Evaluating Tissue Mechanical Properties Using Quantitative Mueller Matrix Polarimetry and Neural Network
Abstract
1. Introduction
2. Materials and Methods
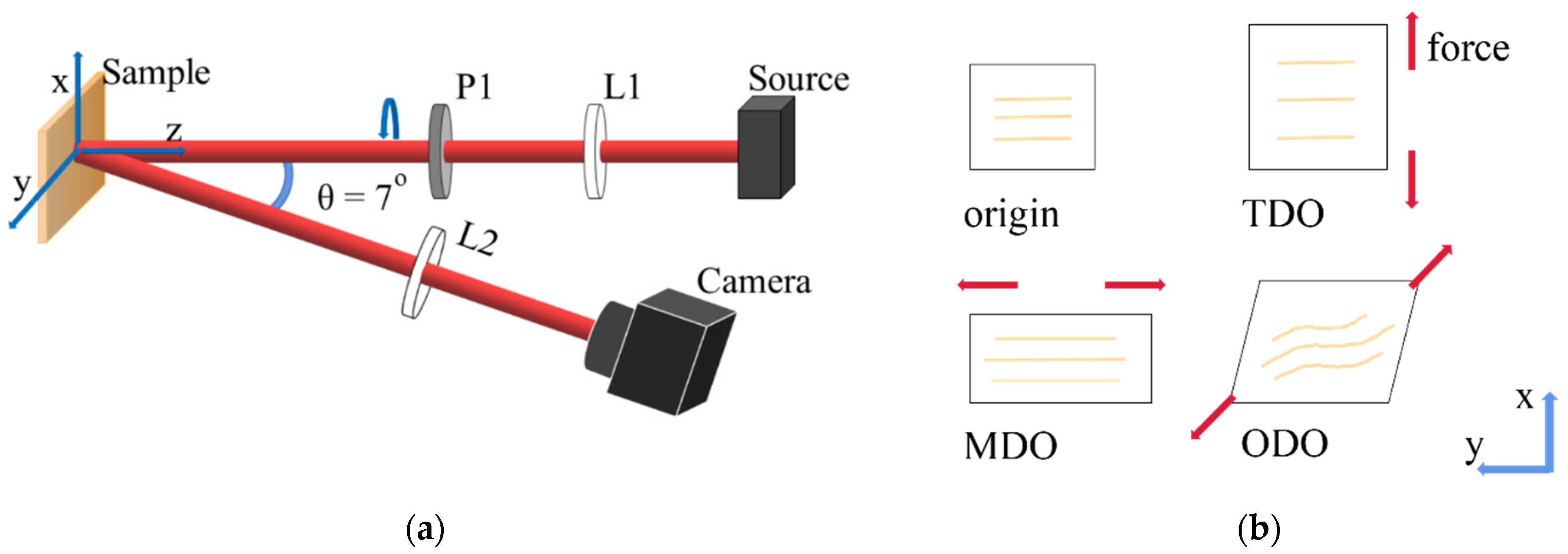
2.1. Experimental Setup and Tendon Tissue Samples
2.2. Frequency Distribution Histograms and Mueller Matrix Transformation Parameters
2.3. Central Moments
2.4. Neural Networks
3. Results and Discussions
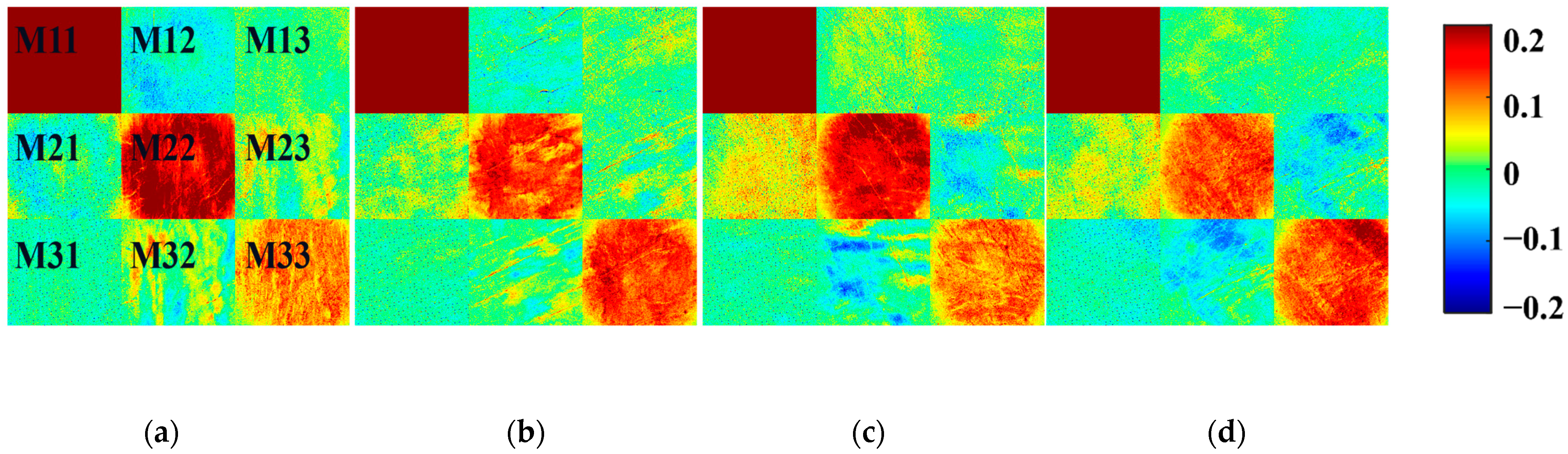
3.1. FDHs of Mueller Matrix Elements for Tendon Tissues in Different States
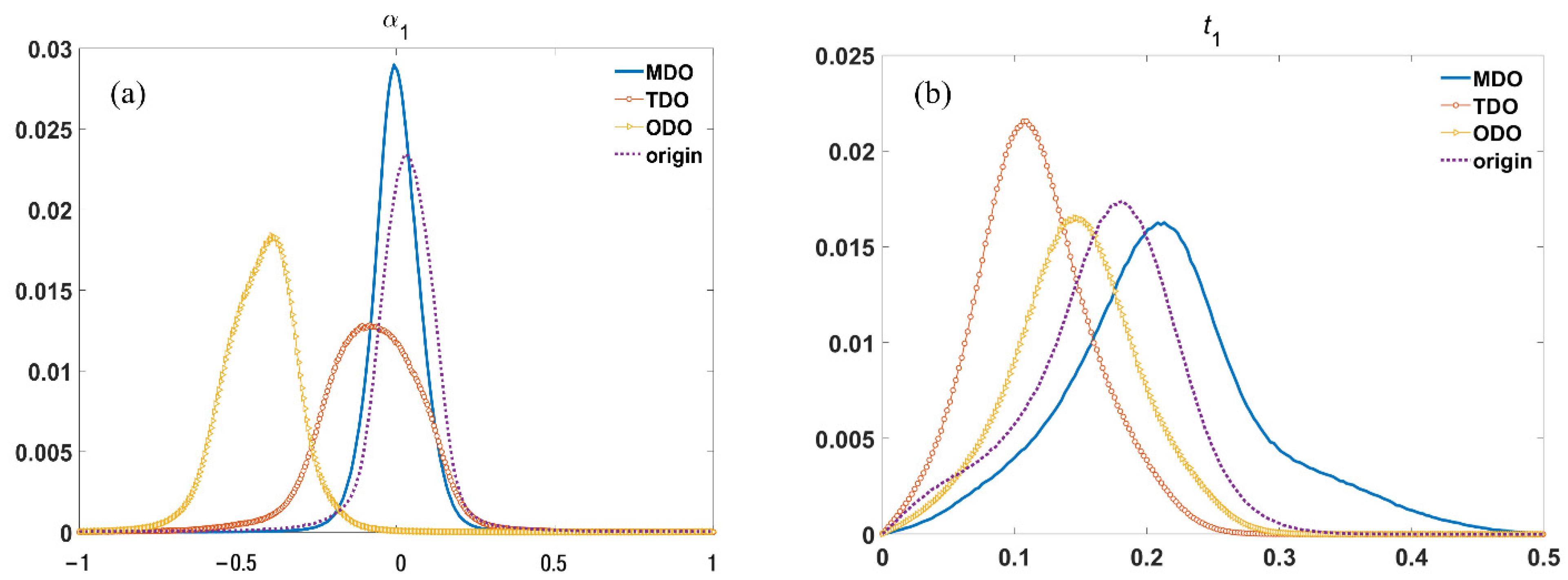
3.2. MMT Parameters for Tendon Tissues in Different States
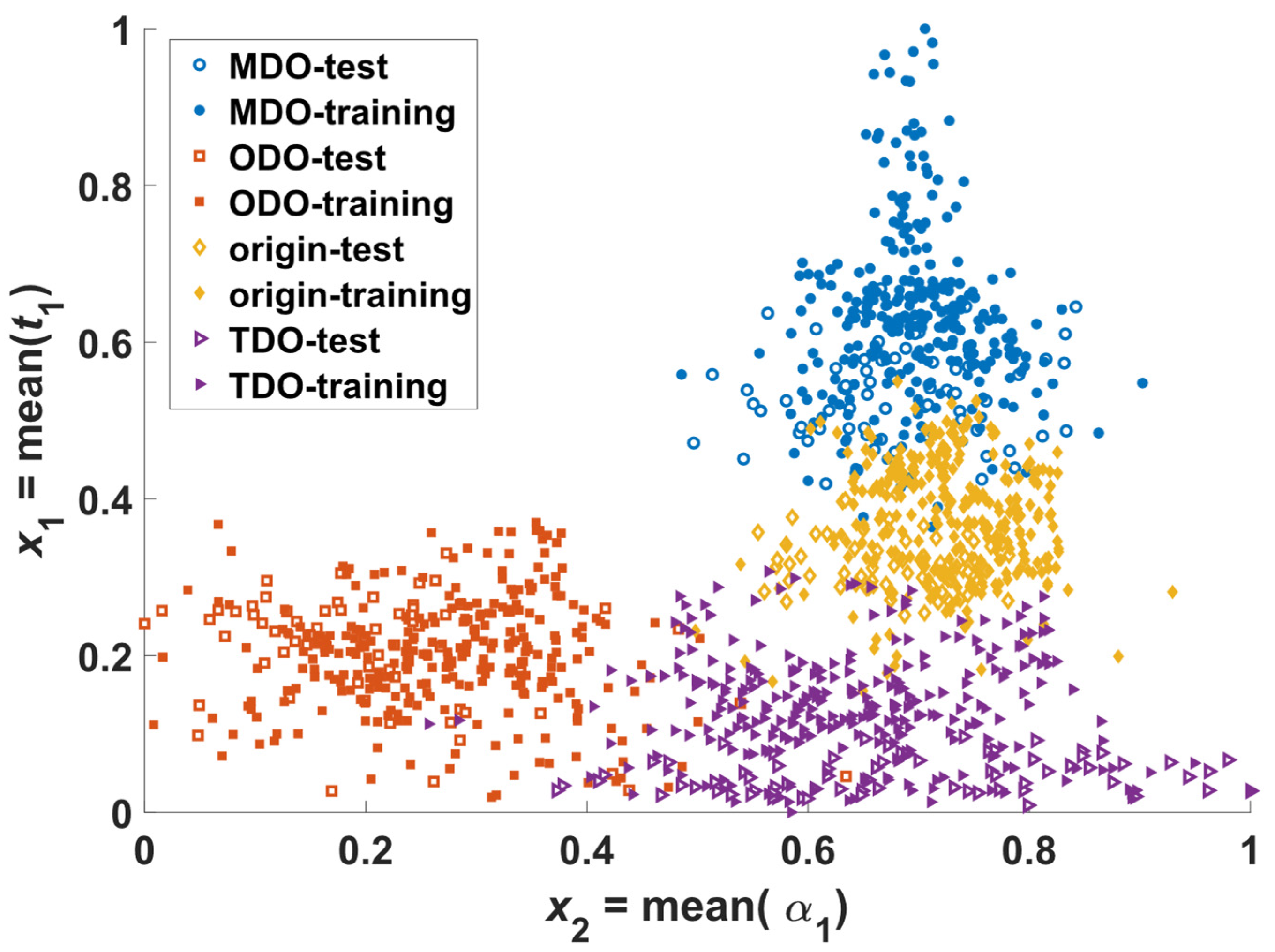
3.3. Separation of Tendon Tissues in Different States Using NN Classifiers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tekieli, M.; De Santis, S.; de Felice, G.; Kwiecień, A.; Roscini, F. Application of Digital Image Correlation to Composite Reinforcements Testing. Compos. Struct. 2017, 160, 670–688. [Google Scholar] [CrossRef]
- Liu, C.; Dai, Y.T.; Dai, M.L.; Liu, X.; Zhu, C.; Shao, X.; He, X. Deformation measurement by two-dimensional multi-camera full-field digital image correlation. Acta Opt. Sin. 2016, 36, 97–112. [Google Scholar]
- Shunqing, Z.; Chenjia, G.A.O.; Long, Z. The Development and Latest Applications of Digital Image Correlation in Stress and Strain Measurement. Imaging Sci. Photochem. 2017, 35, 193. [Google Scholar]
- Innocenti, B. Chapter 2—Mechanical Properties of Biological Tissues. In Human Orthopaedic Biomechanics; Academic Press: Cambridge, MA, USA, 2022; pp. 9–24. [Google Scholar]
- Pan, B.; Wang, B. Research Progress in Digital Volume Correlation Method. Chin. Sci. Bull. 2017, 62, 1671–1681. [Google Scholar] [CrossRef]
- Huňady, R.; Hagara, M. A New Procedure of Modal Parameter Estimation for High-Speed Digital Image Correlation. Mech. Syst. Signal Process. 2017, 93, 66–79. [Google Scholar] [CrossRef]
- Kennedy, B.F.; Wijesinghe, P.; Sampson, D.D. The Emergence of Optical Elastography in Biomedicine. Nat. Photonics 2017, 11, 215–221. [Google Scholar] [CrossRef]
- Konarski, W.; Poboży, T.; Kotela, A.; Hordowicz, M.; Poboży, K. Ultrasound in the Differential Diagnosis of Medial Epicondylal gia and Medial Elbow Pain-Imaging Findings and Narrative Literature Review. Healthcare 2022, 10, 1529. [Google Scholar] [CrossRef]
- He, H.; Liao, R.; Zeng, N.; Li, P.; Chen, Z.; Liu, X.; Ma, H. Mueller Matrix Polarimetry—An Emerging New Tool for Characterizing the Microstructural Feature of Complex Biological Specimen. J. Light. Technol. 2019, 37, 2534–2548. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, B.; He, C.; He, H.; Guo, J.; Wu, J.; Elson, D.S.; Ma, H. Polarization Aberrations in High-Numerical-Aperture Lens Systems and Their Effects on Vectorial-Information Sensing. Remote Sens. 2022, 14, 1932. [Google Scholar] [CrossRef]
- Azzam, R.M. Photopolarimetric Measurement of the Mueller Matrix by Fourier Analysis of a Single Detected Signal. Opt. Lett. 1978, 2, 148. [Google Scholar] [CrossRef]
- He, C.; He, H.; Chang, J.; Chen, B.; Ma, H.; Booth, M.J. Polarisation Optics for Biomedical and Clinical Applications: A Review. Light Sci. Appl. 2021, 10, 194. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Meng, R.; Qi, J.; Liu, Y.; Wang, X.; Chen, Y.; Liao, R.; Ma, H. Fast Mueller Matrix Microscope Based on Dual DoFP Polarimeters. Opt. Lett. 2021, 46, 1676. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; He, H.; Chen, Z.; Wang, Y.; Ma, H. Modulus Design Multiwavelength Polarization Microscope for Transmission Mueller Matrix Imaging. J. Biomed. Opt. 2018, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Lan, Y.; Zhai, H.; Deng, L.; He, H.; Mao, H.; Ma, H. Comparative Study of Modified Mueller Matrix Transformation and Polar Decomposition Parameters for Transmission and Backscattering Tissue Polarimetries. Appl. Sci. 2021, 11, 10416. [Google Scholar] [CrossRef]
- Liu, T.; Sun, T.; He, H.; Liu, S.; Dong, Y.; Wu, J.; Ma, H. Comparative Study of the Imaging Contrasts of Mueller Matrix Derived Parameters between Transmission and Backscattering Polarimetry. Biomed. Opt. Express 2018, 9, 4413. [Google Scholar] [CrossRef]
- He, H.; Sun, M.; Zeng, N.; Du, E.; Liu, S.; Guo, Y.; Wu, J.; He, Y.; Ma, H. Mapping Local Orientation of Aligned Fibrous Scatterers for Cancerous Tissues Using Backscattering Mueller Matrix Imaging. J. Biomed. Opt. 2014, 19, 106007. [Google Scholar] [CrossRef]
- Sun, T.; Liu, T.; He, H.; Wu, J.; Ma, H. Distinguishing anisotropy orientations originated from scattering and birefringence of turbid media using Mueller matrix derived parameters. Opt. Lett. 2018, 43, 4092–4095. [Google Scholar] [CrossRef]
- Qi, J.; Elson, D.S. Mueller polarimetric imaging for surgical and diagnostic applications: A review. J. Biophotonics 2017, 10, 950–982. [Google Scholar] [CrossRef]
- He, H.; Zeng, N.; Du, E.; Guo, Y.; Li, D.; Liao, R.; He, Y.; Ma, H. Two-Dimensional and Surface Backscattering Mueller Matrices of Anisotropic Sphere-Cylinder Scattering Media: A Quantitative Study of Influence from Fibrous Scatterers. J. Biomed. Opt. 2013, 18, 046002. [Google Scholar] [CrossRef]
- Li, P.; Dong, Y.; Wan, J.; He, H.; Tariq, A.; Ma, H. Polaromics: Deriving polarization parameters from a Mueller matrix for quantitative characterization of biomedical specimen. J. Phys. D Appl. Phys. 2021, 55, 034002. [Google Scholar] [CrossRef]
- Chen, B.; Li, W.; He, H.; He, C.; Guo, J.; Shen, Y.; Liu, S.; Sun, T.; Wu, J.; Ma, H. Analysis and Calibration of Linear Birefringence Orientation Parameters Derived from Mueller Matrix for Multi-Layered Tissues. Opt. Lasers Eng. 2021, 146, 106690. [Google Scholar] [CrossRef]
- Alali, S.; Vitkin, I.A. Polarized light imaging in biomedicine: Emerging Mueller matrix methodologies for bulk tissue assessment. J. Biomed. Opt. 2015, 20, 061104. [Google Scholar] [CrossRef]
- He, C.; He, H.; Li, X.; Chang, J.; Wang, Y.; Liu, S.; Zeng, N.; He, Y.; Ma, H. Quantitatively Differentiating Microstructures of Tissues by Frequency Distributions of Mueller Matrix Images. J. Biomed. Opt. 2015, 20, 105009. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; He, H.; Sheng, W.; Wu, J.; Ma, H. A quantitative and non-contact technique to characterise microstructural variations of skin tissues during photo-damaging process based on Mueller matrix polarimetry. Sci. Rep. 2017, 7, 14702. [Google Scholar] [CrossRef] [PubMed]
- Grimmett, G.; Stirzaker, D. Probability and Random Processes; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Ushenko, V.A.; Dubolazov, O.V.; Karachevtsev, A.O. Two Wavelength Mueller Matrix Reconstruction of Blood Plasma Films Polycrystalline Structure in Diagnostics of Breast Cancer. Appl. Opt. 2014, 53, B128. [Google Scholar] [CrossRef] [PubMed]
- Aulestia, P.S.; Talahua, J.S.; Andaluz, V.H.; Benalcázar, M.E. Real-Time Face Detection Using Artificial Neural Networks. In Proceedings of the Artificial Neural Networks and Machine Learning—ICANN, Alghero, Italy, 11–14 September 2017; Springer: Cham, Switzerland, 2017; pp. 590–599. [Google Scholar]
- Cavalin, P.; Oliveira, L.S. A Review of Texture Classification Methods and Databases. In Proceedings of the 2017 30th SIBGRAPI Coference on Graphics, Patterns and Images Tutorials (SIBGRAPI-T), Niteroi, Brazil, 17–18 October 2017; pp. 1–8. [Google Scholar]
- Bera, K.; Schalper, K.A.; Rimm, D.L.; Velcheti, V.; Madabhushi, A. Artificial Intelligence in Digital Pathology—New Tools for Diagnosis and Precision Oncology. Nat. Rev. Clin. Oncol. 2019, 16, 703–715. [Google Scholar] [CrossRef]
- Otter, D.W.; Medina, J.R.; Kalita, J.K. A Survey of the Usages of Deep Learning for Natural Language Processing. IEEE Trans. Neural Netw. Learn. Syst. 2021, 32, 604–624. [Google Scholar] [CrossRef]
- Dong, Y.; Wan, J.; Wang, X.; Xue, J.-H.; Zou, J.; He, H.; Li, P.; Hou, A.; Ma, H. A Polarization-Imaging-Based Machine Learning Framework for Quantitative Pathological Diagnosis of Cervical Precancerous Lesions. IEEE Trans. Med. Imaging 2021, 40, 3728–3738. [Google Scholar] [CrossRef]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778. [Google Scholar]
- He, C.; Chang, J.; Hu, Q.; Wang, J.; Antonello, J.; He, H.; Liu, S.; Lin, J.; Dai, B.; Elson, D.S.; et al. Complex Vectorial Optics through Gradient Index Lens Cascades. Nat. Commun. 2019, 10, 4264. [Google Scholar] [CrossRef]
- He, C.; Chang, J.; Salter, S.P.; Shen, Y.; Dai, B.; Li, P.; Jin, Y.; Thodika, S.C.; Li, M.; Tariq, A.; et al. Revealing complex optical phenomena through vectorial metrics. Adv. Photon. 2022, 4, 026001. [Google Scholar] [CrossRef]
- Shen, Y.; Huang, R.; He, H.; Liu, S.; Dong, Y.; Wu, J.; Ma, H. Comparative study of the influence of imaging resolution on linear retardance parameters derived from the Mueller matrix. Biomed. Opt. Express 2021, 12, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Uijlings, J.R.R.; van de Sande, K.E.A.; Gevers, T.; Smeulders, A.W.M. Selective Search for Object Recognition. Int. J. Comput. Vis. 2013, 104, 154–171. [Google Scholar] [CrossRef]
- Rivenson, Y.; Wang, H.; Wei, Z.; de Haan, K.; Zhang, Y.; Wu, Y.; Günaydın, H.; Zuckerman, J.E.; Chong, T.; Sisk, A.E.; et al. Virtual Histological Staining of Unlabelled Tissue-Autofluorescence Images via Deep Learning. Nat. Biomed. Eng. 2019, 3, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Cuingnet, R.; Rosso, C.; Chupin, M.; Lehéricy, S.; Dormont, D.; Benali, H.; Samson, Y.; Colliot, O. Spatial Regularization of SVM for the Detection of Diffusion Alterations Associated with Stroke Outcome. Med. Image Anal. 2011, 15, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Youngentob, S.L.; Markert, L.M.; Mozell, M.M.; Hornung, D.E. A Method for Establishing a Five Odorant Identification Confusion Matrix Task in Rats. Physiol. Behav. 1990, 47, 1053–1059. [Google Scholar] [CrossRef]
- Tang, W.; Hu, J.; Zhang, H.; Wu, P.; He, H. Kappa Coefficient: A Popular Measure of Rater Agreement. Shanghai Arch. Psychiatry 2015, 27, 62–67. [Google Scholar]
- Huang, J.; Rathod, V.; Sun, C.; Zhu, M.; Korattikara, A.; Fathi, A.; Fischer, I.; Wojna, Z.; Song, Y.; Guadarrama, S.; et al. Speed/Accuracy Trade-Offs for Modern Convolutional Object Detectors. In Proceedings of the 2017 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Honolulu, HI, USA, 21–26 July 2017; pp. 3296–3297. [Google Scholar]


| Origin | TDO | MDO | ODO | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.04 | 0.00 | 0.03 | 0.03 | 0.03 | −0.07 | −0.08 | −0.01 | 0.00 | −0.39 | −0.52 | −0.42 | |
| 0.02 | 0.01 | 0.02 | 0.33 | 0.16 | 0.03 | 0.05 | 0.02 | 0.01 | 0.24 | 0.05 | 0.02 | |
| −0.35 | −0.16 | −0.49 | −0.19 | −0.02 | −0.07 | 0.49 | 0.20 | −0.11 | 1.50 | 3.11 | 1.76 | |
| 7.82 | 5.35 | 16.71 | 1.80 | 2.84 | 6.40 | 5.97 | 5.59 | 18.46 | 4.37 | 18.51 | 19.96 | |
| Origin | TDO | MDO | ODO | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.10 | 0.20 | 0.17 | 0.03 | 0.09 | 0.12 | 0.08 | 0.24 | 0.21 | 0.05 | 0.21 | 0.15 | |
| 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 | 0.00 | 0.01 | 0.00 | |
| 0.13 | −0.23 | −0.31 | 1.43 | 0.84 | 0.33 | 0.19 | −0.17 | 0.42 | 0.70 | −0.15 | 0.03 | |
| 2.93 | 3.23 | 3.28 | 7.95 | 4.41 | 3.20 | 3.31 | 2.78 | 3.54 | 3.82 | 3.07 | 3.08 | |
| R | H | W | |||||||||||
| −0.858 | 2.267 | 1.933 | −2.096 | 2.455 | 1.813 | 1.863 | 0.724 | ||||||
| 1.829 | −3.686 | 1.103 | 2.323 | −2.277 | 0.248 | −1.640 | −1.023 | ||||||
| 1.270 | 2.833 | −0.436 | 0.946 | 3.476 | 1.090 | 1.991 | 0.494 | 2.348 | −0.838 | 1.459 | −0.292 | −6.215 | −1.836 |
| 0.217 | 1.851 | 0.165 | 1.112 | 0.052 | −0.331 | −0.670 | 0.034 | −0.630 | 1.462 | −1.537 | −0.884 | 2.807 | −0.937 |
| 1.922 | 0.926 | −1.955 | −0.449 | −1.472 | −4.153 | 0.440 | −2.365 | 0.835 | −3.253 | 1.144 | −2.456 | 1.596 | 1.355 |
| −0.143 | 0.083 | −2.326 | 1.494 | 1.210 | −2.034 | 0.850 | 0.132 | −3.301 | 1.408 | 1.408 | 1.732 | −0.834 | 0.808 |
| −0.454 | 0.186 | |
| 0.678 | 1.547 | |
| −0.901 | −0.648 | −0.462 |
| −1.058 | −0.381 | 1.405 |
| −0.636 | 2.597 | −0.066 |
| −0.815 | −0.229 | 0.065 |
| Real Predict | 0 (MDO) | 1 (ODO) | 2 (Origin) | 3 (TDO) |
|---|---|---|---|---|
| 0(MDO) | 70 | 1 | 0 | 0 |
| 1(ODO) | 0 | 71 | 0 | 0 |
| 2(origin) | 1 | 0 | 69 | 1 |
| 3(TDO) | 0 | 3 | 0 | 68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mi, C.; Shao, C.; He, H.; He, C.; Ma, H. Evaluating Tissue Mechanical Properties Using Quantitative Mueller Matrix Polarimetry and Neural Network. Appl. Sci. 2022, 12, 9774. https://doi.org/10.3390/app12199774
Mi C, Shao C, He H, He C, Ma H. Evaluating Tissue Mechanical Properties Using Quantitative Mueller Matrix Polarimetry and Neural Network. Applied Sciences. 2022; 12(19):9774. https://doi.org/10.3390/app12199774
Chicago/Turabian StyleMi, Changjiang, Conghui Shao, Honghui He, Chao He, and Hui Ma. 2022. "Evaluating Tissue Mechanical Properties Using Quantitative Mueller Matrix Polarimetry and Neural Network" Applied Sciences 12, no. 19: 9774. https://doi.org/10.3390/app12199774
APA StyleMi, C., Shao, C., He, H., He, C., & Ma, H. (2022). Evaluating Tissue Mechanical Properties Using Quantitative Mueller Matrix Polarimetry and Neural Network. Applied Sciences, 12(19), 9774. https://doi.org/10.3390/app12199774








