Abstract
Three-dimensional (3D) printing was introduced firstly for industrial use, but it gained popularity in different medical fields, including orthopedic surgeries. Particularly, 3D-printed models have been used in the pre-operative planning for spine surgery, oncology, acetabular fracture treatment and complex primary total hip arthroplasty (THA) or revision THA. In knee surgery, some authors described good accuracy with 3D-printed wedge for Opening Wedge High Tibial Osteotomy (OWHTO), but there are no studies describing its application in Total Knee Arthroplasty (TKA). In both primary and revision TKA, a 3D-printed model may be useful to better evaluate knee morphology and deformity, implants, bone losses and the compatibility between different components used. Furthermore, some companies provide a bone thickness evaluation, which may be useful to identify zones at risk of intra-operative fracture, especially in those cases in which a cone or sleeve must be used. The first aim of this manuscript was to evaluate possible application of 3D-printed model in pre-operative planning of both complex primary and revision TKA, compared to standard planning. Two clinical cases will also be described to show how these models can be used for planning purposes.
1. Introduction
Three-dimensional (3D) printing was first introduced in the 1980s, mainly for industrial use. However, since early 2000, it gained popularity in different medical fields, such as maxillofacial, dental, orthopedic and oncologic surgery [1,2].
In orthopedic surgeries 3D-printed models can be used for both educational purposes, pre-operative planning and surgery simulation. Furthermore, it can be used to create custom guides or implants [1,3,4]. A hands-on model can increase the visualization for bone pathology and deformity, especially for less skilled surgeons. Different studies focused on 3D printing technologies’ utility in orthopedic surgeries, with a dramatic increase in publications between 2014 and 2020 [5].
Patient-specific 3D-printed models can be obtained from both Computer Tomography (CT) scans and Magnetic Resonance Imaging (MRI) slices, with the latter being less used [6].
There are two 3D printing techniques in orthopedic surgery. In the subtractive technique, the model is milled from a foam (e.g., polyurethane), with low costs and poor geometric accuracy, and with no possibility to be sterilized for intra-operative use. Conversely in additive techniques the models are produced through a layer-by-layer process, with a powder, liquid, or plastic material. With this technique, the 3D-printed model can reproduce more complex structures and cavities, with higher accuracy compared to subtractive techniques [7]. Three additive technologies are mainly used in orthopedic fields: stereolithography, selective laser sintering (SLS) and fused deposition modelling (FDM). Stereolithography is based on an optical light energy source scanning over a resin, which turns solid specific areas of the liquid’s surface. SLS is based on a high-power laser which fuses plastic, metal, ceramic, or glass powder into a physical model designed on 3D images by a computer-aided design. This type of manufacturing can provide high geometry accuracy, but with high costs. FDM is based on extraction and solidification of materials in layers made by the deposition of a polymer. As this technique allows the use of different materials (and colors), it is probably the most versatile, and also provides a high geometric accuracy and the possibility to use sterilizable materials [7,8].
Three-dimensionally-printed models have been used in different orthopedic fields, including traumatology, shoulder surgeries, oncology, spine and, mainly, complex hip arthroplasty and acetabular fractures [9]. However, there are no studies or case reports on the application of 3D printing in the pre-operative planning of primary and revision Total Knee Arthroplasty (TKA). The aim of this manuscript was to summarize the application of 3D-printed models in the pre-operative planning for both primary and revision TKA. For this purpose, two cases from our institution will be described as an example.
2. Materials and Methods
A literature search was performed including all the studies published until June 2022 regarding application of 3D printing in orthopedics. Initially, all the studies describing application of 3D-printed models in the pre-operative planning of different orthopedic fields were included. Studies were screened and more relevant studies were briefly described.
However, to better understand the role of 3D-printed models in Knee replacement surgeries, two cases were reported. In both the cases a 3D printed model, based on a CT scan, was produced by Medics® (Turin, Italy). The 3D model was printed with FDM additive technology and was provided with a further document with all measurements performed. Furthermore, the model can be printed separable in two parts, especially in revision cases: the bone (after implant removal) and the implant. The surgeon can decide to make bone, implant and cement in different colors in the document, as well as in the 3D-printed model. Finally, the manufacturer can provide the surgeon with a bone thickness map, which turns out useful in planning more delicate zones.
3. Results
3.1. Applications of 3D-Printed Models
Three-dimensionally-printed models have been extensively used in different orthopedic fields. Campana et al. summarized the application of 3D-printed models in shoulder surgery, particularly for Total Shoulder Arthroplasty (TSA), either by creating a 3D model to allow a more precise pre-operative planning, especially in case of severe glenoid deformities, or to create a Patient-Specific Instrumentation (PSI) in more complex cases [10]. Several studies reported good results and possible implant positioning improvement with both PSI and pre-operative planning with 3D-printed models, especially in complex TSA [11,12]. Other authors evaluated the utility of 3D-printed models in both shoulder instability and traumatological surgery, concluding that these models may help surgeons to make an accurate pre-operative planning and to simulate the surgery, leading to better outcomes [13,14,15,16].
Some researchers described 3D-printed models’ utility in spine surgery, especially for correction of deformities (scoliosis) or complex fractures. This technology may help surgeons with both pre-operative planning, surgical simulations, doctor-patient communication, development of intra-operative PSI and even implantable devices. In particular, 3D technology’s efficacy in improving screw placement was evaluated and confirmed by different studies [8,17,18].
Three-dimensionally-printed models can also be successfully applied to complex traumatology, in which pre-operative planning and good visualization of fracture morphology is mandatory and not always easy with standard images modalities, such as X-rays or CT scans. Good outcomes are described using 3D-printed models in pre-operative planning for complex proximal tibial plateau fractures, with better visualization of fracture’s morphology, decreased operative time, intraoperative blood loss and fluoroscopy time [19,20,21,22,23]. Sun et al. recently published their data on 50 patients with hip or knee fracture who underwent a THA/TKA, concluding that 3D-printed models can help surgeons in pre-operative planning as well as in improving accuracy and safety [24].
However, most of the studies on trauma surgery and 3D-printed models focused on acetabular fractures, confirming that 3D-printed models help in better defining morphology of acetabular fractures, leading to reduced operative time, blood loss and intra-operative use of fluoroscopy. However, there is no consensus that these advantages can lead to better functional outcomes [25,26,27]. A recent systematic review and meta-analysis evaluated the effect of 3D-printed models on pre-operative planning and outcomes in orthopedic trauma surgery. The authors included 17 studies with 922 patients with different fracture sites and confirmed in all cases a reduction in operative time, blood loss and fluoroscopy use when pre-operative planning with 3D-printed models was performed [28].
Many researchers focused on the utility of 3D technology in total hip arthroplasty (THA) or revision THA. In particular, THA in dysplastic, traumatic or post-septic cases can be challenging for the surgeon due to altered anatomy. In these cases, 3D-printed models may help to identify pelvic structure, acetabular deformity, bone defects and to plan some surgical needs such as ideal extent of reaming and possible use of augments [29,30,31,32]. Several researchers also evaluated the outcomes of PSI in complex primary or revision hip replacement cases, with good results. However, this technology implies high costs, and it should be reserved to only difficult cases [33,34].
Few papers have evaluated 3D-printed models for pre-operative planning in knee surgery, mostly describing the outcomes using a 3D-printed model wedge to improve alignment accuracy in Opening Wedge High Tibial Osteotomy (OWHTO). Kim et al. evaluated the outcomes in 20 patients treated using the 3D-printed model (a plastic sterilized wedge equal to the amount of opening required) compared to 20 patients with standard pre-operative planning. The authors concluded that a more accurate correction can be achieved using the 3D-printed model compared to standard pre-operative planning [35]. In a similar study, same authors evaluated the efficacy of the same technology not only in achieving a satisfactory correction, but also in respecting the posterior tibial slope (PTS) [36].
Different studies evaluated the utility of PSI in Total Knee Arthroplasty (TKA), focusing both on implant alignment and cost-effectiveness. However, no studies were published reporting 3D-printed models in pre-operative planning for both primary and revision TKA. Furthermore, it must be mentioned that 3D-printed models cost between EUR500 and EUR1000, depending on model’s complexity, and it is cheaper compared to other technologies, such as PSI.
3.2. Application of 3D-Printed Models in Pre-Operative Planning of Primary Total Knee Arthroplasty
A careful pre-operative planning is mandatory in primary TKA to obtain a well-positioned, stable implant and, consequently, good outcomes [37,38,39].
Pre-operatively, surgeons should focus on comorbidities, patient’s history and characteristics (e.g., previous surgery, level of activities, etc.) and a complete knee evaluation. In particular, clinical evaluation should include Range of Motion (ROM), skin (previous scars) evaluation, patients’ gait, neurovascular status, patellar tracking and stability evaluation, as well as the complete lower limb alignment. Furthermore, presence of foot abnormalities, especially in valgus knee, should be evaluated and, in some cases, addressed pre-operatively [37,38,39,40]. Evaluating all these characteristics is useful to choose the correct implant design and the level of constraint in primary TKA.
For a correct pre-operative planning, including implant’s size and positioning, a complete imaging is also mandatory. An X-ray panel should include a knee antero-posterior weight-bearing view (AP), a weight-bearing lateral view (LL), a full-length hip-to-ankle AP weight-bearing view and a patellar view [37]. Radiographic planning should include evaluation of the Hip-Knee Angle (HKA), with consequent description of the type (varus or valgus) and amount of the deformity. The second step is to define the femoral Valgus Cut Angle (VCA), which may be standardized (e.g., 3°, 5° or 6°), or based on HKA [41].
A femoral cut is then simulated based on VCA, and a tibial cut is also simulated based on the chosen alignment (mechanical or Kinematic). At the time of pre-operative planning, osteophytes are also recognized on AP and LL view, and the patella is evaluated. Lastly, implant size and positioning can be simulated according to dedicated templates. Recently, different software for digital templating has been proposed, with improvement in pre-operative planning accuracy [42]. Table 1 summarizes the current pre-operative planning.

Table 1.
Summary of information available to the surgeons with standard pre-operative planning for primary TKA.
However, some primary TKA can be defined as “complex”, due to severe ligament deficiency, post-traumatic bone losses, extra-articular deformities and comorbidities (e.g., poliomyelitis) [37]. In these cases, an accurate pre-operative planning can be challenging, so different authors proposed using a Patient Specific Instrumentation (PSI) to improve implant positioning and outcomes [43,44]. However, PSI should be reserved to complex primary TKA to improve its cost-effectiveness [45].
There may be another option to simplify pre-operative planning and visualization in primary TKA: a 3D-printed knee model for pre-operative planning, as demonstrated by the following case.
3.3. Clinical Case: Primary TKA in Poliomyelitis
A 58-year-old female patient suffered of poliomyelitis in her childhood, with subsequent left lower limb muscular deficiency. She came to our attention complaining about a left knee pain. The patient was a small lady, 150 cm in height, 47 kg in weight, with a Body mass Index (BMI) of 21 kg/m2. Due to poliomyelitis, she underwent three different surgeries for lower limb elongation and a hindfoot fusion. She was diagnosed with left knee osteoarthritis, and, after failure of initial conservative treatment, she was recommended for TKA. Figure 1 shows pre-operative X-rays.
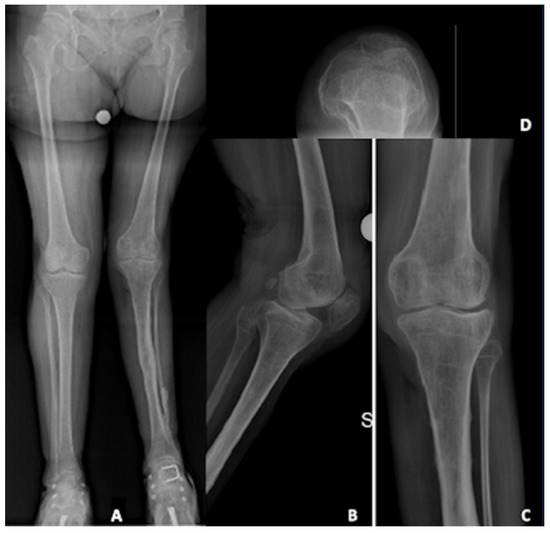
Figure 1.
Pre-operative X-rays evaluation: (A) long-leg weight-bearing view; (B) lateral view; (C) antero-posterior view; (D) patellar view.
At the clinical evaluation, the patient was able to fully extend the knee, with a flexion limited to 100° due to pain. There was a partially reducible valgus deformity, with severe lower limb muscular deficiency, a positive valgus stress and recurvatum and valgus thrust during gait. Furthermore, there was a mild-to-moderate extension lag mainly due to quadricep muscle deficiency.
The main difficulties to be considered approaching this case were: (1) ligamentous deficiency with possible need for high constrain; (2) issues related to poliomyelitis, such as bone deformities, ligament laxities, recurvatum and quadricep deficiency [46]; and (3) the patient’s small size, as detected with a digital templating. The patient’s size was particularly worrying in this case, because digital templating already demonstrated very small distal femur and proximal tibia, which were not compatible with some available implants.
For these reasons, a 3D-printed model, based on a CT scan, was produced by Medics® (Turin, Italy). In this case, as shown in Figure 2A, available data on the femur were Posterior Condylar Offset (PCO = 28.7 mm), medio-lateral femur diameter (59.8 mm), antero-posterior medial (44.2 mm) and lateral (64.6 mm) diameter and distal and proximal medullary canal diameter (distal 15.3 mm × 29.1 mm, proximal 12.08 mm × 17.4 mm). On the tibial side (Figure 2B), available data were antero-posterior axis (36.9 mm), medio-lateral axis (53.2 mm), tibial slope (9.6°) and distal and proximal medullary canal diameter (distal 9.7 mm × 15.4 mm, proximal 18.7 mm × 30.5 mm).
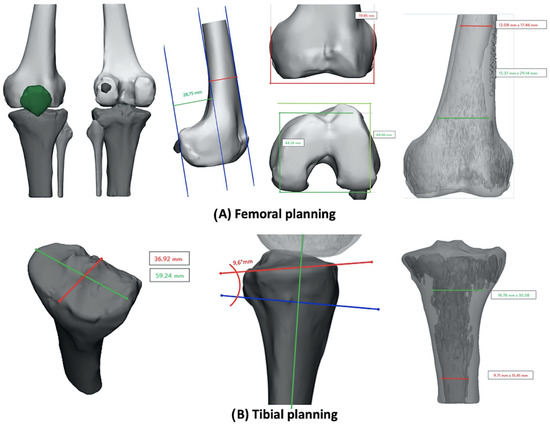
Figure 2.
Pre-operative planning based on 3D-printed model: (A) femoral measurements; (B) tibial measurements.
A bone thickness analysis was also included (Figure 3), revealing poor bone thickness in the distal femur (on both condyles, more on medial than on lateral condyle) and in the medial proximal tibia. In Figure 3, red zones correspond to 0.1 mm thickness, while green zones correspond to a maximum thickness of 6 mm.
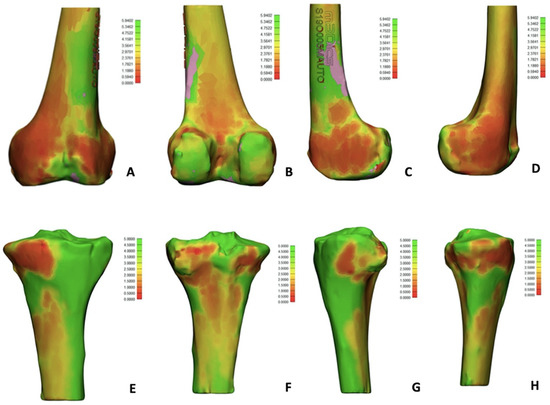
Figure 3.
Bone thickness evaluation: red zones correspond to lower thickness (0.1 mm) while green zones correspond to higher thickness (6 mm). (A) Anterior femoral side; (B) posterior femoral side; (C) medial femoral side; (D) lateral femoral side; (E) anterior tibial side; (F) posterior tibial side; (G) medial tibial side; (H) lateral femoral side.
In this case, the 3D-printed model turned out to be extremely useful to plan the surgery. Considering knee dimension and the need for a constraint, the only implant that could fit these characteristics was the extra-small size of Endomodell® (Waldemar Link GmbH, Hamburg, Germany), which guaranteed both the right level of constraint and a very small dimension. With all this information, an accurate pre-operative planning, sizing, and surgery simulation was performed (Figure 4).

Figure 4.
Three-dimensionally-printed model for pre-operative planning and implant size evaluation. (A) Comparison between tibial component size and tibial bone model; (B) comparison between femoral component size and femoral bone model.
Post-operative X-rays for this patient are shown in Figure 5.
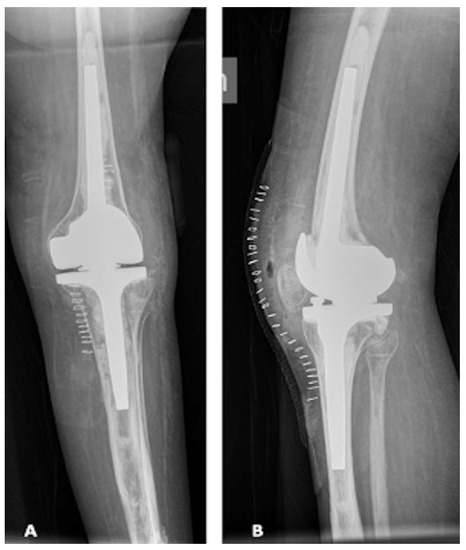
Figure 5.
Post-operative X-ray. (A) Antero-posterior view; (B) lateral view.
Six months after surgery, the patient was satisfied with complete ROM, stable knee, no pain and no sign of loosening at the X-rays.
3.4. Application of 3D-Printed Models in Pre-Operative Planning of Revision Total Knee Arthroplasty
The goals of revision TKA are the same as for primary TKA, but there are often more challenges to face. In some cases, it may be difficult to obtain good stability, treat bone loss, achieve good fixation and restore knee kinematic at the same time. An accurate pre-operative planning is mandatory in revision TKA to correctly address all these aspects. The first step in pre-operative planning for revision TKA is to make a diagnosis. Firstly, it is mandatory to rule out infection [47]. Recently, the new International Consensus Meeting (ICM) of Philadelphia has been published, with a new algorithm for periprosthetic Joint Infection (PJI) diagnosis [48]. A patient’s history and careful and complete lower limb clinical evaluation are mandatory to identify the cause of failure [47]. A complete X-ray evaluation should be obtained, including long-leg weight bearing view, AP and LL weight bearing view and patellar view [47]. X-rays can provide a lot of information, including implant position, bone loss, implant alignment, patellar height and morphology, presence of cement and implant size. Particularly, bone loss should be accurately evaluated pre-operatively, and, in some cases, a Computer Tomography (CT) scan can be useful to better evaluate bone loss amount and location and to determine how to treat it, even if it can be difficult to visualize due to metal artifacts [49,50].
However, not only the amount of bone loss, but also the quality of the remaining bone should be evaluated to predict which treatment choice should best achieve a good fixation in at least two out of three zones as described by Morgan-Jones (epiphysis, metaphysis and diaphysis) [51], as well as bone filling [52].
Standard pre-operative planning still has and important role in evaluating implant size, need for stems, augments, cones or sleeve and correct component positioning. Digital planning may help in terms of accuracy and to better understand the relationship between the different revision implant components. Table 2 summarizes key points for pre-operative planning in revision TKA.

Table 2.
Summary of information available to the surgeons with standard pre-operative planning for revision TKA.
However, 3D-printed models, obtained from CT scans, can be useful in the pre-operative planning, particularly in complex revision cases, in which bone loss can be a huge issue. The usefulness of 3D-printed models in pre-operative planning was previously published only in revision THA, while there are no studies regarding revision TKA [53].
The following clinical case will show how 3D knee models can be used in pre-operative setting of complex revision TKA.
3.5. Clinical Case: Complex Revision TKA
A female patient, 66 years-old, slightly overweight (BMI 27 kg/m2) and without significant comorbidities, underwent right and left revision TKA for aseptic loosening with a rotating hinge implant. A couple of years later, she developed progressive left knee pain, in which an Endomodell® (Waldemar Link GmbH, Hamburg, Germany) rotating hinge was implanted. The pain was mainly located at the tibial side. At the clinical evaluation, there was one single anterior scar, no signs of infection, ROM was 0–100°, tibial pain was evocated during palpation and the knee was stable. PJI was firstly ruled out with negative lab test and negative white blood cells (WBC) and polymorphonucleate (PMN%) count in the synovial liquid.
Standard X-rays demonstrated a varus lower limb alignment, tibial aseptic loosening, previous augments on the tibial side, full stem cementation, acceptable patellar height and a huge bone loss was expected, especially on the tibial side. A CT scan was also performed, and bone loss was confirmed on the tibial side, even if it was difficult to evaluate due to the presence of multiple artifacts. Figure 6 shows pre-operative X-rays and CT scan.
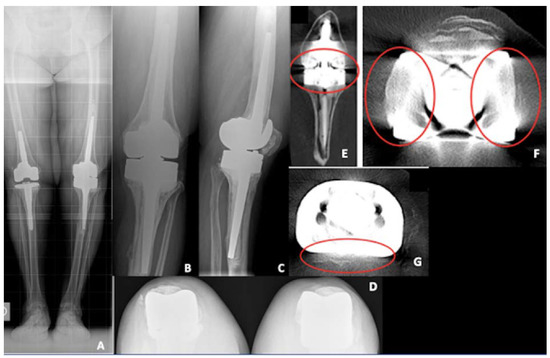
Figure 6.
Pre-operative X-rays of the re-revision TKA case. (A) Long-leg weight bearing view; (B) antero-posterior view; (C) lateral view; (D) patellar view; (E) coronal view of a computer tomography (CT) scan demonstrating tibial bone loss (red circle); (F) coronal view of a CT scan demonstrating femoral artifacts (red circle); (G) axial view of a CT scan demonstrating tibial artifacts (red circle).
Pre-operative planning was performed as usual using a digital template, including implant (size and position), stem (size, type, and position), augments and cones for both bone loss filling and implant fixation.
The predictable difficulties in this re-revision TKA were removal of the implant (rotating hinge), removal of the cement (full stem cementation), bone loss treatment, obtaining a good implant fixation considering predictable poor bone quality, and restoring tibial joint line and correct patellar height. Considering all these difficulties, a 3D-printed model was obtained from the CT scan (Medics®, Turin, Italy). The surgeon chose to print implant and cement together in the 3D model of the tibial component, and to isolate bone loss in the tibia, which was the main concern. Figure 7 shows the 3D-printed model.
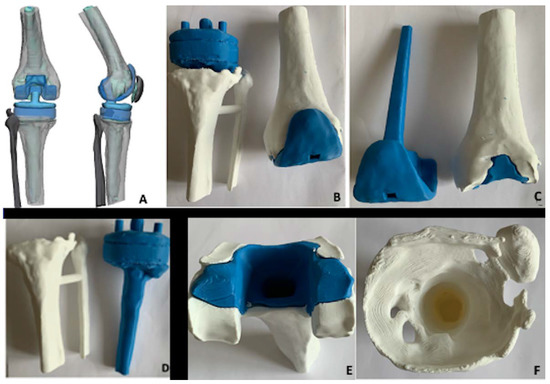
Figure 7.
Three-dimensionally-printed model of the case. (A) Image by the company (grey bone, blue implant, light blue cement); (B) 3D model femoral and tibial component; (C) 3D femoral model with implant separated from bone-cement; (D) 3D tibial model with implant separated from bone without cement; (E) 3D femoral model showing bone (white) and cement (blue); (F) 3D tibial model showing tibial bone loss.
In the provided document, different information was collected, such as femoral to tibial gap (26 mm); femoral component lateral positioning (9.3° flexion); mediolateral (ML) and antero-posterior (AP) diameter for the femoral side, both with and without cement, to better evaluate implant size (ML 67.6 mm, AP medial 47.5 mm, AP lateral 49.6 mm); ML and AP diameter for the tibial side (respectively, 72.2 mm and 54.13 mm); and intramedullary canal diameter both on femoral and tibial side (respectively, distal diameter 16.2 mm × 20.1 mm and 15.7 mm × 17.8 mm, proximal diameter 19.5 mm × 28.4 mm and 26.4 mm × 27.6 mm). All these features are shown in Figure 8.
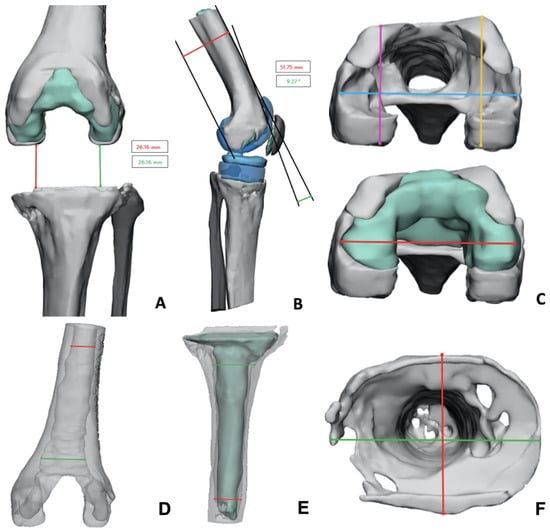
Figure 8.
Data provided with the 3D-printed model. (A) Femoral-tibial gap (red and green line); (B) femoral component lateral positioning; (C) femoral diameters (mediolateral diameter for cement red line, mediolateral diameter for bone light blue line, antero-posterior medial diameter purple line, antero-posterior lateral orange line); (D) intramedullary femoral canal diameter (distal red line, proximal green line); (E) intramedullary tibial canal diameter (distal red line, proximal green line); (F) tibial bone loss and diameter evaluation (mediolateral green line, anteroposterior red line).
Similar to the primary TKA 3D-printed model, in this case, the manufacturer provided a bone thickness evaluation (minimum thickness = red, maximum thickness = green). On the femoral size, the bone was very thin in the anterior and posterior femoral cortex (about 0.86 mm), while distal femoral condyles showed good bone thickness. On the tibial side, basically all the metaphyseal and proximal diaphysis were extremely thin (around 0.62 mm), except for the anterior tibial cortex (10 mm thickness). Figure 9 showed these results.
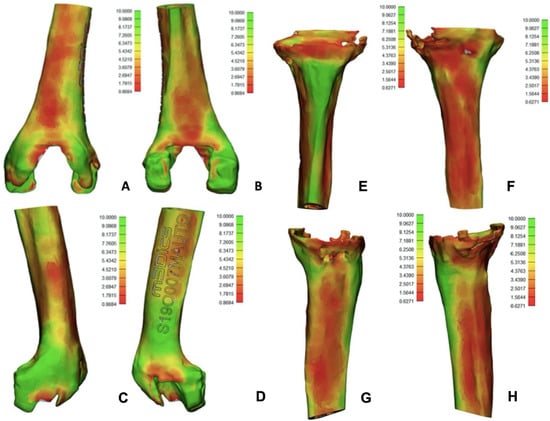
Figure 9.
Data provided with the 3D model regarding bone thickness (low thickness red color, high thickness green color). (A) Anterior femur; (B) posterior femur; (C) medial femur; (D) lateral femur. Al the femoral images showed low bone thickness at the femoral cortex. (E) anterior tibia; (F) posterior tibia; (G) medial tibia; (H) lateral tibia. All tibial images show poor bone thickness except for anterior tibial cortex.
With these data on both bone morphology, bone loss and bone thickness, a careful surgery simulation was performed, including size of cone (used both to fill tibial bone loss and to achieve a good fixation in the metaphysis) and zones of safe position, without damaging femoral or tibial cortex, as well as size of the chosen implant (Endomodel®, Waldemar Link GmbH, Hamburg, Germany). Figure 10 shows X-rays one year after surgery, with good implant fixation, no signs of loosening in a satisfied patient.
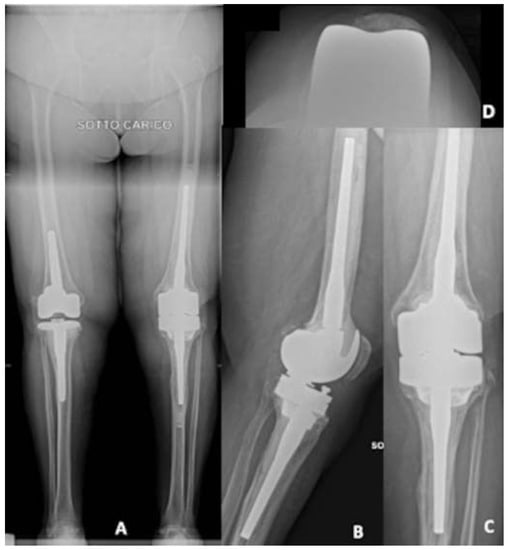
Figure 10.
X-rays one year after surgery. (A) Long-leg weight bearing view; (B) lateral view; (C) antero-posterior view, (D) patellar view.
4. Discussion
A 3D-printed knee model may add different advantages to standard digital templating in both primary and revision TKA. First, it allows for direct visualization of the knee, including bone defect and neurovascular structures, which can be reproduced with the 3D printing. This feature may turn out to be extremely useful in certain primary setting, such as poliomyelitis sequalae. Furthermore, surgeons can decide to print the entire lower limb, to better visualize extra-articular deformities in three dimensions, including rotational deformities, which may be difficult to be visualized with standard imaging techniques. The real dimensions of the knee can also be directly experienced with the 3D model, allowing the surgeon for a precise implant sizing. Lastly, the surgeon can simulate implant positioning in the 3D model pre-operatively, with a good accuracy compared to the surgery. All these advantages were also previously described for complex primary THA [29]. However, in both primary and revision TKA, 3D printed models cannot replace a careful clinical evaluation and complete radiological examination, which are mandatory and give different information, such as degree of constraint needed, need for extensive approach, coronal and sagittal alignment and many others in both primary and revision TKA.
In the revision setting, 3D printing can add more information to the standard pre-operative planning. For example, the bone and implant can be printed separately for a better evaluation of bone losses. Furthermore, compared to CT scans, 3D-printed models have no artifacts due to images manipulation, allowing again for a better bone loss evaluation. As well as in primary cases, the 3D-printed model can be used to “simulate” the surgery, and it allows a better visualization of the relationship between stem, augments, and cones or sleeves. In some implants, the internal diameter of cones is independent from the size, and it can match only a few stems’ diameter. If augments are added in the epiphyseal zone, the relationship between tibial component and cone or sleeve changes causing, in some cases, an incompatibility between presence of both augments, stems and cones/sleeves. Furthermore, if an offset stem is used, it may not fit into the cone because the diameter is bigger due to the offset. All these aspects can be only partially evaluated on digital templating, while a 3D-printed model may help in visualizing them.
Finally, as previously described, some 3D printing companies also allow for a bone thickness evaluation. This information cannot be fully obtained from CT scan due to artifacts, and it may be useful in pre-operative setting, especially if a cone or sleeve must be implanted, because it may conflict with the anterior, posterior, medial or lateral cortex, with subsequent risk of intra-operative fracture.
Three-dimensionally-printed models are pretty “low cost” compared to other technologies possibly used in primary or revision TKA, such as PSI: a model costs between EUR500 and EUR1000, depending on its complexity. Obviously, PSI and other similar technologies have different advantages compared to 3D-printed models, but the latter seems to have a good cost-effectiveness in pre-operative planning of complex cases.
It must be mentioned that manufacturers involved in 3D-printed models are studying new solutions, including bone quality evaluation through Bone Mineral Density (BMD). Some of them can define bone “quantity”, intended as bone thickness, as shown in the cases, and reproduce it with a colored scale. This feature can add new information, and the surgeon may be able to know pre-operatively where the risk of intra-operative fractures is very high.
This study has different limitations. First, it is a narrative review of the available literature on this emerging technology. Considering the heterogeneity of the study and the anatomical districts involved, a more systematic approach with a meta-analysis of data was not feasible. Furthermore, only two cases were reported. More cases have been performed with this technology in our hospital, but collecting concrete data of the advantages compared to standard planning (i.e., accuracy of implant size and position) would be very hard due to the complexity and variety of surgeries. For this reason, the authors decided to just describe the usefulness of the 3D printed models to give the reader a first sight into this new application of this relatively new technology. However, more data has to be collected with a more systematic approach to better evaluate cost-effectiveness of this technology in primary and revision TKA.
5. Conclusions
Three-dimensional printing technology is gaining popularity in different orthopedic fields. Three-dimensional models are successfully used for pre-operative planning in spine surgery, oncology, trauma (especially acetabular fracture) and hip replacement/revision. In all these cases, 3D models were reported to help surgeons to better visualize anatomy, fracture morphology and to simulate the reconstruction. Three-dimensional printing technology is also involved in development of PSI, which can be used for hip or knee replacement.
However, there is no literature regarding the application of 3D-printed models in pre-operative planning for complex primary or revision TKA. In these cases, 3D-printed models may be useful to better visualize the deformity, to accurately plan implant size and positioning and to simulate the surgery in complex cases. Furthermore, especially in complex revision TKA, a 3D model may help surgeons to better visualize bone losses, and to evaluate the compatibility between different systems used, such as augments, cones, sleeves and stems. Some manufacturers can provide surgeons also with further information regarding bone thickness, which may be useful to pre-operatively know which zones are at risk of fracture during surgery.
In conclusion, 3D-printed models may be a useful tool in pre-operative setting of complex primary and revision TKA, also considering the relative low cost compared to other technologies, but it cannot replace a careful clinical and radiological evaluation. However, more studies will be useful to better evaluate clinical advantages of this relatively new technology.
Author Contributions
Conceptualization: F.R. and R.R.; Methodology: F.R. and U.C.; Investigation: F.R. and U.C.; Writing—original draft preparation: F.R. and D.E.B.; Writing—review and editing: F.D., M.B. and D.E.B.; Formal analysis: R.R., M.B. and F.D.; Supervision: R.R. and D.E.B.; Project administration roles: R.R. and D.E.B. All authors have read and agreed to the published version of the manuscript.
Funding
No funding for this manuscript has been requested.
Institutional Review Board Statement
This is a narrative review, so no IRB approval is needed. However, it includes two clinical cases. Considering that patients underwent the same pre-operative evaluation to all other patients (including a CT scan for 3D printed model), and the only difference was that a 3D printed model of the involved knee has been produced, the Institutional Board consider this study “exempt from IRB approval”.
Informed Consent Statement
All the patients included in the study gave verbal and written consent to the surgery and to use images of it. No further consent was needed.
Data Availability Statement
Data available on request due to restrictions eg privacy or ethical. The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical reasons.
Conflicts of Interest
R.R., MD, is paid teaching consultant for Arthrex, Zimmer-Biomet, Lima, Medacta, Smith and Nephew, DePuy. D.B., MD, is a paid teaching consultant for Arthrex and Zimmer-Biomet, received editorial royalties for Elsevier and Springer and he is part of the editorial board “The Knee” journal, Elsevier. M.B. is a Teaching consultant and marketing consultant for Johnson & Johnson. The other authors have no financial or no-financial conflict of interest.
References
- Shah, D.; Naik, L.; Paunipagar, B.; Rasalkar, D.; Chaudhary, K.; Bagaria, V. Setting Up 3D Printing Services for Orthopaedic Applications: A Step-by-Step Guide and an Overview of 3DBioSphere. Indian J. Orthop. 2020, 54, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Bagaria, V.; Bhansali, R.; Pawar, P. 3D printing-creating a blueprint for the future of orthopedics: Current concept review and the road ahead! J. Clin. Orthop. Trauma 2018, 9, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Edelmers, E.; Kazoka, D.; Pilmane, M. Creation of Anatomically Correct and Optimized for 3D Printing Human Bones Models. Appl. Syst. Innov. 2021, 4, 67. [Google Scholar] [CrossRef]
- Edelmers, E.; Kazoka, D.; Bolocko, K.; Pilmane, M. Different Techniques of Creating Bone Digital 3D Models from Natural Specimens. Appl. Syst. Innov. 2022, 5, 85. [Google Scholar] [CrossRef]
- Vaishya, R.; Patralekh, M.K.; Vaish, A.; Agarwal, A.K.; Vijay, V. Publication trends and knowledge mapping in 3D printing in orthopaedics. J. Clin. Orthop. Trauma 2018, 9, 194–201. [Google Scholar] [CrossRef]
- Parthasarathy, J.; Krishnamurthy, R.; Ostendorf, A.; Shinoka, T.; Krishnamurthy, R. 3D printing with MRI in pediatric applications. J. Magn. Reson. Imaging 2020, 51, 1641–1658. [Google Scholar] [CrossRef]
- Wong, T.M.; Jin, J.; Lau, T.W.; Fang, C.; Yan, C.H.; Yeung, K.; To, M.; Leung, F. The use of three-dimensional printing technology in orthopaedic surgery. J. Orthop. Surg. 2017, 25, 2309499016684077. [Google Scholar] [CrossRef]
- Garg, B.; Mehta, N. Current status of 3D printing in spine surgery. J. Clin. Orthop. Trauma 2018, 9, 218–225. [Google Scholar] [CrossRef]
- Skelley, N.W.; Smith, M.J.; Ma, R.; Cook, J.L. Three-dimensional Printing Technology in Orthopaedics. J. Am. Acad. Orthop. Surg. 2019, 27, 918–925. [Google Scholar] [CrossRef]
- Campana, V.; Cardona, V.; Vismara, V.; Monteleone, A.S.; Piazza, P.; Messinese, P.; Mocini, F.; Sircana, G.; Maccauro, G.; Saccomanno, M.F. 3D printing in shoulder surgery. Orthop. Rev. 2020, 12, 8681. [Google Scholar] [CrossRef]
- Iannotti, J.; Baker, J.; Rodriguez, E.; Brems, J.; Ricchetti, E.; Mesiha, M.; Bryan, J. Three-Dimensional Preoperative Planning Software and a Novel Information Transfer Technology Improve Glenoid Component Positioning. J. Bone Jt. Surg. 2014, 96, 71. [Google Scholar] [CrossRef]
- Walch, G.; Vezeridis, P.S.; Boileau, P.; Deransart, P.; Chaoui, J. Three-dimensional planning and use of patient-specific guides improve glenoid component position: An in vitro study. J. Shoulder Elb. Surg. 2015, 24, 302–309. [Google Scholar] [CrossRef]
- Willemsen, K.; Berendes, T.D.; Geurkink, T.; Bleys, R.L.; Leeflang, M.A.; Weinans, H.; Castelein, R.M.; Nelissen, R.G.; van der Wal, B.C. A Novel Treatment for Anterior Shoulder Instability. J. Bone Jt. Surg. 2019, 101, 68. [Google Scholar] [CrossRef]
- Chen, Y.; Jia, X.; Qiang, M.; Zhang, K.; Chen, S. Computer-Assisted Virtual Surgical Technology Versus Three-Dimensional Printing Technology in Preoperative Planning for Displaced Three and Four-Part Fractures of the Proximal End of the Humerus. J. Bone Jt. Surg. 2018, 100, 1960–1968. [Google Scholar] [CrossRef]
- Lal, H.; Patralekh, M.K. 3D printing and its applications in orthopaedic trauma: A technological marvel. J. Clin. Orthop. Trauma 2018, 9, 260–268. [Google Scholar] [CrossRef]
- Puglisi, G.; Montemagno, M.; Denaro, R.; Condorelli, G.; Caruso, V.F.; Vescio, A.; Testa, G.; Pavone, V. 3D-Printed Models versus CT Scan and X-Rays Imaging in the Diagnostic Evaluation of Proximal Humerus Fractures: A Triple-Blind Interobserver Reliability Comparison Study. Adv. Orthop. 2022, 2022, 5863813. [Google Scholar] [CrossRef]
- Senkoylu, A.; Daldal, I.; Cetinkaya, M. 3D printing and spine surgery. J. Orthop. Surg. 2020, 28, 2309499020927081. [Google Scholar] [CrossRef]
- Sun, Z.; Yin, M.; Sun, Y.; Cheng, M.; Fang, M.; Huang, W.; Ma, J.; Yan, W. Customized Multilevel 3D Printing Implant for Reconstructing Spine Tumor: A Retrospective Case Series Study in a Single Center. Orthop. Surg. 2022, 14, 2016–2022. [Google Scholar] [CrossRef]
- Lou, Y.; Cai, L.; Wang, C.; Tang, Q.; Pan, T.; Guo, X.; Wang, J. Comparison of traditional surgery and surgery assisted by three dimensional printing technology in the treatment of tibial plateau fractures. Int. Orthop. 2017, 41, 1875–1880. [Google Scholar] [CrossRef]
- Shen, S.; Wang, P.; Li, X.; Han, X.; Tan, H. Pre-operative simulation using a three-dimensional printing model for surgical treatment of old and complex tibial plateau fractures. Sci. Rep. 2020, 10, 6044. [Google Scholar] [CrossRef]
- Wu, W.; Xu, W.; Wan, C.; Fang, M. Preoperative Plan with 3D Printing in Internal and External Fixation for Complex Tibial Plateau Fractures. Orthop. Surg. 2019, 11, 560–568. [Google Scholar] [CrossRef]
- Dust, T.; Hartel, M.J.; Henneberg, J.-E.; Korthaus, A.; Ballhause, T.M.; Keller, J.; Ohlmeier, M.; Maas, K.-J.; Frosch, K.-H.; Krause, M. The influence of 3D printing on inter- and intrarater reliability on the classification of tibial plateau fractures. Eur. J. Trauma Emerg. Surg. 2022. [Google Scholar] [CrossRef]
- Huitema, J.M.; van der Gaast, N.; Brouwers, L.; Jaarsma, R.L.; Doornberg, J.N.; Edwards, M.J.R.; Hermans, E. Are 3D-printed Models of Tibial Plateau Fractures a Useful Addition to Understanding Fractures for Junior Surgeons? Clin. Orthop. Relat. Res. 2022, 480, 1170–1177. [Google Scholar] [CrossRef]
- Sun, S.; Xi, Y.; Shi, X.; Zhao, L.; Ma, F.; Qian, J. Study on the Application Value of CT Thin-Layer Scan Data Assisted 3D Printing Technology in Hip and Knee Replacement. J. Health Eng. 2021, 2021, 3491509. [Google Scholar] [CrossRef]
- Huang, Z.; Song, W.; Zhang, Y.; Zhang, Q.; Zhou, D.; Zhou, X.; He, Y. Three-dimensional printing model improves morphological understanding in acetabular fracture learning: A multicenter, randomized, controlled study. PLoS ONE 2018, 13, e0191328. [Google Scholar] [CrossRef]
- Ansari, S.; Barik, S.; Singh, S.K.; Sarkar, B.; Goyal, T.; Kalia, R.B. Role of 3D printing in the management of complex acetabular fractures: A comparative study. Eur. J. Trauma Emerg. Surg. 2020, 47, 1291–1296. [Google Scholar] [CrossRef]
- Wang, P.; Kandemir, U.; Zhang, B.; Fei, C.; Zhuang, Y.; Zhang, K. The effect of new preoperative preparation method compared to conventional method in complex acetabular fractures: Minimum 2-year follow-up. Arch. Orthop. Trauma Surg. 2020, 141, 215–222. [Google Scholar] [CrossRef]
- Morgan, C.; Khatri, C.; A Hanna, S.; Ashrafian, H.; Sarraf, K.M. Use of three-dimensional printing in preoperative planning in orthopaedic trauma surgery: A systematic review and meta-analysis. World J. Orthop. 2020, 11, 57–67. [Google Scholar] [CrossRef]
- Woo, S.-H.; Sung, M.-J.; Park, K.-S.; Yoon, T.-R. Three-dimensional-printing Technology in Hip and Pelvic Surgery: Current Landscape. Hip Pelvis 2020, 32, 1–10. [Google Scholar] [CrossRef]
- Yan, L.; Wang, P.; Zhou, H. 3D Printing Navigation Template Used in Total Hip Arthroplasty for Developmental Dysplasia of the Hip. Indian J. Orthop. 2020, 54, 856–862. [Google Scholar] [CrossRef]
- Xiao, C.; Zhang, S.; Gao, Z.; Tu, C. Custom-made 3D-printed porous metal acetabular composite component in revision hip arthroplasty with Paprosky type III acetabular defects: A case report. Technol. Health Care 2022, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.; Chua, C.; Chen, Y.; Murphy, D.; O’Neill, G. Utility of 3D printed models as adjunct in acetabular fracture teaching for Orthopaedic trainees. BMC Med. Educ. 2022, 22, 595. [Google Scholar] [CrossRef] [PubMed]
- Xia, R.; Zhai, Z.; Chang, Y.; Li, H. Clinical Applications of 3-Dimensional Printing Technology in Hip Joint. Orthop. Surg. 2019, 11, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Okolie, O.; Stachurek, I.; Kandasubramanian, B.; Njuguna, J. 3D Printing for Hip Implant Applications: A Review. Polymers 2020, 12, 2682. [Google Scholar] [CrossRef]
- Kim, H.-J.; Park, J.; Shin, J.-Y.; Park, I.-H.; Park, K.-H.; Kyung, H.-S. More accurate correction can be obtained using a three-dimensional printed model in open-wedge high tibial osteotomy. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 3452–3458. [Google Scholar] [CrossRef]
- Kim, H.-J.; Park, J.; Park, K.-H.; Park, I.-H.; Jang, J.-A.; Shin, J.-Y.; Kyung, H.-S. Evaluation of Accuracy of a Three-Dimensional Printed Model in Open-Wedge High Tibial Osteotomy. J. Knee Surg. 2018, 32, 841–846. [Google Scholar] [CrossRef]
- Tanzer, M.; Makhdom, A.M. Preoperative Planning in Primary Total Knee Arthroplasty. J. Am. Acad. Orthop. Surg. 2016, 24, 220–230. [Google Scholar] [CrossRef]
- Rossi, R.; Cottino, U.; Bruzzone, M.; Dettoni, F.; Bonasia, D.E.; Rosso, F. Total knee arthroplasty in the varus knee: Tips and tricks. Int. Orthop. 2019, 43, 151–158. [Google Scholar] [CrossRef]
- Rossi, R.; Rosso, F.; Cottino, U.; Dettoni, F.; Bonasia, D.E.; Bruzzone, M. Total knee arthroplasty in the valgus knee. Int. Orthop. 2013, 38, 273–283. [Google Scholar] [CrossRef]
- Mullaji, A.; Shetty, G.M. Persistent Hindfoot Valgus Causes Lateral Deviation of Weightbearing Axis after Total Knee Arthroplasty. Clin. Orthop. Relat. Res. 2011, 469, 1154–1160. [Google Scholar] [CrossRef]
- Davis, J.A.; Hogan, C.; Dayton, M. Postoperative Coronal Alignment After Total Knee Arthroplasty: Does Tailoring the Femoral Valgus Cut Angle Really Matter? J. Arthroplast. 2015, 30, 1444–1448. [Google Scholar] [CrossRef]
- Kniesel, B.; Konstantinidis, L.; Hirschmüller, A.; Südkamp, N.; Helwig, P. Digital templating in total knee and hip replacement: An analysis of planning accuracy. Int. Orthop. 2013, 38, 733–739. [Google Scholar] [CrossRef]
- Gaukel, S.; Vuille-Dit-Bille, R.N.; Schläppi, M.; Koch, P.P. CT-based patient-specific instrumentation for total knee arthroplasty in over 700 cases: Single-use instruments are as accurate as standard instruments. Knee Surg. Sports Traumatol. Arthrosc. 2020, 30, 447–455. [Google Scholar] [CrossRef]
- Thomas, S.; Patel, A.; Patrick, C.; Delhougne, G. Total Hospital Costs and Readmission Rate of Patient-Specific Instrument in Total Knee Arthroplasty Patients. J. Knee Surg. 2020, 35, 113–121. [Google Scholar] [CrossRef]
- Teeter, M.G.; Marsh, J.D.; Howard, J.L.; Yuan, X.; Vasarhelyi, E.M.; McCalden, R.W.; Naudie, D.D.R. A randomized controlled trial investigating the value of patient-specific instrumentation for total knee arthroplasty in the Canadian healthcare system. Bone Jt. J. 2019, 101-B, 565–572. [Google Scholar] [CrossRef]
- Prasad, A.; Donovan, R.; Ramachandran, M.; Dawson-Bowling, S.; Millington, S.; Bhumbra, R.; Achan, P.; Hanna, S.A. Outcome of total knee arthroplasty in patients with poliomyelitis. EFORT Open Rev. 2018, 3, 358–362. [Google Scholar] [CrossRef]
- Cottino, U.; Rosso, F.; Pastrone, A.; Dettoni, F.; Rossi, R.; Bruzzone, M. Painful knee arthroplasty: Current practice. Curr. Rev. Musculoskelet. Med. 2015, 8, 398–406. [Google Scholar] [CrossRef]
- Parvizi, J.; Tan, T.L.; Goswami, K.; Higuera, C.; Della Valle, C.; Chen, A.F.; Shohat, N. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J. Arthroplast. 2018, 33, 1309–1314. [Google Scholar] [CrossRef]
- Lei, P.-F.; Hu, R.-Y.; Hu, Y.-H. Bone Defects in Revision Total Knee Arthroplasty and Management. Orthop. Surg. 2019, 11, 15–24. [Google Scholar] [CrossRef]
- Sheth, N.P.; Bonadio, M.B.; Demange, M. Bone Loss in Revision Total Knee Arthroplasty. J. Am. Acad. Orthop. Surg. 2017, 25, 348–357. [Google Scholar] [CrossRef]
- Morgan-Jones, R.; Oussedik, S.I.S.; Graichen, H.; Haddad, F.S. Zonal fixation in revision total knee arthroplasty. Bone Jt. J. 2015, 97-B, 147–149. [Google Scholar] [CrossRef]
- Rosso, F.; Cottino, U.; Dettoni, F.; Bruzzone, M.; Bonasia, D.E.; Rossi, R. Revision total knee arthroplasty (TKA): Mid-term outcomes and bone loss/quality evaluation and treatment. J. Orthop. Surg. Res. 2019, 14, 280. [Google Scholar] [CrossRef]
- Kavalerskiy, G.M.; Murylev, V.Y.; Rukin, Y.A.; Elizarov, P.M.; Lychagin, A.V.; Tselisheva, E.Y. Three-Dimensional Models in Planning of Revision Hip Arthroplasty with Complex Acetabular Defects. Indian J. Orthop. 2018, 52, 625–630. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).