Adsorption of Emerging Pollutant by Pecan Shell-Based Biosorbent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adsorbate
2.2. Adsorbent
2.3. Batch Adsorption
3. Results and Discussion
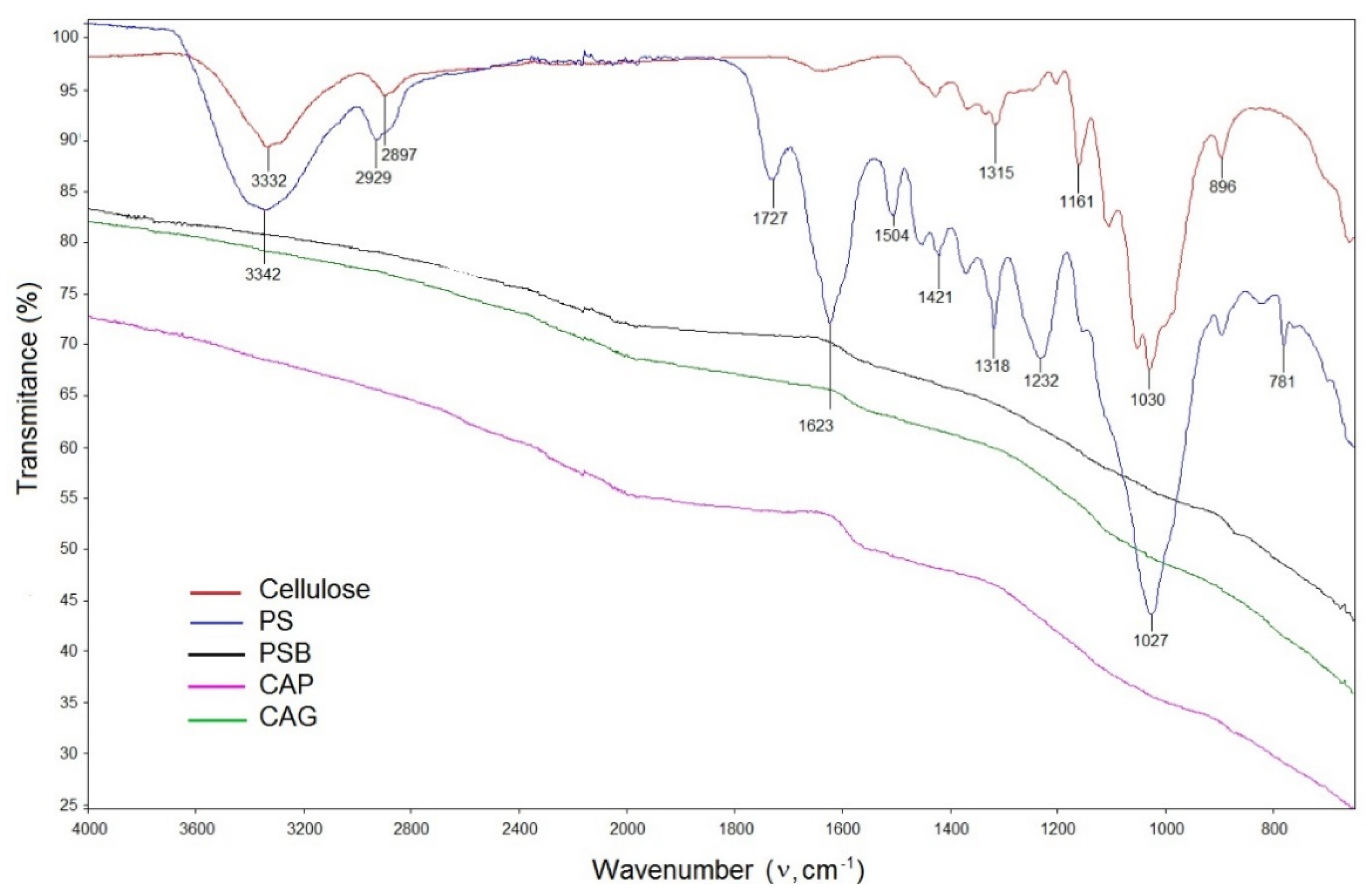
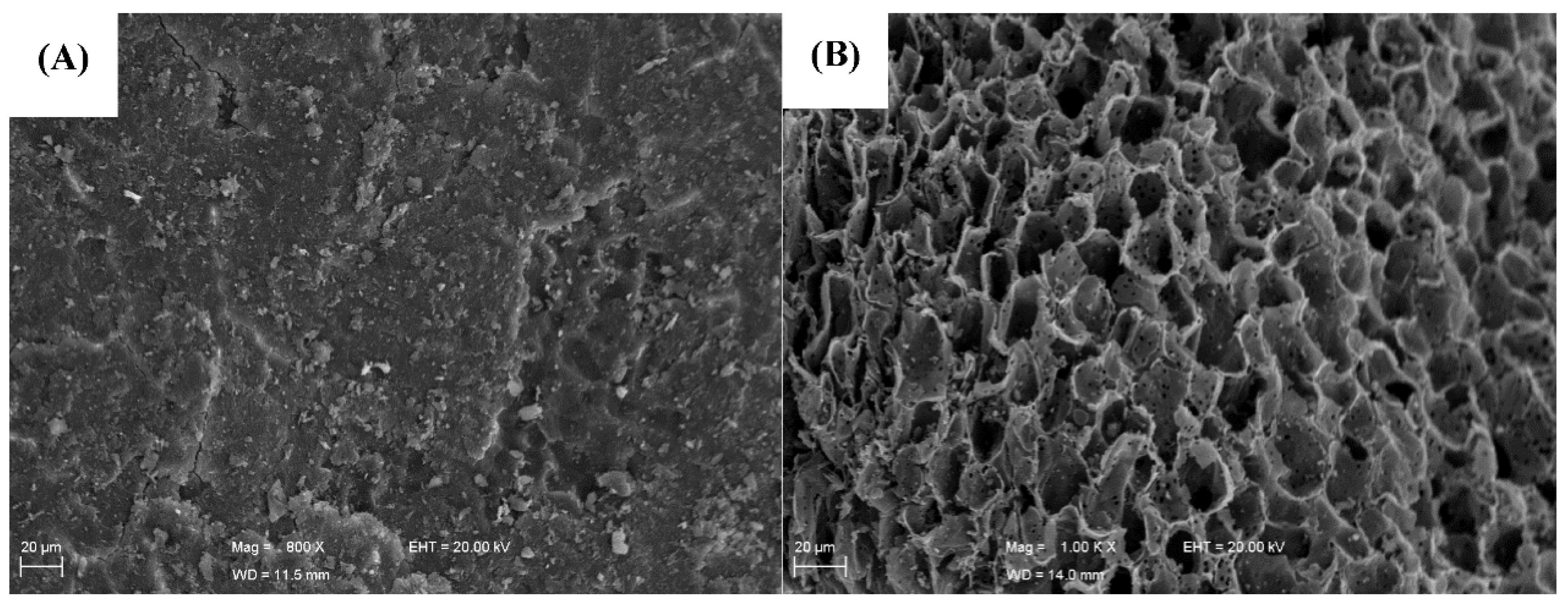
3.1. Characterization of the Biosorbent
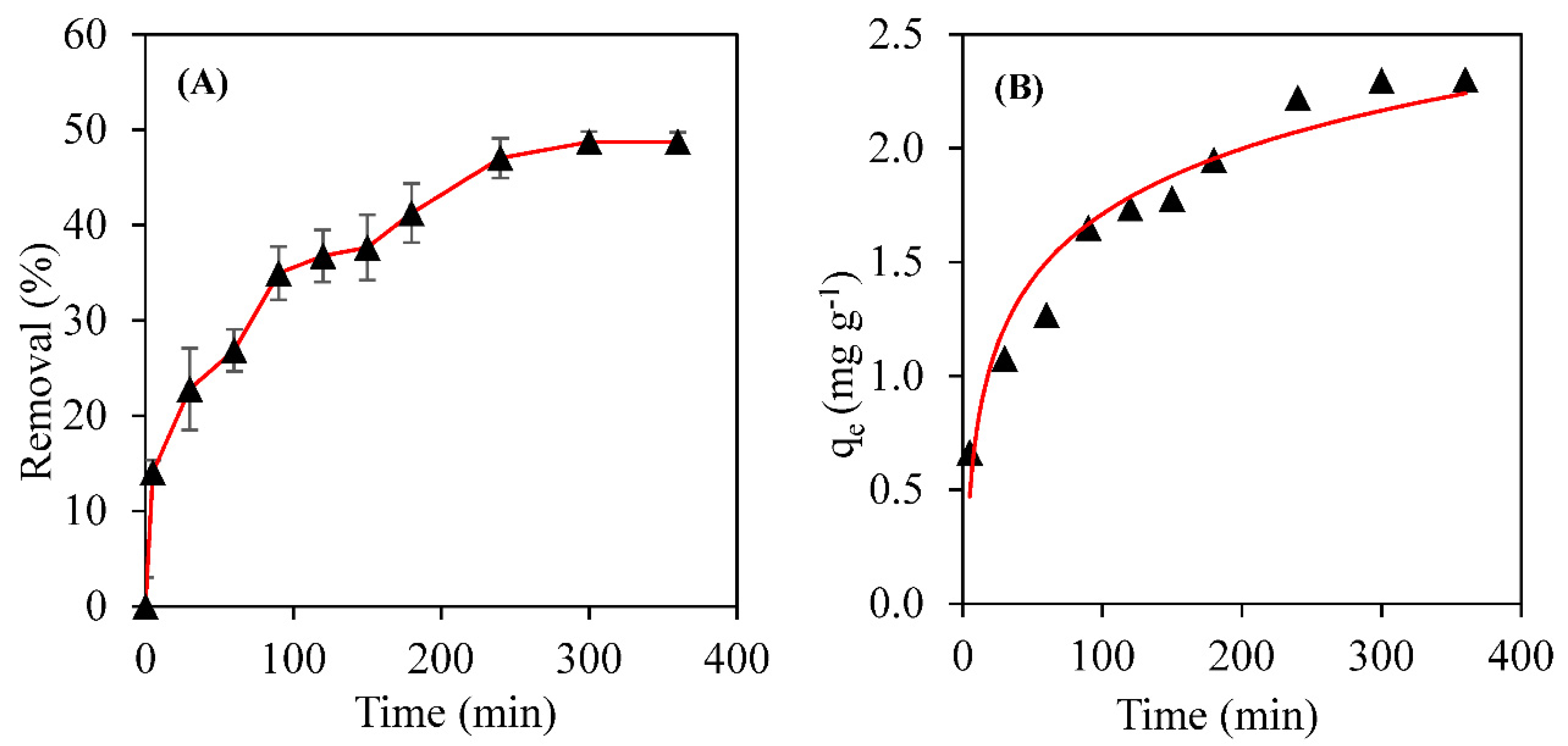
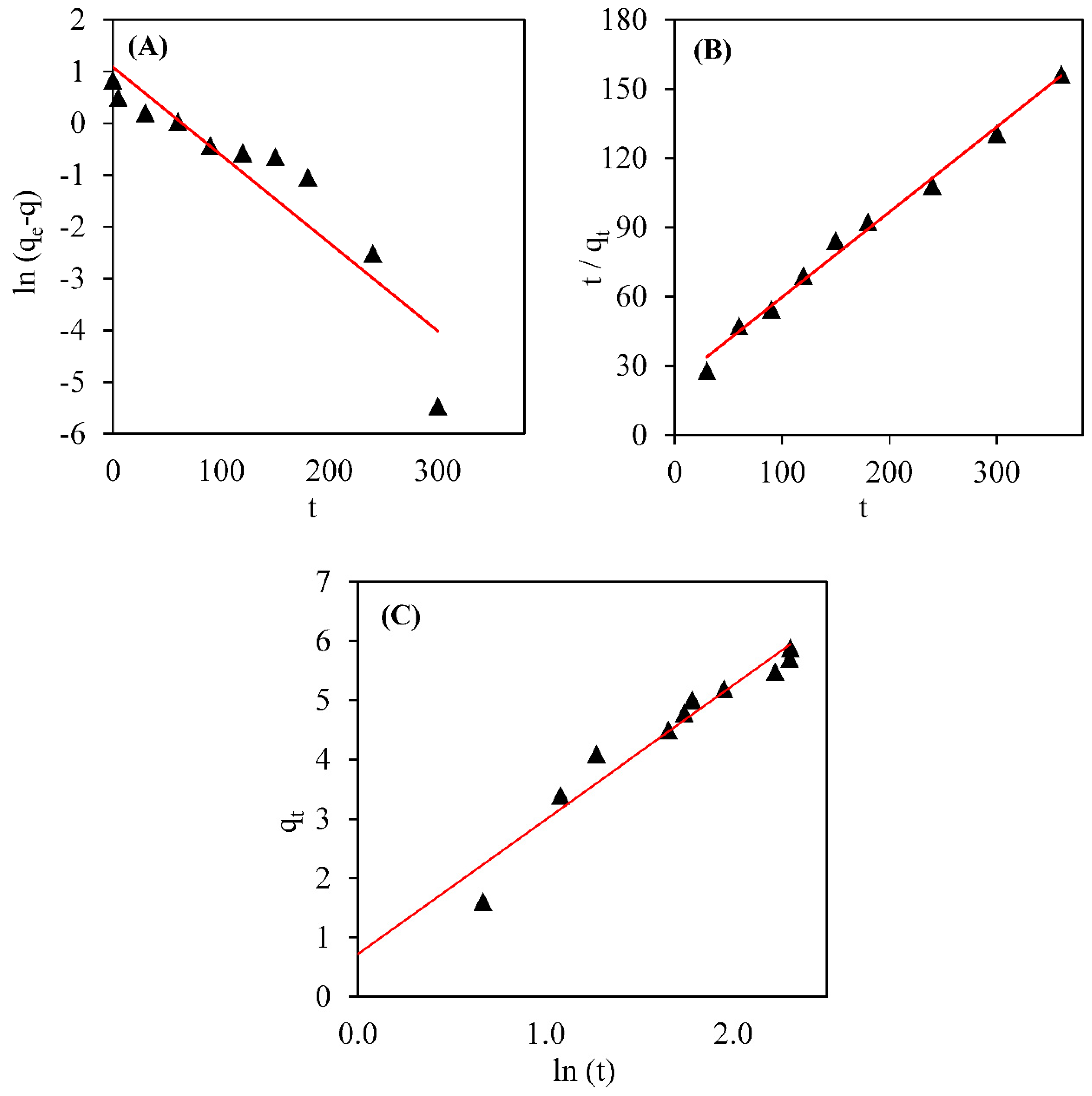
3.2. Biosorption Kinetics
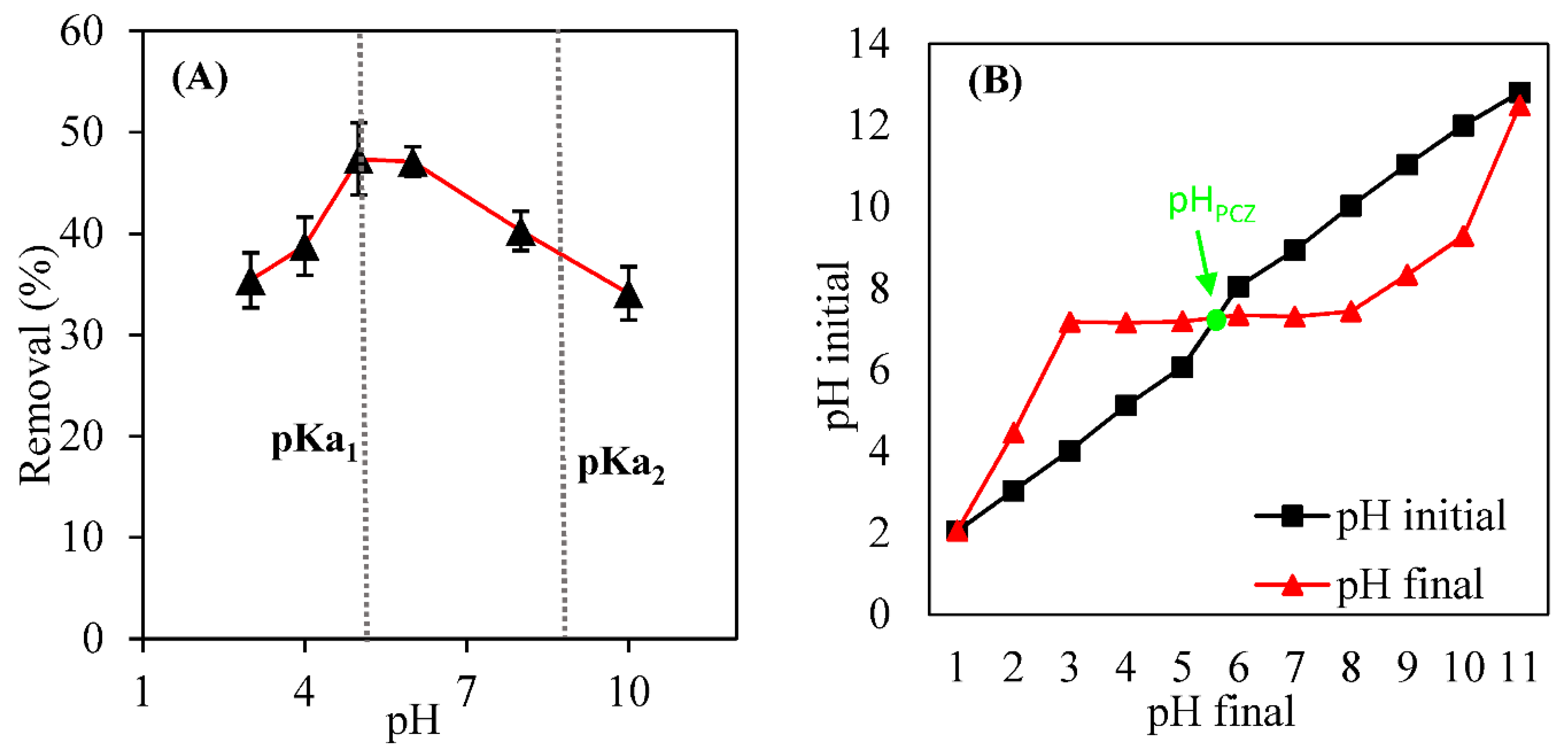
3.3. Effect of pH and the Zero-Charge Point pH
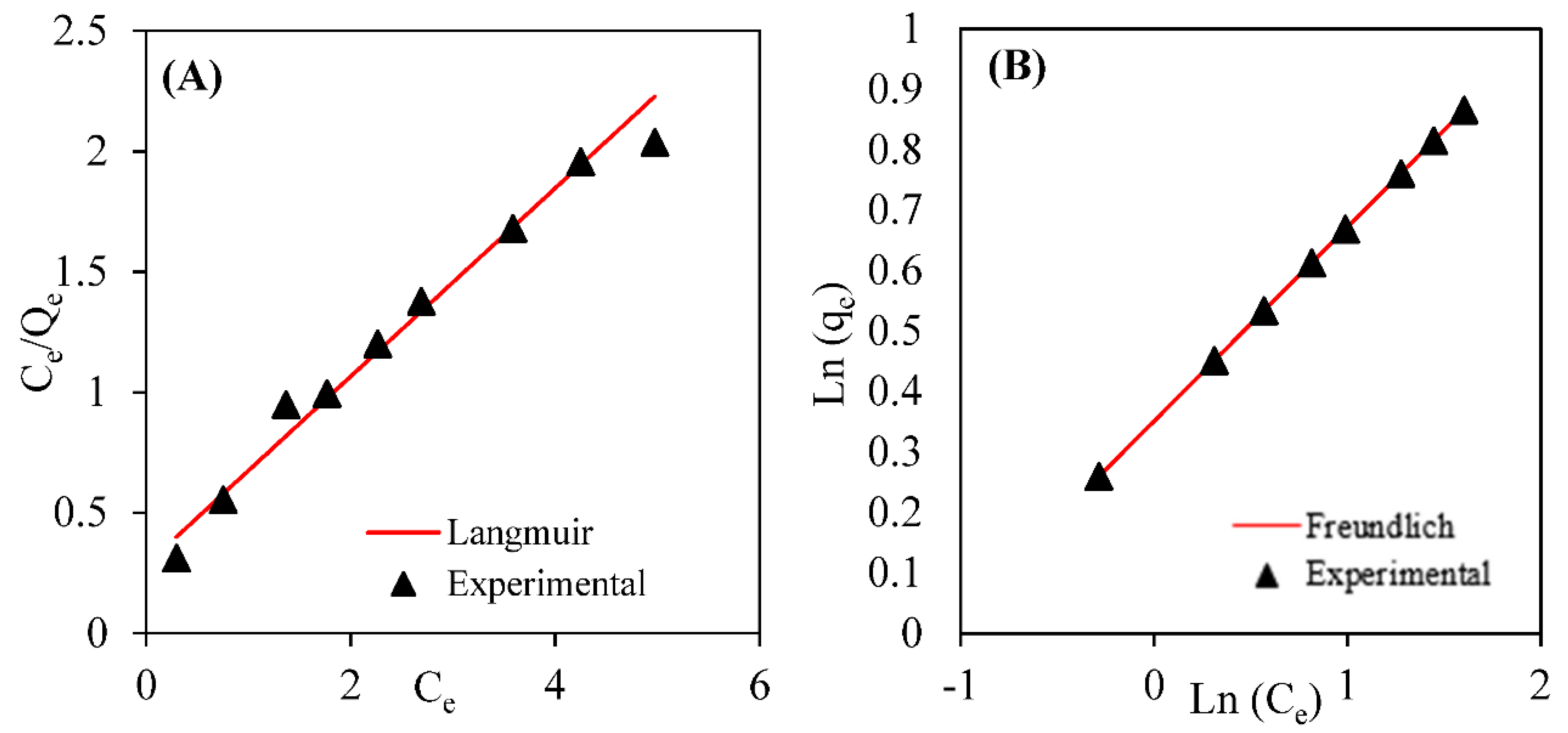
3.4. Biosorption Equilibrium Isotherms
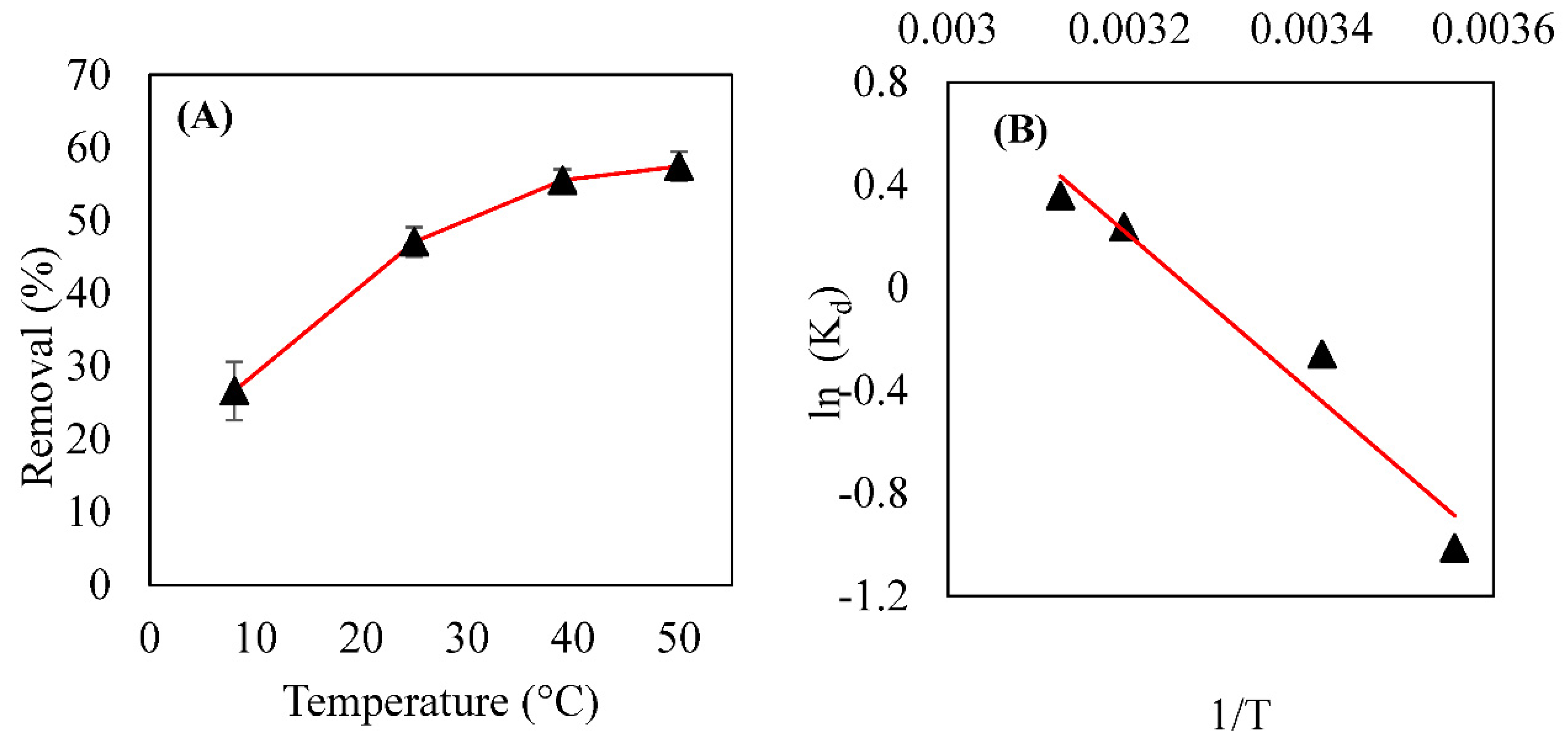
3.5. Thermodynamics of Adsorption and Its Modeling
4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mashayekh-Salehi, A.; Moussavi, G. Removal of acetaminophen from the contaminated water using adsorption onto carbon activated with NH4Cl. Desalination Water Treat. 2016, 57, 12861–12873. [Google Scholar] [CrossRef]
- Yassine, M.H.; Rifai, A.; Hoteit, M.; Mazellier, P.; Al Iskandarani, M. Study of the degradation process of ofloxacin with free chlorine by using ESI-LCMSMS: Kinetic study, by-products formation pathways and fragmentation mechanisms. Chemosphere 2017, 189, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.B.; Garcia, L.F.; Caetano, M.P.; Lobón, G.S.; de Oliveira, M.T.; de Oliveira, R.; Torres, I.M.S.; Yepez, A.; Vaz, B.G.; Luque, R.; et al. Electrochemical remediation of amoxicillin: Detoxification and reduction of antimicrobial activity. Chem. Biol. Interact. 2018, 291, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Wardenier, N.; Vanraes, P.; Nikiforov, A.; Van Hulle, S.W.H.; Leys, C. Removal of micropollutants from water in a continuous-flow electrical discharge reactor. J. Hazard. Mater. 2019, 362, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Khan, A.A.; Chowdhury, A.; Bhakta, A.K.; Mekhalif, Z.; Hussain, S. Efficient and highly selective adsorption of cationic dyes and removal of ciprofloxacin antibiotic by surface modified nickel sulfide nanomaterials: Kinetics, isotherm and adsorption mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124264. [Google Scholar] [CrossRef]
- Richard, J.; Boergers, A.; vom Eyser, C.; Bester, K.; Tuerk, J. Toxicity of the micropollutants Bisphenol A, Ciprofloxacin, Metoprolol and Sulfamethoxazole in water samples before and after the oxidative treatment. Int. J. Hyg. Environ. Health 2014, 217, 506–514. [Google Scholar] [CrossRef]
- Gros, M.; Rodríguez-Mozaz, S.; Barceló, D. Rapid analysis of multiclass antibiotic residues and some of their metabolites in hospital, urban wastewater and river water by ultra-high-performance liquid chromatography coupled to quadrupole-linear ion trap tandem mass spectrometry. J. Chromatogr. A 2013, 1292, 173–188. [Google Scholar] [CrossRef]
- Reis-Santos, P.; Pais, M.; Duarte, B.; Caçador, I.; Freitas, A.; Pouca, A.S.V.; Barbosa, J.; Leston, S.; Rosa, J.; Ramos, F.; et al. Screening of human and veterinary pharmaceuticals in estuarine waters: A baseline assessment for the Tejo estuary. Mar. Pollut. Bull. 2018, 135, 1079–1084. [Google Scholar] [CrossRef]
- Dionísio, R.; Daniel, D.; de Alkimin, G.D.; Nunes, B. Multi-parametric analysis of ciprofloxacin toxicity at ecologically relevant levels: Short- and long-term effects on Daphnia magna. Environ. Toxicol. Pharmacol. 2020, 74, 103295. [Google Scholar] [CrossRef]
- Ferreira, R.C.; de Lima, H.H.C.; Cândido, A.A.; Junior, O.M.C.; Arroyo, P.A.; Gauze, G.F.; de Carvalho, K.Q.; Barros, M.A.S.D. Adsorption of Paracetamol Using Activated Carbon of Dende and Babassu Coconut Mesocarp. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. 2015, 9, 575–580. [Google Scholar]
- Niaei, H.A.; Rostamizadeh, M. Adsorption of metformin from an aqueous solution by Fe-ZSM-5 nano-adsorbent: Isotherm, kinetic and thermodynamic studies. J. Chem. Thermodyn. 2020, 142, 106003. [Google Scholar] [CrossRef]
- Guo, Z.; Yan, N.; Lapkin, A.A. Towards circular economy: Integration of bio-waste into chemical supply chain. Curr. Opin. Chem. Eng. 2019, 26, 148–156. [Google Scholar] [CrossRef]
- Nazari, G.; Abolghasemi, H.; Esmaieli, M. Batch adsorption of cephalexin antibiotic form aqueous solution by walnut shell-based activated carbon. J. Taiwan Inst. Chem. Eng. 2016, 58, 357–365. [Google Scholar] [CrossRef]
- Peñafiel, M.E.; Matesanz, J.M.; Vanegas, E.; Bermejo, D.; Mosteo, R.; Ormad, M.P. Comparative adsorption of ciprofloxacin on sugarcane bagasse from Ecuador and on commercial powdered activated carbon. Sci. Total Environ. 2021, 750, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; Porter, J.F.; McKay, G. Equilibrium Isotherm Studies for the Sorption of Divalent Metal Ions onto Peat: Coper, Nickel and Lead Single Component Systems. Water Air Soil Pollut. 2002, 141, 1–33. [Google Scholar] [CrossRef]
- Avcı, A.; Inci, I.; Baylan, N. Adsorption of Ciprofloxacin Hydrochloride on Multiwall Carbon Nanotube. J. Mol. Struct. 2020, 1206, 127711. [Google Scholar] [CrossRef]
- Kilislioglu, A.; Binay, B. Thermodynamic and Kinetic Investigations of Uranium Adsorption on Amberlite IR-118H Resin. Appl. Radiat. Isot. 2003, 58, 155–160. [Google Scholar] [CrossRef]
- González-García, P. Activated carbon from lignocellulosics precursors: A review of the synthesis methods, characterization techniques and applications. Renew. Sustain. Energy Rev. 2018, 82, 1393–1414. [Google Scholar] [CrossRef]
- Jalil, M.E.R.; Baschini, M.; Sapag, K. Influence of pH and antibiotic solubility on the removal of ciprofloxacin from aqueous media using montmorillonite. Appl. Clay Sci. 2015, 114, 69–76. [Google Scholar] [CrossRef]
- Maeda, C.H.; Araki, C.A.; Moretti, A.L.; de Barros, M.A.S.D.; Arroyo, P.A. Adsorption and desorption cycles of reactive blue BF-5G dye in a bone char fixed-bed column. Environ. Sci. Pollut. Res. 2019, 26, 28500–28509. [Google Scholar] [CrossRef]
- Angulo, E.; Bula, L.; Mercado, I.; Montaño, A.; Cubillán, N. Bioremediation of Cephalexin with non-living Chlorella sp., biomass after lipid extraction. Bioresour. Technol. 2018, 257, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Cherkasov, N. Liquid-phase adsorption: Common problems and how we could do better. J. Mol. Liq. 2020, 301, 112378. [Google Scholar] [CrossRef]
- Nascimento, R.F.D.; Lima, A.C.A.D.; Vidal, C.B.; Melo, D.D.Q.; Raulino, G.S.C. Adsorção: Aspectos teóricos e aplicações ambientais. In Adsorção Aspesctos Teóricos e Aplicações Ambientais; Imprensa Universitária da Universidade Federal do Ceará: Rio Grande do Norte, Brazil, 2014. [Google Scholar] [CrossRef]
| Model | Equation | Variables | References | |
|---|---|---|---|---|
| Kinetic models | Pseudo-first order | qe; k1 | [15] | |
| Pseudo-second order | qe; k2 | [15] | ||
| Elovich | α; β | [16] | ||
| Equilibrium models | Langmuir | qmax; KL | [17] | |
| Freundlich | Kf; n | [17] |
| Kinetics Model | Kinetics Model Parameter and Regression Coefficient | ||
|---|---|---|---|
| Pseudo-first order | k1 (h−1) | qe (mg g−1) | R2 |
| 0.0155 | 2.19 | 0.8553 | |
| Pseudo-second order | k2 (g mg h−1) | qe (mg g−1) | R2 |
| 0.0082 | 2.25 | 0.9915 | |
| Elovich | β | α | R2 |
| 2.05 | 0.15 | 0.9391 | |
| Equilibrium Model | Isotherm Parameter | |
|---|---|---|
| Langmuir | qmax (mg g−1) | 2.5592 |
| KL (L mg−1) | 1.3747 | |
| R2 | 0.9892 | |
| RL | ||
| Freundlich | KF (mg g−1) | 1.4207 |
| N | 3.1184 | |
| R2 | >0.9999 | |
| Temperature (K) | ∆G (J mol−1) | ∆S (J mol−1) | ∆H (kJ mol−1) |
|---|---|---|---|
| 281 | 2366.72 | −82.88 | 25.37 |
| 293 | 628.66 | ||
| 313 | −620.43 | ||
| 320 | −953.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordeiro, S.G.; Weber, A.C.; Costa, B.; Dahmer, B.R.; Kuhn, D.; Ethur, E.M.; Corbellini, V.A.; Hoehne, L. Adsorption of Emerging Pollutant by Pecan Shell-Based Biosorbent. Appl. Sci. 2022, 12, 9211. https://doi.org/10.3390/app12189211
Cordeiro SG, Weber AC, Costa B, Dahmer BR, Kuhn D, Ethur EM, Corbellini VA, Hoehne L. Adsorption of Emerging Pollutant by Pecan Shell-Based Biosorbent. Applied Sciences. 2022; 12(18):9211. https://doi.org/10.3390/app12189211
Chicago/Turabian StyleCordeiro, Sabrina Grando, Ani Caroline Weber, Bruna Costa, Bruno Rampanelli Dahmer, Daniel Kuhn, Eduardo Miranda Ethur, Valeriano Antonio Corbellini, and Lucélia Hoehne. 2022. "Adsorption of Emerging Pollutant by Pecan Shell-Based Biosorbent" Applied Sciences 12, no. 18: 9211. https://doi.org/10.3390/app12189211
APA StyleCordeiro, S. G., Weber, A. C., Costa, B., Dahmer, B. R., Kuhn, D., Ethur, E. M., Corbellini, V. A., & Hoehne, L. (2022). Adsorption of Emerging Pollutant by Pecan Shell-Based Biosorbent. Applied Sciences, 12(18), 9211. https://doi.org/10.3390/app12189211








