1. Introduction
Edible films and coatings are thin layers of edible materials that are formed directly on the surface of the food to enhance preservation, and they are consumed as part of the whole product. This is why their application is considered a physical and environmentally friendly method of food preservation [
1,
2,
3]. Various materials are used for the preparation of edible films or coatings, which are classified into three basic categories: polysaccharides, proteins and lipids [
4,
5].
These materials are desired to possess specific properties, which can be classified into the following categories: barrier, mechanical, microstructural, optical, physicochemical and thermal properties. Of these, the barrier, mechanical and microstructural properties are considered more important and therefore should be examined before the use/application of each material.
Edible films and coatings can also act as carriers for additives (antimicrobial, antioxidant and antifungal), which may further extend the shelf life of the coated products by reducing or inhibiting the microbial growth or oxidation on the food surfaces [
6,
7]. Especially, chitosan has attracted attention as a potential food preservative due to its antimicrobial activity against a wide range of fungi, yeasts and bacteria. For example, films containing chitosan can exhibit antimicrobial activity against
Staphylococcus aureus [
6,
8].
Each category of edible materials can impart specific properties to the final formed film or coating. Thus, their choice should be made based on the desired characteristics for a certain food application. Polysaccharides, such as cellulose, starch, pectin and chitosan, are widely available materials which possess good mechanical properties but poor water barrier properties [
9]. Protein materials, such as gelatin, casein and whey protein, provide effective barriers against O
2 and CO
2, but not for water. Proteins have satisfactory mechanical properties as well. Lipids, such as paraffin wax and beeswax, are excellent barriers against moisture migration, but they present some disadvantages such as fragility, lack of homogeneity and presence of holes and cracks on the surface of the coating [
4,
10].
Ιt is clear from the above, that edible films or coatings consisting of only one component may present deficiencies in some of their properties. Therefore, the development of composite films is proposed, as the combination of two or more components, preferably from different categories, can significantly improve their individual properties [
4,
5,
10,
11,
12]. For example, the low mechanical strength of lipids can be enhanced by the addition of water-soluble proteins or polysaccharides (hydrocolloids) [
9]. More specifically, mixing of gelatin with chitosan results in improved barrier properties [
13]. The addition of a second component may, also, be selected to provide antimicrobial or/and antioxidant activity [
14].
Based on the above, the current study investigates the use of chitosan (CH), beta-cyclodextrin (CD) and cellulose nanocrystals (CNC) for the development of efficient composite edible films and coatings.
Chitosan has the ability to form edible films and coatings with advanced mechanical properties. Beta-cyclodextrin, on the other hand, can provide homogeneity, transparency and higher mechanical properties [
6,
7,
15,
16,
17].
In the recent years, the use of nanomaterials is also investigated, in order to provide enhanced or even new properties to the edible films and coatings [
18,
19,
20,
21]. The nanomaterials have specific physical, optical and chemical properties. In addition, they can be synthesized to improve the performance of conventional materials, due to their particle size and their large surface area in respect to their shape. More specifically, the addition of nanomaterials, such as nano-starch or cellulose nanocrystals, may modify several properties such as flexibility, durability, thermal stability, barrier properties, and mechanical properties [
3,
22,
23,
24]. The nanomaterials, due to their size, offer a new way of modifying the gas transport in natural products, while increasing the mechanical resistance, transparency, functionality, and antioxidant and antimicrobial activity. Nano-systems are more stable and biologically active, allowing the incorporation of hydrophobic and/or active substances, without particularly affecting the final appearance or transparency [
24,
25,
26]. Nanomaterials in the food industry can altogether provide: (i) sensory enhancement (taste/color improvement or texture modification), (ii) increased absorption and targeted supply of nutrients and bioactive compounds, (iii) stabilization of food, (iv) packaging and product innovation to increase shelf life, (v) food safety, and (vi) antimicrobial protection against foodborne pathogenic bacteria [
27,
28,
29,
30].
The current study first examines the use of cellulose nanocrystals or beta-cyclodextrin in composite edible films and coatings containing chitosan as their basic material. More specifically, the effect on the properties of the resulting CH/CNC and CH/CD films is examined. In addition, the basic characteristics of the films formed by the combination of all three materials (CH–CNC–CD) is examined. These three components have been investigated in the literature alone or along with various other materials [
31,
32,
33,
34,
35]. For example, Xu et al. [
34] and Ye et al. [
35] used gelatin as their main film forming material and incorporated CD or CNC as an additive respectively.
The innovation of the current study lies in the investigation of the CH/CNC, CH/CD and CH–CNC–CD combinations. Chitosan was chosen as the basic material due to its ease of use, its wide availability, its ability to form a film, as well as its high biocompatibility and biodegradation. Moreover, according to the literature review, the triple combination of CH–CNC–CD materials has not yet been studied.
Additionally, most of the available studies, so far, focus on the effect of edible films and coatings on the properties of food products [
36]. For example, Fang et al. [
31] and Reyna et al. [
37] investigated the effect of chitosan edible films on papaya and broccoli, respectively. However, the current study focuses on the properties of films and coatings themselves. Before being applied to a food product, the optimal proportions of the components of edible films and coatings, as well as their resulting properties should be examined, in order to enhance their functionality and characteristics according to the intended food application. For that reason, this study aims to fully investigate the properties of the final formed films and the solutions from which the films are formed.
2. Materials and Methods
2.1. Materials
For the preparation of the edible films, the following materials were used: high molecular weight CH (Deacetylated chitin, ≥75% deacetylated, molecular weight 310,000–375,000 Da, Poly[D-glucosamine]) from Sigma-Aldrich (St. Louis, MO, USA), acetic acid (glacial 99–100% a.r.) from Chem-Lab NV (Zedelgem, Belgium), CNC from CelluForce (Montreal, QC, Canada) and CD from Acros Organics (Geel, Belgim). The specifications of CNC and CD used in the current study are presented in the
Supporting Material.
2.2. Preparation of Edible Films
CH/CNC and CH/CD Edible Films
Initially, the concentration of the primary film solutions was selected based on preliminary experiments. The films were prepared as follows: solutions of CH 1% w/v were produced by dispersing chitosan and acetic acid in distilled water (1 g chitosan/100 mL water and 1 mL acetic acid/100 mL water) and stirring with a magnetic stirrer for 2 h at 80 °C and subsequently overnight at room temperature. The CNC and CD solutions were prepared by dispersing cellulose nanocrystals and beta-cyclodextrin, respectively, in distilled water (both 1 g/100 mL water). Following, part of the CH 1% w/v solutions was mixed with the CNC 1% w/v or CD 1% w/v solutions in proportions of 75/25, 50/50 and 25/75. CH/CNC and CH/CD solutions were degassed using an Elmasonic Ultrasonic device S30H (Elma Schmidbauer GmbH, Singen, Germany) (280 W/60 Hz) for 15 min at 30 °C. After that, 20 mL of the final solutions were poured into Petri dishes (with diameter of 9 cm) and allowed to dry in a vacuum oven at 50 °C for 24 h. Eventually, the dried films were kept at 20% relative humidity and room temperature.
CH–CNC–CD Edible Films
Solutions of CH/CNC and CH/CD (both in proportion of 50/50) were used. These were prepared as described in the previous paragraph. The CH/CNC and CH/CD solutions were mixed together using a magnetic stirrer, in proportions of 75/25, 50/50 and 25/75. Thus, three new solutions were produced, having the following proportions of CH–CNC–CD, respectively: 50–37.5–12.5, 50–25–25, 50–12.5–37.5. Finally, they were degassed, poured into Petri dishes, allowed to dry and stored as described above.
2.3. Measurements
2.3.1. Density and pH
In order to determine the density (g/mL) of the solutions, the weight of 20 mL samples was measured (seven repetitions for each solution at 25 °C). The samples were taken using a laboratory pipette. Each weight was divided by 20 mL and the average density values were calculated.
The pH of the solutions was measured using a digital pH-meter 3310 (Willis Towers Watson, London, UK) at 25 °C. This measurement was repeated three times.
2.3.2. Surface Tension
The surface tension of each solution was measured with the wihelmy plate method, using a Sigma 700 digital Force Tensionmeter (Attention, Biolin Scientific AB, Västra Frölunda, Sweden). Ten measurements were performed for each sample solution at 25 °C and the average values were calculated.
2.3.3. Viscosity and Rheological Parameters
The rheological parameters: consistency index (k) and flow behavior index (n), were analyzed by means of a RC1 rotational rheometer (Rheotec GmbH, Radeburg, Germany). The rheological curves (shear stress, τ–shear rate, γ) were obtained after a stabilization time of 5 min at 25 °C. Shear stress was determined as a function of shear rate between 0 and 300 s−1, with the following procedure: 3 min to attain the maximum shear rate and 2 min at the maximum shear rate.
Finally,
k and
n were exported by applying the following equation to the rheological curves obtained through the rheometer’s software:
where
τ is the shear stress,
γ is the shear rate,
k is the consistency index and
n is the flow behavior index.
The procedure was repeated three times for each case at 25 °C and the final values of k and n were calculated as an average of the individual values. Apparent viscosity was calculated at 256 s−1.
2.3.4. Thickness
The thickness of the films (mm) was determined using a stack of 7 films per sample. Measurements were made with a hand-held micrometer at five different points for each film.
2.3.5. Color
The color of the films was determined with a CR-200 Colorimeter (Konica Minolta, UK). The L (Luminosity), a (Red-Green) and b (Yellow-Blue) color parameters of the Cielab scale were used for the calculations. A white plate was used also, as a standard (L0, a0 and b0).
The following calculations were made [
38]:
Yellow index:
where Δ
L =
L −
L0, Δ
a =
a −
a0 and Δ
b =
b −
b0.
2.3.6. Transparency
The transparency of the films was determined by measuring their absorbance with a U-2900 UV-Vis spectrophotometer (Hitachi, Tokyo, Japan) at 550 nm through the following formula:
where
Abs550 is the absorbance’s value at 550 nm and
l is the film’s thickness (mm).
2.3.7. Moisture
The moisture of the films was determined by measuring their weight loss (2 cm × 2 cm) upon drying in an oven at 110 °C for 24 h (dry sample weight). Moisture (g H
2O/100 g film) was calculated as follows [
39]:
2.3.8. Mechanical Properties
For the study of the mechanical properties, a TA-XT2i Texture Analyzer (Stable Micro Systems, Godalming, UK) was used, with a cylindrical probe having a diameter of 5 mm. The initial grip separation and the crosshead speed were set at 20 mm and 10 mm/s, respectively. Measurements were made on circular samples with a diameter of 5 cm each. The Texture Analysis software provides the curves of force (N) versus deformation (mm).
Based on the ASTM D882-10 [
40] standard, the following parameters can be obtained from force versus deformation curves:
- -
maximum breaking force (N);
- -
breaking factor (maximum breaking force divided by film thickness, N·mm−1);
- -
deformation at break (extension at the moment of rupture, mm);
- -
percent of elongation at break (deformation divided by the initial probe length and multiplied by 100%);
- -
elastic modulus (slope of force–deformation curve, N·mm−1).
Breaking stress (MPa) was calculated by dividing maximum force by the film cross-section (thickness × width).
2.3.9. Oxygen Permeability
The oxygen permeability was measured as follows: an edible film is sealed between two specially designed metallic cups, each of which has a diameter of 6 cm and a depth of 3 cm. Both cups have two channels. Oxygen enters the lower cup from the down left entrance and exits from the down-right channel of the same cup. A stream of nitrogen enters the upper cup from the up-right entrance and comes out from the up-left exit of the same cup. The nitrogen acts as a carrier that transfers the oxygen permeating through the film (from its one side to the other) into a wet analysis system. The design of this system is mainly based on iodimetry according to the ASTM D3985-05 [
41] standard. It consists of a conical flask containing an aqueous manganese II sulphate and alkaline iodide solution [
42]. The gas mixture (N
2 and permeated O
2) passes through the wet system for a specific time period and, in the presence of oxygen, manganese II hydroxide is, rapidly and quantitatively, converted to manganese III hydroxide by the following reaction:
4 Mn(OH)2 + O2 + 2 H2O → 4 Mn(OH)3 ↓
The brown precipitate formed, is rapidly dissolved causing the oxidation of the iodide ions present to iodine:
Mn(OH)3 + I− + 3 H+ → Mn2+ + ½ I2 + 3 H2O
The liberated iodine is then titrated with a standard thiosulphate solution:
2 S2O32− + I2 → S4O62− + 2 I−
Finally, the Oxygen Permeability (
OP) of the film is calculated by the equation:
where
m is the mass of O
2 permeated through the film with a thickness of
d and an area of
A, over the measured time interval
t, and Δ
P is the difference in the O
2 pressure between the two sides of the film.
2.3.10. Water Vapor Permeability
The method of Bertuzzi et al. [
43] was used to determine water vapor permeability. The films were sealed in glass cups containing 10 mL of distilled water. Each glass cup was placed in a desiccator and kept at 40 °C and 75% relative humidity, using a saturated sodium chloride solution. The water vapors’ transfer through the films was determined by measuring cups’ weight periodically for 24 h. The weight’s changes of the glass cups versus time were calculated and plotted. Linear regression was used to calculate the slope of the fitted straight line, representing the Δ
m/Δ
t ratio.
The Water Vapor Permeability (
WVP) was calculated by the following equation:
where Δ
m is the amount of water vapor transferred through a film of area
A and thickness
l during a finite time Δ
t, and Δ
P is the vapor’s pressure difference across the film.
2.3.11. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
The FTIR measurements were performed to evaluate the structural interactions of the films. The spectrum of the films was recorded using an ATR-4200 FTIR instrument (Jasco, Easton, MD, USA) at a wavenumber range of 700–4000 cm−1 and resolution of 1 cm−1.
2.3.12. Scanning Electronic Microscopy (SEM) Analysis
The morphology of the surface and the cross-section of the films were examined using scanning electron microscopy (SEM) (Edwards Sputter Coater, Crawley, UK) at an accelerated electron energy of 25 kV. Prior to scanning, the films were coated with a thin layer of gold.
2.4. Statistical Analysis
Experiments for edible films’ preparations were carried out twice, as well as the measurement of all their properties (unless otherwise indicated), and the results were expressed as mean ± standard deviation (SD).
The statistical processing of the results was carried out with the Statistica 13.0 software (StatSoft, Inc., Tulsa, OK, USA). The significance of each experimental factor (the addition of CNC or CD to CH, the proportion of CH/CNC or CH/CD and the proportion of CH–CNC–CD) was assessed through the ANOVA variance analysis. Significant differences were considered at the p < 0.05 level. In such cases, the Duncan’s Test was further applied.
3. Results and Discussion
In the current study, the effect of CNC or CD addition to CH edible films and coatings, in proportions of 75/25, 50/50 and 25/75 (CH/CNC or CH/CD), was examined. Their combined effect was subsequently examined in various CH–CNC–CD mixtures, in order to achieve the best possible results for specific properties of the composite films. In each case, the properties of the primary solutions (from which the edible films were formed), as well as those of the final films, were studied. The parameters examined are analyzed extensively in the following paragraphs.
3.1. CH/CNC and CH/CD Edible Films
The properties of the primary solutions and the final formed edible films for CH and the CH/CNC and CH/CD mixtures are presented and evaluated in the following paragraphs.
3.1.1. Thickness and Physicochemical Properties
Both the formation of edible films and coatings and their application in foods, are affected by physicochemical properties [
44].
The effect of CNC or CD incorporation on the thickness and physicochemical properties of the CH samples is reported in
Table 1 and
Table 2. The physicochemical properties measured were density (ρ), viscosity (η), consistency index (k), flow behavior index (n), pH, surface tension and moisture. Thickness and moisture were studied in the final formed edible films, while the rest properties were studied in the primary solutions.
Results show that the standard CH solution exhibited high surface tension (52.017 mN/m,
Table 1), which is desirable for a coating, but, on the other hand, it presented a significantly high viscosity (167.34 mPa·s,
Table 1), which may hinder its handling. In general, the combination of high surface tension and low viscosity is considered ideal for a coating [
45]. The high viscosity of the CH sample explains the high values of the other two rheological parameters (k and n). As it is obvious, it is required to reduce the viscosity of the solutions in order to improve the coating process.
The addition of CNC or CD to the CH solutions changed their physicochemical properties and, especially, their rheological parameters (
Table 1). In particular, they both reduced the consistency of the CH solutions and led to reduced viscosity values (from 167.34 mPa·s to 90.67–54.83 mPa·s), which facilitates handling and processing. This can be easily explained as CNC and CD form solutions with lower viscosity than CH. Furthermore, the surface tension presented small changes and remained in high values, which is beneficial for the coating procedure [
45].
The final formed edible films with only CH had relatively high moisture (11.0875 g H
2O/100 g film,
Table 2), probably because chitosan is a hydrophilic substance and holds a considerable amount of water in its three predominant absorption sites: hydroxyl group, amino group and polymer chain end [
14]. High moisture can favor the growth of microorganisms acting negatively on the antimicrobial activity of the edible films and coatings and consequently on the shelf life of the food product it is applied to [
46]. On the other hand, the thickness of the CH films was relatively low (0.04 mm,
Table 2), which is a positive attribute. It should be noted that edible films with thickness less than 0.25 mm are considered thin [
47].
In contrast to the addition of CNC to CH, which maintained the moisture content of the final formed edible films at the same levels, the addition of CD to CH reduced films’ moisture. This can be explained by the final structure of the CH/CD films. More specifically, they consist of hollow truncated cone structures with an external hydrophilic character and an internal hydrophobic character, which means that the interactions between CD and CH include hydrophobic forces [
48]. When compared to the studies of Xu et al. [
34] and Ye et al. [
35], where the basic film forming material was gelatin (another widely used basic material), lower moisture values were observed in the present study, which is obviously considered beneficial. Finally, the thickness of the final formed edible films was not significantly changed by the addition of either CNC or CD to the CH, regardless of the level of addition, with all films presenting a thickness between 0.03 mm and 0.04 mm.
The above results show that both CNC and CD, provide composite films with improved physicochemical properties. With the only exception in the case of humidity, CD proved slightly more advantageous than CNC.
3.1.2. Barrier Properties
Barrier properties are extensively studied in edible films as they are involved in reactions that may result in food deterioration. OP and WVP were measured for this purpose. These are both affected by the materials used, the preparation method, the type, level and dispersion quality of additives, and the voids, cracks and chains’ order of polymers [
44,
49].
Oxygen and water vapor can be transferred from the indoor/outdoor environment through the polymer, resulting in appreciable changes in the products’ quality and shelf life. The target is to achieve low values in both OP and WVP because this leads to lower mass transfer between the food product and the environment, resulting in lower weight loss, oxidation, and microbial contamination.
Water vapor barrier properties can be quantified by WVP, which indicates the amount of permeating water per unit area and time (kg/m·s·Pa) [
8,
44]. Additionally, the oxygen barrier properties can be quantified by OP, which indicates the amount of permeating oxygen per unit area and time (kg/m·s·Pa) [
50,
51].
The effect of CNC or CD on the barrier properties of the final formed edible films is shown in
Table 2.
The final formed edible films with only CH presented low OP values and, despite their hydrophilic nature, they exhibited low WVP values, as well (
Table 2). Nevertheless, the addition of CNC or CD led to a further significant reduction (
p < 0.05) in these values, which in some cases exceeded 50%. These results are in agreement with related studies claiming that the addition of nanomaterials to edible films improves their barrier properties [
24,
52]. This is attributed to the high crystallinity of CNC [
53]. Oxygen and water vapor diffuse more easily through the amorphous areas of the polymer matrix, so the increase of the crystalline region, formed by a network of hydrogen bonds, results in a stable polymer which improves the block permeability behavior of the nanocomposite edible films [
54]. In the case of the CH/CD films, the effect on the barrier properties is due to hydrophobic nature of these materials, which provides higher moisture barrier and water resistance, and due to the forces between CD and CH, which enhance the stability of the composite films [
6].
Finally, when comparing results from the addition of CNC or CD, CD led to superior barrier properties, which makes it a better choice between the two materials in question.
3.1.3. Optical Properties
Optical properties are crucial features that affect the suitability, the appearance and the marketability of edible films and coatings for various applications. They include color and transparency and can be easily detected by human vision. They characterize the surface and affect certain aspects of foods’ quality [
43,
55]. Therefore, it is desirable to produce films with lighter color and increased transparency. For this reason, the difference of color with a white plate (ΔE), the white index (WI), the chrome (C*), the yellow index (YI) and the transparency was measured in all film samples.
The effect of CNC or CD on the optical properties of the final formed edible films is shown in
Table 3.
The ΔE values of the films indicate differences that can be perceived with a naked eye, as ΔE > 1 [
56]. In particular, the final formed edible films with only CH presented the highest ΔE value, which was expected as these films had a light-yellow color. For the same reason, these films presented the lowest WI value. The incorporation of CNC and CD caused significant changes (
p < 0.05) in most of the optical properties, leading to the improvement of the final formed films (
Table 3). Both CNC and CD reduced the color difference between the final formed edible film and the white plate (ΔE). This is indicated by the increased WI and the decreased YI values, as well as the increased transparency, which is desired for edible films and coatings. In general, the transparency of the films was increased with the addition of both CNC and CD. The C* value, which is indicative of the color intensity, also remained low in all cases [
38].
The above experiments show that both materials (CNC and CD) contributed to the improvement of the optical properties with comparable results. Nevertheless, the CH/CD samples showed slightly lower C* values giving a small advantage in choosing CD over CNC.
3.1.4. Mechanical Properties
Mechanical properties are very important as they provide a direct indication of the strength and cohesion of the films or coatings. They are related to the structural coherence and the mechanical resistance against the destruction of the food product during its transport, storage or preservation. Commonly, the mechanical resistance of films is studied according to (i) the breaking stress (σ), which shows the maximum traction force per film’s cross section required to break the film, and (ii) the elongation at break (ε), which provides the degree to which a film can be stretched before it breaks [
55,
57,
58].
The mechanical properties of the films measured in this study were the maximum breaking force (F), the breaking factor (Puncture Strength), the deformation at break (D), the percent of elongation at break (ε) and the elastic modulus and the breaking stress (σ). Mechanical properties concern not only the hardness of the films but also their stability and homogeneity. The target was to produce edible films with the ideal combination of these characteristics.
Table 4 shows the effect of CNC and CD addition on the mechanical properties of the formed edible films.
Results show that the films with only CH were flexible and presented high mechanical strength. In fact, they presented high values in all the properties examined (
Table 4). This is in agreement with similar studies, according to which chitosan is a material that creates edible films and coatings with good mechanical properties [
6,
59]. This is also confirmed by comparison with other popular film forming materials, such as gelatin, which exhibit significantly lower values in their mechanical properties [
34,
35].
Results show that the addition of CNC or CD to the CH samples slightly degraded the mechanical properties of the composite films. This degradation, however, was not statistically significant. An exception was the sample with CH/CD (50/50) which presented similar or higher values in its mechanical properties compared to the control sample. This is attributed to the same ratio of the two components (CH and CD), which allows them to be more evenly distributed on the surface of the film and results in a structure that facilitates the uniform distribution of the entered stress across the film and reduces stress concentration areas [
60].
Additionally, results indicated that the addition of CD had a significant influence (
p < 0.05) on the elastic modulus (
Table 4). This is associated with the interactions between CH and CD and the final structure of the formed edible films. The comparison of the two materials in question shows that in some cases the CNC containing films presented higher mechanical strength than the CD containing films. This is probably due to the ability of nanocrystals to facilitate load transfer and stress distribution, thus increasing the stability of the edible films [
61].
3.1.5. Microstructure Properties
The understanding of the microstructure of a film or coating is very useful as it determines its mechanical, physicochemical and barrier properties, and furthermore specifies its application. The structure of edible films can be studied by: (i) scanning electron microscopy (SEM), to investigate the structure changes of the films and obtain their surface and cross-sectional topography and (ii) Fourier-transform infrared spectroscopy (FTIR) analysis, to examine the interactions between the components of the films [
45,
55].
FTIR Analysis
The types of bonds between (i) CH and CNC and (ii) CH and CD, were studied with FTIR measurements on the final formed edible films.
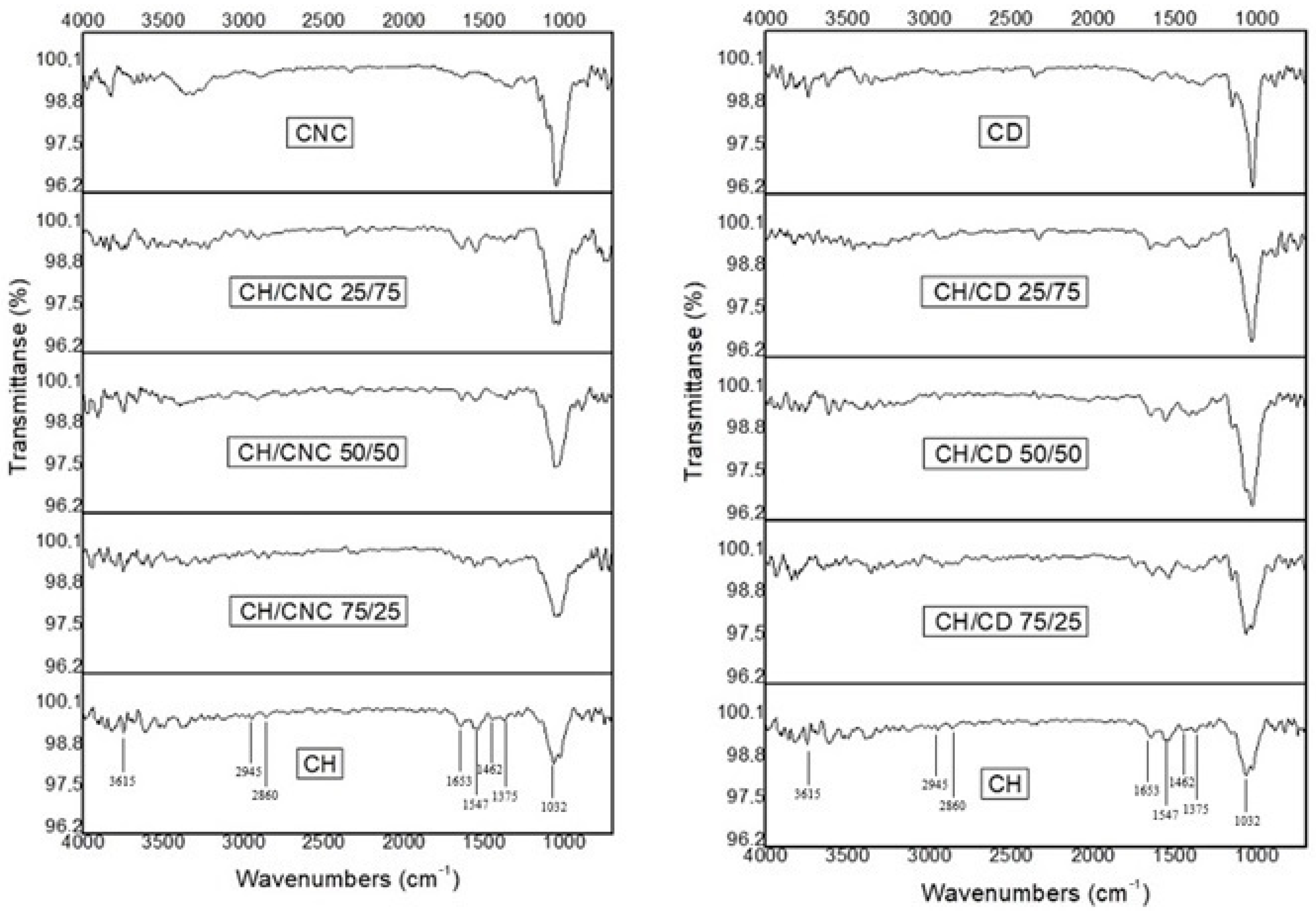
Figure 1 depicts the FTIR spectra of the different final formed edible films.
The FTIR spectrum of pure CH film, shows a broad absorption peak, with its center at about 3615 cm
−1, which was related to the stretching vibration of O−H groups and to the intermolecular and intramolecular hydrogen bonds. Two absorption peaks appeared at 2945 and 2860 cm
−1, which were attributed to the symmetrical and asymmetric stretching vibrations of the carboxylate group (−CH
2) respectively. The absorption peaks at 1462, 1547 and 1653 cm
−1 were attributed to the amides III (HN–CO), II (NH) and I (−C=O). The absorption peak at 1375 cm
−1 was due to the bending vibrations of O−H. Finally, the absorption peak at 1032 cm
−1 was attributed to the C–O–C stretching vibration [
61].
Results in
Figure 1 indicate that no chemical reactions occurred between CH and CNC or CH and CD during the production of composite films. There were only peaks around the absorption peak of O−H (centered at 3615 cm
−1), indicating the formation of intermolecular hydrogen bonds between the components. Moreover, a significant increase in the intensity of the absorption peaks at 1032 cm
−1 occurred by the addition of both CNC and CD to the CH samples.
SEM Analysis
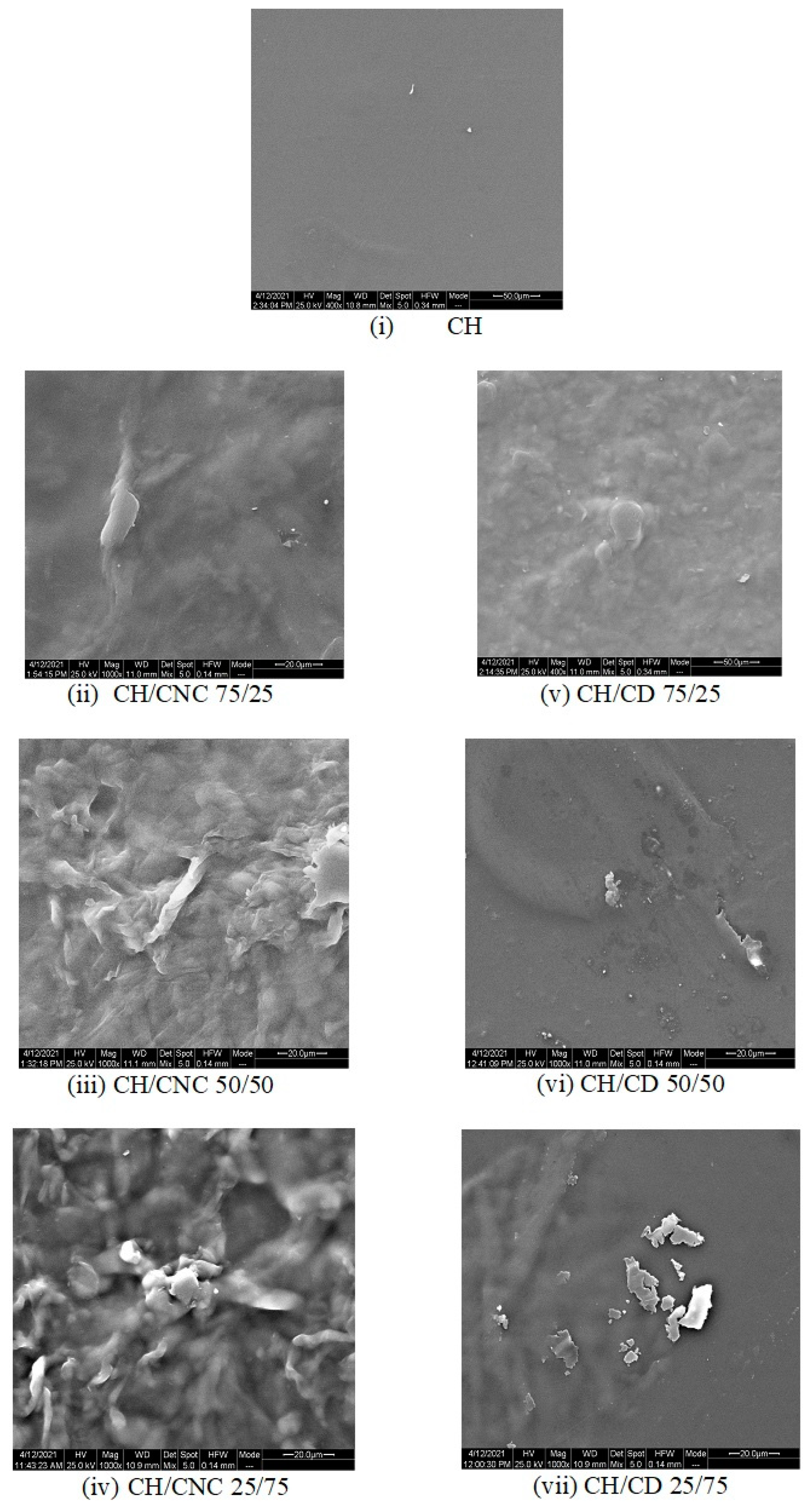
The SEM images of the CH, CH/CNC and CH/CD final formed edible films depict the distribution of the composing materials on the surface of the films (
Figure 2).
The surface of the CH/CNC films was rougher compared to those with only CH, probably due to the formation of a polyelectrolyte–macroion complex (PMC) between CNC and CH [
61,
62]. The 75/25 and 50/50 proportions of the CH/CNC films showed lower heterogeneity and a more dense structure compared to the 25/75 ones, with less CNC agglomerates and/or PMC crystals on the film surface, indicating a better dispersion of the CNC within the CH matrix at the lower CNC concentration [
61,
62]. Additionally, the surface of the 25/75 CH/CNC films contained more crystals, apparently due to the increased CNC and/or PMCs concentration. On the other hand, the addition of CD to the CH samples created a heterogeneous structure in which crystals of CD were entrapped in the continuous polymer network. It is obvious that the CD crystals became more intense as the amount of CD was increasing.
3.1.6. Summary of Results for CH/CNC and CH/CD Edible Films
The overall evaluation of the above results shows that the addition of CNC as well as CD had an enhancing effect on the CH films, both decreased the viscosity, leading to better coating applicability. Moreover, they both improved the barrier properties of the final formed edible films. Furthermore, the addition of CNC led to films with a more stable structure and higher mechanical strength, while CD improved their barrier and optical properties by increasing the transparency and reducing the color index. Finally, the 50/50 proportions for both CH/CNC and CH/CD films provided the best overall results.
All the above-mentioned findings supported the idea of combining the two materials (CNC and CD) in a ternary mixture with CH, in order to examine whether each material retains its beneficial properties in the final mixture. The results of this research are analyzed below.
3.2. CH–CNC–CD Mixture Characteristics
In order to study the combined effect of CNC and CD, samples of CH/CNC and CH/CD, both in proportion of 50/50, were mixed at three levels (75/25, 50/50 and 25/75). Therefore, the final proportions of the CH–CNC–CD samples were 50–37.5–12.5, 50–25–25 and 50–12.5–37.5 respectively. The following properties were studied: viscosity, surface tension, thickness, oxygen permeability, water vapor permeability, color difference, elastic modulus and breaking stress (σ). Viscosity and surface tension were evaluated in the primary solutions and the rest in the final formed edible films.
It was observed that most properties (viscosity, oxygen permeability, water vapor permeability, color difference, elastic modulus and breaking stress) underwent statistically significant changes (
p < 0.05) in the composite films. Samples with CH–CNC–CD in the proportion of 50–37.5–12.5 displayed the best combination of viscosity and surface tension (
Table 5) for coating (low viscosity and high surface tension) and better mechanical properties. As far as the barrier properties (
Table 5) are concerned, samples with CH–CNC–CD in the proportions of 50–25–25 and 50–12.5–37.5 showed better results compared to samples with CH–CNC–CD in the proportion of 50–37.5–12.5. As expected, the color difference (
Table 6) decreased as the CD proportion increased, due to the higher transparency of the CH/CD solution. Therefore, samples with CH–CNC–CD in the proportion of 50–12.5–37.5 excelled in their optical properties. Correspondingly, films with increased CNC proportions, presented higher mechanical properties (
Table 6), as nanocrystal compounds create more stable structures.
Apparently, there is no ideal ratio of CH–CNC–CD to achieve the best results in all properties. Thus, the choice of the most suitable combination depends on the priorities set in each case. More specifically, the 50–25–25 mixture is considered optimal when it comes to barrier properties. The 50–37.5–12.5 mixture provided enhanced coating characteristics and high mechanical properties. Finally, regarding optical properties, the 50–12.5–37.5 mixture offers the most appropriate solution.
4. Conclusions
The results of the present work highlight the positive contribution of both CNC and CD to the improvement of the properties of CH films (both in the primary solutions and in the final formed edible films). They both led to enhanced coating characteristics by decreasing the viscosity by more than 50%. They also improved the barrier properties of the occurring films. Specifically, CD decreased the oxygen permeability by more than 50%, as well as the color of the final formed edible films at a significant level, thus improving their optical properties. The films that contained CNC had higher mechanical strength as nanocrystals create more efficient load transfer. In general, the addition of CNC to the CH samples increased the stability of the final formed edible films. For both materials, the 50/50 ratio with CH gave the best overall results.
In the case of the CH–CNC–CD mixture, no proportion appeared to clearly provide the optimal properties, compared to the other proportions. However, the addition of both CNC and CD to the basic material CH, in all proportions, improved most of the properties which were examined. Increasing the CD content led to better optical properties (lighter color and increased transparency), while increasing the CNC content led to better coating characteristics (increase of surface tension and decrease of viscosity) and superior mechanical properties (elastic modulus and breaking stress).
According to the results analyzed above, the application of the triple combination of CH–CNC–CD for the formation of edible films and coatings leads to improved film properties and therefore can be generally recommended.
The results of the present study were a trigger for further investigation, especially as far as the combination of CH–CNC–CD is concerned. In this context, further specialized study of the CH–CNC–CD mixture is underway, including comparison with the triple mixture of hydroxypropyl methylcellulose (HPMC), CNC and CD (HPMC–CNC–CD).