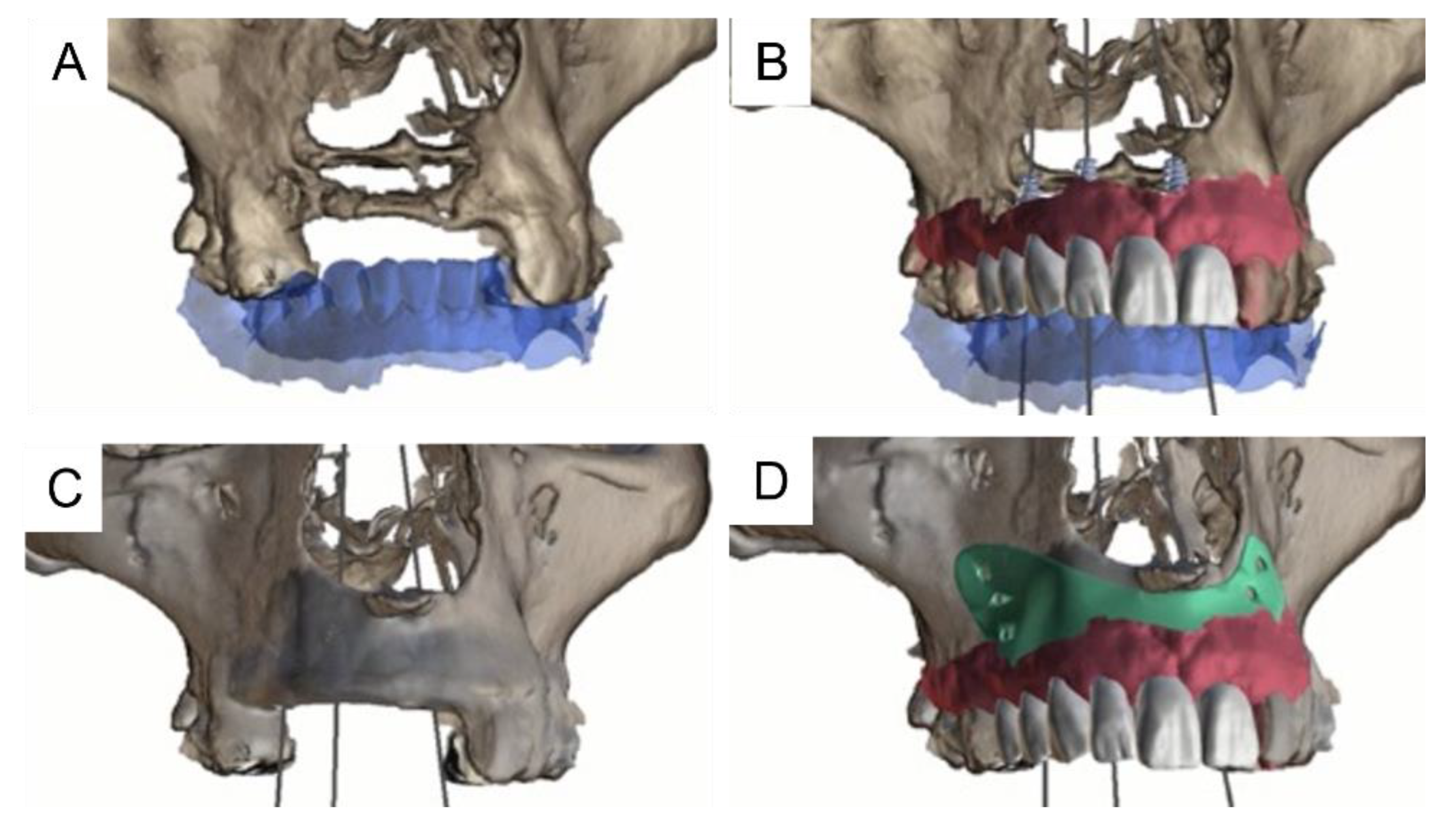
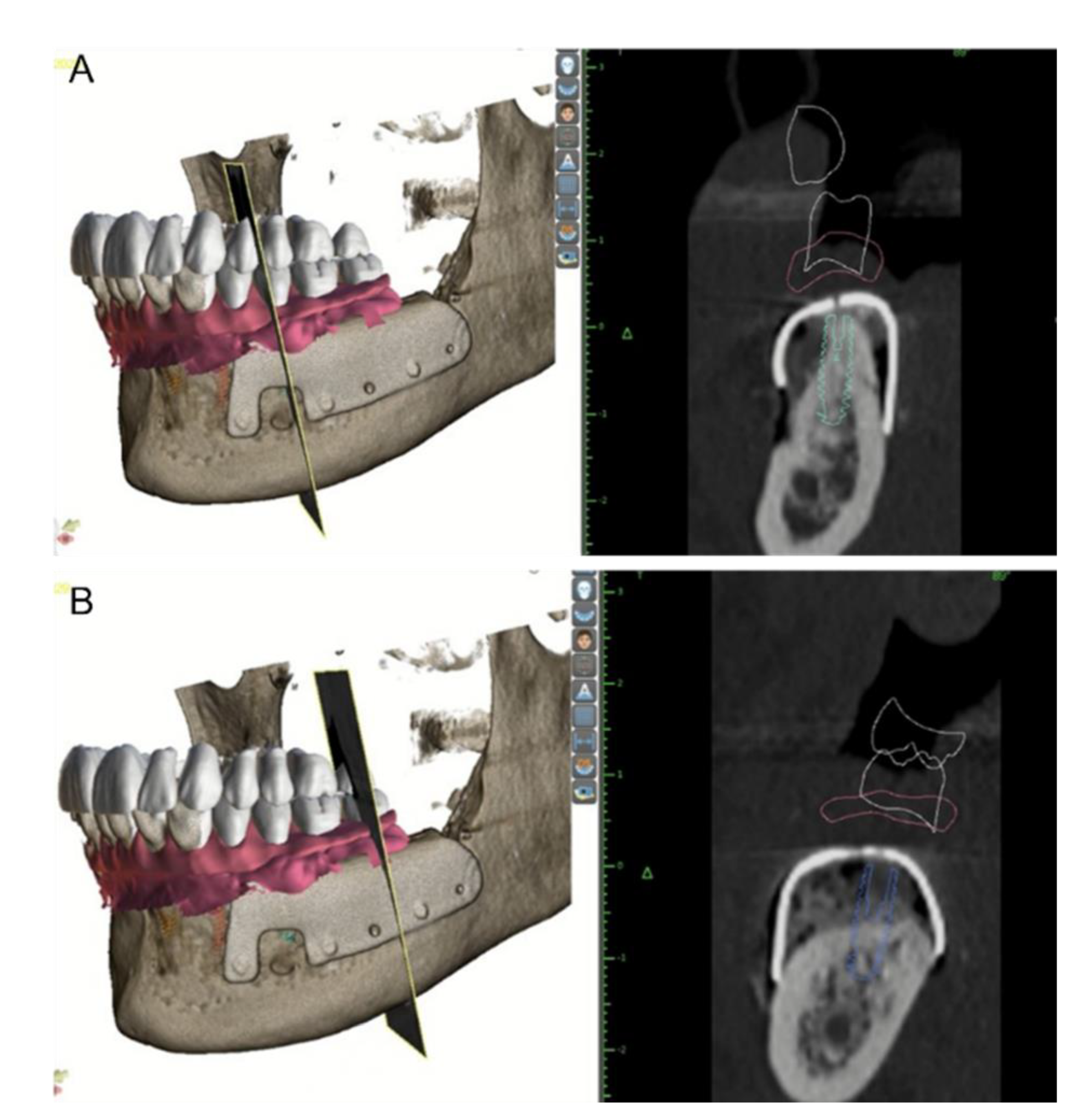
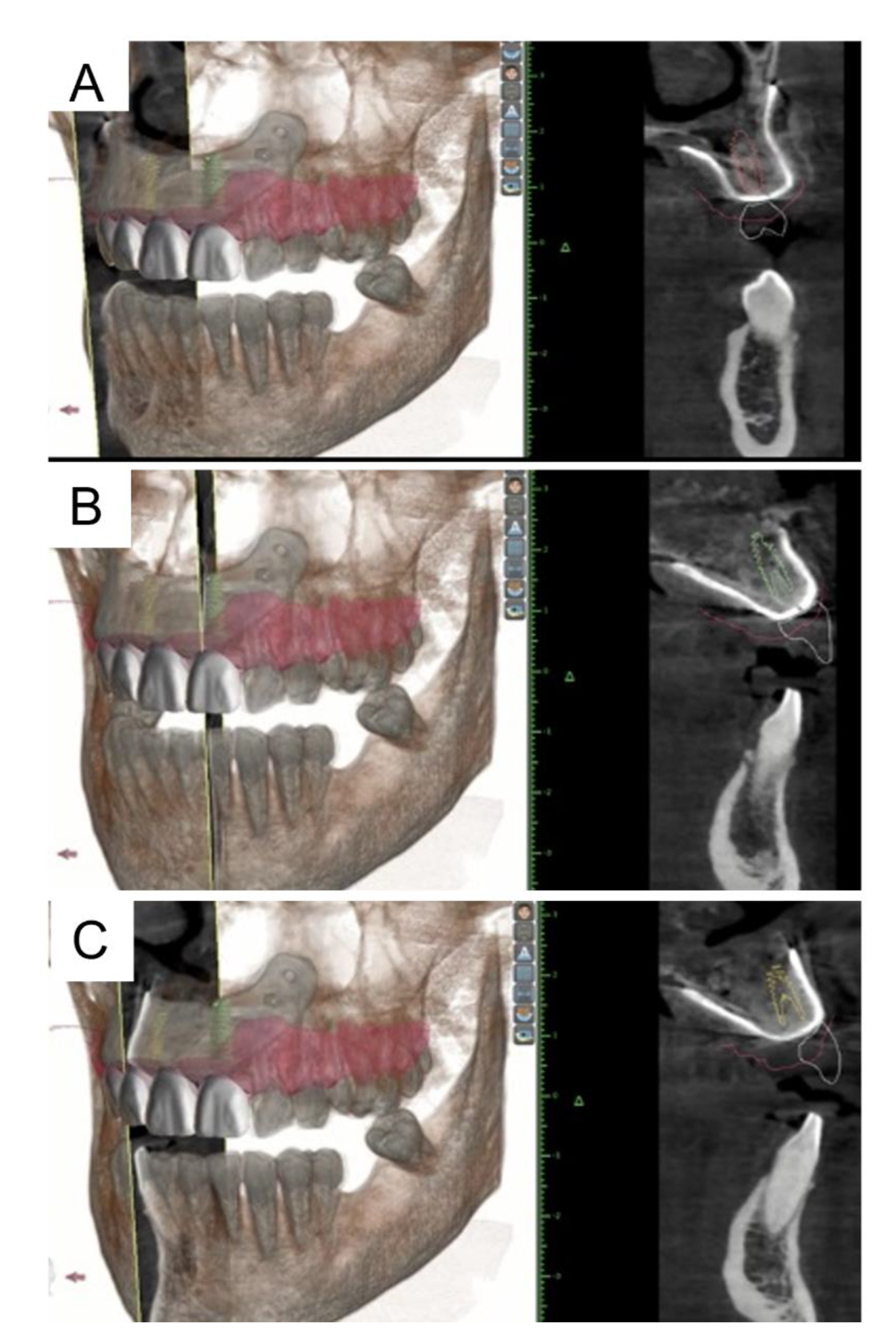
2. Materials and Methods
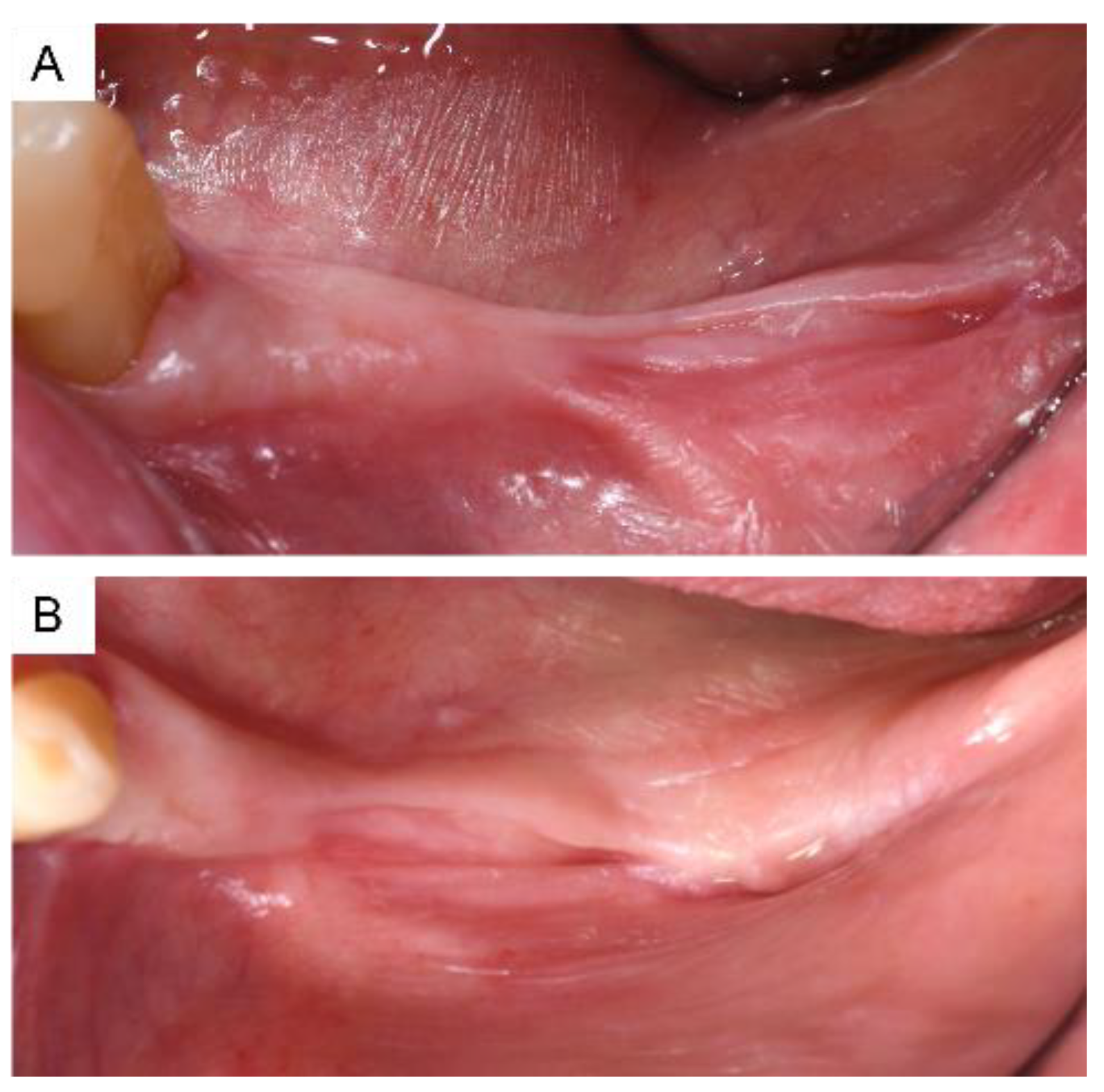
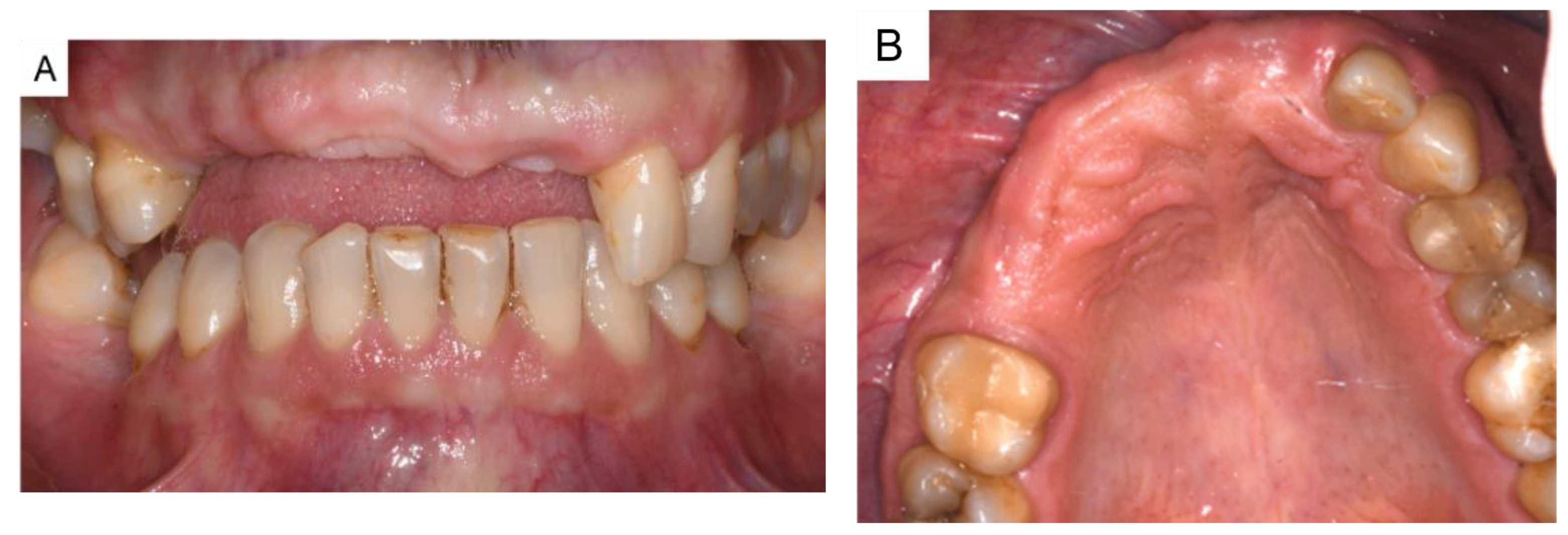
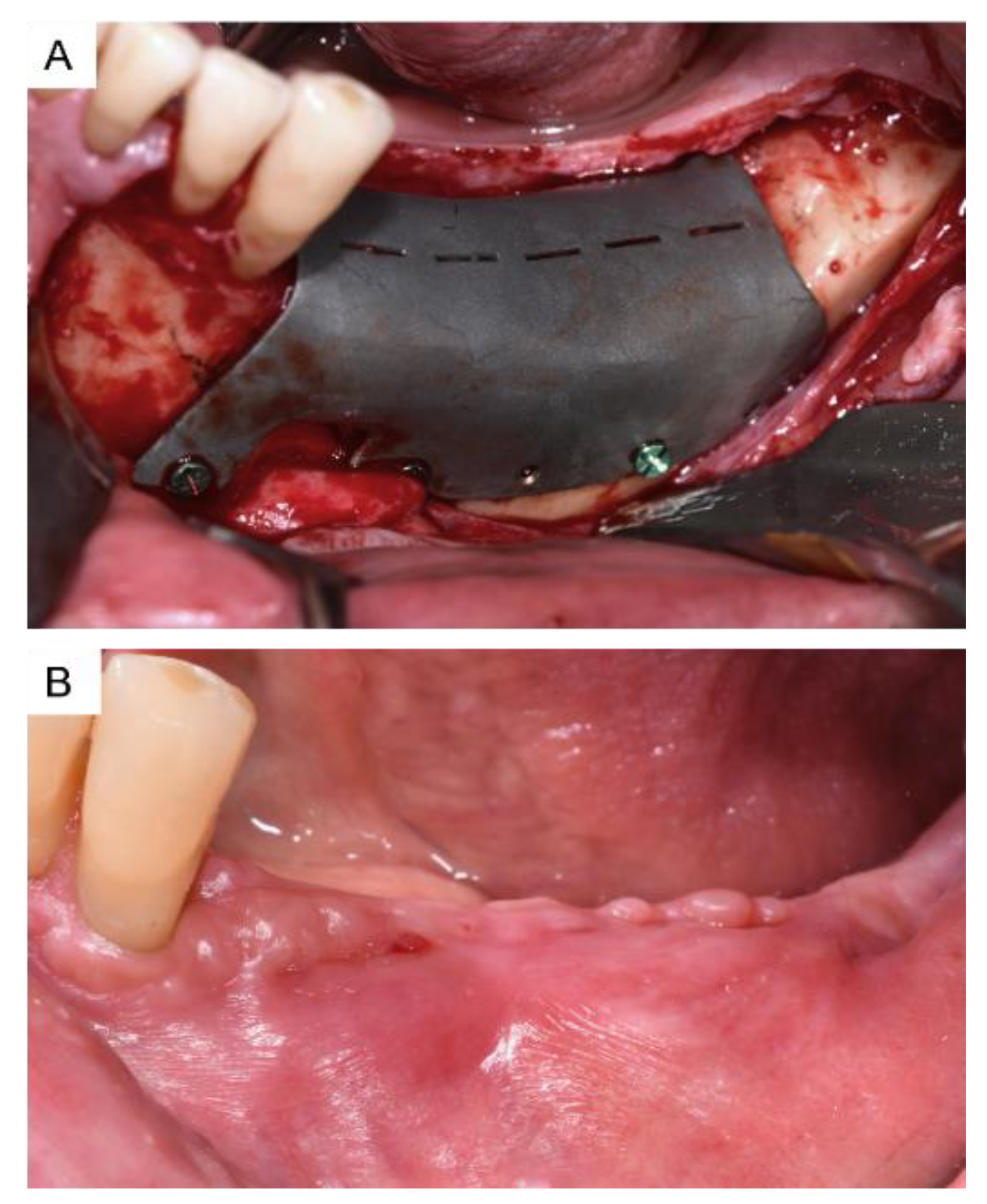
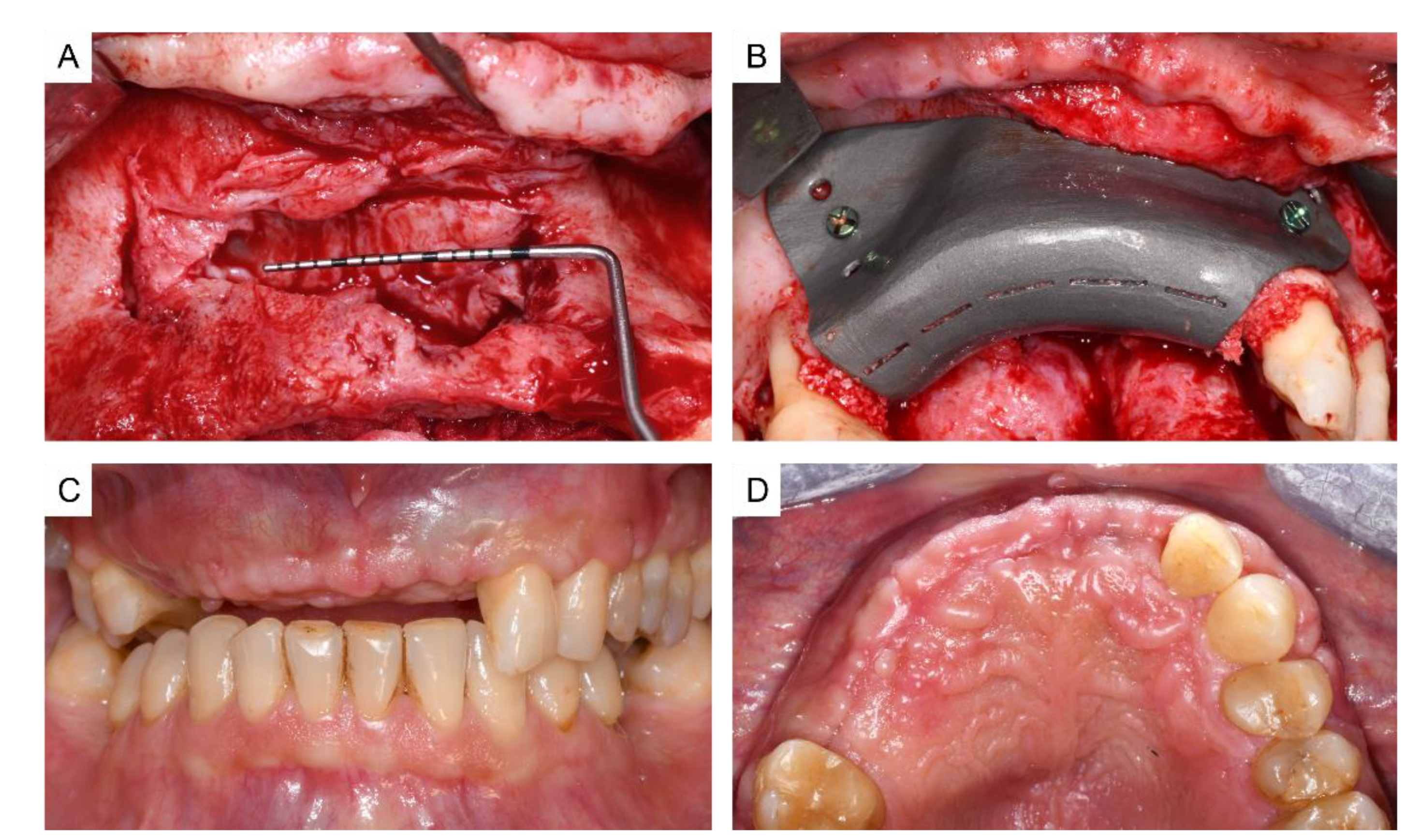
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GBR | Guided Bone Regeneration |
| 3D | Three-Dimensional |
| CAD/CAM | Computer-Aided Design/Computer-Aided Manufacturing |
| CBCT | Cone-Beam Computerized Tomography |
| CT | Computerized Tomography |
| STL | Standard Tessellation Language |
| CA (s-CAI; d-CAI) | Computer-Aided Implantology (static and dynamic) |
| DMLS | Digital Machine Laser Sintering |
References
- Ciocca, L.; Lizio, G.; Baldissara, P.; Sambuco, A.; Scotti, R.; Corinaldesi, G. Prosthetically CAD-CAM–Guided Bone Augmentation of Atrophic Jaws Using Customized Titanium Mesh: Preliminary Results of an Open Prospective Study. J. Oral Implant. 2018, 44, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Lizio, G.; Pellegrino, G.; Corinaldesi, G.; Ferri, A.; Marchetti, C.; Felice, P. Guided bone regeneration using titanium mesh to augment 3-dimensional alveolar defects prior to implant placement. A pilot study. Clin. Oral Implant. Res. 2022, 33, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Maiorana, C.; Santoro, F.; Rabagliati, M.; Salina, S. Evaluation of the use of iliac cancellous bone and anorganic bovine bone in the reconstruction of the atrophic maxilla with titanium mesh: A clinical and histologic investigation. Int. J. Oral Maxillofac. Implant. 2001, 16, 427–432. [Google Scholar]
- Chiapasco, M.; Casentini, P.; Tommasato, G.; Dellavia, C.; Del Fabbro, M. Customized CAD/CAM titanium meshes for the guided bone regeneration of severe alveolar ridge defects: Preliminary results of a retrospective clinical study in humans. Clin. Oral Implant. Res. 2021, 32, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Ciocca, L.; Ragazzini, S.; Fantini, M.; Corinaldesi, G.; Scotti, R. Work flow for the prosthetic rehabilitation of atrophic patients with a minimal-intervention CAD/CAM approach. J. Prosthet. Dent. 2015, 114, 22–26. [Google Scholar] [CrossRef]
- Sumida, T.; Otawa, N.; Kamata, Y.; Kamakura, S.; Mtsushita, T.; Kitagaki, H.; Mori, S.; Sasaki, K.; Fujibayashi, S.; Takemoto, M.; et al. Custom-made titanium devices as membranes for bone augmentation in implant treatment: Clinical application and the comparison with conventional titanium mesh. J. Cranio-Maxillofac. Surg. 2015, 43, 2183–2188. [Google Scholar] [CrossRef]
- Cucchi, A.; Bianchi, A.; Calamai, P.; Rinaldi, L.; Mangano, F.; Vignudelli, E.; Corinaldesi, G. Clinical and volumetric outcomes after vertical ridge augmentation using computer-aided-design/computer-aided manufacturing (CAD/CAM) customized titanium meshes: A pilot study. BMC Oral Health 2020, 20, 219. [Google Scholar] [CrossRef]
- Li, L.; Wang, C.; Li, X.; Fu, G.; Chen, D.; Huang, Y. Research on the dimensional accuracy of customized bone augmentation combined with 3D -printing individualized titanium mesh: A retrospective case series study. Clin. Implant Dent. Relat. Res. 2020, 23, 5–18. [Google Scholar] [CrossRef]
- Li, S.; Zhang, T.; Zhou, M.; Zhang, X.; Gao, Y.; Cai, X. A novel digital and visualized guided bone regeneration procedure and digital precise bone augmentation: A case series. Clin. Implant Dent. Relat. Res. 2021, 23, 19–30. [Google Scholar] [CrossRef]
- Aslan, S.; Tolay, C.; Gehrke, P. Computer-aided planning of soft tissue augmentation with prosthetic guidance for the establishment of a natural mucosal contour in late implant placement. J. Esthet. Restor. Dent. 2019, 31, 553–560. [Google Scholar] [CrossRef]
- Tahmaseb, A.; Wu, V.; Wismeijer, D.; Coucke, W.; Evans, C. The accuracy of static computer-aided implant surgery: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29 (Suppl. 16), 416–435. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, I.; Funaki, K.; Yamauchi, K.; Kodama, T.; Takahashi, T. Alveolar ridge reconstruction with titanium mesh and autogenous particulate bone graft: Computed tomography-based evaluations of augmented bone quality and quantity. Clin. Implant. Dent. Relat. Res. 2012, 14, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Seiler, M. Minimizing risk of customized titanium mesh exposures—A retrospective analysis. BMC Oral Health 2020, 20, 36. [Google Scholar] [CrossRef]
- Tallarico, M.; Ceruso, F.M.; Muzzi, L.; Meloni, S.M.; Kim, Y.-J.; Gargari, M.; Martinolli, M. Effect of Simultaneous Immediate Implant Placement and Guided Bone Reconstruction with Ultra-Fine Titanium Mesh Membranes on Radiographic and Clinical Parameters after 18 Months of Loading. Materials 2019, 12, 1710. [Google Scholar] [CrossRef]
- Tallarico, M.; Park, C.-J.; Lumbau, A.I.; Annucci, M.; Baldoni, E.; Koshovari, A.; Meloni, S.M. Customized 3D-Printed Titanium Mesh Developed to Regenerate a Complex Bone Defect in the Aesthetic Zone: A Case Report Approached with a Fully Digital Workflow. Materials 2020, 13, 3874. [Google Scholar] [CrossRef] [PubMed]
- Cucchi, A.; Chierico, A.; Fontana, F.; Mazzocco, F.; Cinquegrana, C.; Belleggia, F.; Rossetti, P.; Soardi, C.M.; Todisco, M.; Luongo, R.; et al. Statements and Recommendations for Guided Bone Regeneration: Consensus Report of the Guided Bone Regeneration Symposium Held in Bologna, October 15 to 16, 2016. Implant. Dent. 2019, 28, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Lizio, G.; Corinaldesi, G.; Marchetti, C. Alveolar ridge reconstruction with titanium mesh: A three-dimensional evaluation of factors affecting bone augmentation. Int. J. Oral Maxillofac. Implant. 2014, 29, 1354–1363. [Google Scholar] [CrossRef]
- Poomprakobsri, K.; Kan, J.Y.; Rungcharassaeng, K.; Lozada, J. Exposure of Barriers Used in GBR: Rate, Timing, Management, And Its Effect on Grafted Bone. A Retrospective Analysis. J. Oral Implantol. 2021, 48, 27–36. [Google Scholar] [CrossRef]
- Urban, I.A.; Saleh, M.H.A.; Ravidà, A.; Forszter, A.; Wang, H.; Barath, Z. Vertical bone augmentation utilizing a titanium-reinforced PTFE mesh: A multi-variate analysis of influencing factors. Clin. Oral Implant. Res. 2021, 32, 828–839. [Google Scholar] [CrossRef]
- De Bruyckere, T.; Cosyn, J.; Younes, F.; Hellyn, J.; Bekx, J.; Cleymaet, R.; Eghbali, A. A randomized controlled study comparing guided bone regeneration with connective tissue graft to re-establish buccal convexity: One-year aesthetic and patient-reported outcomes. Clin. Oral Implant. Res. 2020, 31, 507–516. [Google Scholar] [CrossRef]
- Avila-Ortiz, G.; Couso-Queiruga, E.; Pirc, M.; Chambrone, L.; Thoma, D.S. Outcome measures and methods of assessment of soft tissue augmentation interventions in the context of dental implant therapy: A systematic review of clinical studies published in the last 10 years. Clin. Oral Implant. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Fortin, Y.; Sullivan, R.M. Terminal Posterior Tilted Implants Planned as a Sinus Graft Alternative for Fixed Full-Arch Implant-Supported Maxillary Restoration: A Case Series with 10- to 19-Year Results on 44 Consecutive Patients Presenting for Routine Maintenance. Clin. Implant. Dent. Relat. Res. 2017, 19, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Pieri, F.; Corinaldesi, G.; Fini, M.; Aldini, N.N.; Giardino, R.; Marchetti, C. Alveolar ridge augmentation with titanium mesh and a combination of autogenous bone and anorganic bovine bone: A 2-year prospective study. J. Periodontol. 2008, 79, 2093–2103. [Google Scholar] [CrossRef]
- Hartmann, A.; Hildebrandt, H.; Schmohl, J.U.; Kämmerer, P.W. Evaluation of Risk Parameters in Bone Regeneration Using a Customized Titanium Mesh: Results of a Clinical Study. Implant. Dent. 2019, 28, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Sagheb, K.; Schiegnitz, E.; Moergel, M.; Walter, C.; Al-Nawas, B.; Wagner, W. Clinical outcome of alveolar ridge augmentation with individualized CAD-CAM-produced titanium mesh. Int. J. Implant Dent. 2017, 3, 36. [Google Scholar] [CrossRef]
- Briguglio, F.; Falcomatà, D.; Marconcini, S.; Fiorillo, L.; Briguglio, R.; Farronato, D. The Use of Titanium Mesh in Guided Bone Regeneration: A Systematic Review. Int. J. Dent. 2019, 2019, 9065423. [Google Scholar] [CrossRef]
- Xie, Y.; Li, S.; Zhang, T.; Wang, C.; Cai, X. Titanium mesh for bone augmentation in oral implantology: Current application and progress. Int. J. Oral Sci. 2020, 12, 37. [Google Scholar] [CrossRef]
- Senoo, M.; Hasuike, A.; Yamamoto, T.; Ozawa, Y.; Watanabe, N.; Furuhata, M.; Sato, S. Comparison of Macro-and Micro-porosity of a Titanium Mesh for Guided Bone Regeneration: An In Vivo Experimental Study. In Vivo 2021, 36, 76–85. [Google Scholar] [CrossRef]
- Van Steenberghe, D.; Johansson, C.; Quirynen, M.; Molly, L.; Albrektsson, T.; Naert, I. Bone augmentation by means of a stiff occlusive titanium barrier. Clin. Oral Implant. Res. 2003, 14, 63–71. [Google Scholar] [CrossRef]
- Abduo, J.; Lau, D. Accuracy of static computer-assisted implant placement in long span edentulous area by novice implant clinicians: A cross-sectional in vitro study comparing fully-guided, pilot-guided, and freehand implant placement protocols. Clin. Implant. Dent. Relat. Res. 2021, 23, 361–372. [Google Scholar] [CrossRef]
- Derksen, W.; Wismeijer, D.; Flügge, T.; Hassan, B.; Tahmaseb, A. The accuracy of computer-guided implant surgery with tooth-supported, digitally designed drill guides based on CBCT and intraoral scanning. A prospective cohort study. Clin. Oral Implant. Res. 2019, 30, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Franchina, A.; Stefanelli, L.V.; Maltese, F.; Mandelaris, G.A.; Vantaggiato, A.; Pagliarulo, M.; Pranno, N.; Brauner, E.; De Angelis, F.; Di Carlo, S. Validation of an Intra-Oral Scan Method Versus Cone Beam Computed Tomography Superimposition to Assess the Accuracy between Planned and Achieved Dental Implants: A Randomized In Vitro Study. Int. J. Environ. Res. Public Health 2020, 17, 9358. [Google Scholar] [CrossRef] [PubMed]
- Monaco, C.; Arena, A.; Corsaletti, L.; Santomauro, V.; Venezia, P.; Cavalcanti, R.; Di Fiore, A.; Zucchelli, G. 2D/3D accuracies of implant position after guided surgery using different surgical protocols: A retrospective study. J. Prosthodont. Res. 2020, 64, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Putra, R.H.; Yoda, N.; Astuti, E.R.; Sasaki, K. The accuracy of implant placement with computer-guided surgery in partially edentulous patients and possible influencing factors: A systematic review and meta-analysis. J. Prosthodont. Res. 2022, 66, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Frizzera, F.; Calazans, N.N.N.; Hooper Pascoal, C.; Elen Martins, M.; Mendonça, G. Flapless Guided Implant Surgeries Compared with Conventional Surgeries Performed by Nonexperienced Individuals: Randomized and Controlled Split-Mouth Clinical Trial. Int. J. Oral Maxillofac. Implants 2021, 36, 755–761. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Felice, P.; Lizio, G.; Barausse, C.; Roccoli, L.; Bonifazi, L.; Pistilli, R.; Simion, M.; Pellegrino, G. Reverse Guided Bone Regeneration (R-GBR) Digital Workflow for Atrophic Jaws Rehabilitation. Appl. Sci. 2022, 12, 9947. https://doi.org/10.3390/app12199947
Felice P, Lizio G, Barausse C, Roccoli L, Bonifazi L, Pistilli R, Simion M, Pellegrino G. Reverse Guided Bone Regeneration (R-GBR) Digital Workflow for Atrophic Jaws Rehabilitation. Applied Sciences. 2022; 12(19):9947. https://doi.org/10.3390/app12199947
Chicago/Turabian StyleFelice, Pietro, Giuseppe Lizio, Carlo Barausse, Lorenzo Roccoli, Lorenzo Bonifazi, Roberto Pistilli, Massimo Simion, and Gerardo Pellegrino. 2022. "Reverse Guided Bone Regeneration (R-GBR) Digital Workflow for Atrophic Jaws Rehabilitation" Applied Sciences 12, no. 19: 9947. https://doi.org/10.3390/app12199947
APA StyleFelice, P., Lizio, G., Barausse, C., Roccoli, L., Bonifazi, L., Pistilli, R., Simion, M., & Pellegrino, G. (2022). Reverse Guided Bone Regeneration (R-GBR) Digital Workflow for Atrophic Jaws Rehabilitation. Applied Sciences, 12(19), 9947. https://doi.org/10.3390/app12199947








