Deep Learning Model for Classifying Periodontitis Stages on Dental Panoramic Radiography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dataset
2.1.1. CBNUH Dataset
2.1.2. AIHub Dataset
2.2. Methods and Algorithm
2.2.1. Data Augmentation
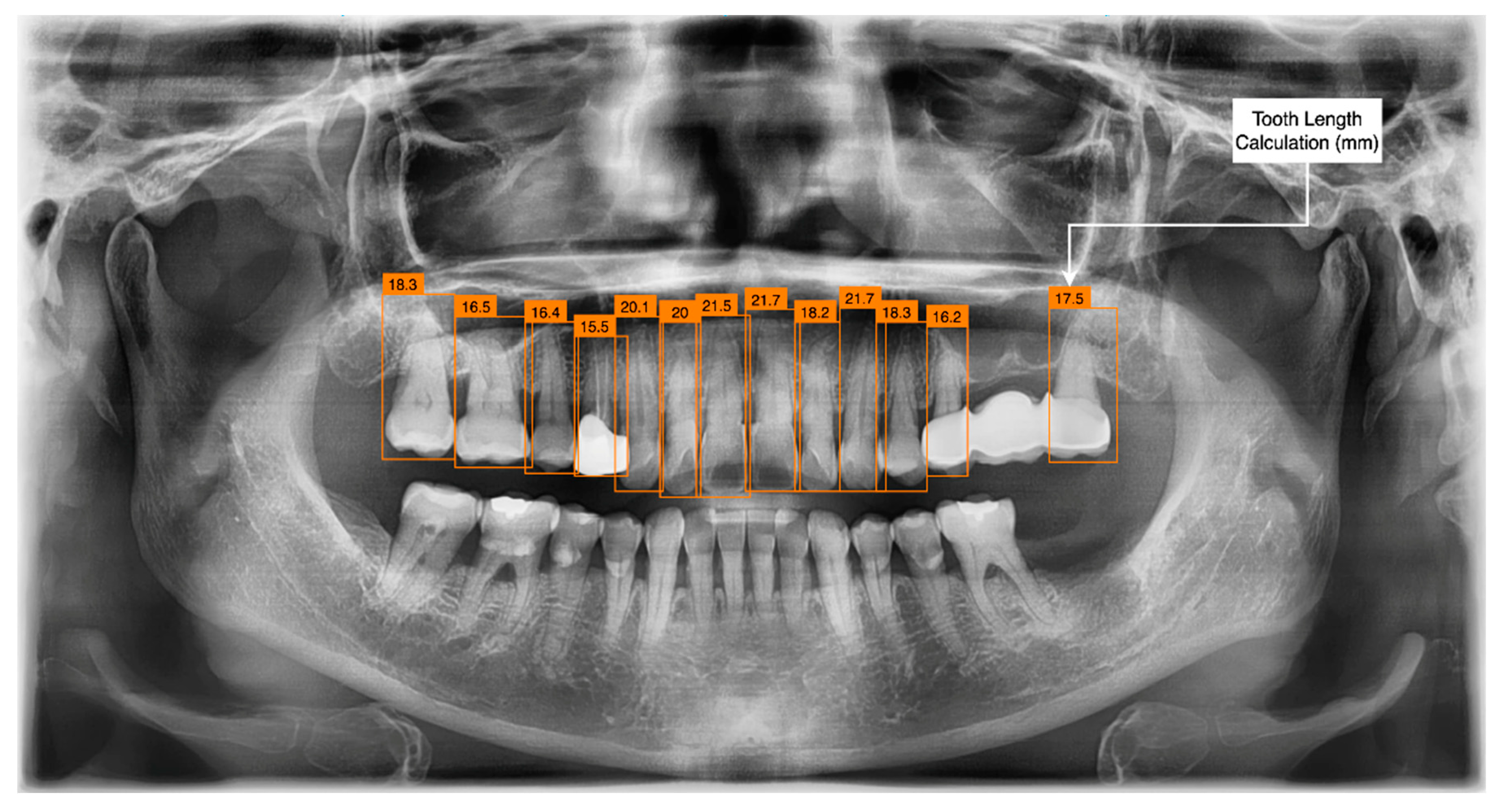
2.2.2. Deep Learning Algorithm
2.2.3. Model Evaluation Metrics
3. Experiments and Results
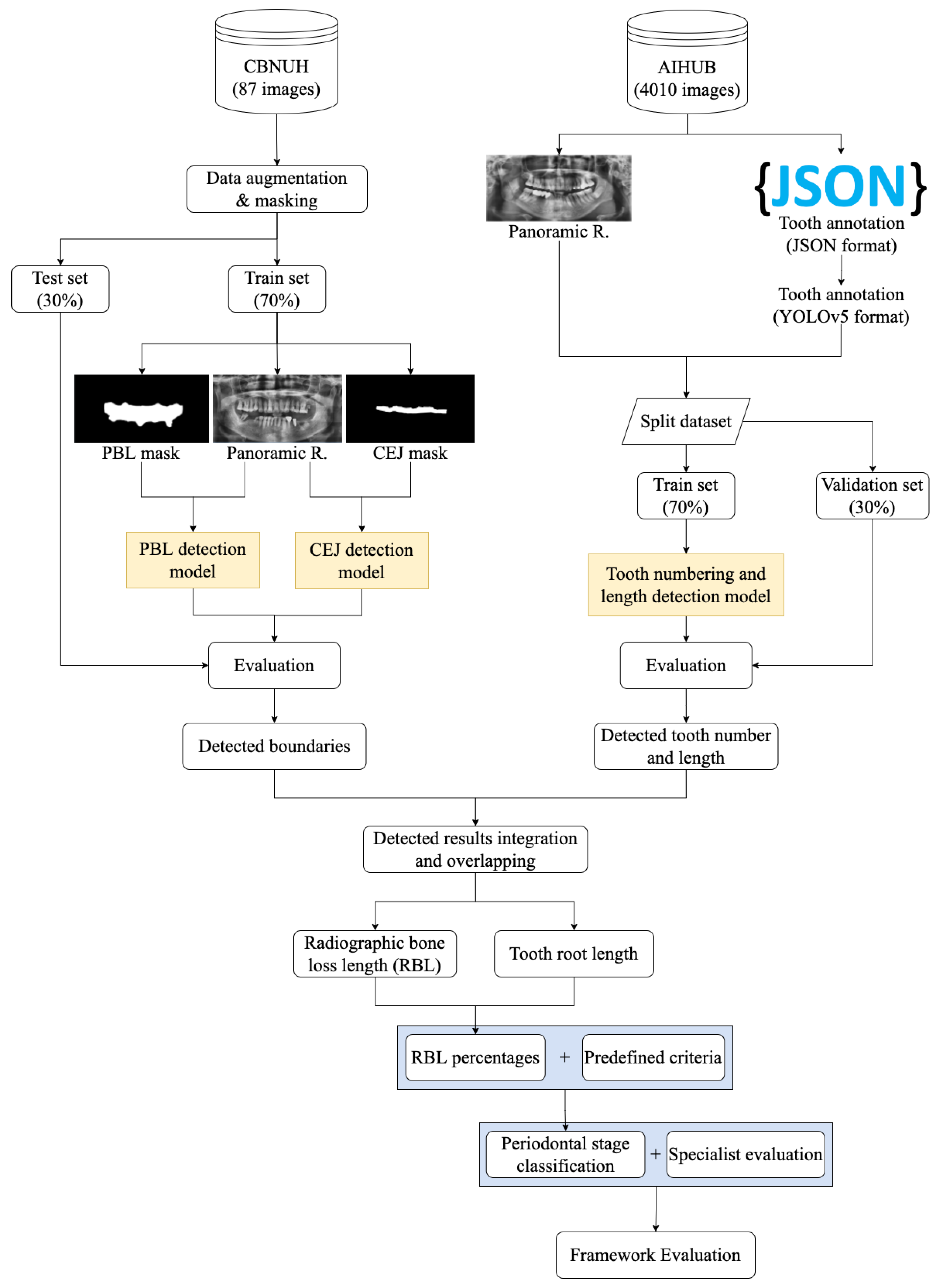
3.1. Experimental Flow
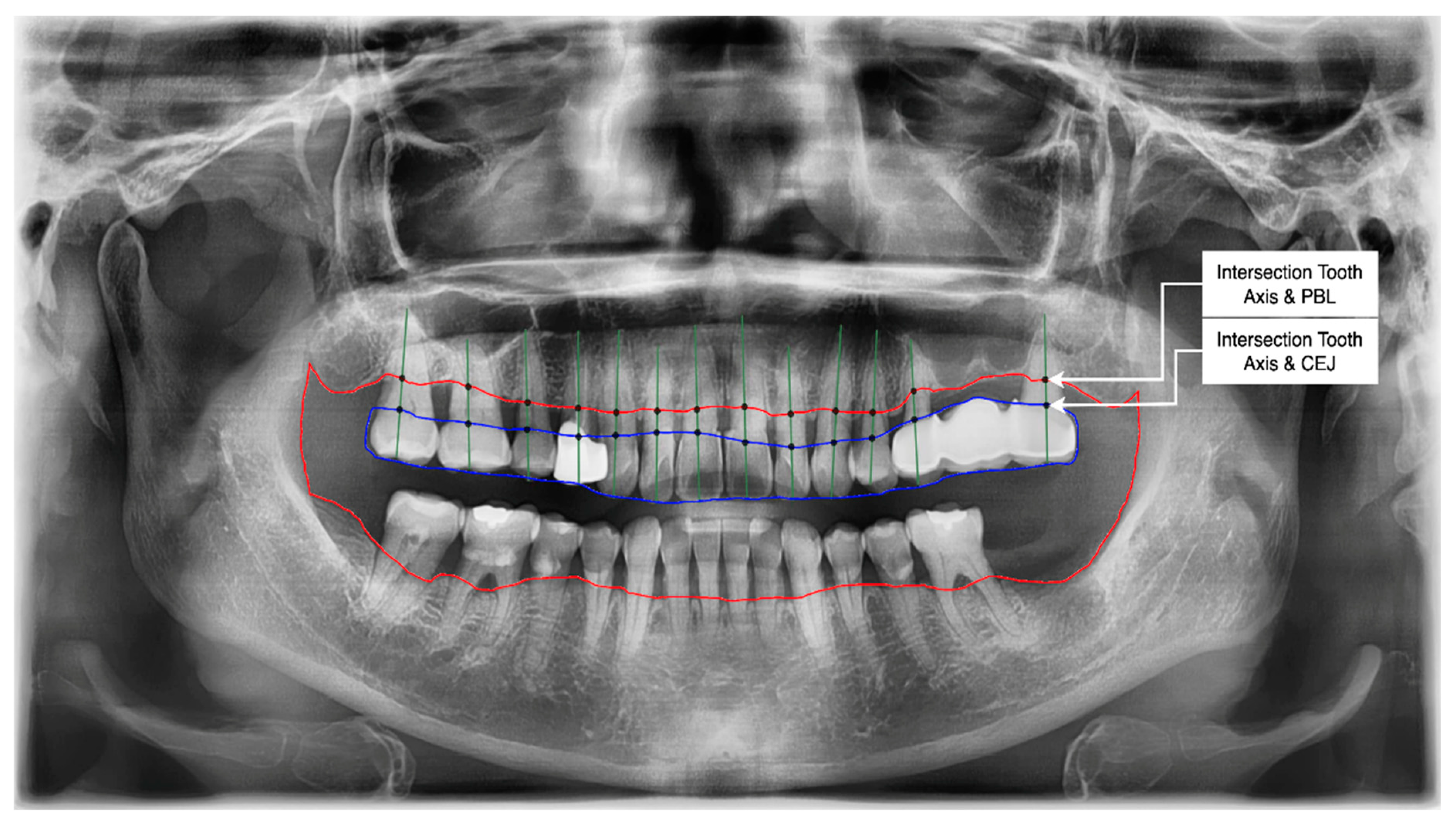
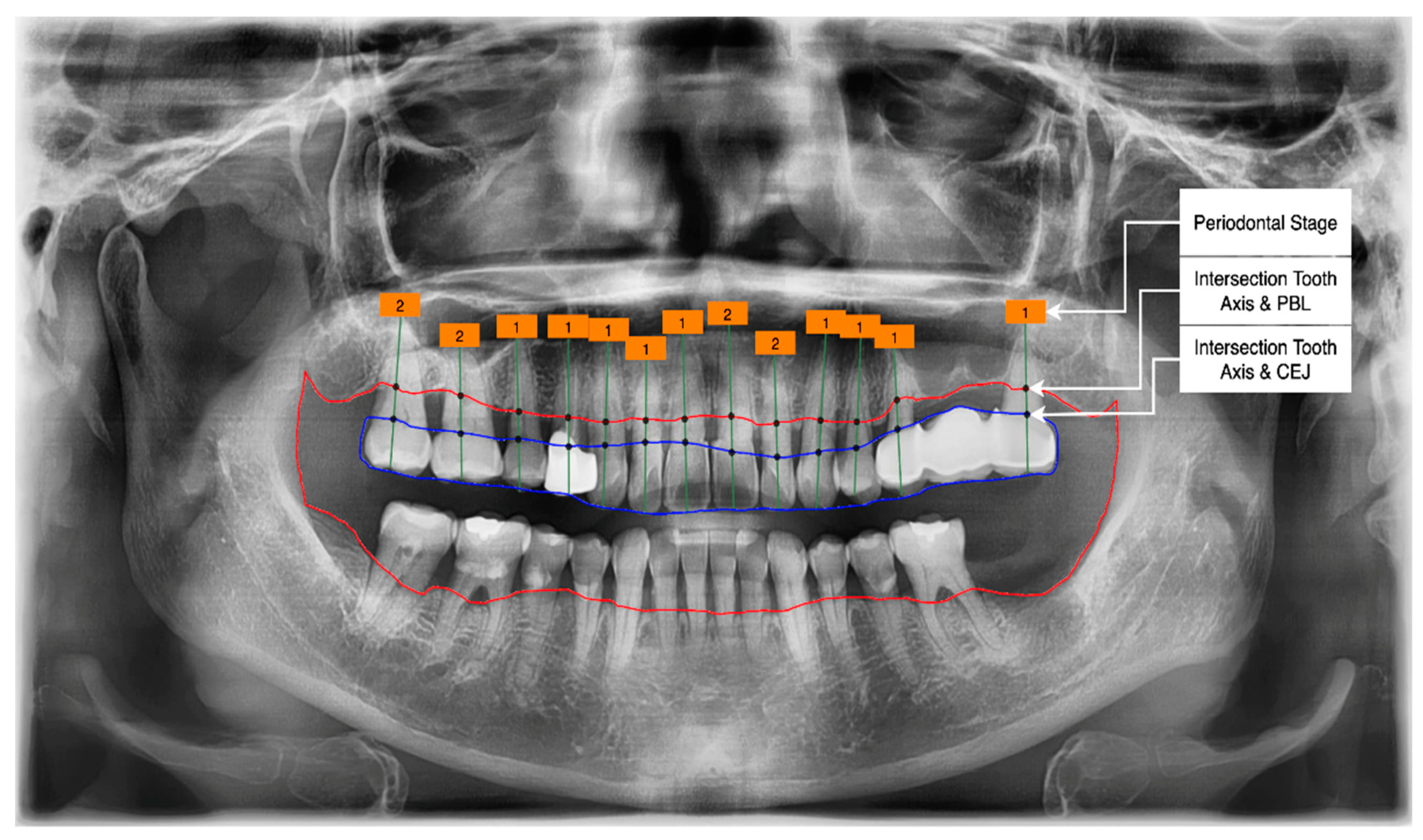
- Get the intersection of the PBL’s upper side and the tooth’s middle axis.
- Get the intersection of the CEJ’s upper side and the tooth’s middle axis.
- Calculate the length from those two intersections (known as the RBL length).
- Calculate the RBL percentage from the RBL length and tooth root.
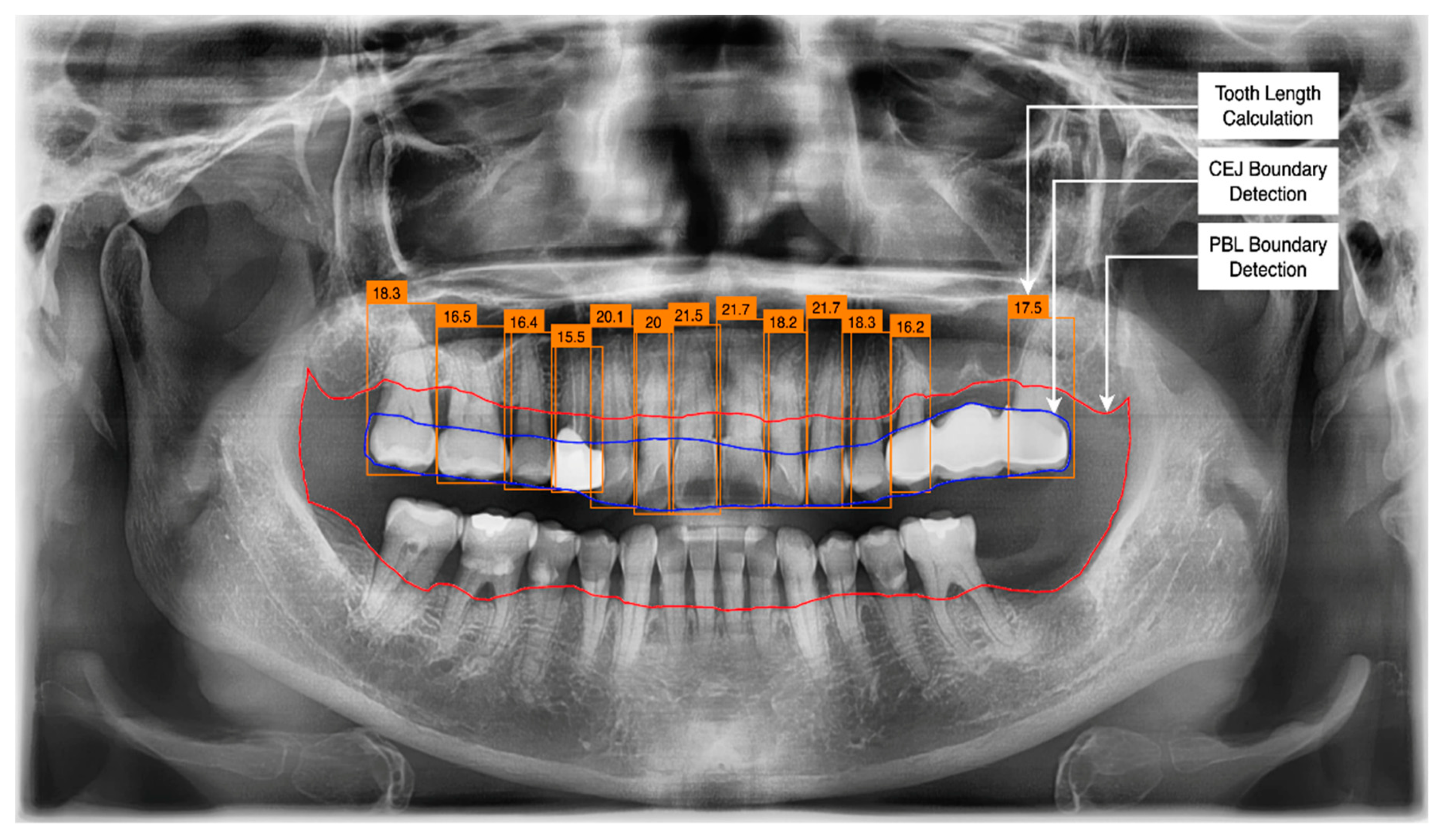
3.2. PBL and CEJ Boundary Detection through U-Net
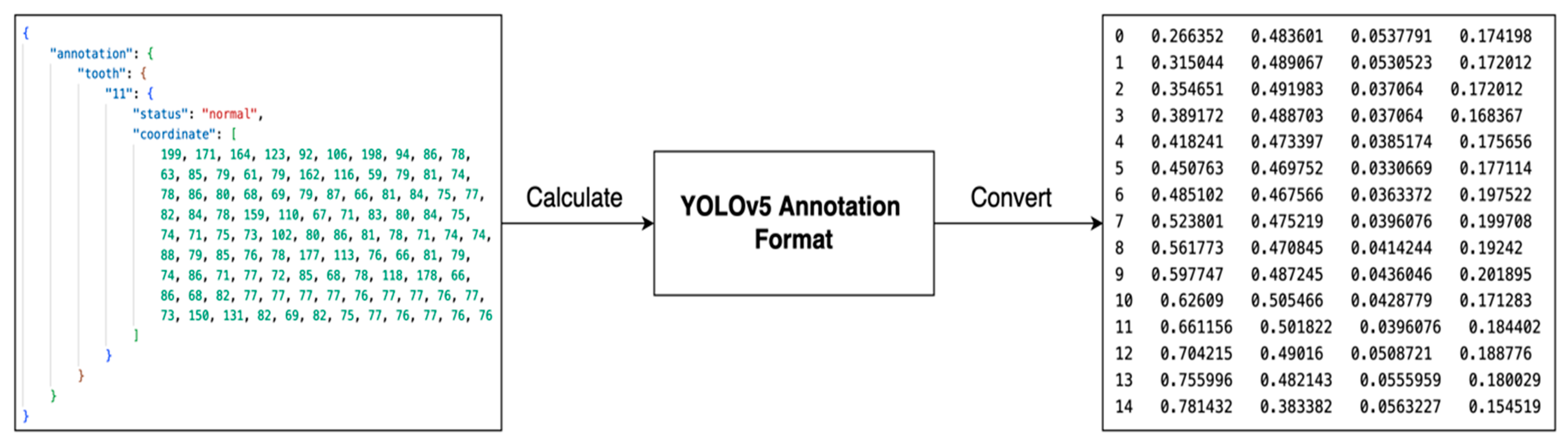
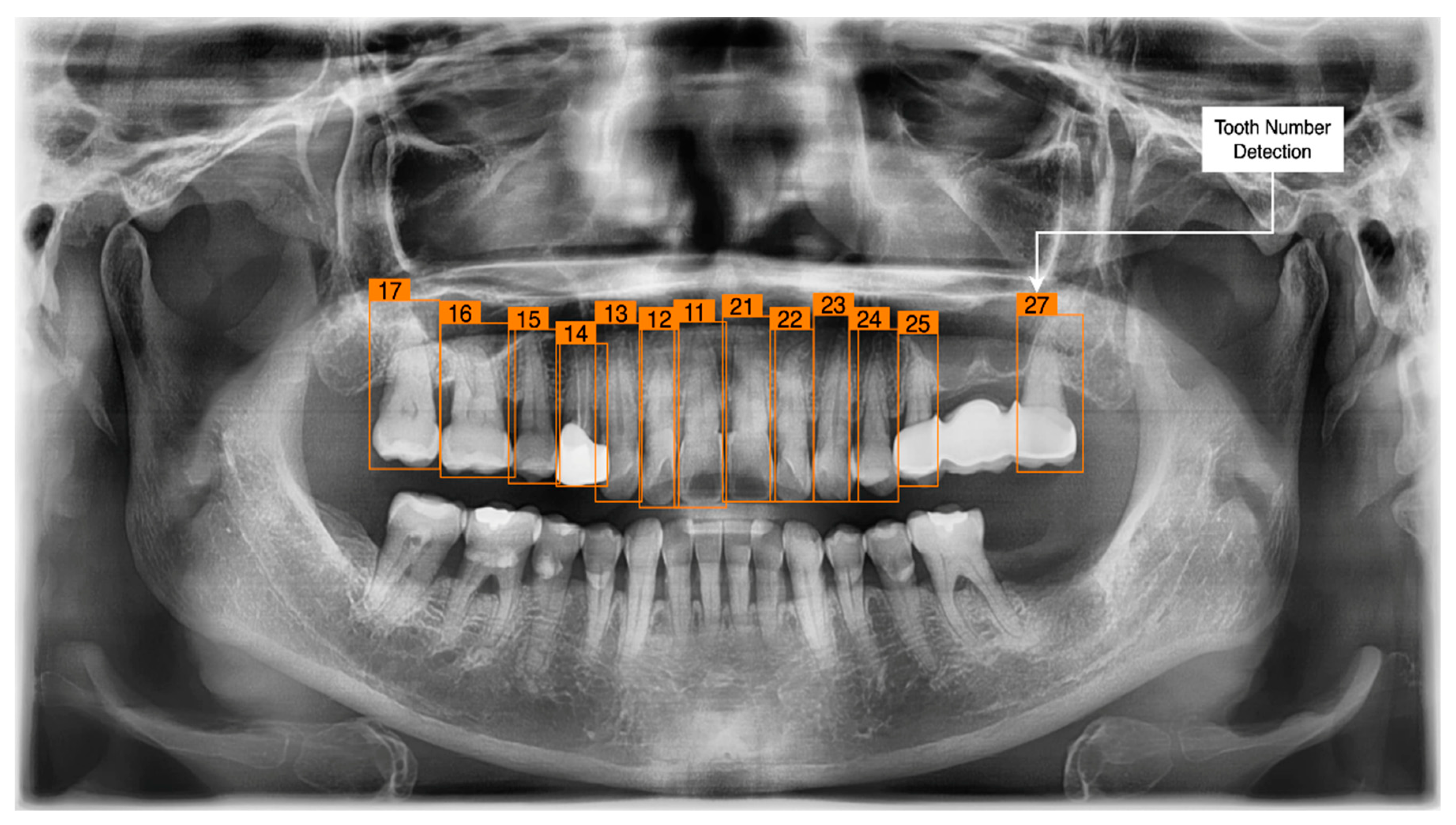
3.3. Tooth Identification through YOLOv5
3.4. Determination of Tooth Stage through U-Net and YOLOv5 Integration
Classification of the PBL by Percentage Rate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jung, H.; Yang, J.; Park, H.J. Research trend of health life expectancy using oral health indicators (2010–2020). J. Korea Soc. Sch. Community Health Educ. 2021, 22, 75–91. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otomo-Corgel, J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017, 44, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Clin. Periodontol. 2018, 45, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, R.; Nishio, M.; Do, R.K.; Togashi, K. Convolutional neural networks: An overview and application in radiology. Insights Imaging 2018, 9, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.J. Deep learning hybrid method to automatically diagnose periodontal bone loss and stage periodontitis. Sci. Rep. 2020, 10, 7531. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O. Automatic diagnosis for cysts and tumors of both jaws on panoramic radiographs using a deep convolution neural network. Dentomaxillofacial Radiol. 2020, 49, 20200185. [Google Scholar] [CrossRef] [PubMed]
- Hannun, A.Y. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat. Med. 2019, 25, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Ronneberger, O.; Fischer, P.; Brox, T. Dental X-ray image segmentation using a U-shaped Deep Convolutional network. In Proceedings of the 2015 IEEE International Symposium on Biomedical Imaging, Bridge, NY, USA, 16–19 April 2015; Volume 1, pp. 1–13. [Google Scholar]
- Miki, Y. Classification of teeth in cone-beam CT using deep convolutional neural network. Comput. Biol. Med. 2017, 80, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Hiraiwa, T. A deep-learning artificial intelligence system for assessment of root morphology of the mandibular first molar on panoramic radiography. Dentomaxillofacial Radiol. 2019, 48, 20180218. [Google Scholar] [CrossRef] [PubMed]
- Murata, M. Deep-learning classification using convolutional neural network for evaluation of maxillary sinusitis on panoramic radiography. Oral Radiol. 2018, 35, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Krois, J. Deep learning for the radiographic detection of periodontal bone loss. Sci. Rep. 2019, 9, 8495. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. DeNTNet: Deep neural transfer network for the detection of periodontal bone loss using panoramic dental radiographs. Sci. Rep. 2019, 9, 17615. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, D.H.; Jeong, S.N.; Choi, S.H. Detection and diagnosis of dental caries using a deep learning-based convolutional neural network algorithm. J. Dent. 2018, 77, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Yeon, H.G.; Shin, W.S.; Yun, J.P.; Jeong, S.H.; Lim, H.J. Deep learning-based prediction of extraction difficulty for mandibular third molars. Sci. Rep. 2021, 11, 1954. [Google Scholar] [CrossRef]
- Farook, T.H.; Jamayet, N.B.; Abdullah, J.Y.; Alam, M.K. Machine learning and intelligent diagnostics in dental and orofacial pain management: A systematic review. Pain Res. Manag. 2021, 2021, 6659133. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.aihub.or.kr/aihubdata/data/view.do?dataSetSn=203 (accessed on 24 January 2022).
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. Available online: https://lmb.informatik.uni-freiburg.de/people/ronneber/u-net/ (accessed on 2 February 2022).
- Russakovsky, O.; Deng, J.; Su, H.; Krause, J.; Satheesh, S.; Ma, S.; Huang, Z.; Karpathy, A.; Khosla, A.; Bernstein, M.; et al. ImageNet large scale visual recognition challenge. Int. J. Comput. Vis. (IJCV) 2015, 115, 211–252. [Google Scholar] [CrossRef]
- Ren, S.; He, K.; Girshick, R.; Sun, J. Faster r-cnn: Towards real-time object detection with region proposal networks. arXiv 2015, arXiv:1506.01497. [Google Scholar] [CrossRef] [PubMed]
- Redmon, J.; Divvala, S.; Girshick, R.; Farhadi, A. You Only Look Once: Unified, Real-Time Object Detection. 2016. Available online: https://pjreddie.com/darknet/yolo/ (accessed on 26 January 2022).
- Han, J.; Kamber, M.; Pei, J. Data Mining: Concepts and Techniques, 3rd ed.; Morgan Kaufmann: Waltham, MA, USA, 2012; pp. 327–391. ISBN 978-12-391479-1. [Google Scholar]
- Jiang, L.; Chen, D.; Cao, Z.; Wu, F.; Zhu, H.; Zhu, F. A two-stage deep learning architecture for radiographic staging of periodontal bone loss. BMC Oral Health 2022, 22, 106. [Google Scholar] [CrossRef] [PubMed]


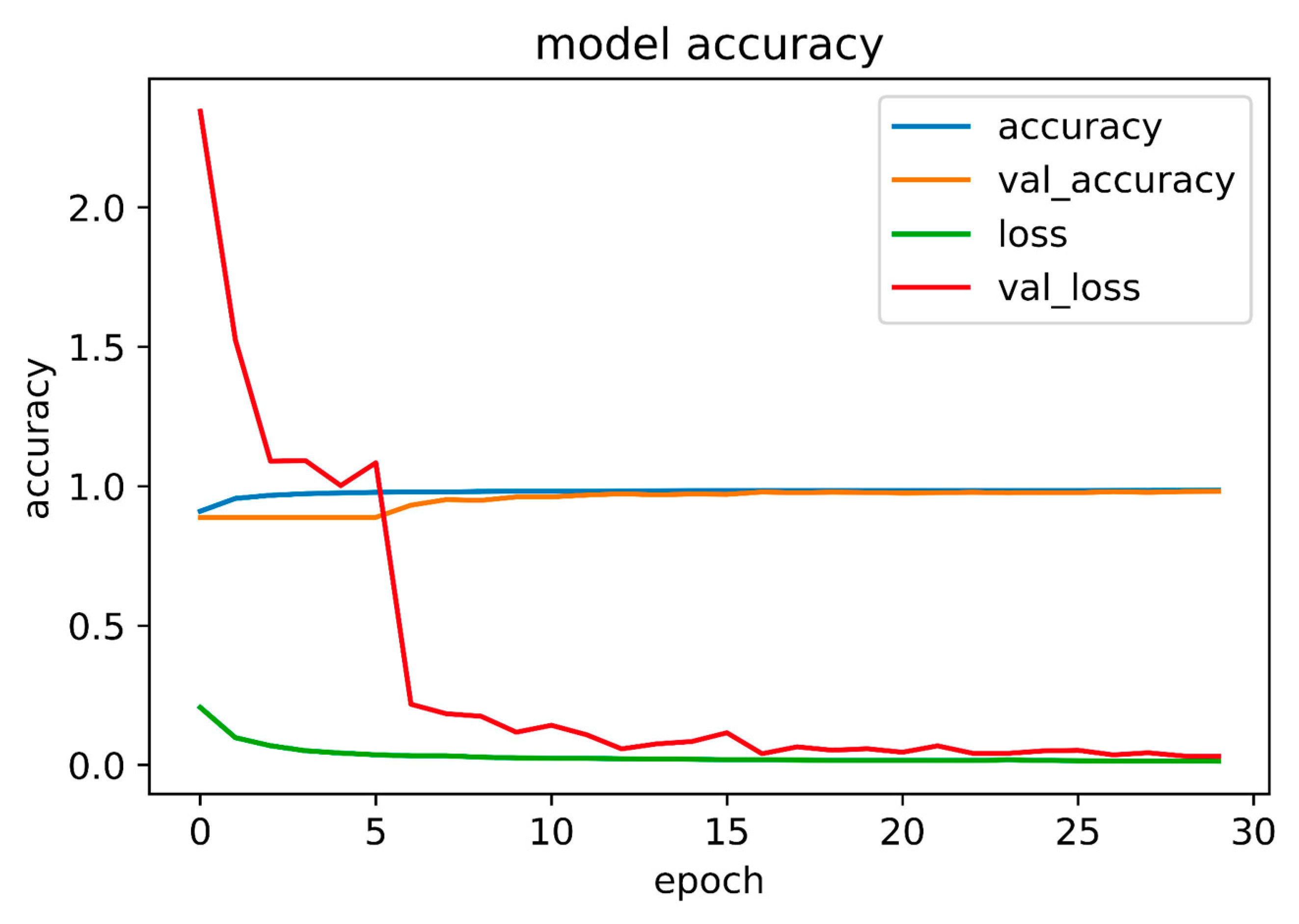
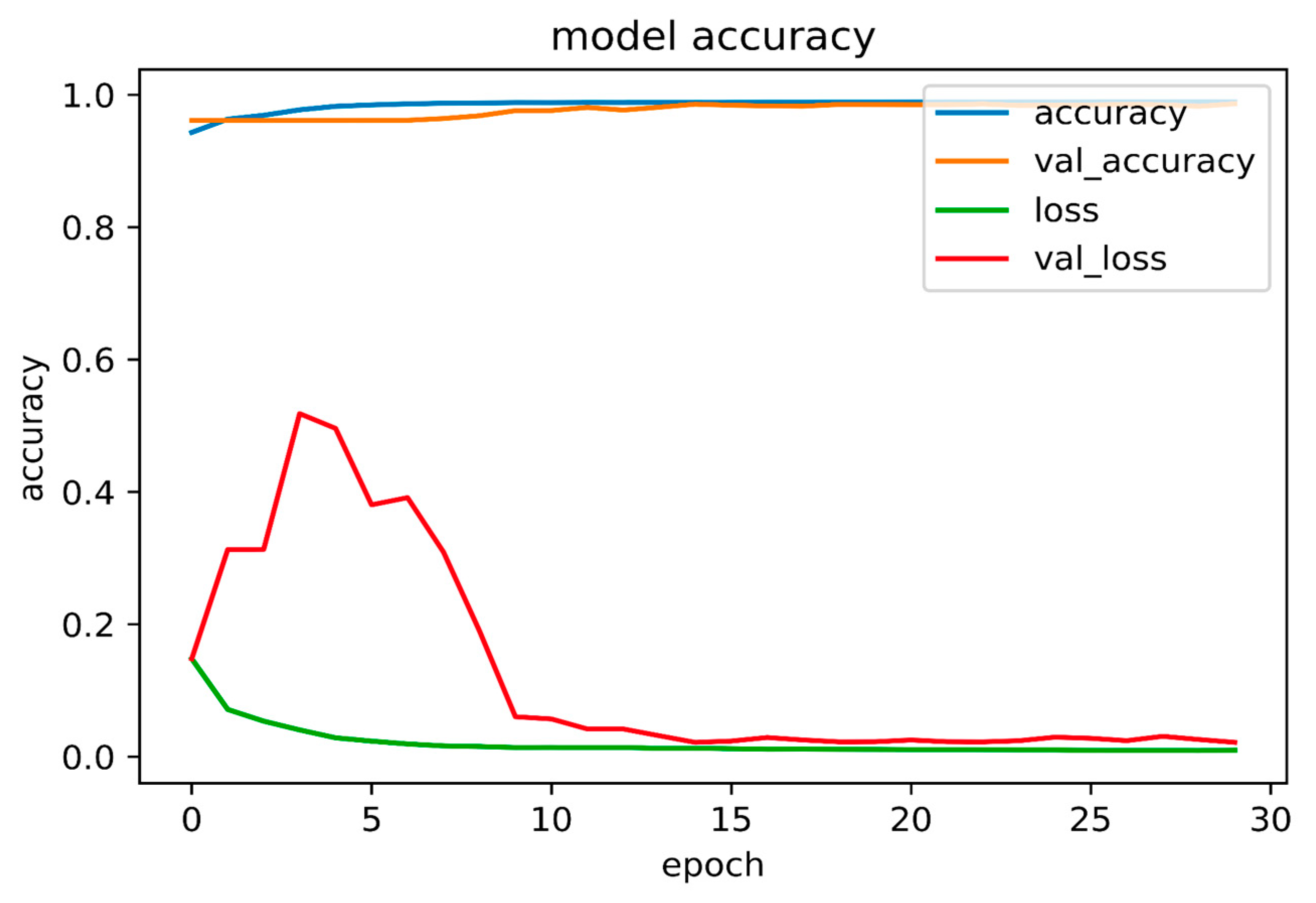
| Accuracy | Loss | Validation Accuracy | Validation Loss | |
|---|---|---|---|---|
| PBL | 0.9856 | 0.0132 | 0.9813 | 0.0306 |
| CEJ | 0.9891 | 0.0096 | 0.9864 | 0.0213 |
| Stage | Stage Count of Test Dataset | Stage Count of Prediction |
|---|---|---|
| 1 | 97 | 101 |
| 2 | 34 | 31 |
| 3 | 8 | 4 |
| 4 | 1 | 4 |
| Stage | Recall (95% CI) | Precision (95% CI) | F1-Score (95% CI) |
|---|---|---|---|
| 1 | 1.000 (1.000–1.000) | 0.962 (0.948–0.975) | 0.979 (0.97–0.989) |
| 2 | 0.857 (0.85–0.862) | 0.941 (0.924–0.957) | 0.897 (0.886–0.907) |
| 3 | 0.374 (0.364–0.384) | 0.772 (0.753–0.79) | 0.504 (0.483–0.525) |
| 4 | 1.000 (1.000–1.000) | 0.253 (0.241–0.265) | 0.404 (0.388–0.419) |
| Mean | 0.805 (0.799–0.811) | 0.732 (0.716–0.745) | 0.696 (0.681–0.709) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shon, H.S.; Kong, V.; Park, J.S.; Jang, W.; Cha, E.J.; Kim, S.-Y.; Lee, E.-Y.; Kang, T.-G.; Kim, K.A. Deep Learning Model for Classifying Periodontitis Stages on Dental Panoramic Radiography. Appl. Sci. 2022, 12, 8500. https://doi.org/10.3390/app12178500
Shon HS, Kong V, Park JS, Jang W, Cha EJ, Kim S-Y, Lee E-Y, Kang T-G, Kim KA. Deep Learning Model for Classifying Periodontitis Stages on Dental Panoramic Radiography. Applied Sciences. 2022; 12(17):8500. https://doi.org/10.3390/app12178500
Chicago/Turabian StyleShon, Ho Sun, Vungsovanreach Kong, Jae Sung Park, Wooyeong Jang, Eun Jong Cha, Sang-Yup Kim, Eun-Young Lee, Tae-Geon Kang, and Kyung Ah Kim. 2022. "Deep Learning Model for Classifying Periodontitis Stages on Dental Panoramic Radiography" Applied Sciences 12, no. 17: 8500. https://doi.org/10.3390/app12178500
APA StyleShon, H. S., Kong, V., Park, J. S., Jang, W., Cha, E. J., Kim, S.-Y., Lee, E.-Y., Kang, T.-G., & Kim, K. A. (2022). Deep Learning Model for Classifying Periodontitis Stages on Dental Panoramic Radiography. Applied Sciences, 12(17), 8500. https://doi.org/10.3390/app12178500







