Abstract
Screening new Clostridium strains that can efficiently utilize lignocellulose to produce hydrogen is extremely important for dark fermentative hydrogen production. In this study, a mesophilic hydrogen-producing bacterium, identified as Clostridium populeti FZ10, was newly isolated from compost acclimated by microcrystalline cellulose. The strain could produce hydrogen from various cellulosic substrates. The performances of hydrogen production from microcrystalline cellulose (MCC) and corn stalk (CS) were especially investigated. The maximum hydrogen yield and hydrogen production rate from MCC were 177.5 ± 4.8 mL/g and 7.7 ± 0.2 mL·g−1·h−1, respectively. Furthermore, scanning electron microscopy (SEM) images showed that the structure of CS was destroyed after fermentation, which could be attributed to the presence of exoglucanase, endoglucanase, β-glucosidase and xylanase produced by Clostridium populeti FZ10. The maximum hydrogen yield and hydrogen production rate from CS were 92.5 ± 3.7 mL/g and 5.9 ± 0.2 mL·g−1·h−1,respectively, with a cellulose degradation of 47.2 ± 2.3% and a hemicellulose degradation of 58.1 ± 2.0%. This study demonstrates that Clostridium populeti FZ10 is an ideal candidate for directly converting lignocellulose into biohydrogen under mesophilic conditions. The discovery of strain C. populeti FZ10 has special significance in the field of bioenergy.
1. Introduction
With the advent of the new energy economy, hydrogen has been considered a promising alternative energy source due to its cleanliness and renewability [1,2,3]. Compared with the conventional hydrogen-producing processes (thermochemical and electrochemical), the biological process for hydrogen production through dark fermentation has shown many advantages, such as being more environmentally friendly, less energy intensive and having a wide range of substrates [4,5].
Lignocellulosic biomass is considered an attractive material for hydrogen production, owing to its abundance and renewability on earth. Unfortunately, hydrogen-fermentative microbes cannot utilize lignocellulose effectively because of its highly polymeric structure and hard biodegradability [6,7]. Hence, several pretreatment methods are performed to break down the lignocellulosic biomass into soluble sugar, which is easily utilized by hydrogen-fermentative bacteria. Pretreatment processes can be classified as chemical, physical and biological, etc. [8,9,10,11]. During pretreatment processes, there are disadvantages that should not be ignored, such as equipment corrosion, high energy consumption, the generation of microbial growth inhibitors, the time-consuming nature of the process, etc. Undoubtedly, these processes will lead to higher costs and block the commercialization of hydrogen production from cellulose-based biomass.
Microorganisms play a pivotal role in the hydrogen fermentation system. During the hydrogen fermentation process, specific microbes could produce enzymes such as endoglucanase, exoglucanase, cellobiohydrolase, xylanase, β-xylosidases, etc., which are responsible for complex substrate hydrolysis and converting hydrolysates into biohydrogen [12,13,14]. This integrated process is more economical for hydrogen production from lignocellulose [15]. As a proficient hydrogen producer, the genus Clostridium has been widely researched in dark fermentative hydrogen production because of its high hydrogen productivity, wide range of substrate utilization and high resistance to adverse conditions [16,17,18]. Moreover, the Clostridium species is usually the dominant hydrogen-producing genus which exists in anaerobic mixed cultures [19]. Some species of Clostridium were found to be capable of producing hydrogen from cellulosic substrates [20,21,22,23]. However, most Clostridium species seem to prefer oligosaccharides for hydrogen production rather than lignocellulose, and several disadvantages such as a low hydrogen yield and substrate conversion efficiency limit their application. Lo et al. isolated a mesophilic Clostridium which could effectively utilize sucrose and xylose to produce hydrogen, and the hydrogen yield reached 129.1 mL/g and 172.9 mL/g, respectively; however, the strain could not directly utilize cellulose to produce hydrogen [24]. Magnusson et al. found a thermophilic Clostridium that also faced the same problem of low hydrogen yield from cellulosic biomasses. The maximum hydrogen yield of this strain from grain was only 23.97 mL/g [25]. Fermentative hydrogen production using lignocellulose as a substrate can effectively reduce the production cost. This will lead to the further development of hydrogen production from renewable resources. Although much work has been undertaken to obtain hydrogen from dark fermentation processes, the hydrogen yield produced by the Clostridium species from cellulosic-based biomasses is still considered to be insufficiently high. In view of this point, screening new Clostridium strains that can efficiently utilize natural lignocellulose to produce hydrogen is of value.
If hydrogen-producing Clostridium could efficiently convert cellulosic substrates into hydrogen, which could then be isolated through microbial acclimation, the limitation stated above would be overcome. In the present research, a mesophilic hydrogen-producing strain was newly isolated from an anaerobic bioreactor and was acclimated from cow dung compost with microcrystalline cellulose as the substrate. The isolated strain could directly convert various cellulosic substrates into hydrogen. To assess the hydrogen production ability of the new strain, the hydrogen production performances of microcrystalline cellulose and corn stalk were investigated in a batch culture.
2. Materials and Methods
2.1. Seed Microorganism
The seed microorganism used during this study was isolated from cow dung compost. The compost was obtained from a dairy in Xinzheng, China. Prior to use, the compost was pretreated with 1.5 min of microwave irradiation to enrich the hydrogen-producing, spore-forming bacteria [26].
2.2. Microbial Acclimation
Microbial acclimation was conducted in a 1.5 L anaerobic bioreactor with microcrystalline cellulose (MCC) as the sole carbon source, 4.0 g/L NH4HCO3 as the nitrogen source, 1.0 g/L KH2PO4 as the medium pH buffer and an initial pH of 7.0 at 35 ± 1 °C. During the acclimation process, MCC concentration increased from 0.5 to 10 g/L along with the stable hydrogen that was to be obtained constantly. At the end of each round of acclimation, half the working volume of the reactor was replaced with fresh culture medium. The process of acclimation was performed for at least 30 rounds until the color of MCC changed from white to faint yellow, the culture period was reduced from 7 days to 3 days and a high hydrogen yield was detected.
2.3. Strain Isolation and Identification
A hydrogen-producing bacterium was isolated from the hydrogen-producing bioreactor, which was feeding the acclimated compost and using microcrystalline cellulose as the carbon source. A basal medium (initial pH 7.0) was used to isolate the bacteria, and the chemical composition of the basal medium is shown in Table 1. The obtained colonies were re-streaked multiple times on the fresh, solid basal medium until pure isolated colonies were obtained. An anaerobic glove box was used to perform the isolation of the colonies to ensure anaerobic conditions. The well-formed single colonies were inoculated in a liquid anaerobic basal medium and then cultivated at 35 ± 1 °C in a constant-temperature oscillator (120 rpm). The purified isolates with the highest hydrogen production potential from MCC were selected for further experimental investigation.

Table 1.
Chemical composition of the basal medium.
The morphology of the isolated strain was examined with a scanning electron microscope (SEM) (Hitachi, Japan). The isolated strain was subjected to Gram staining tests following the Hucker method [27].
The genomic DNA of the isolated strain was extracted using the method described by Sambrook et al. and amplified with polymerase chain reaction [28]. A BLAST search with the GenBank database was used to analyze the 16S rDNA gene of the isolated strain [29]. The program CLUSTAL X was used to compare similar DNA sequences [30]. The program MEGA 5.0 was used to construct the phylogenetic tree, employing the neighbor-joining method [31,32,33].
2.4. Batch Hydrogen Production
The above isolated strain was precultured first in basal medium, except MCC was replaced with 10 g/L glucose. The obtained early-exponential culture broth was incubated at 10% (v/v) in the basal fermentation medium. The chemical composition of the basal fermentation medium is shown in Table 2 and Table 3. In addition, a KH2PO4-Na2HPO4 buffer (0.1 M, pH 6.98) was added as the pH buffer agent. An amount of 10 g of microcrystalline cellulose (MCC), carboxymethyl cellulose (CMC), xylan, xylose, cellobiose, glucose, corn stalk (CS), wheat straw (WS) and rice straw (RS) was used as the carbon source. Prior to use, the naturally dried CS, WS and RS were comminuted to a powder and passed through a 40-mesh sieve. Batch hydrogen fermentation was operated in 140 mL anaerobic bioreactors with working volumes of 50 mL at 35 ± 1 °C in a constant-temperature oscillator (120 rpm).

Table 2.
Chemical composition of the basal fermentation medium.

Table 3.
Chemical composition of the nutrient solution.
2.5. Analytical Methods
Hydrogen content was measured with a gas chromatograph (GC, Agilent 7890B) equipped with a thermal conductivity detector (TCD) and a Porapak Q stainless column. The temperatures of the injection port, the oven and the detector were 100 °C, 80 °C and 150 °C, respectively. Nitrogen served as the carrier gas at a flow rate of 20 mL/min. The concentrations of volatile fatty acids and alcohols were detected using another GC with a flame ionization detector (FID) and an 8 ft stainless column packed with 10% PEG-20 M and 2% H3PO4 (80/100 mesh). The temperatures of the injection port, FID detector, and oven were 220 °C, 140 °C and a programmed column temperature of 115–170 °C, respectively. Nitrogen was used as the carrier gas at a flow rate of 20 mL/min [34].
The cell density of the insoluble microcrystalline was monitored indirectly by determining the protein content of the culture [35]. The morphology of corn stalk was observed with a scanning electron microscope (SEM) (Hitachi, Japan). All samples were dried at 60 °C to a constant weight, and samples were affixed using conductive tape. In order to enhance the electric conduction of the samples, the samples were sputtered with a layer of gold. The contents of lignin, cellulose and hemicellulose in the raw materials were determined with the detergent fiber method described by Van Soest et al. [36].
Cellulase activity was measured with the filter paper assay method. The assay system had a total volume of 2 mL, consisting of 1 mL of culture supernatant, 1 mL of citrate acid buffer (0.05 M, pH 5.0) and 50 mg of Whatman filter paper No. 1, and was incubated for 60 min at 50 °C. The unit of cellulase activity was defined as the amount of enzyme that produced micromoles of reducing sugar per min. The endoglucanase, exoglucanase, β-glucosidase and xylanase were measured with the method described by Rattanachomsri et al. [37]. In brief, 1 mL of culture supernatant was added to a 25 mL test tube, containing 1.5 mL of 1.0% of the corresponding substrate in 1 mL of citrate acid buffer (0.05 M, pH 5.0). After incubation at 50 °C for 60 min, the absorbance at 540 nm was determined to confirm the content of reducing sugar. The unit of enzyme activity was presented as the quantity of enzyme that produced micromoles of reducing sugar per min.
2.6. Kinetic Modeling
Cumulative H2 production was calculated using a modified Gompertz equation:
H denotes the cumulative H2 production (mL); Rm denotes the maximum H2 production rate (mL/h); λ denotes the lag time (h); e denotes the constant of 2.718281828; and P denotes the H2 production potential (mL). The values of P, Rm and λ were estimated with Excel 14.0 software, employing a Newtonian algorithm. In this research, Rm and Ps are defined as mL·g−1-substrate·h−1 and mL/g-substrate [36].
3. Results and Discussion
3.1. Isolation and Identification of Bacterial Strain
A strain designated as FZ10 was isolated from an anaerobic fermentative hydrogen-producing bioreactor, which was inoculated with acclimated compost and used MCC as feedstock. FZ10 is a rod-shaped, Gram-negative bacterium with a size of 0.7–1.0 × 1.5–5.0 μm (Figure 1). Spores could be observed when the environment became unfavorable. The 16S rDNA gene sequence of 1490 bp was compared with other sequences with a BLAST search of the GenBank data. The results showed that FZ10 had a 99% homology with Clostridium populeti (X71853) (Figure 2). A phylogenetic tree for FZ10 with the most closely related taxonomic species was constructed. Based on its morphology and physiological characters, the strain FZ10 was identified as Clostridium populeti FZ10. Furthermore, the gene sequence of C. populeti FZ10 was submitted to the GenBank with the accession no. KT278845.
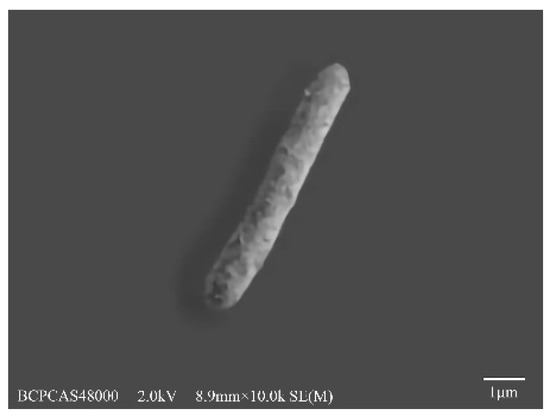
Figure 1.
Scanning electron microscopy (SEM) image of strain FZ10.
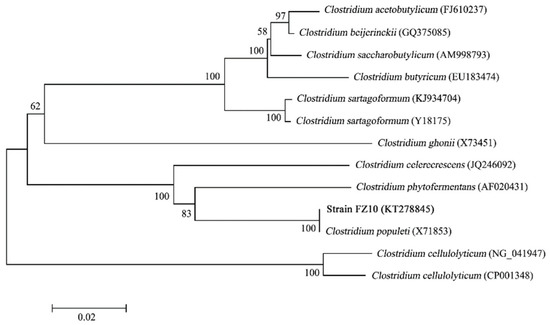
Figure 2.
Phylogenetic tree between strain FZ10 and closely related species, according to 16S rDNA sequences.
3.2. Hydrogen Production Performance of C. populeti FZ10
3.2.1. Hydrogen Production Using Defined Carbohydrates
In order to assess the hydrogen production performance of C. populeti FZ10, various defined carbohydrates present in cellulosic biomasses, including microcrystalline cellulose (MCC), carboxymethyl cellulose (CMC), xylan, xylose, cellobiose and glucose could be used as feedstock. As shown in Figure 3 and Table 4, C. populeti FZ10 could effectively utilize glucose, xylose and cellobiose to generate hydrogen, which resulted in the complete degradation of these simple sugars. Xylan could also promote intensive cell growth and hydrogen production by C. populeti FZ10. At the end of fermentation, almost all the xylan was degraded. It is worth noting that the strain C. populeti FZ10 could also directly utilize microcrystalline cellulose (MCC) and carboxymethyl cellulose (CMC), and a considerable amount of hydrogen was obtained during the fermentation process. In all the substrates, acetate and butyrate were the main liquid metabolites of hydrogen fermentation, followed by a small amount of ethanol and butanol. These observations indicated that C. populeti FZ10 had a great capability for utilizing various polymers presented in the lignocellulosic biomasses to produce hydrogen under mesophilic conditions.
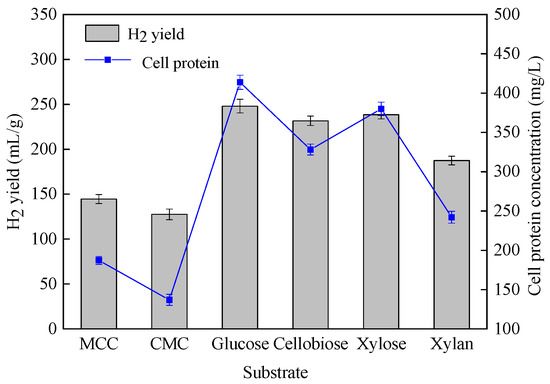
Figure 3.
Comparison of H2 production ability against different carbohydrates produced by C. populeti FZ10.

Table 4.
Liquid fermentation products of C. populeti FZ10 from different carbohydrates.
Based on the results of hydrogen production from various carbohydrates, the dynamics of hydrogen fermentation by C. populeti FZ10 with 10 g/L MCC were further investigated. As presented in Figure 4a, a lag time of 15 h was observed. When the fermentation time was between 20 and 45 h, the cumulative hydrogen yield (Ps) and hydrogen production rate (Rm) increased rapidly. Thereafter, Ps remained essentially constant, while Rm decreased sharply. The maximum Ps and Rm were 177.5 ± 4.8 mL/g and 7.7 ± 0.2 mL·g−1·h−1, respectively. As fermentation progressed, the medium pH changed significantly. The pH value dropped from an initial 6.98 to 5.12 during the first 50 h. Subsequently, alongside hydrogen production and the formation of acidic metabolites, the pH value gradually stabilized at about 4.91. These results were similar to previous reports, in which cellulose degradation and hydrogen production did not occur when the medium pH was lower than 5.5 [35].
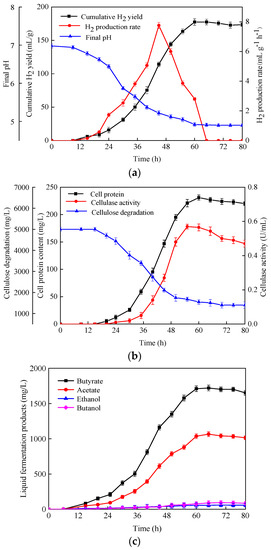
Figure 4.
Characteristics of H2 fermentation from MCC by C. populeti FZ10. (a) Cumulative H2 yield, H2 production rate and medium pH, (b) cell protein, cellulase activity and cellulose degradation, (c) liquid fermentation products.
Furthermore, an exponential growth of cells was detected after cultivation for 24 h, and cell protein peaked at 231 mg/L at 60 h (Figure 4b). A similar trend was observed between cell protein and cellulase activity, where the maximum cellulase activity of 0.57 ± 0.01 U/mL occurred with a cultivate time of 55 h. Consistent with the increase in cellulase activity, the cellulose promptly hydrolyzed after 20 h of incubation, and then remained at a constant level at 65–80 h. At the end of fermentation, 72 ± 2.4% of cellulose was degraded by C. populeti FZ10.
As with the degradation of MCC, both butyrate and acetate were the main metabolites (Figure 4c), indicating that the hydrogen production by C. populeti FZ10 from MCC was a butyrate-type fermentation. Several studies have found that butyrate-type fermentation is favorable for efficient hydrogen production; the high hydrogen yield is usually associated with a high production of butyrate and acetate during the fermentation process [16].
3.2.2. Hydrogen Production Using Natural Lignocellulosic Biomass
In light of the above findings, the ability to utilize natural lignocellulosic biomasses was further investigated for C. populeti FZ10. The hydrogen production capacities of three different untreated lignocellulosic substrates (10 g/L), i.e., corn stalk (CS), wheat straw (WS) and rice straw (RS), were assessed.
As can be seen in Figure 5, the maximum hydrogen yield (Ps) for all three substrates appeared at 70–90 h. Consistent with the degradation of the substrates, the Ps of CS was slightly higher than that of WS and RS. It should be noted that hydrogen could be detected within 10 h of inoculation for corn stalk (λ = 9), while no hydrogen was detected until 15 h after inoculation for WS and RS (Table 5). These phenomena show that CS is more favorable for hydrogen-producing bacteria growth than other substrates during the start-up phase of fermentation. The hydrogen production rates (Rm) of WS and RS were similar, but somewhat lower than that of CS. At the end of fermentation, the degradation of CS, WS and RS were 61.5 ± 3.4%, 57.4 ± 2.8% and 52.8 ± 2.6%, respectively. Similar to the growth on the defined substrates, both acetate and butyrate were the main liquid metabolic by-products during the fermentation process.
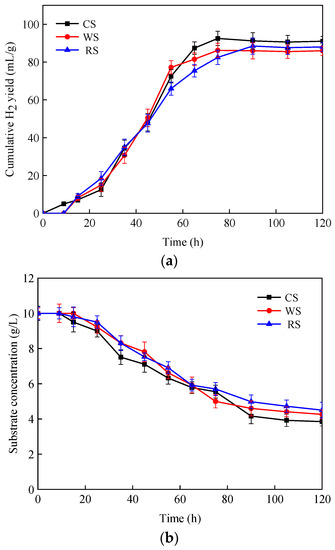
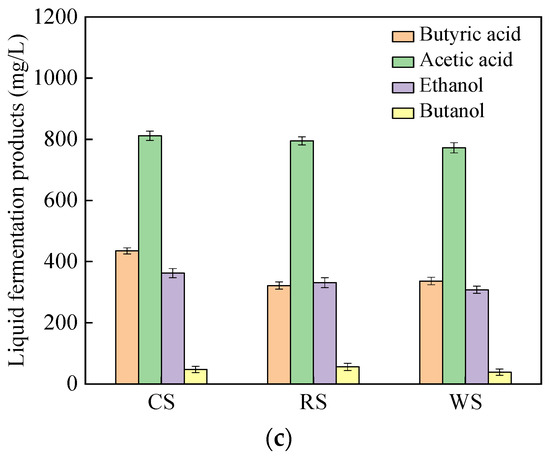
Figure 5.
H2 fermentation from lignocellulosic biomasses by C. populeti FZ10. (a) Cumulative H2 yield, (b) substrate concentration, (c) liquid fermentation products.

Table 5.
Kinetic parameters for hydrogen production from lignocellulosic biomasses.
It can be seen from the above results that C. populeti FZ10 could directly utilize untreated lignocellulosic biomasses (such as CS, WS and RS) to produce hydrogen. However, compared with the pure celluloses (such as MCC or CMC), the hydrogen yields of raw lignocellulosic biomasses were lower and the fermentation processes were slower. The main components of lignocellulosic biomasses are lignin, cellulose and hemicellulose. Lignin connects with hemicellulose through covalent bonds and encases cellulose to form a dense barrier, which hinders the degradation of cellulose and hemicellulose by fermentative microbes, resulting in low hydrogen production during the fermentation process [38].
To further elucidate the degradation characteristics of C. populeti FZ10 on lignocellulosic substrates, corn stalk was used as a representative for batch fermentation tests. The changes in the surface morphology and microstructure of corn stalk before and after fermentation by C. populeti FZ10 were determined with SEM. Before fermentation, a smooth and complete surface could be observed on the epidermis of corn stalk (Figure 6). After fermentation by C. populeti FZ10, the surface morphology of CS was changed dramatically. The initial dense and regular structure was destroyed and replaced by a loose and porous structure. The waxy layer on the surface was almost removed, and pits and cracks could be observed clearly. These results indicated that the barrier structure of lignin was disintegrated.
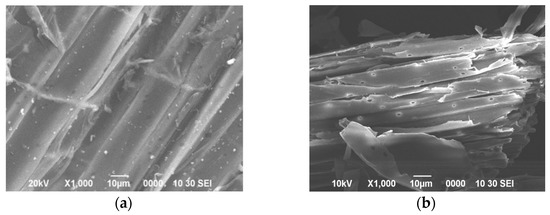
Figure 6.
SEM image of corn stalk. (a) Unfermented corn stalk, (b) corn stalk after fermentation.
In addition to the observation of surface morphology, the changes in the chemical components of CS and the enzyme activity of the fermentation broth were also analyzed. As presented in Figure 7a, the hemicellulose content decreased significantly after cultivation for 25 h, while the cellulose content did not drop until cultivation for 35 h. The reason might be that hemicellulose, with its lower polymerization degree, is more easily degraded than cellulose by C. populeti FZ10. We noticed that the lignin content had no obvious change, indicating that C. populeti FZ10 could not degrade lignin efficiently. At the end of fermentation, 47.2 ± 2.3% of cellulose and 58.1 ± 2.0% of hemicelluloses in corn stalk were degraded by C. populeti FZ10. Consistent with the degradation of cellulose and hemicellulose, the activity of exoglucanase, endoglucanase and xylanase increased rapidly after cultivation for 25 h, reaching the maximum values of 0.37 ± 0.02 U/mL, 0.46 ± 0.01 U/mL and 0.54 ± 0.02 U/mL, respectively (Figure 7b). β-glucosidase activity was not obviously detected until cultivation for 45 h, achieving a maximum activity of 0.13 ± 0.02 U/mL. These results further indicated the degradation of cellulose and hemicellulose in untreated corn stalk by C. populeti FZ10.
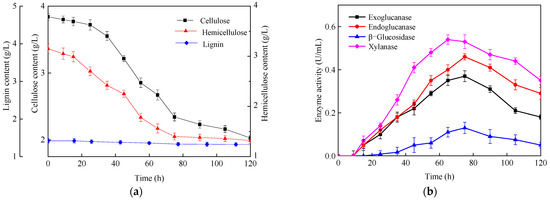
Figure 7.
Characteristics of H2 fermentation from corn stalk by C. populeti FZ10. (a) Chemical components of corn stalk, (b) enzyme activity of fermentation broth.
The hydrogen production capability from various untreated cellulosic substrates (pure cellulose and lignocellulosic biomasses) by C. populeti FZ10 was also compared with that of several other Clostridium species. As illustrated in Table 6, when using pure cellulose as the fermentation substrate for hydrogen production, the hydrogen yield of C. populeti FZ10 was much higher than that found in the existing literature. This may be due to microbial acclimation by MCC, making strain C. populeti FZ10 more adaptable to cellulose than other strains. When using various other lignocellulosic biomasses as substrates, the strain C. populeti FZ10 displayed an excellent hydrogen production performance. Particularly when compared with the previous isolated strain C. sartagoforme FZ11, C. populeti FZ10 demonstrated a higher degradation of cellulose and hemicellulose in raw corn stalk [26]. Although Song et al. reported that C. butyricum FS3 displayed a slightly higher hydrogen yield for raw corn stalk than the present strain C. populeti FZ10, it could not directly utilize pure cellulose to produce hydrogen [34]. Moreover, the hydrogen yield from raw corn stalk by C. populeti FZ10 was comparable to some reported data on the hydrogen fermentation of pretreated corn stalk. For example, Li et al. reported that C. butyricum AS1.209 could utilize steam-exploded corn stalk to generate hydrogen, and the hydrogen yield reached 68 mL/g [39]. Lu et al. introduced C. paraputrificum into the hydrolysis stage of corn stalk under acidic conditions, and the hydrogen yield was only 63.7 mL/g [40].

Table 6.
Comparison of biohydrogen production by different Clostridium species from cellulosic substrates.
Many studies have also reported on hydrogen production with C. thermocellum under thermophilic conditions. For example, Magnusson et al. reported that the maximum hydrogen yield was only 23.97mL/g from dried distillers grain using C. thermocellum 27,405 [25]. Cheng et al. used C. thermocellum 7072 to degrade corn stalk, and the maximum hydrogen yield was able to reach 74.4 mL/g from corn stalk [46]. Compared with C. thermocellum, the present mesophilic strain C. populeti FZ10 had a higher hydrogen yield and hydrogen production rate from raw corn stalk. Due to the different fermentation conditions, it is difficult to compare the hydrogen yields between different strains. Fermentative hydrogen production from cellulosic biomasses under mesophilic conditions is a more promising technology that can reduce the production cost and improve the operating economics. Obtaining high-efficiency hydrogen-producing strains is the key to improving the efficiency of hydrogen production. In this study, the methods of microbial pretreatment and microbial acclimation were used for the screening of hydrogen-producing bacteria. These are effective strategies to obtain hydrogen-producing strains that efficiently convert lignocellulosic biomasses into hydrogen.
4. Conclusions
In this research, a mesophilic anaerobic hydrogen-producing Clostridium was successfully isolated from cow dung compost, acclimated using microcrystalline cellulose in an anaerobic bioreactor and identified as Clostridium populeti FZ10. The strain can secrete various hydrolytic enzymes (such as endoglucanase, exoglucanase, β-glucosidase and xylanase) that can effectively utilize pure cellulose and lignocellulosic biomasses for hydrogen production. The maximum hydrogen yield and hydrogen production rate from MCC were 177.5 ± 4.8 mL/g and 7.7 ± 0.2 mL·g−1·h−1, respectively. The loose and porous structure of corn stalk after fermentation was observed with SEM, and the maximum hydrogen yield and hydrogen production rate from CS were 92.5 ± 3.7 mL/g and 5.9 ± 0.2 mL·g−1·h−1, respectively, with a cellulose degradation of 47.2 ± 2.3% and a hemicellulose degradation of 58.1 ± 2.0%. The main liquid metabolic products were acetate and butyrate for both MCC and CS fermentation. These observations indicate that C. populeti FZ10 is a promising candidate for efficient hydrogen production from lignocellulose under mesophilic conditions.
Author Contributions
Writing—original draft, J.Z., B.J. and H.Z.; reviewing and editing, J.Z., Z.Z., L.S. and T.W.; methodology, J.Z., X.Y., B.J. and T.W.; supervision, J.Z. and T.W.; data collection and conceptualization, J.Z., B.J., H.Z. and S.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Nature Science Foundation of China (No. 21706244) and Key Research Projects of the Science and Technology Department of Henan Province (No. 182102210162; 212102310077; 222102320331) and Zhongyuan Science and Technology Innovation Leading Talent Project (No. 224200510017).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors gratefully acknowledge the Collaborative Innovation Center for Food Production and Safety of Henan Province, China, for their support of this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lenz, O. Hydrogen comes alive. Nat. Energy 2020, 5, 426–427. [Google Scholar] [CrossRef]
- Martinez-Burgos, W.J.; de Souza Candeo, E.; Pedroni Medeiros, A.B.; Cesar de Carvalho, J.; Oliveira de Andrade Tanobe, V.; Soccol, C.R.; Sydney, E.B. Hydrogen: Current advances and patented technologies of its renewable production. J. Clean. Prod. 2021, 286, 124970. [Google Scholar] [CrossRef]
- Wu, X.Y.; Luo, Y.; Hess, F.; Lipinski, W. Sustainable hydrogen for energy, fuel and commodity applications. Front. Energy Res. 2021, 9, 231. [Google Scholar] [CrossRef]
- Abdel-Kader, H.A.A.; Abdel-Basset, R.; Danial, A.W. Yeast and enzymatic hydrolysis in converting Chlorella biomass into hydrogen gas by Rhodobacter sp. and Rhodopseudomonas palustris. Int. J. Hydrogen Energy 2022, 47, 1516–1528. [Google Scholar] [CrossRef]
- Hassan, N.S.; Jalil, A.A.; Vo, D.V.N.; Nabgan, W. An overview on the efficiency of biohydrogen production from cellulose. Biomass Convers. Bior. 2020. [CrossRef]
- Baik, J.H.; Jung, J.H.; Sim, Y.B.; Park, J.H.; Kim, S.M.; Yang, J.; Kim, S.H. High-rate biohydrogen production from xylose using a dynamic membrane bioreactor. Bioresour. Technol. 2022, 344, 126205. [Google Scholar] [CrossRef]
- Lu, X.H.; Li, F.; Zhou, X.; Hu, J.R.; Liu, P. Biomass, lignocellulolytic enzyme production and lignocellulose degradation patterns by Auricularia auricula during solid state fermentation of corn stalk residues under different pretreatments. Food Chem. 2022, 384, 13262. [Google Scholar] [CrossRef]
- Beig, B.; Riaz, M.; Raza Naqvi, S.; Hassan, M.; Zheng, Z.; Karimi, K. Current challenges and innovative developments in pretreatment of lignocellulosic residues for biofuel production: A review. Fuel 2021, 287, 119670. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Gupta, R.K.; Das, D.; Lee, J.K.; Kalia, V.C. Continuous biohydrogen production from poplar biomass hydrolysate by a defined bacterial mixture immobilized on lignocellulosic materials under non-sterile conditions. J. Clean. Prod. 2021, 287, 125037. [Google Scholar] [CrossRef]
- Usman, M.; Kavitha, S.; Kannah, Y.; Yogalakshmi, K.N.; Sivashanmugam, P.; Bhatnagar, A.; Kumar, G. A critical review on limitations and enhancement strategies associated with biohydrogen production. Int. J. Hydrogen Energy 2021, 46, 16565–16590. [Google Scholar]
- Jomnonkhaow, U.; Sittijunda, S.; Reungsang, A. Influences of size reduction, hydration, and thermal-assisted hydration pretreatment to increase the biogas production from Napier grass and Napier silage. Bioresour. Technol. 2021, 331, 125034. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, D.; Lee, D.J.; Chang, J.S. Recent insights into consolidated bioprocessing for lignocellulosic biohydrogen production. Int. J. Hydrogen Energy 2019, 44, 14362–14379. [Google Scholar] [CrossRef]
- Ratti, R.P.; Botta, L.S.; Sakamoto, I.K.; Varesche, M.B.A. Microbial diversity of hydrogen-producing bacteria in batch reactors fed with cellulose using leachate as inoculums. Int. J. Hydrogen Energy 2013, 38, 9707–9717. [Google Scholar] [CrossRef]
- Lo, Y.C.; Su, Y.C.; Chen, C.Y.; Chen, W.M.; Lee, K.S.; Chang, J.S. Biohydrogen production from cellulosic hydrolysate produced via temperature-shift-enhanced bacterial cellulose hydrolysis. Bioresour. Technol. 2009, 100, 5802–5807. [Google Scholar] [CrossRef] [PubMed]
- Zagrodnik, R.; Duber, A.; Seifert, K. Hydrogen production during direct cellulose fermentation by mixed bacterial culture: The relationship between the key process parameters using response surface methodology. J. Clean. Prod. 2021, 314, 127971. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y. Clostridium species for fermentative hydrogen production: An overview. Int. J. Hydrogen Energy 2021, 46, 34599–34625. [Google Scholar] [CrossRef]
- RamKumar, N.; Anupama, P.D.; Nayak, T.; Subudhi, S. Scale up of biohydrogen production by a pure strain; Clostridium butyricum TM-9A at regulated pH under decreased partial pressure. Renew. Energy 2021, 170, 1178–1185. [Google Scholar] [CrossRef]
- Koo, J. Enhanced aerobic H2 production by engineering an [FeFe] hydrogenase from Clostridium pasteurianum. Int. J. Hydrogen Energy 2020, 45, 10673–10679. [Google Scholar] [CrossRef]
- Guerrero, K.; Gallardo, R.; Paredes, I.; Quintero, J.; Mau, S.; Conejeros, R.; Gentina, J.C.; Aroca, G. Continuous biohydrogen production by a degenerated strain of Clostridium acetobutylicum ATCC 824. Int. J. Hydrogen Energy 2022, 46, 5100–5111. [Google Scholar] [CrossRef]
- Srimawong, C.; Chulalaksananukul, W. Evaluating biohydrogen production by Clostridium hydrogenum sp. nov. strain CUEA01 isolated from mangrove sediments in Thailand. Int. J. Hydrogen Energy 2022, 47, 9169–9182. [Google Scholar] [CrossRef]
- Constante, F.B.; Giovanni, D.; Cress, G.V.; Sibeli, C.; Parras, M.L.; Latorre, Z.A.L.R.; Melo, F.R.P.; Rita, T.D.; Valeria, R. Use of algae biomass obtained by single-step mild acid hydrolysis in hydrogen production by the beta-glucosidase-producing Clostridium beijerinckii Br21. Waste Biomass Valorization 2020, 11, 1393–1402. [Google Scholar]
- An, Q.; Cheng, J.; Wang, Y.; Zhu, M. Performance and energy recovery of single and two stage biogas production from paper sludge: Clostridium thermocellum augmentation and microbial community analysis. Renew. Energy 2020, 148, 214–222. [Google Scholar] [CrossRef]
- Morales-Martinez, T.K.; Medina-Morales, M.A.; Ortiz-Cruz, A.L.; Rodriguez-De La Garza, J.A.; Moreno-Davila, M.; Lopez-Badillo, C.M.; Rios-Gonzalez, L. Consolidated bioprocessing of hydrogen production from agave biomass by Clostridium acetobutylicum and bovine ruminal fluid. Int. J. Hydrogen Energy 2020, 45, 13707–13716. [Google Scholar] [CrossRef]
- Lo, Y.C.; Chen, W.M.; Hung, C.H.; Chen, S.D.; Chang, J.S. Dark H2 fermentation from sucrose and xylose using H2-producing indigenous bacteria: Feasibility and kinetic studies. Water Res. 2008, 42, 827–842. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, L.; Islam, R.; Sparling, R.; Levin, D.; Cicek, N. Direct hydrogen production from cellulosic waste materials with a single-step dark fermentation process. Int. J. Hydrogen Energy 2008, 33, 5398–5403. [Google Scholar] [CrossRef]
- Zhang, J.N.; Li, Y.H.; Zheng, H.Q.; Fan, Y.T.; Hou, H.W. Direct degradation of cellulosic biomass to bio-hydrogen from a newly isolated strain Clostridium sartagoforme FZ11. Bioresour. Technol. 2015, 192, 60–67. [Google Scholar] [CrossRef]
- Doetsch, R.N. Determinative methods of light microscopy. In Manual of Methods for General Bacteriology; Gerhardt, P., Ed.; American Society for Microbiology: Washington, DC, USA, 1981; pp. 29–30. [Google Scholar]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- An, D.; Li, Q.; Wang, X.Q.; Yang, H.H.; Guo, L.J. Characterization on hydrogen production performance of a newly isolated Clostridium beijerinckii YA001 using xylose. Int. J. Hydrogen Energy 2014, 39, 19928–19936. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Hall, B.G. Building phylogenetic trees from molecular data with MEGA. Mol. Biol. Evol. 2013, 30, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.X.; Li, X.H.; Li, W.W.; Bai, Y.X.; Fan, Y.T.; Hou, H.W. Direct bioconversion of raw corn stalk to hydrogen by a new strain Clostridium sp. FS3. Bioresour. Technol. 2014, 157, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.L.; Zhao, L.; Wang, A.J.; Wang, Z.Y.; Ren, N.Q. Single-step bioconversion of lignocellulose to hydrogen using novel moderately thermophilic bacteria. Biotechnol. Biofuels 2014, 7, 82. [Google Scholar] [CrossRef]
- Zhang, J.N.; Sun, H.; Pan, C.M.; Fan, Y.T.; Hou, H.W. Optimization of process parameters for directly converting raw corn stalk to biohydrogen by Clostridium sp. FZ11 without substrate pretreatment. Energy Fuels 2016, 30, 311–317. [Google Scholar] [CrossRef]
- Rattanachomsri, U.; Tanapongpipat, S.; Eurwilaichitr, L.; Champreda, V. Simultaneous non-thermal saccharification of cassava pulp by multi-enzyme activity and ethanol fermentation by Candida tropicalis. J. Biosci. Bioeng. 2009, 107, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Elbeshbishy, E.; Dhar, B.R.; Nakhla, G.; Lee, H.S. A critical review on inhibition of dark biohydrogen fermentation. Renew. Sustain. Energy Rev. 2017, 79, 656–668. [Google Scholar] [CrossRef]
- Li, D.M.; Chen, H.Z. Biological hydrogen production from steam-exploded straw by simultaneous saccharification and fermentation. Int. J. Hydrogen Energy 2007, 32, 1742–1748. [Google Scholar] [CrossRef]
- Lu, Y.; Lai, Q.H.; Zhang, C.; Zhao, H.X.; Ma, K.; Zhao, X.B.; Chen, H.Z.; Liu, D.H.; Xing, X.H. Characteristics of hydrogen and methane production from cornstalks by an augmented two- or three-stage anaerobic fermentation process. Bioresour. Technol. 2009, 100, 2889–2895. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Li, Y.; Liu, X.S.; Ren, N.Q.; Ding, J. Lignocellulosic hydrogen production using dark fermentation by Clostridium lentocellum strain Cel10 newly isolated from Ailuropoda melanoleuca excrement. RSC Adv. 2019, 9, 11179–11185. [Google Scholar] [CrossRef]
- Ren, N.Q.; Wang, A.J.; Gao, L.F.; Liang, X.; Lee, D.J.; Su, A. Bioaugmented hydrogen production from carboxymethyl cellulose and partially delignified corn stalks using isolated cultures. Int. J. Hydrogen Energy 2008, 33, 5250–5255. [Google Scholar] [CrossRef]
- Ramachandran, U.; Wrana, N.; Cicek, N.; Sparling, R.; Levin, D.B. Hydrogen production and end-product synthesis patterns by Clostridium termitidis strain CT1112 in batch fermentation cultures with cellobiose or a-cellulose. Int. J. Hydrogen Energy 2008, 33, 7006–7012. [Google Scholar] [CrossRef]
- Ren, Z.; Ward, T.E.; Logan, B.E.; Regan, J.M. Characterization of the cellulolytic and hydrogen-producing activities of six mesophilic Clostridium species. J. Appl. Microbiol. 2007, 103, 2258–2266. [Google Scholar] [CrossRef]
- Cheng, X.Y.; Liu, C.Z. Enhanced coproduction of hydrogen and methane from cornstalks by a three-stage anaerobic fermentation process integrated with alkaline hydrolysis. Bioresour. Technol. 2012, 104, 373–379. [Google Scholar] [CrossRef]
- Cheng, X.Y.; Liu, C.Z. Hydrogen production via thermophilic fermentation of cornstalk by Clostridium thermocellum. Energy Fuels 2011, 25, 1714–1720. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).