1. Introduction
Since the 1990s, the gross inland energy consumption of biogas has increased 25-fold, exceeding 163 MWh in 2019 in the European Union [
1]. This increase has been supported by technological development and policy guidelines and strategies promoting this type of energy production. Germany, one of the leading biogas producers in Europe, is already generating 1 MWh per capita, and similar or even higher levels are considered possible in many countries [
2]. The biogas plants in Croatia generated 316.5 GWh in 2018 [
3], which roughly equals 0.08 MWh per capita, indicating significant improvements in this sector are desirable and necessary. The European Union demands such improvements, outlined in the new regulative, REPowerEU [
4], and sets new targets for the member states to produce 20% more biogas and biomethane by 2030.
In the EU, the technically and economically most important substrate for biogas production is maize silage, which is considered the benchmark in terms of the biomass volume per hectare and biogas produced [
5]. However, corn silage is also used as livestock feed, so demands are increasing, leading to a sharp rise in silage prices in the EU market. Therefore, gradually replacing silage with alternative biomass sources is both a long and short-term goal. Lignocellulose waste, such as crop residues, energy crops, municipal solid waste, or forest waste, are abundant and renewable resources [
6]. They are rich in sugars and other energy-valuable structural components and are considered one of the alternative biomass sources for biogas production. In Europe, especially in Mediterranean countries, one of the sectors producing large amounts of lignocellulose waste is the olive (
Olea europaea) industry. As olive is mainly cultivated for olive oil extraction, this sector is a major producer of olive cake, as the primary residue and pruning residues. Disposal of these waste, in many cases, causes environmental problems such as odor production and change in the soil microbiome.
On the other hand, these wastes have considerable potential for further utilization as an energy source [
7]. Carbohydrate components of lignocellulose biomass (cellulose and hemicellulose) are fermentable, making it a suitable substrate for bioenergy and biofuel production. However, lignocellulose biomass is highly resistant to microorganism enzymatic biodegradation. Therefore, optimized (pre)treatments that make the carbohydrates available for further processing are often performed [
6,
8], increasing the time and money cost of the processes. Another option for boosting biogas production during anaerobic digestion is bioaugmentation, a process that implies the addition of targeted and desired microorganisms in bioreactors. In the case of biogas production, these microorganisms, mainly bacteria and methanogenic archaea, can enhance the primary degradation of substrate, fermentation, or methane production. These can be added as pure cultures, i.e.,
Acetobacteroides hydrogenigenes [
9], a hydrogen-producing acetate-type fermenter, or
Methanoculleus bourgensis [
10], methanogenic archaea. Another option is to add a microbial consortium that combines methanogens, hydrolytic, acidogenic, or acetogenic bacteria [
11,
12,
13]. Finally, the added microorganisms can be purchased from banks of cell cultures [
9,
10] or previously isolated from a suitable environment and propagated [
12,
14].
In the presented study, the idea was to isolate the targeted consortium of microorganisms from the inoculum to be used for anaerobic digestion. The isolated bacteria are intended to be specifically optimized to use the olive waste (olive cake and pruned biomass) as a substrate for biodegradation. These microorganisms augmented the biogas reactors. To enhance the process, isolated microorganisms are to be immobilized onto a suitable biocarrier. Microbial cell immobilization can propagate the process stability, intensify biochemical transformations, protect the microbial cells from unfavorable influences, and optimize the cost-effectiveness [
14,
15]. As a carrier, expanded perlite was used, a naturally occurring aluminosilicate material that is lightweight, cost-effective, highly porous, and shows great capacity for bacterial immobilization [
16].
2. Materials and Methods
2.1. Olive Waste
In this study, olive (Olea europaea) waste was used; both pruning residues (PR) and olive cake (OC) were investigated. The substrates originated from the same olive plantation in the Mediterranean part of Croatia (N 44.033315, E 15.611384). OC was collected after the cold oil extraction at the local oil mill in November 2020, whereas the PB was collected in June 2021. The plantation and agricultural production methods are in accordance with those commonly used for Croatian olive cultivation. The OC was frozen until the experiment, and PR was kept in dry conditions at room temperature.
2.2. Preparation of Bioparticles
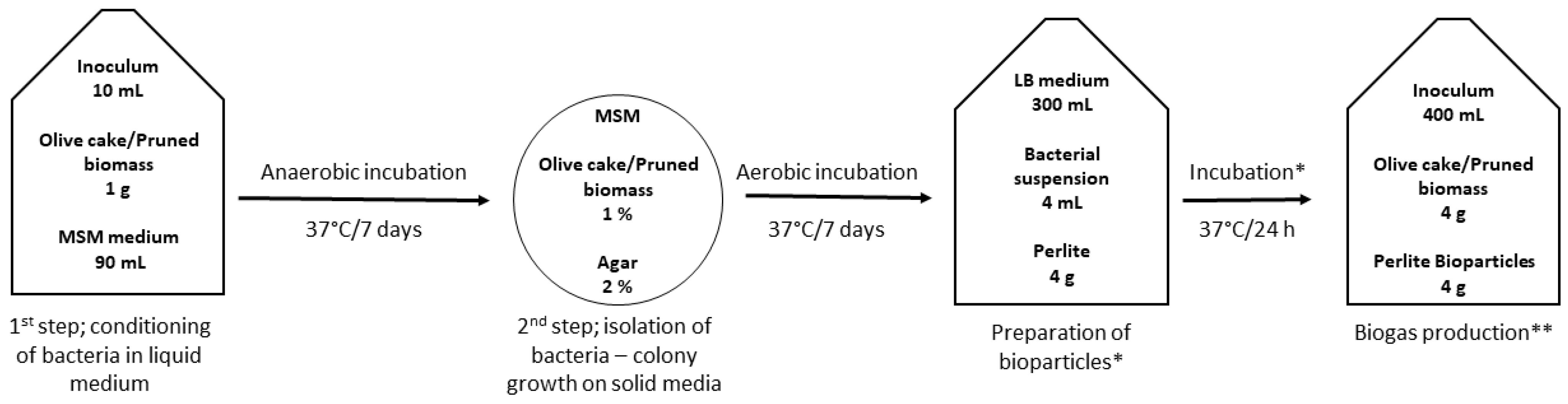
The preparation of the bioparticles was a two-step process; first, we isolated the bacteria that are capable of producing colonies on solid media with olive cake (OC) or pruned biomass (PB) as the sole carbon source, presuming these organisms are capable of enzymatic degradation of the organic matter. Next, we immobilized the isolated bacteria onto perlite particles, testing aerated and anaerobic incubation setups. Finally, bioparticles were added to biogas-producing reactors, and the amount of produced gas was monitored. The experimental scheme is shown in
Figure 1.
2.3. Isolation of Augmenting Bacteria
The isolation of bacteria to be used for bioaugmentation was also a two-step process; first, bacteria were conditioned in liquid media and subsequently grown on agar plates. In the first step (
Figure 1), 1 g of either sterilized OC or PB and 10 mL of inoculum were added to a 100-mL Winkler bottle. The bottle was then filled to the top with sterile MSM and capped to prevent airflow and enable anaerobic conditions. The bottles were incubated for one week at 37 °C on an orbital shaker.
In the second step (
Figure 1), a 1 mL aliquot was taken from Winkler bottles, serially diluted (10
−1 to 10
−4), and 0.1 mL was inoculated on beforehand-prepared minimal agar plates. The minimal agar plates were prepared by adding 2% of agar and 1% mass weight of either OC or PB to liquid MSM prior to autoclaving (121 °C/20 min). The plates were incubated for one week at 37 °C. After the incubation, distinguishable single colonies were scraped off the surface of the plates and transferred to a plastic vial with a sterile 0.3% saline solution. This suspension was the microbial consortium to be used for augmentation. The composition of MSM was (in g L
−1 of tap water): NaCl 2.5, K
2HPO
4 0.47, KH
2PO
4 0.56, MgSO
4·7H
2O 0.5, CaCl
2·2H
2O 0.1, NH
4NO
3 2.5, pH 7.0 ± 0.2.
2.4. Immobilization of Bacteria onto Perlite
Bioparticles for analysis were prepared by adding 1 g of sterilized perlite (105 °C/6 h in a dry oven) and 1 mL of consortium suspension to Schott bottles of 250 mL volume that contained 100 mL of sterile LB medium (tryptone 10 g, yeast extract 5 g, NaCl 5 g, deionized water 1L, pH 7.0 ± 0.2). The bottles were then incubated for 24 h at 37 °C with aeration (1 L min−1 of sterile air). For anaerobic conditions, 0.5 g of perlite and 0.5 mL of consortium suspension were added to sterile plastic vials of 50 mL, which were filled to the top with LB and tightly capped. The vials were incubated for 24 h at 37°C on a rotatory shaker (Stuart rotator SB3, 4 rpm).
After the incubation, the number of bacteria immobilized on perlite particles was determined by aseptically transferring perlite particles to sterile plastic vials (Falcon type of 50 mL volume) containing 20 mL of sterile saline. The particles were washed beforehand three times with sterile saline to remove the bacteria that were not tightly attached. Next, the vials were shaken for 3 min on a vortex shaker (Kartell, 50 Hz) to detach the immobilized cells remaining in the saline supernatant. The supernatant was serially diluted, inoculated on LB agar plates, and incubated at 37 °C for 48 h, after which the grown colonies were counted, and the number of immobilized cells was expressed as CFU g−1 of perlite. The perlite was a commercially available substrate intended to be used for gardening (Special Mix B.V. manufactured by Gold Label, Aalsmeer, The Netherlands).
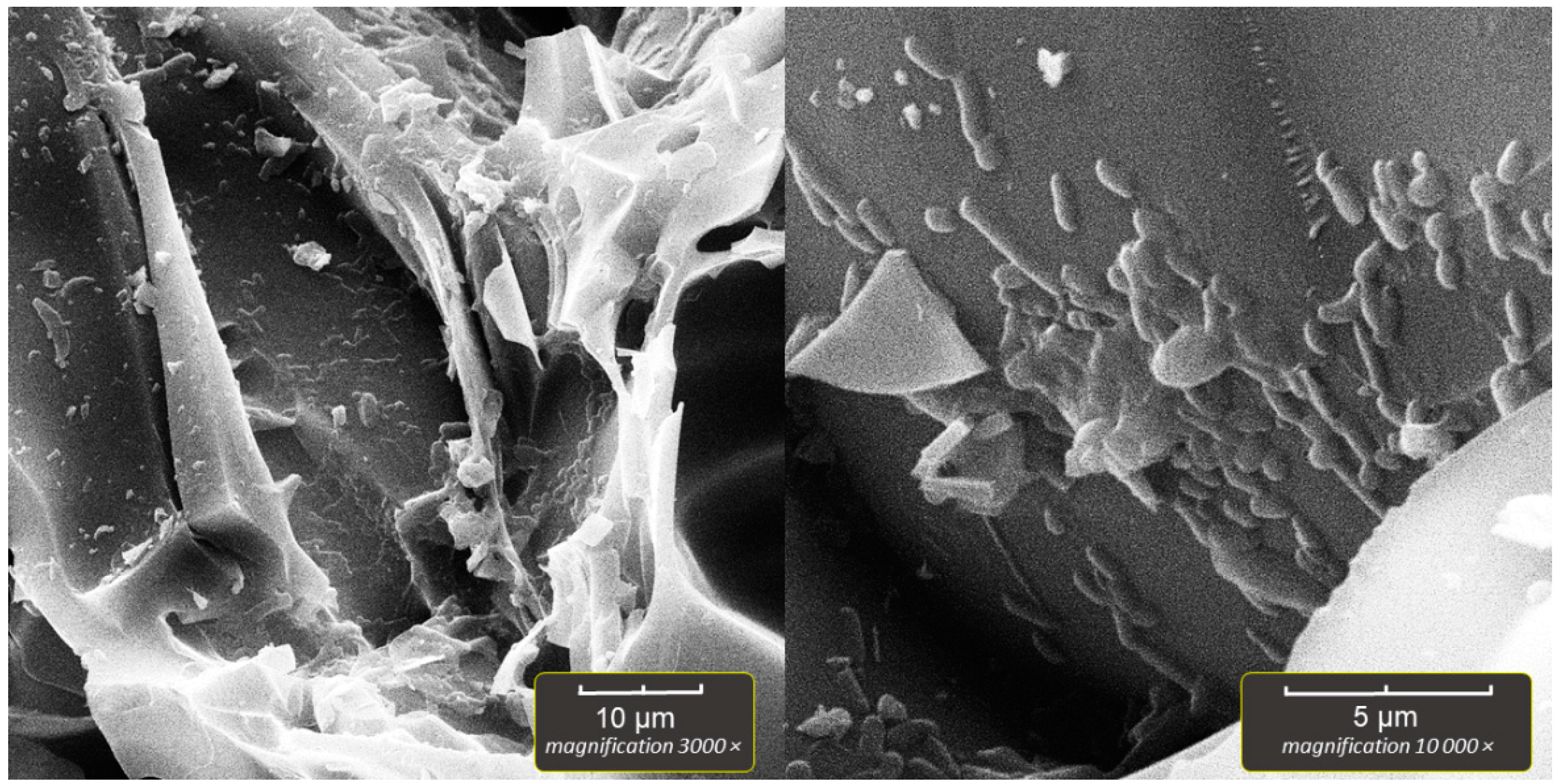
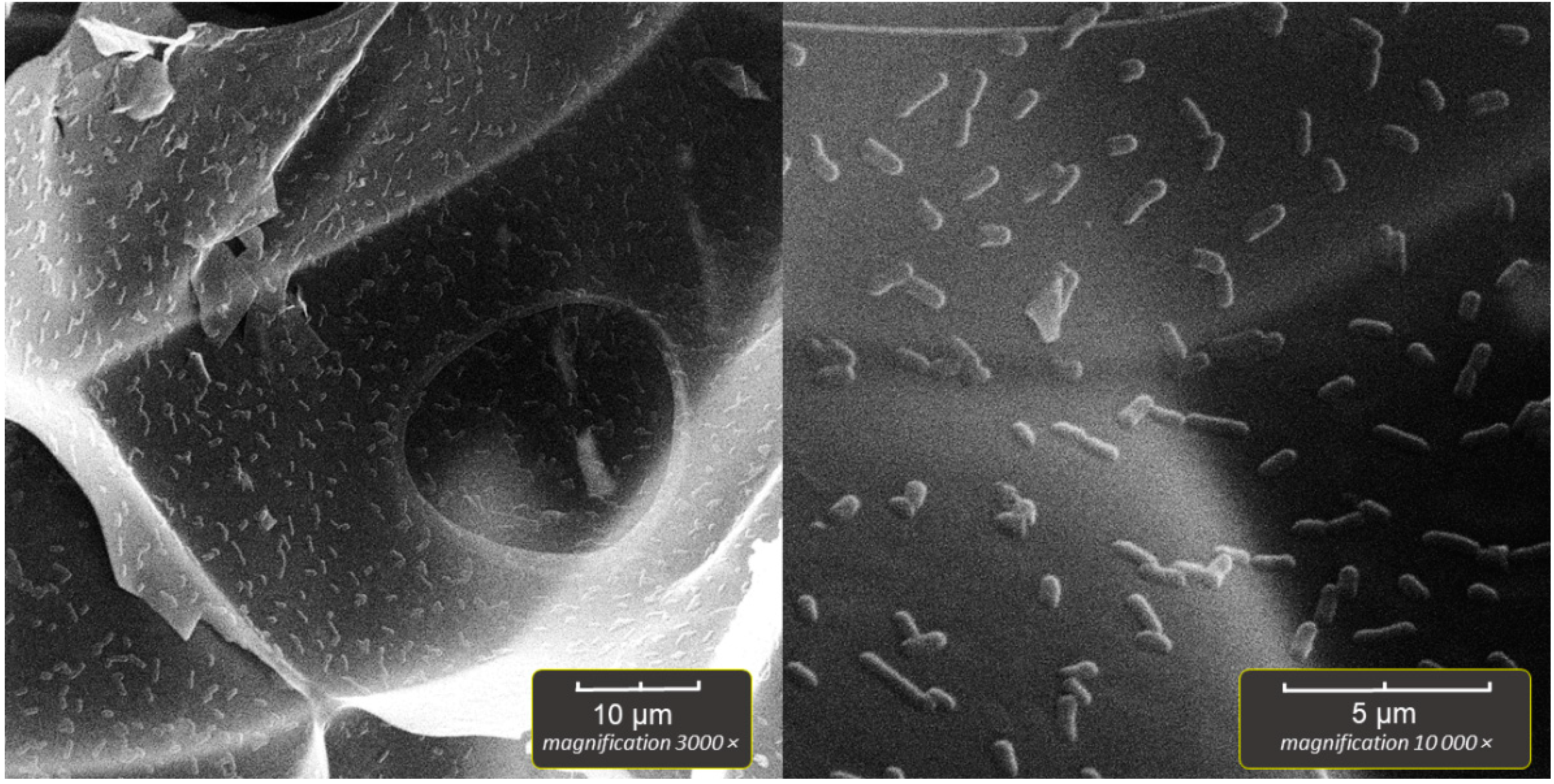
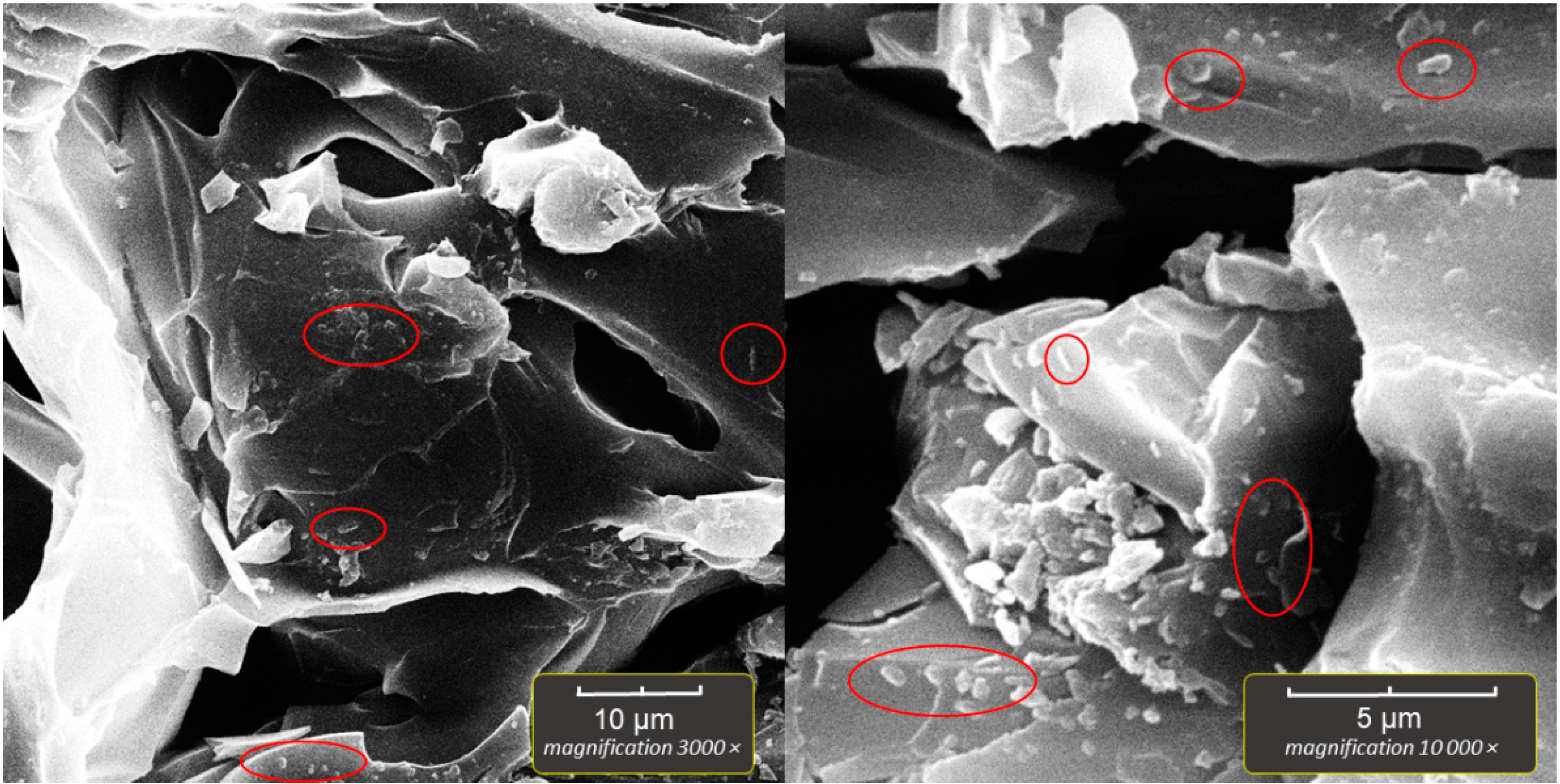
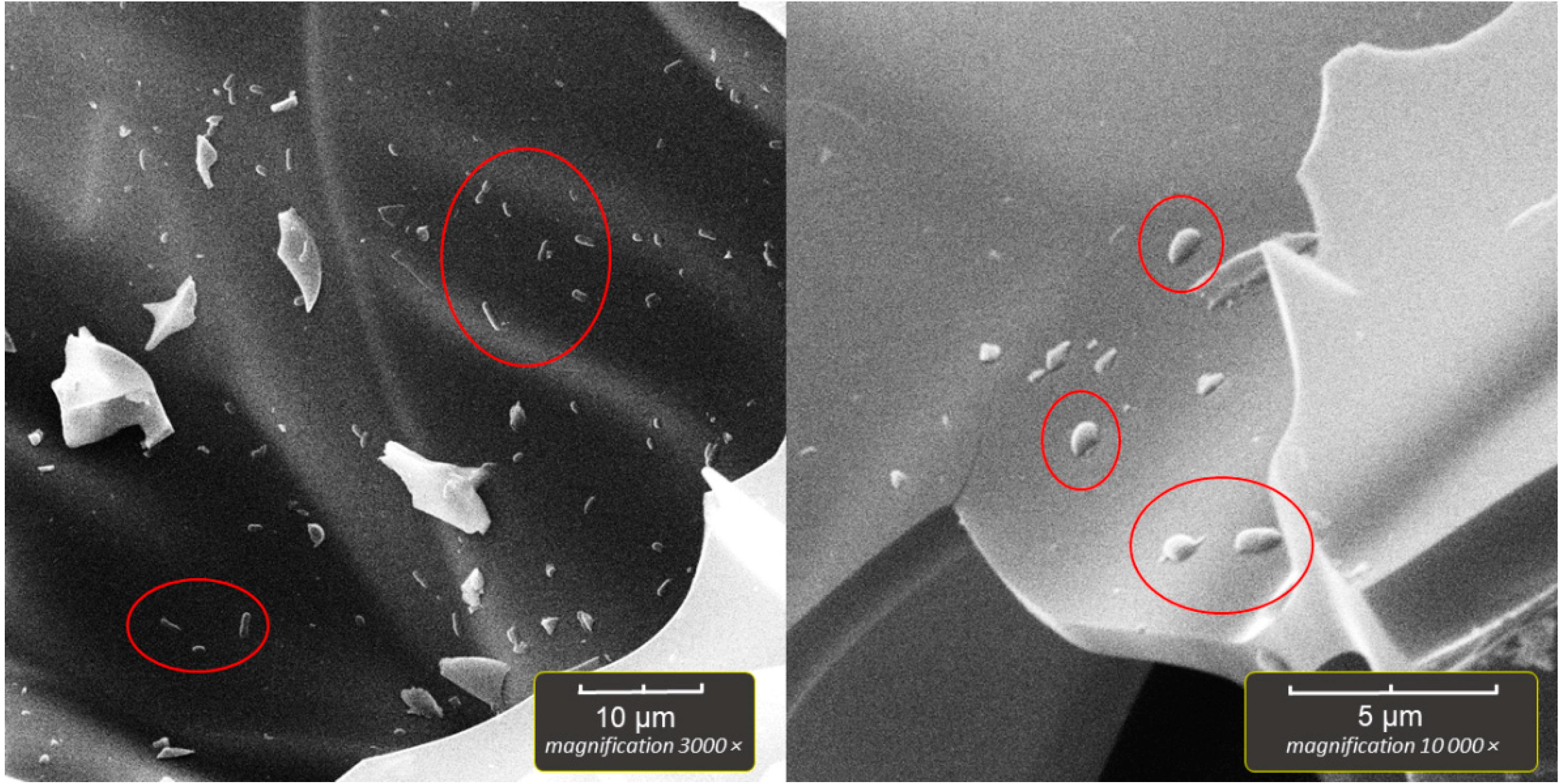
2.5. Scanning Electron Microscopy of Bioparticles (SEM)
The bioparticles were prepared for SEM by aseptically transferring several perlite particles, after the incubation and washing with sterile saline, to sterile Falcon-type tubes. The 2% solution of paraformaldehyde in PBS was then poured into the vials, which were kept in a refrigerator (4–8 °C) overnight to fixate the immobilized bacteria. Next, dehydration was performed in a series of ethanol solutions as follows; 30% EtOH/2 min, 50%/2 min, 70%/5 min, 96%/5 min, 100%/5 min, 100%/5 min. The final step was drying the bioparticles in a dry oven for 30 min at 50 °C. After drying, a single bioparticle was coated with a plasma of gold and palladium for 180 s and imaged by TESCAN Vega3 EasyProbe SEM at an electron beam energy of 7 keV.
2.6. Augmentation of Biogas-Producing Reactors
The biogas reactors were laboratory glass bottles filled with 400 mL of inoculum (
Figure 2). The inoculum was a sludge from anaerobic digestors and was collected at the Osilovac Ltd. biogas plant (Fericanci, Croatia), which uses cattle manure and corn silage as primary input feedstocks, as well as certain types of non-hazardous waste of plant and animal origin. In bottles filled with inoculum,
Table 1 lists the added material. The bottles were connected to a biogas monitoring system. The study of the potential of biogas production, i.e., anaerobic digestion, was carried out in mesophilic conditions (37 °C), controlled by a hot bath for 24 days. The complete laboratory scale bioreactor system was developed by CROTEH Ltd. (Croatia), and it consisted of 15 bioreactors with associated mixers, rotation speed and mixing regime control, a biogas purification section, and a flow meter. The glass bottles used for the anaerobic digestion were connected to a pre-calibrated flow meter via the tubing system. As the bioreactor produces biogas, it flows irreversibly through the system, where the flow meter records the volume of biogas produced and stores readings hourly in the internal memory (
Figure 2). Biogas potential measurements of olive waste were performed in triplicate by the volumetric method.
4. Discussion
Based on the results showing a high affinity for cell immobilization, the perlite proved to be a promising biocarrier for bacterial cells to augment biogas reactors. Perlite, also referred to as expanded perlite, is a naturally occurring aluminosilicate glassy volcanic rock. When this volcanic glass is heated to 760–1200 °C, it expands due to the evaporation of 2–5% of contained constitutive water, increasing the volume to 10–20 times its original volume. The obtained product is commercially called “perlite”. It is a lightweight, frothy material with low bulk density, low thermal conductivity, high sound adsorption, and is fire resistant [
16]. Due to such properties, perlite is widely used in the construction industry as an additive in insulation boards, concrete, or plaster.
As perlite is a non-toxic and relatively cheap natural material, it was proposed as a biocarrier in a number of studies showing that microorganisms attach to perlite in high numbers and remain metabolically active. To mention a few, Ivankovic et al. [
16] used perlite as a carrier of phosphate-accumulating bacteria
Acinetobacter junii intended to augment the tertiary phase of the wastewater treatment process. Foroughi et al. [
17] immobilized the yeast
Saccharomyces cerevisiae on perlite beads and improved the removal of aflatoxin M1 from milk. Darmayati et al. [
18] used perlite as a carrier of microbial consortium for oil degradation, and Nimnoi et al. [
19] successfully introduced several bacterial strains immobilized on perlite into soil.
In our experiment, the highest number of immobilized cells was obtained in systems with aeration, but in the system without aeration, the number of immobilized cells was also high (~10
10 cells per gram). Technologically, the perlite particles floated on the water surface and were floating during incubation in the aeration system, so we assumed this could decrease the degree of immobilization. In the second system, the particles were tightly sealed in the vial and rotated during the incubation, thus maximizing the water media/particle surface contact. It turned out that aeration significantly boosted the immobilization regardless of the reduced particle/water media contact. Two setups were evaluated, as they allow the cultivation of different bacteria types. With aeration, the attachment of facultative anaerobes (the obligate aerobes were eliminated during the anaerobic first step of isolation described in
Section 2.3 and
Figure 1) was propagated, and without aeration, the enrichment with obligate anaerobes is possible. As both bioparticle preparation systems give good results, in future research, the type of microorganism can be chosen, immobilized, and introduced into the biogas reactors.
The biocarriers have been described to be used in biogas production, although not with precolonized microorganisms as in this study. Weiβ et al. [
12] introduced zeolite particles to anaerobic bioreactors producing biogas from grass silage. The SEM and metagenomics analysis showed preferential attachment of certain types of bacteria and methanogenic archaea onto zeolite during the 84 days of incubation. The immobilized cells were viable and enzymatically active, but the effect of biocarrier addition on biogas production was not reported. On the other hand, Pilarska et al. [
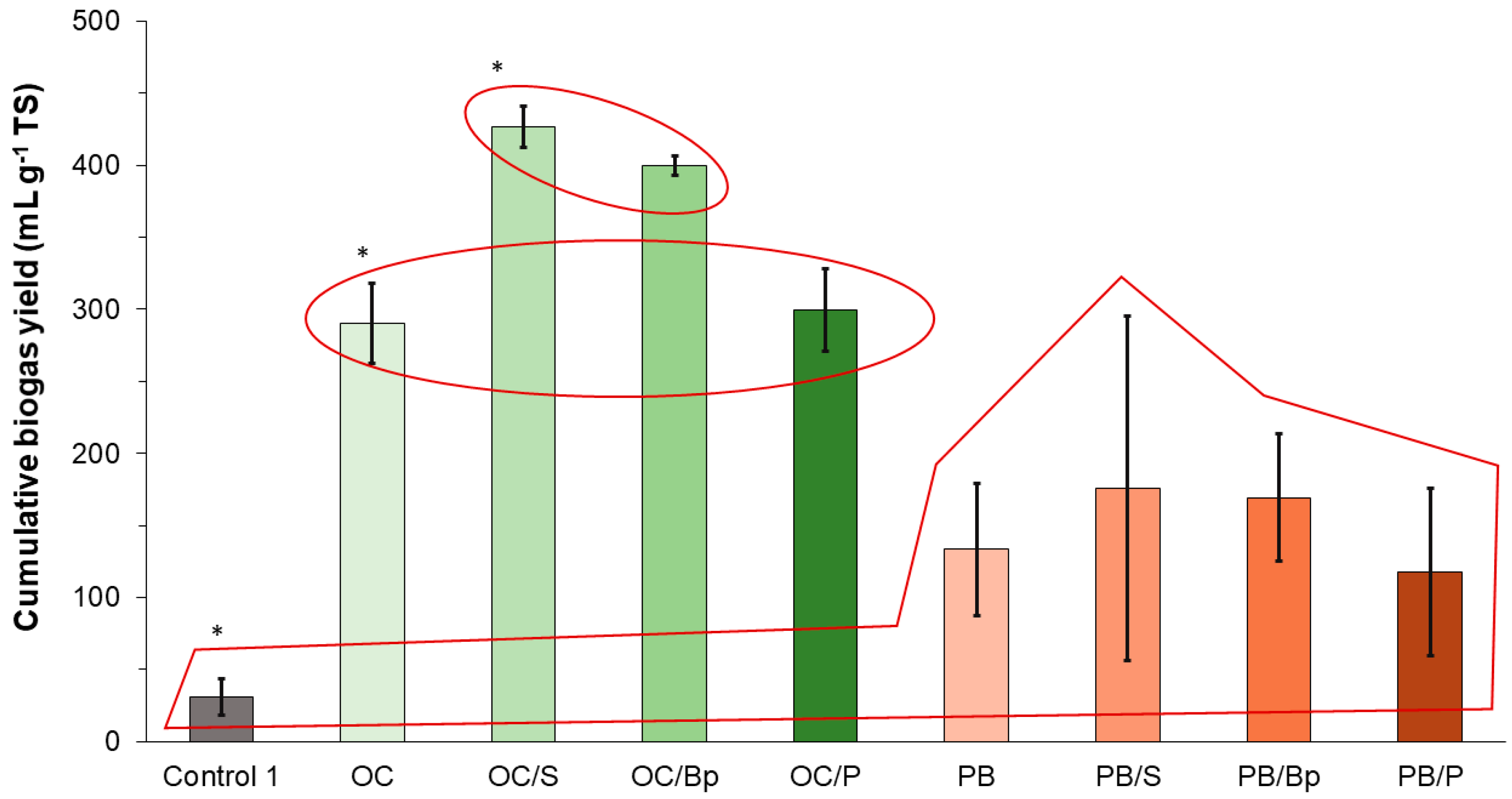
15] added kraft lignin and organic substrate to biogas-producing batch reactors and reported an increased cumulative yield of biogas, process stability, and intensified bacterial proliferation of inoculum sludge. In the case of this study, the addition of biocarrier itself did not affect biogas yield from the specific substrate, but the addition of bioparticles (perlite with immobilized microorganisms) and the suspension of bacterial consortium did. However, the yield was significantly increased only in the experiments with olive cake as a substrate. The bioactive compounds in OC, such as cellulose, organic fats, and phenols, are readily biodegradable [
20], and the volatile compounds are available for fermentation by bacteria [
21]. OC is, therefore, quite suitable as a substrate for anaerobic digestion as the native bacteria capable either for its degradation or fermentation should be abundant in anaerobic sludge. On the other hand, pruned biomass is composed mostly of lignin, which is less prone to biodegradation and is not usually found as a substrate in anaerobic sludge. The majority of microorganisms capable of lignin degradation belong to fungi, although several bacterial species have been identified [
22]. In this study, the bioparticles were prepared with organisms presumably targeted for lignin degradation, but the accent was on bacteria, not fungi. Although successfully cultivated, these bacteria probably have limited capacity for enzymatic lignin breakdown or could have been outgrown when introduced into the biogas-producing reactors. A better approach for future experiments might be the targeted isolation of fungi targeted for lignin degradation and their immobilization onto perlite bioparticles. Such findings indicate that bioaugmentation, either in the form of suspension or in the form of bioparticles made from the suitable carrier, can enhance the biogas production yield if the substrate itself is readily degradable by the indigenous inoculum microorganisms.
5. Conclusions
Perlite could be used as a suitable biocarrier for the augmentation of biogas-producing reactors. With a high affinity for cell attachment, both anaerobic and aerobic cells could be successfully immobilized, allowing the introduction of targeted microorganisms. Optimizing the biocarrier introduction would be desirable through experiments in which one would test more conditions of bioparticle preparation in combination with the anaerobic digestion conditions. Such would include completely anaerobic bioparticles or bioparticles with pure bacterial cultures. The bioparticles precolonized with methanogen archaea could also be prepared and used.
Additional research into the subject should use metagenome analysis of microbial consortium involved in the anaerobic digestion process, various natural carriers, and substrates. From a technological point of view, it should be answered what happens to the bioparticles in commercial reactors. Should they be replaced after each batch, or could the biocarrier immobilize the microorganisms in a manner allowing just the replacement of the substrate, enabling continuous operation? Systematic and comprehensive research of biocarriers in biogas production has the potential to significantly increase biogas yield with economic feasibility.