Protection of Phytoextracts against Rotenone-Induced Organismal Toxicities in Drosophila melanogaster via the Attenuation of ROS Generation
Abstract
1. Introduction
2. Materials and Methods
2.1. Drosophila Strains
2.2. Plant Materials
2.3. Phytoextract Preparation
2.4. Treatment Schedule
2.4.1. Preparation of Tissue Homogenate and Measurement of Intracellular ROS Production
2.4.2. Climbing Assay
2.4.3. Memory Assay
2.4.4. Emergence
2.4.5. Reproductive Capacity
2.5. Statistical Analysis
3. Results
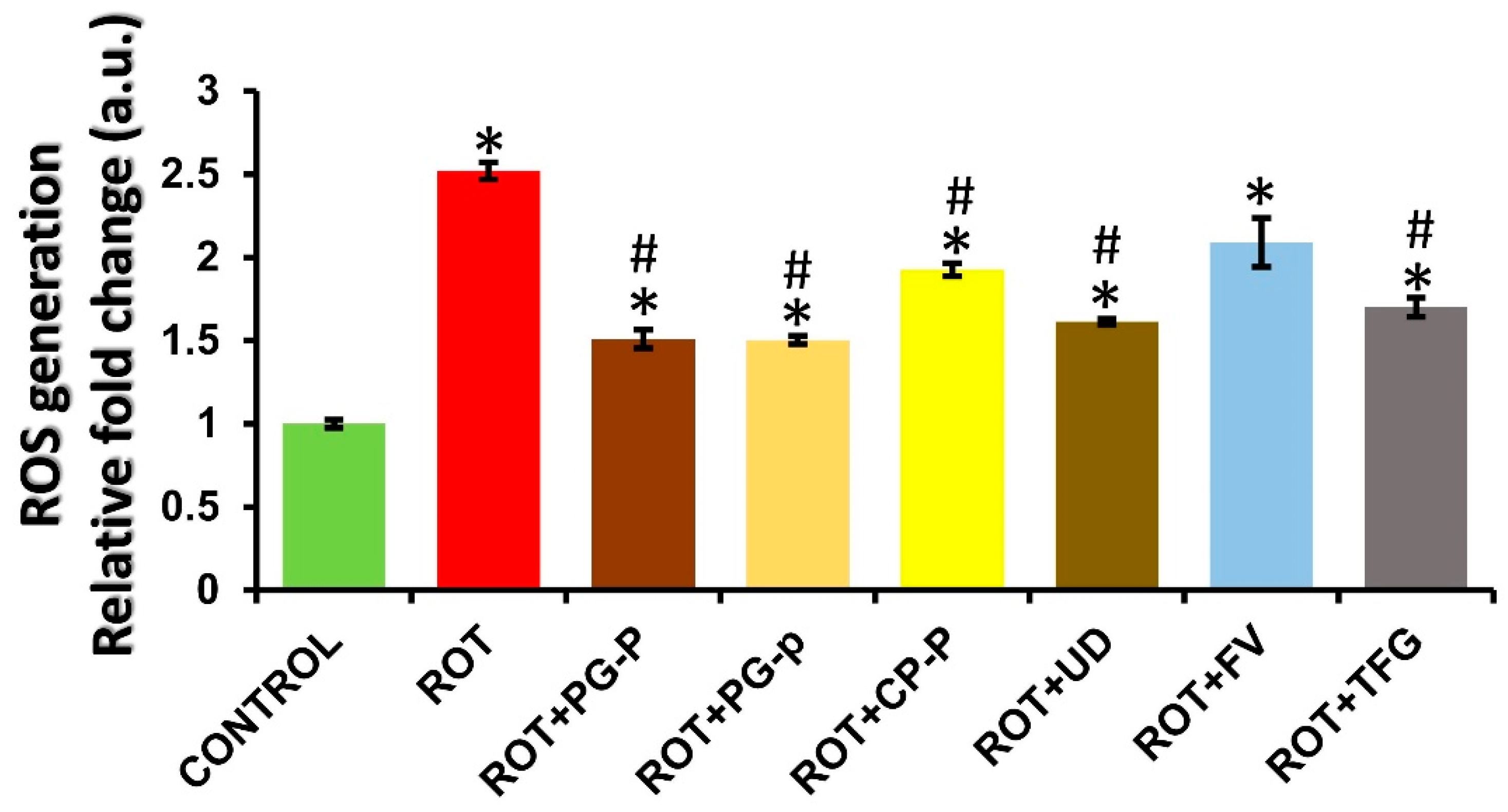
3.1. ROS Measurement in Control, Rotenone, and Rotenone along with Plant-Extract-Fed Groups in Wild-Type Oregon R+
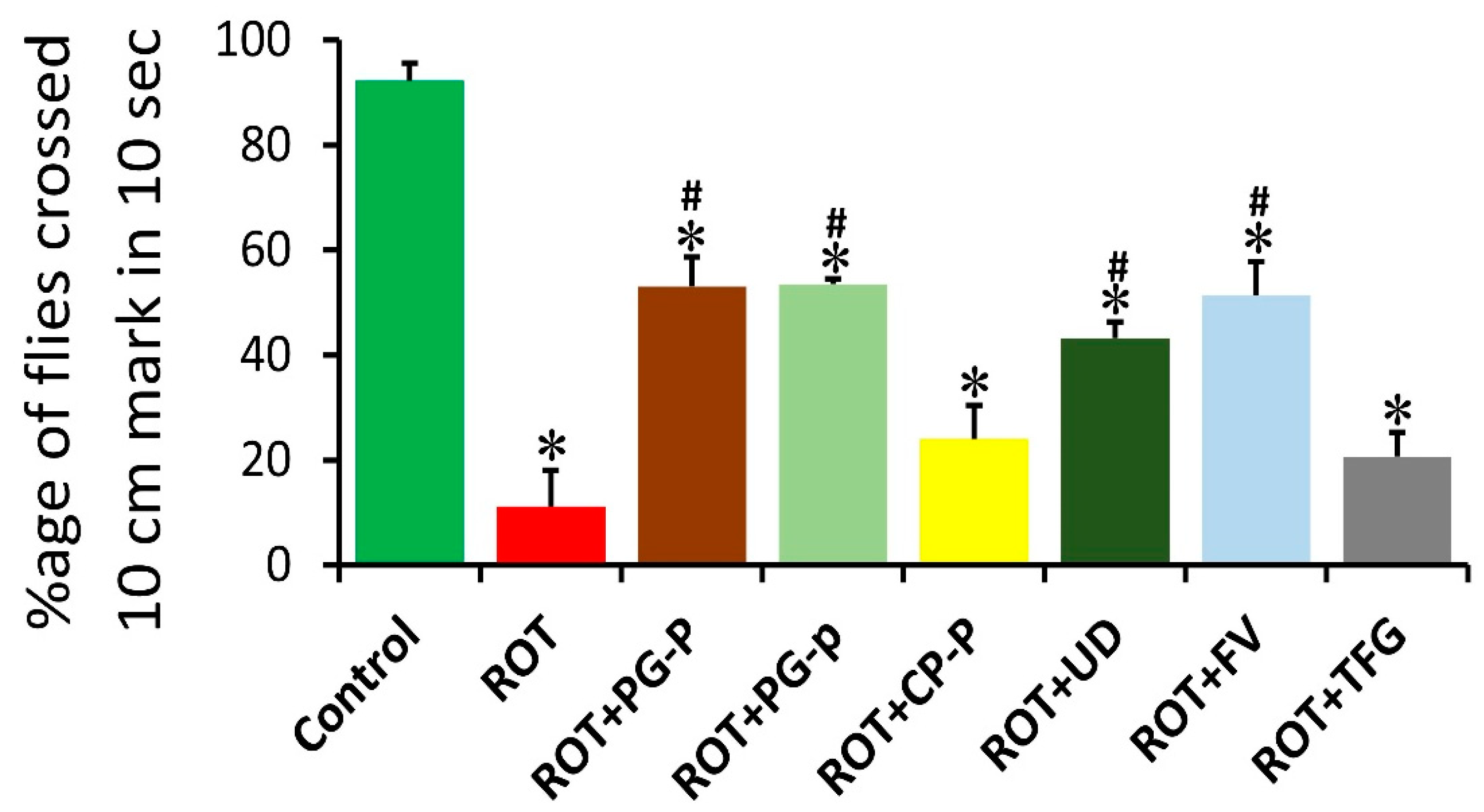
3.2. Climbing Assay of Control, Rotenone, and Rotenone along with Plant-Extract-Fed Groups in Wild-Type Oregon R+
3.3. Memory Assay of Drosophila Exposed to ROT and ROT Cotreated with Plant Extract on Oregon R+
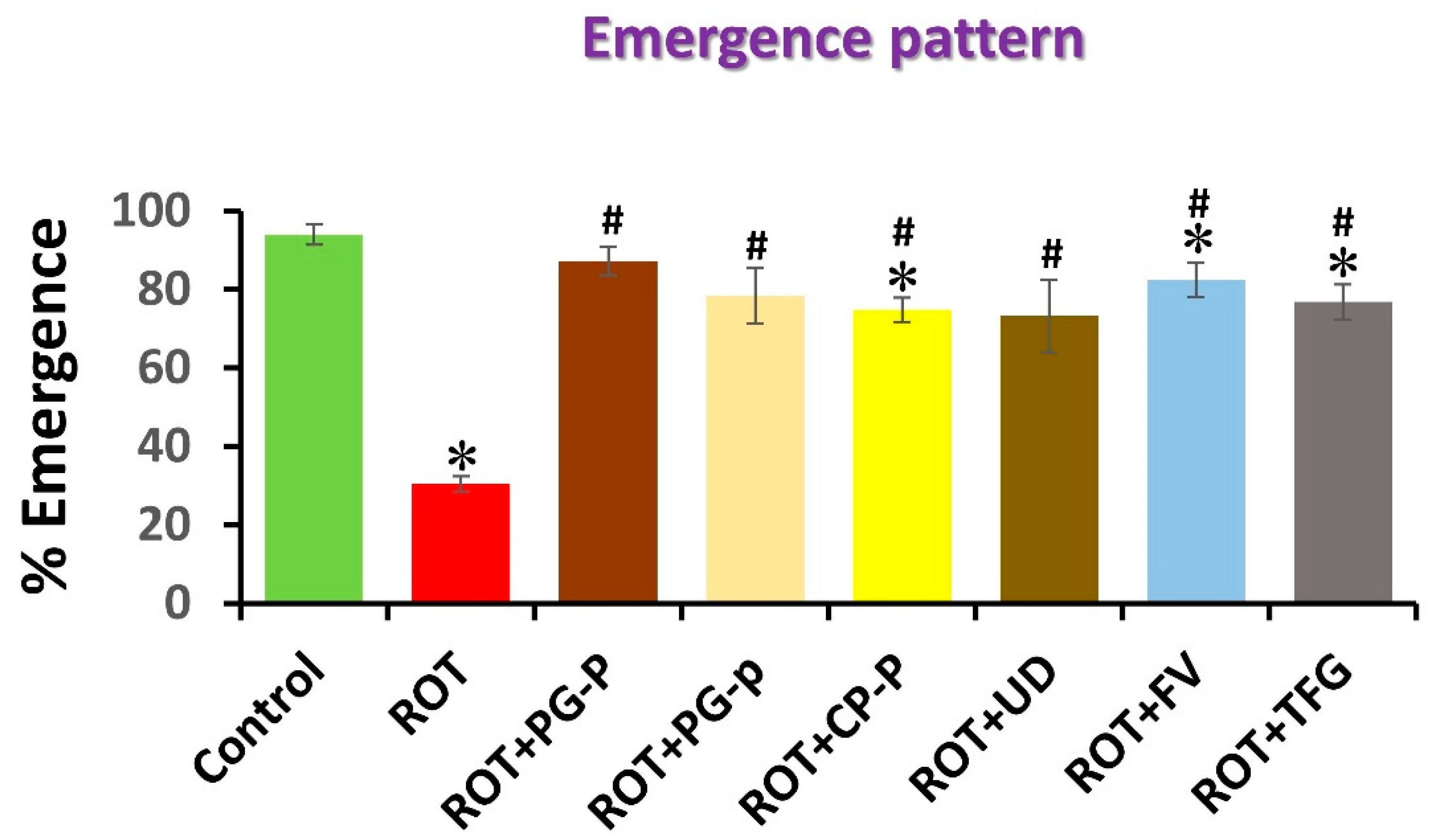
3.4. The Emergence of Drosophila Melanogaster in ROT, and ROT and Phytoextract-Fed Groups on the Wild-Type Strain (Oregon R+)
3.5. Reproductive Capacity of Control, ROT, and ROT Coexposed to Phytoextracts in Oregon R+
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Javed, H.; Meeran, M.F.N.; Azimullah, S.; Eddin, L.B.; Dwivedi, V.D.; Jha, N.K.; Ojha, S. α-Bisabolol, a Dietary Bioactive Phytochemical Attenuates Dopaminergic Neurodegeneration through Modulation of Oxidative Stress, Neuroinflammation and Apoptosis in Rotenone-Induced Rat Model of Parkinson’s disease. Biomolecules 2020, 10, 1421. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.-Y.; Leow, D.M.-K.; Herr, D.R.; Yeo, C.J.-J. What Do Randomized Controlled Trials Inform Us about Potential Disease-Modifying Strategies for Parkinson’s Disease? NeuroMolecular Med. 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, R.; Yousuf, S.; Singh, M.P. Contributive Role of Hyperglycemia and Hypoglycemia Towards the Development of Alzheimer’s Disease. Mol. Neurobiol. 2022, 59, 4274–4291. [Google Scholar] [CrossRef] [PubMed]
- Booth, H.D.; Hirst, W.D.; Wade-Martins, R. The Role of Astrocyte Dysfunction in Parkinson’s Disease Pathogenesis. Trends Neurosci. 2017, 40, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Beckers, M.; Bloem, B.R.; Verbeek, M.M. Mechanisms of peripheral levodopa resistance in Parkinson’s disease. NPJ Park. Dis. 2022, 8, 56. [Google Scholar] [CrossRef]
- Thirumalaisamy, R.; Bhuvaneswari, M.; Haritha, S.; Jeevarathna, S.; Janani, K.; Suresh, K. Curcumin, Naringenin and Resveratrol from Natural Plant Products Hold Promising Solution for Modern World Diseases–A Recent Review. S. Afr. J. Bot. 2022. [Google Scholar] [CrossRef]
- Himalian, R.; Singh, S.K.; Singh, M.P. Ameliorative Role of Nutraceuticals on Neurodegenerative Diseases Using the Drosophila melanogaster as a Discovery Model to Define Bioefficacy. J. Am. Nutr. Assoc. 2021, 41, 511–539. [Google Scholar] [CrossRef]
- Moradi, A.; Nezamoleslami, S.; Nezamoleslami, S.; Clark, C.C.; Sohouli, M.H.; Ghiasvand, R. The association between Dietary total antioxidant capacity with risk of Rheumatoid Arthritis in adults: A case-control study. Clin. Nutr. ESPEN 2022, 2405–4577. [Google Scholar] [CrossRef]
- Dhalaria, R.; Verma, R.; Kumar, D.; Puri, S.; Tapwal, A.; Kumar, V.; Nepovimova, E.; Kuca, K. Bioactive Compounds of Edible Fruits with Their Anti-Aging Properties: A Comprehensive Review to Prolong Human Life. Antioxidants 2020, 9, 1123. [Google Scholar] [CrossRef]
- Kaveh, S.; Mahoonak, A.S.; Ghorbani, M.; Jafari, S.M. Fenugreek seed (Trigonella foenum graecum) protein hydrolysate loaded in nanosized liposomes: Characteristic, storage stability, controlled release, and retention of antioxidant activity. Ind. Crops Prod. 2022, 182, 114908. [Google Scholar] [CrossRef]
- Khammassi, M.; Mighri, H.; Ben Mansour, M.; Amri, I.; Jamoussi, B.; Khaldi, A. Metabolite profiling and potential antioxidant activity of sixteen fennel (Foeniculum vulgare Mill.) populations wild-growing in Tunisia. S. Afr. J. Bot. 2022, 148, 407–414. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Shahidi, F. Herbal beverages: Bioactive compounds and their role in disease risk reduction—A review. J. Traditional Complement. Med. 2018, 8, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, R.; Azam, S.; Cho, D.-Y.; Su-Kim, I.; Choi, D.-K. Natural Phytochemicals as Novel Therapeutic Strategies to Prevent and Treat Parkinson’s Disease: Current Knowledge and Future Perspectives. Oxid. Med. Cell. Longev. 2021, 2021, 6680935. [Google Scholar] [CrossRef]
- Singh, M.P.; Reddy, M.K.; Mathur, N.; Saxena, D.; Chowdhuri, D.K. Induction of hsp70, hsp60, hsp83 and hsp26 and oxidative stress markers in benzene, toluene and xylene exposed Drosophila melanogaster: Role of ROS generation. Toxicol. Appl. Pharmacol. 2009, 235, 226–243. [Google Scholar] [CrossRef]
- Singh, M.P.; Mishra, M.; Sharma, A.; Shukla, A.; Mudiam, M.; Patel, D.; Ram, K.R.; Chowdhuri, D.K. Genotoxicity and apoptosis in Drosophila melanogaster exposed to benzene, toluene and xylene: Attenuation by quercetin and curcumin. Toxicol. Appl. Pharmacol. 2011, 253, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.P.; Ram, K.R.; Mishra, M.; Shrivastava, M.; Saxena, D.K.; Chowdhuri, D.K. Effects of co-exposure of benzene, toluene and xylene to Drosophila melanogaster: Alteration in hsp70, hsp60, hsp83, hsp26, ROS generation and oxidative stress markers. Chemosphere 2010, 79, 577–587. [Google Scholar] [CrossRef]
- Sharma, A.; Mishra, M.; Shukla, A.; Kumar, R.; Abdin, M.; Chowdhuri, D.K. Organochlorine pesticide, endosulfan induced cellular and organismal response in Drosophila melanogaster. J. Hazard. Mater. 2012, 221–222, 275–287. [Google Scholar] [CrossRef]
- Hu, C.; Yang, J.; Qi, Z.; Wu, H.; Wang, B.; Zou, F.; Mei, H.; Liu, J.; Wang, W.; Liu, Q. Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities. MedComm 2022, 3, e161. [Google Scholar] [CrossRef]
- Deepika; Maurya, P.K. Health Benefits of Quercetin in Age-Related Diseases. Molecules 2022, 27, 2498. [Google Scholar] [CrossRef]
- Coulom, H.; Birman, S. Chronic exposure to rotenone models sporadic Parkinson’s disease in Drosophila melanogaster. J. Neurosci. 2004, 24, 10993–10998. [Google Scholar] [CrossRef]
- Hosamani, R.; Ramesh, S.R.; Muralidhara. Attenuation of Rotenone-Induced Mitochondrial Oxidative Damage and Neurotoxicty in Drosophila melanogaster Supplemented with Creatine. Neurochem. Res. 2010, 35, 1402–1412. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.; Yang, L.; Sah, M.; Kannan, K.; Anamani, D.; Vijayan, C.; Kwok, J.; Cantino, M.E.; Beal, M.F.; Fridell, Y.-W.C. A neuroprotective role of the human uncoupling protein 2 (hUCP2) in a Drosophila Parkinson’s Disease model. Neurobiol. Dis. 2012, 46, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Lawal, H.O.; Chang, H.-Y.; Terrell, A.N.; Brooks, E.S.; Pulido, D.; Simon, A.F.; Krantz, D.E. The Drosophila vesicular monoamine transporter reduces pesticide-induced loss of dopaminergic neurons. Neurobiol. Dis. 2010, 40, 102–112. [Google Scholar] [CrossRef]
- Shabir, S.; Yousuf, S.; Singh, S.K.; Vamanu, E.; Singh, M.P. Ethnopharmacological Effects of Urtica dioica, Matricaria chamomilla, and Murraya koenigii on Rotenone-Exposed D. melanogaster: An Attenuation of Cellular, Biochemical, and Organismal Markers. Antioxidants 2022, 11, 1623. [Google Scholar] [CrossRef] [PubMed]
- Sherer, T.; Betarbet, R.; Testa, C.M.; Seo, B.B.; Richardson, J.R.; Kim, J.H.; Miller, G.W.; Yagi, T.; Matsuno-Yagi, A.; Greenamyre, J.T. Mechanism of Toxicity in Rotenone Models of Parkinson’s Disease. J. Neurosci. 2003, 23, 10756–10764. [Google Scholar] [CrossRef]
- Muñoz-Soriano, V.; Paricio, N. Drosophila Models of Parkinson’s Disease: Discovering Relevant Pathways and Novel Therapeutic Strategies. Park. Dis. 2011, 2011, 520640. [Google Scholar] [CrossRef]
- Steffan, J.S.; Bodai, L.; Pallos, J.; Poelman, M.; McCampbell, A.; Apostol, B.L.; Kazantsev, A.; Schmidt, E.; Zhu, Y.-Z.; Greenwald, M.; et al. Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature 2001, 413, 739–743. [Google Scholar] [CrossRef]
- Auluck, P.K.; Chan, H.Y.E.; Trojanowski, J.Q.; Lee, V.M.-Y.; Bonini, N.M. Chaperone Suppression of alpha -Synuclein Toxicity in a Drosophila Model for Parkinson’s Disease. Science 2002, 295, 865–868. [Google Scholar] [CrossRef]
- Whitworth, A.J.; Theodore, D.A.; Greene, J.C.; Beneš, H.; Wes, P.D.; Pallanck, L.J. Increased glutathione S -transferase activity rescues dopaminergic neuron loss in a Drosophila model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2005, 102, 8024–8029. [Google Scholar] [CrossRef]
- Feany, M.B.; Bender, W.W. A Drosophila model of Parkinson’s disease. Nature 2000, 404, 394–398. [Google Scholar] [CrossRef]
- Hirth, F. Drosophila melanogaster in the study of human neurodegeneration. CNS Neurol. Disord. Drug Targets 2010, 9, 504–523. [Google Scholar] [CrossRef] [PubMed]
- Gayathri, M.; Krishnamurthy, N. Studies on the toxicity of the mercurial fungicide Agallol 3 in Drosophila melanogaster. Environ. Res. 1981, 24, 89–95. [Google Scholar] [CrossRef]
- Pendleton, R.G.; Parvez, F.; Sayed, M.; Hillman, R. Effects of Pharmacological Agents upon a Transgenic Model of Parkinson’s Disease in Drosophila melanogaster. J. Pharmacol. Exp. Ther. 2002, 300, 91–96. [Google Scholar] [CrossRef]
- Ali, Y.O.; Escala, W.; Ruan, K.; Zhai, R.G. Assaying Locomotor, Learning, and Memory Deficits in Drosophila Models of Neurodegeneration. J. Vis. Exp. 2011, 49, 2504. [Google Scholar] [CrossRef]
- Madabattula, S.T.; Strautman, J.C.; Bysice, A.M.; O’Sullivan, J.A.; Androschuk, A.; Rosenfelt, C.; Doucet, K.; Rouleau, G.; Bolduc, F. Quantitative Analysis of Climbing Defects in a Drosophila Model of Neurodegenerative Disorders. J. Vis. Exp. JoVE 2015, 100, e52741. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Abramov, A.Y. Mechanism of oxidative stress in neurodegeneration. Oxid. Med. Cell. Longev. 2012, 2012, 428010. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, S.; Shabir, S.; Singh, M.P. Protection Against Drug-Induced Liver Injuries Through Nutraceuticals via Amelioration of Nrf-2 Signaling. J. Am. Nutr. Assoc. 2022, 1–21. [Google Scholar] [CrossRef]
- Himalian, R.; Singh, M.P. A Comparative account on Antioxidant activities, Total phenolic and Flavonoid contents of Punica granatum, Carica papaya, Foeniculum vulgare, Trigonella foenum-graecum, and Urtica dioica: An in vitro Evaluation. Res. J. Pharm. Technol. 2022, 15, 1175–1183. [Google Scholar] [CrossRef]
- Auluck, P.K.; Bonini, N.M. Pharmacological prevention of Parkinson disease in Drosophila. Nat. Med. 2002, 8, 1185–1186. [Google Scholar] [CrossRef]
- Bilen, J.; Bonini, N.M. Drosophila as a Model for Human Neurodegenerative Disease. Annu. Rev. Genet. 2005, 39, 153–171. [Google Scholar] [CrossRef]
- Whitworth, A.J.; Wes, P.D.; Pallanck, L.J. Drosophila models pioneer a new approach to drug discovery for Parkinson’s disease. Drug Discov. Today 2006, 11, 119–126. [Google Scholar] [CrossRef]
- Vallejo, M.J.; Salazar, L.; Grijalva, M. Oxidative Stress Modulation and ROS-Mediated Toxicity in Cancer: A Review on In Vitro Models for Plant-Derived Compounds. Oxid. Med. Cell. Longev. 2017, 2017, 4586068. [Google Scholar] [CrossRef]
- Golla, U.; Bhimathati, S.S.R. Evaluation of Antioxidant and DNA Damage Protection Activity of the Hydroalcoholic Extract of Desmostachya bipinnata L. Stapf. Sci. World J. 2014, 2014, 215084. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Christian, P.K.; Panchal, K.; Guruprasad, B.R.; Tiwari, A.K. Supplementation of Spirulina (Arthrospira platensis) Improves Lifespan and Locomotor Activity in Paraquat-Sensitive DJ-1βΔ93 Flies, a Parkinson’s Disease Model in Drosophila melanogaster. J. Diet. Suppl. 2017, 14, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Hosamani, R.; Muralidhara. Neuroprotective efficacy of Bacopa monnieri against rotenone induced oxidative stress and neurotoxicity in Drosophila melanogaster. Neurotoxicology 2009, 30, 977–985. [Google Scholar] [CrossRef]
- Carey, J.R.; Harshman, L.G.; Liedo, P.; Müller, H.G.; Wang, J.L.; Zhang, Z. Longevity–fertility trade-offs in the tephritid fruit fly, Anastrepha ludens, across dietary-restriction gradients. Aging Cell 2008, 7, 470–477. [Google Scholar] [CrossRef]
- Lee, K.P.; Simpson, S.J.; Clissold, F.J.; Brooks, R.; Ballard, J.W.O.; Taylor, P.W.; Soran, N.; Raubenheimer, D. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc. Natl. Acad. Sci. USA 2008, 105, 2498–2503. [Google Scholar] [CrossRef]
- Skorupa, D.A.; Dervisefendic, A.; Zwiener, J.; Pletcher, S.D. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell 2008, 7, 478–490. [Google Scholar] [CrossRef]
- Bonilla-Ramirez, L.; Jimenez-Del-Rio, M.; Velez-Pardo, C. Acute and chronic metal exposure impairs locomotion activity in Drosophila melanogaster: A model to study Parkinsonism. BioMetals 2011, 24, 1045–1057. [Google Scholar] [CrossRef]
- Lee, D.W.; Andersen, J.K. Iron elevations in the aging Parkinsonian brain: A consequence of impaired iron homeostasis? J. Neurochem. 2010, 112, 332–339. [Google Scholar] [CrossRef]
- Guilarte, T.R. Manganese and Parkinson’s Disease: A Critical Review and New Findings. Environ. Health Perspect. 2010, 118, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Huster, D. Wilson disease. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Margulies, C.; Tully, T.; Dubnau, J. Deconstructing Memory in Drosophila. Curr. Biol. 2005, 15, R700–R713. [Google Scholar] [CrossRef]
- Batool, Z.; Sadir, S.; Liaquat, L.; Tabassum, S.; Madiha, S.; Rafiq, S.; Tariq, S.; Batool, T.S.; Saleem, S.; Naqvi, F.; et al. Repeated administration of almonds increases brain acetylcholine levels and enhances memory function in healthy rats while attenuates memory deficits in animal model of amnesia. Brain Res. Bull. 2016, 120, 63–74. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, H.; Lu, J.; Li, X.; Chen, X.; Tao, D.; Huang, W.; Huang, B. Lifespan extension and elevated hsp gene expression in Drosophila caused by histone deacetylase inhibitors. J. Exp. Biol. 2005, 208, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Singh, M.P.; Vimal, D.; Kumar, S.; Jha, R.R.; Chowdhuri, D.K. Benzene induced resistance in exposed Drosophila melanogaster: Outcome of improved detoxification and gene modulation. Chemosphere 2018, 201, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Finkbeiner, S.; Skibinski, G. Drug discovery in Parkinson’s disease: Update and developments in the use of cellular models. Int. J. High Throughput Screen. 2011, 2, 15–25. [Google Scholar] [CrossRef]
- Adnew, W.; Asmare, B.; Mekuriaw, Y. Review on knowledge gap in Brachiaria grass research and utilization: Ethiopian perspective. AgroLife Sci. J. 2021, 10, 9–26. Available online: https://agrolifejournal.usamv.ro/index.php/scientific-papers/567-review-on-knowledge-gap-in-brachiaria-grass-research-and-utilization-ethiopian-perspective-567#spucontentCitation1 (accessed on 20 September 2022). [CrossRef]


| Groups | Total Fecundity | Mean Daily Egg Laying/Female/10 Days | Fertility Percentage | Reproductive Performance |
|---|---|---|---|---|
| CONTROL | 1186 | 237.2 ± 2.87 | 80.99 ± 1.15 | 96.1 ± 0.44 |
| ROT | 239 | 47.8 ± 1.74 * | 35.96 ± 3.07 * | 8.5 ± 0.08 * |
| ROT + PG-P | 948 | 189.6 ± 2.80 *,# | 40.21 ± 0.70 * | 38.1 ± 0.10 *,# |
| ROT + PG-p | 709 | 141.8 ± 3.63 *,# | 54.22 ± 1.35 *,# | 38.4 ± 0.19 *,# |
| ROT + CP-P | 855 | 171 ± 4.90 *,# | 44.01 ± 1.36 *,# | 37.5 ± 0.03 *,# |
| ROT + ND | 733 | 146.6 ± 2.73 *,# | 49.79 ± 0.08 *,# | 36.5 ± 0.13 *,# |
| ROT + FV | 751 | 150.2 ± 2.28 *,# | 78.61 ± 1.40 # | 59 ± 0.17 *,# |
| ROT + TFG | 588 | 117.6 ± 1.96 *,# | 77.85 ± 1.43 # | 45.8 ± 0.26 *,# |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, M.P.; Himalian, R.; Shabir, S.; Obaid, A.A.; Alamri, A.S.; Galanakis, C.M.; Singh, S.K.; Vamanu, E. Protection of Phytoextracts against Rotenone-Induced Organismal Toxicities in Drosophila melanogaster via the Attenuation of ROS Generation. Appl. Sci. 2022, 12, 9822. https://doi.org/10.3390/app12199822
Singh MP, Himalian R, Shabir S, Obaid AA, Alamri AS, Galanakis CM, Singh SK, Vamanu E. Protection of Phytoextracts against Rotenone-Induced Organismal Toxicities in Drosophila melanogaster via the Attenuation of ROS Generation. Applied Sciences. 2022; 12(19):9822. https://doi.org/10.3390/app12199822
Chicago/Turabian StyleSingh, Mahendra P., Ranjana Himalian, Shabnam Shabir, Ahmad A. Obaid, Abdulhakeem S. Alamri, Charis M. Galanakis, Sandeep Kumar Singh, and Emanuel Vamanu. 2022. "Protection of Phytoextracts against Rotenone-Induced Organismal Toxicities in Drosophila melanogaster via the Attenuation of ROS Generation" Applied Sciences 12, no. 19: 9822. https://doi.org/10.3390/app12199822
APA StyleSingh, M. P., Himalian, R., Shabir, S., Obaid, A. A., Alamri, A. S., Galanakis, C. M., Singh, S. K., & Vamanu, E. (2022). Protection of Phytoextracts against Rotenone-Induced Organismal Toxicities in Drosophila melanogaster via the Attenuation of ROS Generation. Applied Sciences, 12(19), 9822. https://doi.org/10.3390/app12199822










