Evaluation of Performance of Polyacrylamide-Modified Compacted Clay as a Gas Barrier: Water Retention and Gas Permeability and Diffusion Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. In-Site Clay
2.2. Polyacrylamide
2.3. CCC Sample Preparation
2.4. Test Methods
2.4.1. WRC Tests
2.4.2. Clay Gas Permeability Tests
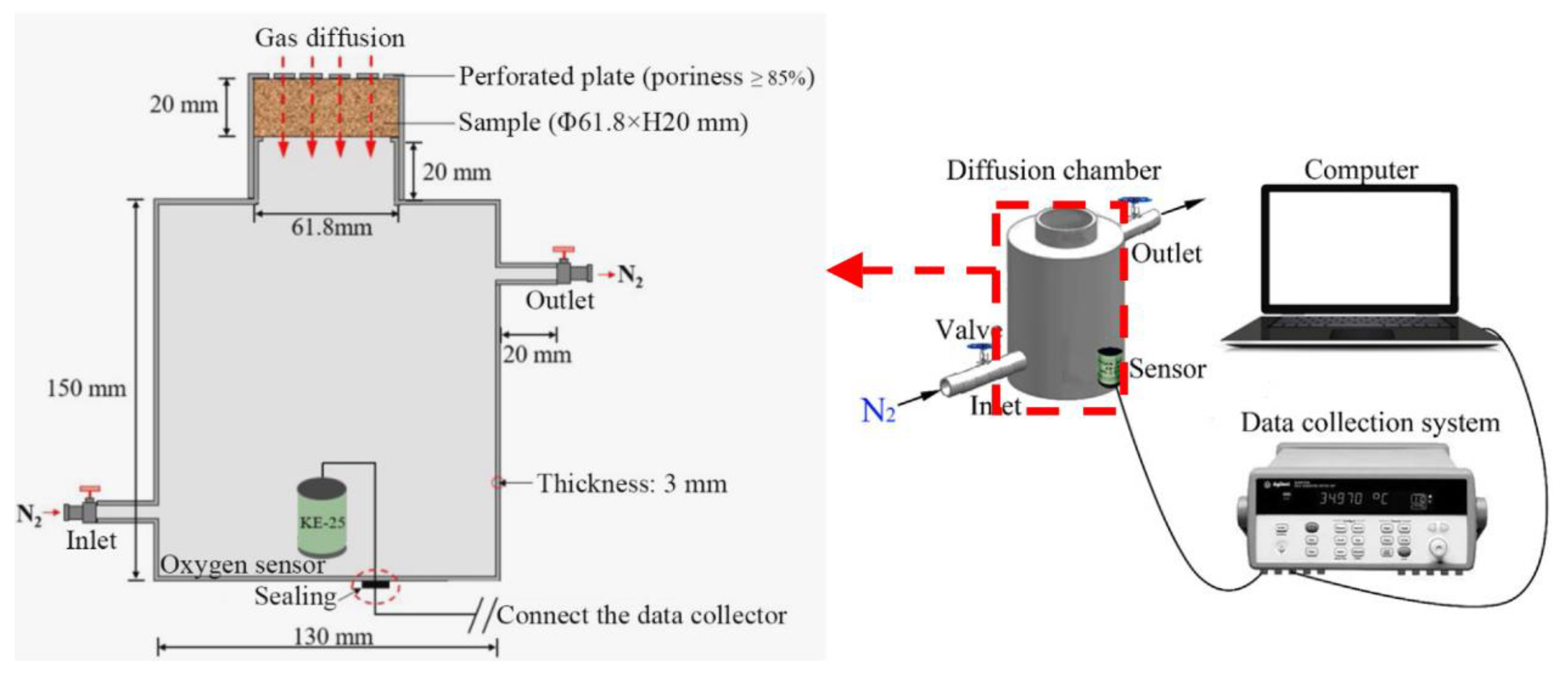
2.4.3. Gas Diffusion Tests
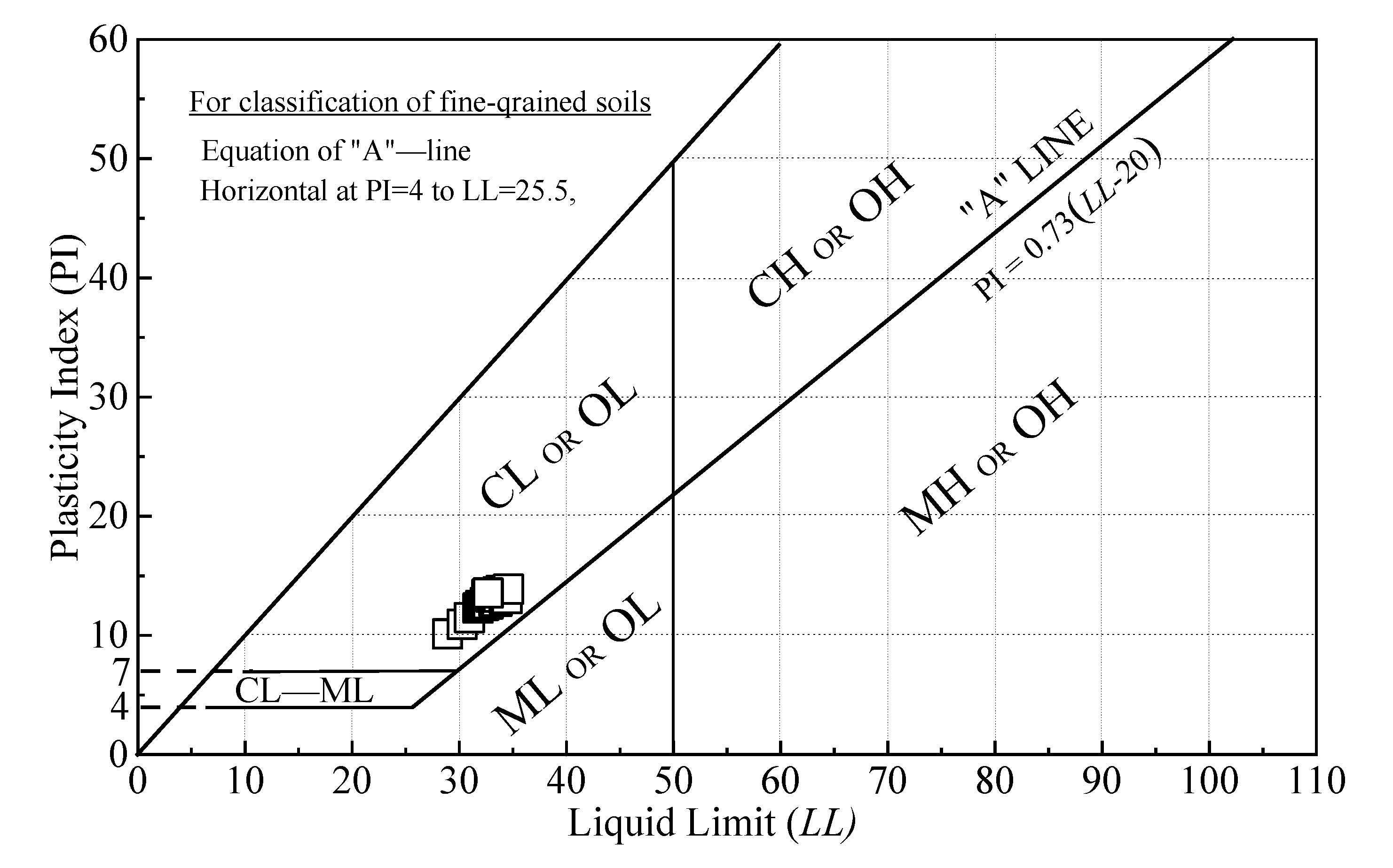
2.4.4. Liquid Limit Test
3. Results and Discussion
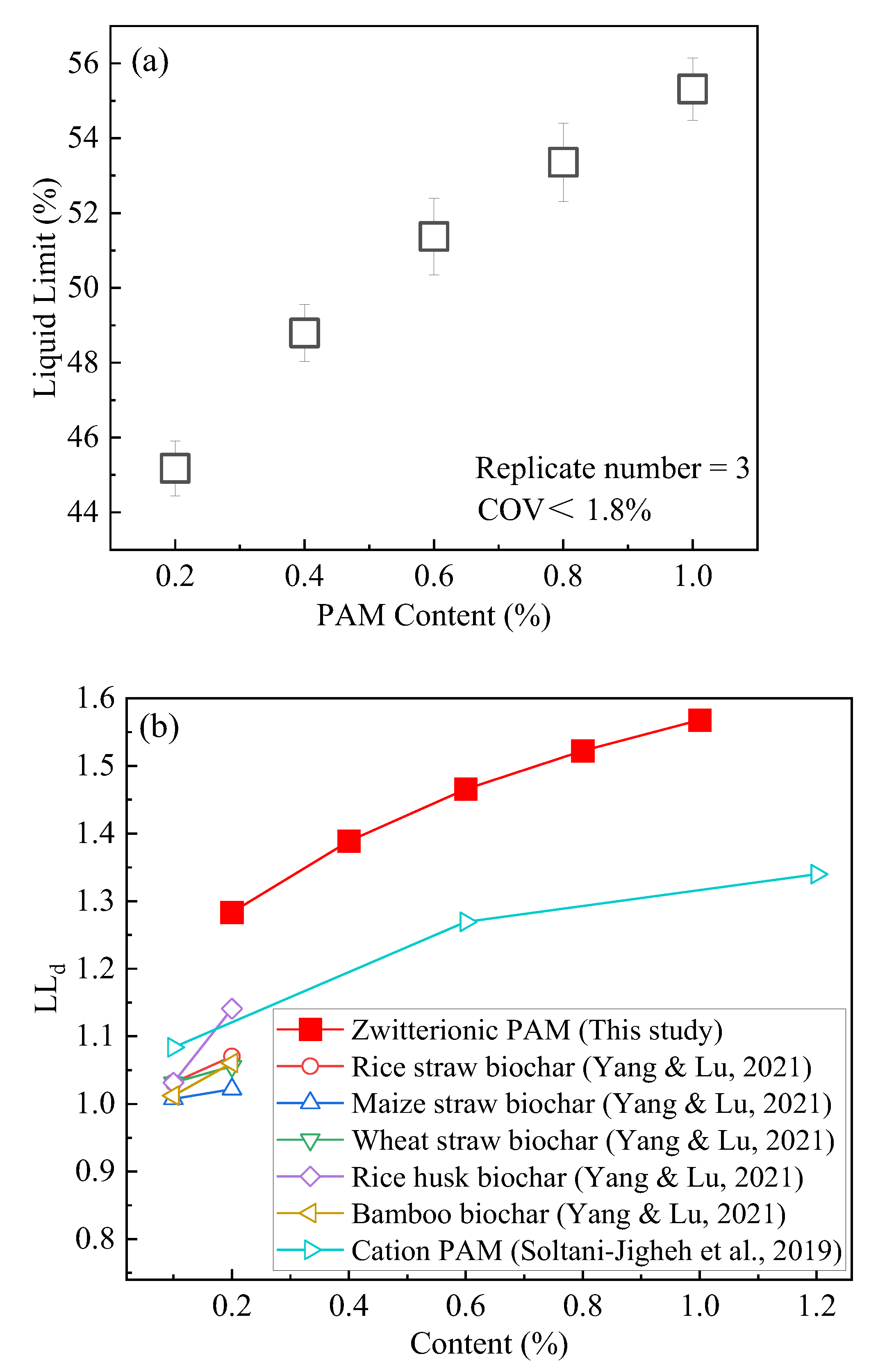
3.1. Liquid Limit
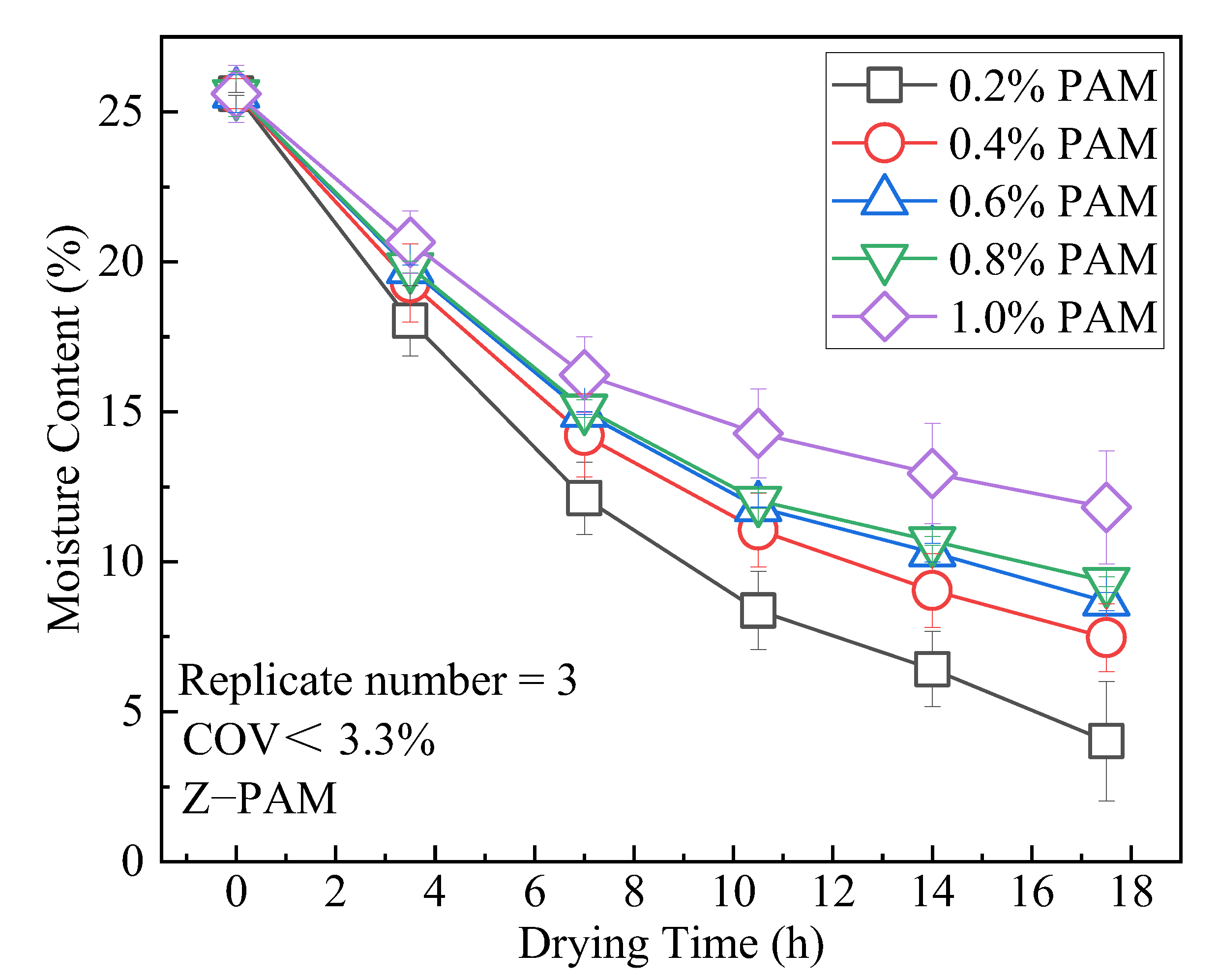
3.2. WRC
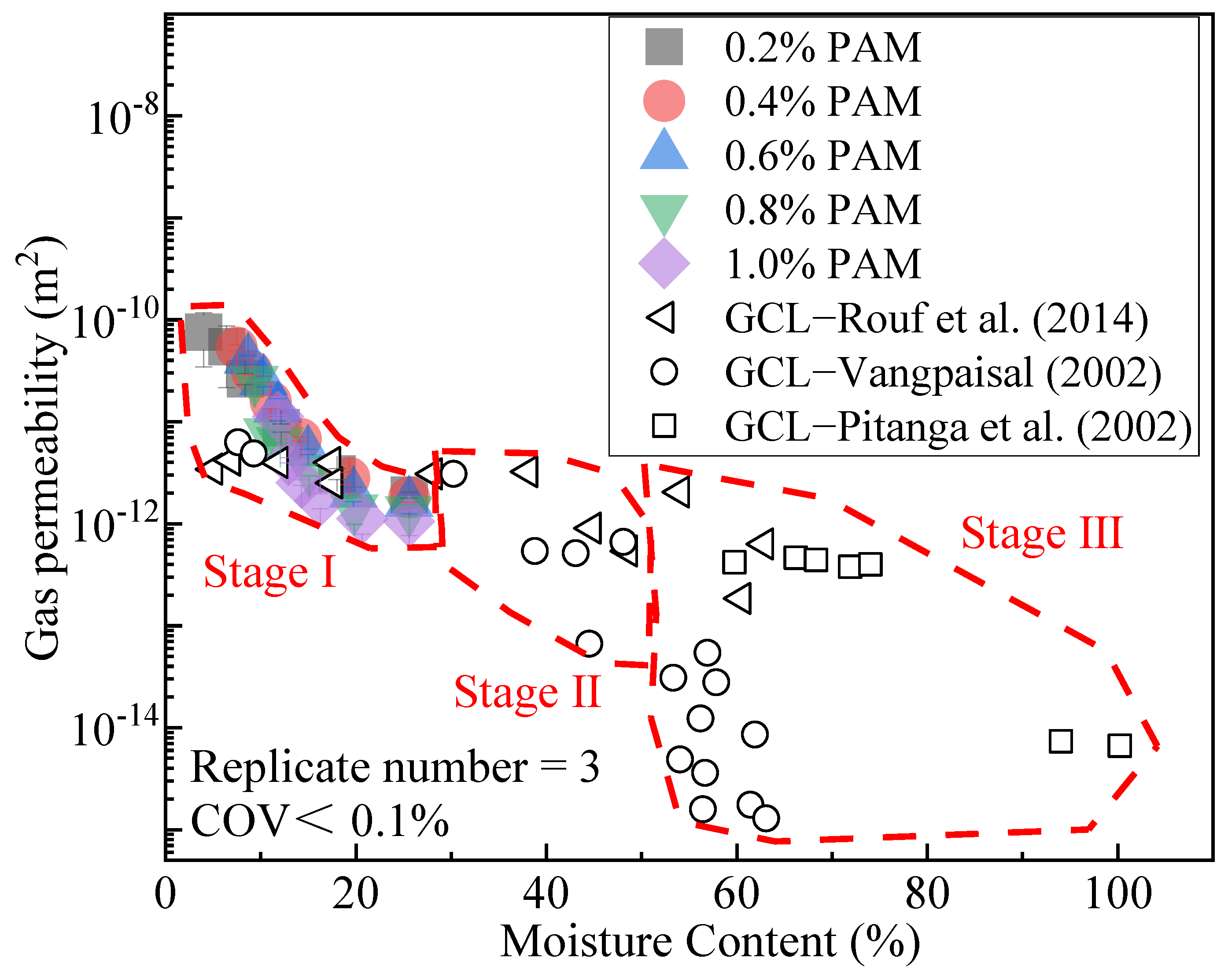
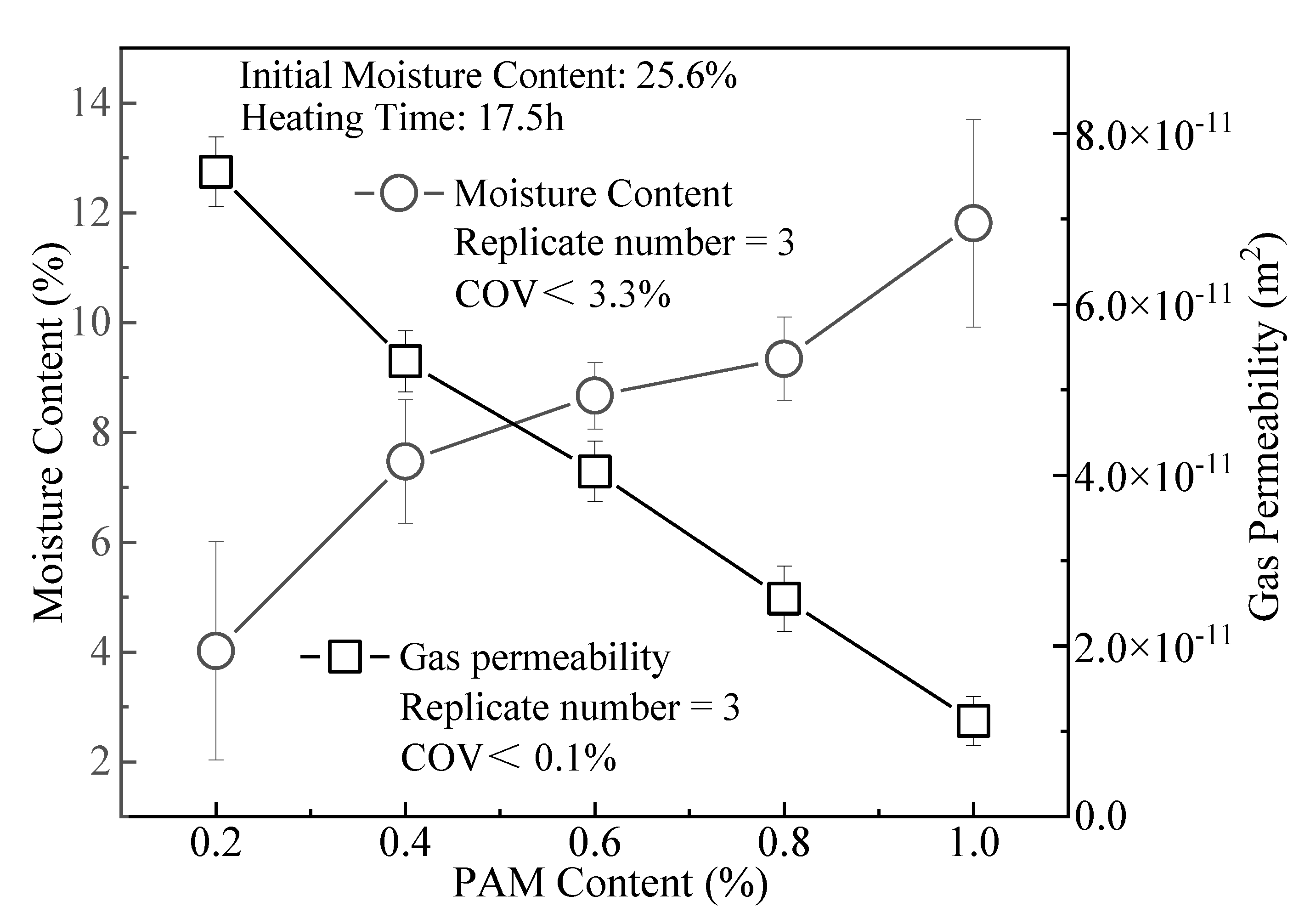
3.3. Gas Permeability
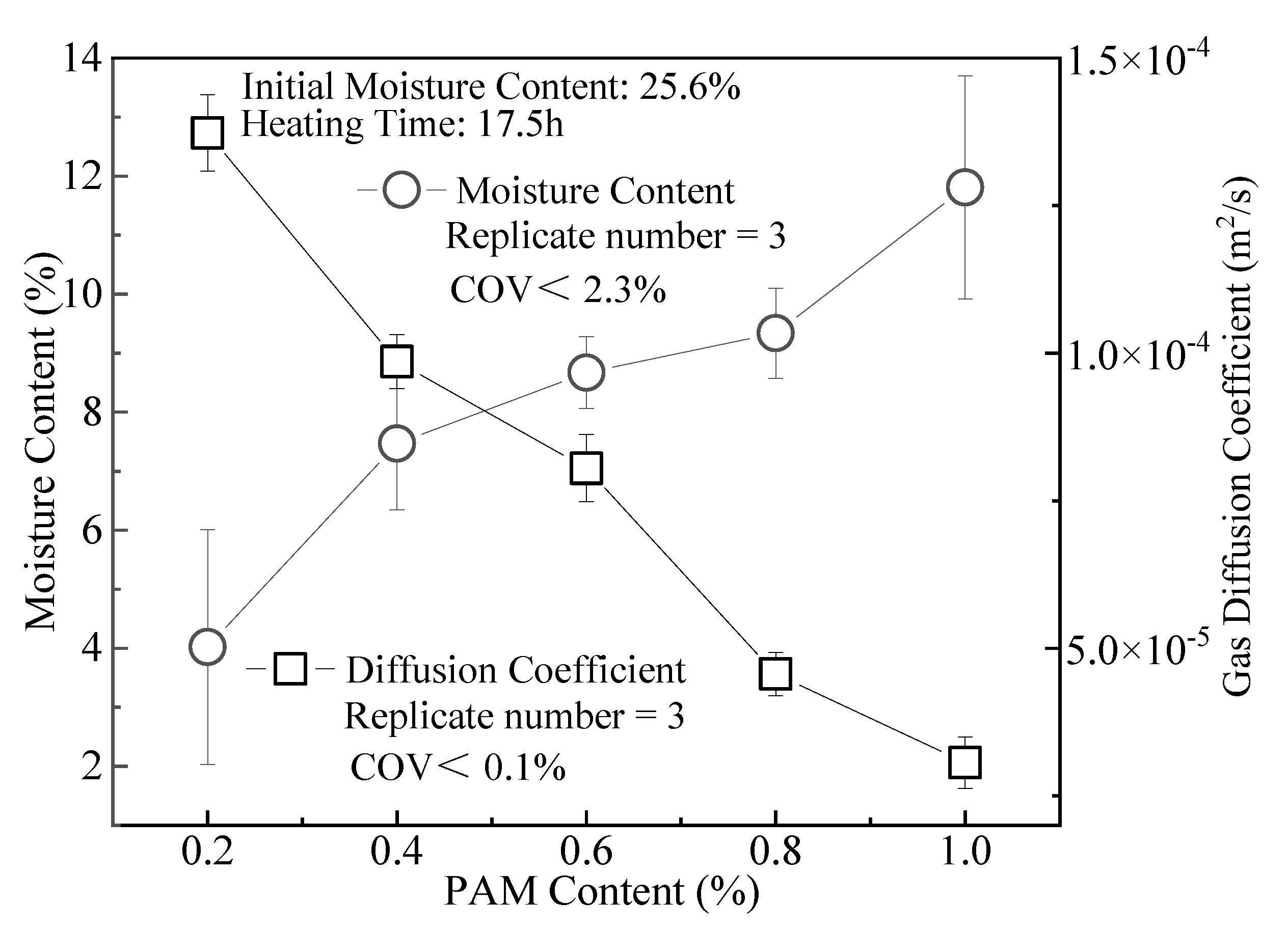
3.4. Gas Diffusion Coefficient
4. Conclusions
- (1)
- ZP could increase the moisture content of the compacted clay. The WRC is related to the linear expansion of polymer molecules after water adsorption. The moisture content of the clay after drying increases with the rise in the ZP admixture content. The modification had a prominent effect at a ZP content of 1%, and the moisture content after drying increased by 193% more than at the ZP content of 0.2%.
- (2)
- The WRC of compacted clay significantly improves after adding ZP. When the admixture content is 0.8% and 1.0%, the water loss rate after the clay is dried for 17.5 h at 100 °C is 64% and 54%. Its WRC is better than ordinary water-retention agents for agriculture and forestry.
- (3)
- The effects of ZP, i.e., reducing the clay gas permeability Kp and gas diffusion coefficient Dp, are not obvious. When the content is 1.0%, the Kp of CCC is about one order of magnitude lower than that at the ZP content of 0.2%. Its Dp is only 35% at the admixture content of 0.2%. For CCC with the same moisture content, the Kp and Dp decrease with the rise in ZP content.
- (4)
- The reasons for the slight improvement in the gas barrier performance of modified clay are as follows. ZP modification enhances the WRC of CCC after drying, thereby reducing the Kp and Dp.
- (5)
- ZP can increase the LL of compacted clay. When the admixture content varies from 0.2 to 1.0%, LL increases by 45~55% more than unmodified clay.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| WRC | Water retention capacity |
| LL | Liquid limit |
| LLd | The dimensionless results of LL |
| LLck | The LL of soil without any additive |
| CCC | Compacted clay cover |
| ADOS | Apparent degree of saturation |
| Ip | Plasticity index |
| CL | Clay with low LL |
| CH | Clay with high LL |
| ML | Silt with low LL |
| MH | Silt with high LL |
| OL | Low liquid limit organic clay or silt |
| OH | High liquid limit organic clay or silt |
| VOC | Volatile organic compound |
| PAM | Polyacrylamide |
| ZP | Zwitterion polyacrylamide |
| GCL | Geosynthetic clay liner |
| CK | Control blank |
| COV | Covariance (it is a measure of the joint variability of two random variables) |
| OC | Oxygen concentration |
| ms | Sum of the mass of the clay and polyacrylamide (g) |
| m0 | The mass of a compacted clay sample before drying (g) |
| mt | The mass of a compacted clay sample after drying for time t (g) |
| WS | The water loss rate (%) |
| Kp | The gas permeability of a clay sample |
| k | The permeability coefficient (m2) |
| ρl | The air density (kg/m3) |
| t | The test time (s) |
| V | The amount of air passing through the sample within time t (m3) |
| L | The thickness of the compacted clay sample to be tested (m) |
| p | The actual pressure value (hPa) |
| A | The bottom area of the sample (m2) |
| Dp | The correction gas diffusion coefficient (m2/s) |
| Dp’ | The gas diffusion coefficient before calibration (m2/s) |
| hs | The height of the compacted clay sample |
| hC | The height of the diffusion chamber (cm) |
| k | The slope of the straight line in the scatter diagram of ln(△Ct/△C0) (t) |
| ΔCt | The difference between OC at both ends of the clay sample at time t |
| ΔC0 | The difference between OC at both ends of the clay sample at time t0 |
| FP | Filter paper |
| SSSM | Saturated salt solution method |
| RH | Relative humidity |
References
- Mahmoodlu, M.G.; Hassanizadeh, S.M.; Hartog, N.; Raoof, A.; van Genuchten, M.T. Evaluation of a horizontal permeable reactive barrier for preventing upward diffusion of volatile organic compounds through the unsaturated zone. J. Environ. Manag. 2015, 163, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.-J.; Wang, Q.; Yan, H.; Chen, Y.-M. Steady-state analytical model for vapour-phase volatile organic compound (VOC) diffusion in layered landfill composite cover systems. Can. Geotech. J. 2017, 54, 1567–1579. [Google Scholar] [CrossRef]
- Su, Y.; Pei, J.; Tian, B.; Fan, F.; Tang, M.; Li, W.; He, R. Potential application of biocover soils to landfills for mitigating toluene emission. J. Hazard. Mater. 2015, 299, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Brusseau, M.L. Transport of organic chemicals by gas advection in structured or heterogeneous porous media: Development of a model and application to column experiments. Water Resour. Res. 1991, 27, 3189–3199. [Google Scholar] [CrossRef]
- Moldrup, P.; Olesen, T.; Gamst, J.; Schjønning, P.; Yamaguchi, T.; Rolston, D. Predicting the Gas Diffusion Coefficient in Repacked Soil Water-Induced Linear Reduction Model. Soil Sci. Soc. Am. J. 2000, 64, 1588–1594. [Google Scholar] [CrossRef]
- Foose, G.J.; Benson, C.H.; Edil, T.B. Comparison of solute transport in three composite liners. J. Geotech. Geoenviron. Eng. 2002, 128, 391–403. [Google Scholar] [CrossRef]
- You, K.; Zhan, H. Comparisons of diffusive and advective fluxes of gas phase volatile organic compounds (VOCs) in unsaturated zones under natural conditions. Adv. Water Resour. 2013, 52, 221–231. [Google Scholar] [CrossRef]
- Falta, R.W.; Javandel, I.; Pruess, K.; Witherspoon, P.A. Density-driven flow of gas in the unsaturated zone due to the evaporation of volatile organic compounds. Water Resour. Res. 1989, 25, 2159–2169. [Google Scholar] [CrossRef]
- Mendoza, C.A.; Frind, E.O. Advective-dispersive transport of dense organic vapors in the unsaturated zone: 2. Sensitivity analysis. Water Resour. Res. 1990, 26, 388–398. [Google Scholar] [CrossRef]
- Conant, B.H.; Gillham, R.W.; Mendoza, C.A. Vapor transport of trichloroethylene in the unsaturated zone: Field and numerical modeling investigations. Water Resour. Res. 1996, 32, 9–22. [Google Scholar] [CrossRef]
- Rouf, M.A.; Singh, R.M.; Bouazza, A.; Rowe, R.K.; Gates, W.P. Gas permeability of partially hydrated geosynthetic clay liner under two stress conditions. Environ. Geotech. 2016, 3, 325–333. [Google Scholar] [CrossRef]
- Mendes, M.; Pierson, P.; Touze-Foltz, N.; Mora, H.; Palmeira, E. Characterisation of permeability to gas of geosynthetic clay liners in unsaturated conditions. Geosynth. Int. 2010, 17, 344–354. [Google Scholar] [CrossRef]
- Suits, L.D.; Sheahan, T.C.; Pitanga, H.N.; Pierson, P.; Vilar, O. Measurement of Gas Permeability in Geosynthetic Clay Liners in Transient Flow Mode. Geotech. Test. J. 2011, 34, GTJ103000. [Google Scholar] [CrossRef]
- Bouazza, A.; Vangpaisal, T. Gas Advective Flux of Partially Saturated Geosynthetic Clay Liners. Advances in Transportation and Geoenvironmental Systems Using Geosynthetics. Geotech. Spec. Publ. 2000, 103, 54–67. [Google Scholar]
- Shan, H.-Y.; Yao, J.-T. Measurement of air permeability of geosynthetic clay liners. Geotext. Geomembr. 2000, 18, 251–261. [Google Scholar] [CrossRef][Green Version]
- Smith, J.A.; Tisdale, A.K.; Cho, H.J. Quantification of natural vapor fluxes of trichloroethene in the unsaturated zone at Pi-catinny Arsenal, New Jersey. Environ. Sci. Technol. 1996, 30, 2243–2250. [Google Scholar] [CrossRef]
- Bouazza, A.; Vangpaisal, T. An apparatus to measure gas permeability of geosynthetic clay liners. Geotext. Geomembr. 2003, 21, 85–101. [Google Scholar] [CrossRef]
- Bi, Y.Z.; Fu, X.L.; Zhou, S.J.; Ni, J.; Du, Y. Field Investigation of Effect of Plants on Cracks of Compacted Clay Covers at a Contaminated Site. Int. J. Environ. Res. Public Health 2022, 19, 7248. [Google Scholar] [CrossRef]
- Rouf, A.; Bouazza, A.; Singh, R.M.; Gates, W.P.; Rowe, R.K. Gas flow unified measurement system for sequential measurement of gas diffusion and gas permeability of partially hydrated geosynthetic clay liners. Can. Geotech. J. 2016, 53, 1000–1012. [Google Scholar] [CrossRef]
- Kerry Rowe, R.; Rayhani, M.T.; Take, W.A. GCL hydration under simulated daily thermal cycles. Geosynth. Int. 2011, 18, 196–205. [Google Scholar] [CrossRef]
- Sjökvist, T.; Niklewski, J.; Blom, Å. Effect of wood density and cracks on the moisture content of coated Norway spruce (Picea abies (L.) Karst). Wood Fiber Sci. 2019, 51, 160–172. [Google Scholar] [CrossRef]
- Drumm, E.C.; Boles, D.R.; Wilson, G.V. Desiccation cracks result in preferential flow. Geotech. News-Vanc. 1997, 15, 22–26. [Google Scholar]
- Friedrich, S. Superabsorbent polymers (SAP). In Application of Super Absorbent Polymers (sap) in Concrete Construction; Springer: Dordrecht, The Netherlands, 2012; pp. 13–19. [Google Scholar]
- Mo, C.; Zhu, S.; Li, H.; Huang, Z. Synthesis of poly (acrylic acid)/sodium humate superabsorbent composite for agricultural use. J. Appl. Polym. Sci. 2006, 102, 5137–5143. [Google Scholar] [CrossRef]
- Theng, B.K.G. Interactions of clay minerals with organic polymers. Some practical applications. Clays Clay Miner. 1970, 18, 357–362. [Google Scholar] [CrossRef]
- Li, Y.; Shao, M.; Horton, R. Effect of Polyacrylamide Applications on Soil Hydraulic Characteristics and Sediment Yield of Sloping Land. Procedia Environ. Sci. 2011, 11, 763–773. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.; Kanungo, D.P.; Song, Z.; Bai, Y.; Wang, Y.; Li, D.; Qian, W. Topsoil reinforcement of sandy slope for preventing erosion using water-based polyurethane soil stabilizer. Eng. Geol. 2019, 252, 125–135. [Google Scholar] [CrossRef]
- Qi, C.; Bai, Y.; Liu, J.; Bu, F.; Kanungo, D.P.; Song, Z.; He, X. Desiccation Cracking Behavior of Polyurethane and Polyacrylamide Admixed Clayey Soils. Polymers 2020, 12, 2398. [Google Scholar] [CrossRef]
- Yu, B.; El-Zein, A.; Rowe, R.K. Effect of added polymer on the desiccation and healing of a geosynthetic clay liner subject to thermal gradients. Geotext. Geomembr. 2020, 48, 928–939. [Google Scholar] [CrossRef]
- ASTM D3441-05; Standard Test Method for Mechanical Cone Penetration Tests of Soil. American Society for Testing and Materials: West Conshohocken, PA, USA, 2005.
- Hunt, R.E. Geotechnical Engineering Investigation Handbook; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Moore, J.E. Field Hydrogeology: A Guide for Site Investigations and Report Preparation; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Hess-Kosa, K. Environmental Site Assessment Phase I: A Basic Guide; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- CJJ-2008; Code for Construction and Quality Acceptance of Road Works in City and Town. China Communications Press: Beijing, China, 2008.
- Kaneko, K.; Nohara, S. Review of effective vegetation mapping using the UAV (Unmanned Aerial Vehicle) method. J. Geogr. Inf. Syst. 2014, 6, 733. [Google Scholar] [CrossRef]
- Wong, W.S.D.; Lee, L. Statistical Analysis of Geographic Information with ArcView GIS and ArcGIS; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Mechtcherine, V.; Gorges, M.; Schroefl, C.; Assmann, A.; Brameshuber, W.; Ribeiro, A.B.; Cusson, D.; Custódio, J.; Da Silva, E.F.; Ichimiya, K.; et al. Effect of internal curing by using superabsorbent polymers (SAP) on autogenous shrinkage and other properties of a high-performance fine-grained concrete: Results of a RILEM round-robin test. Mater. Struct. 2013, 47, 541–562. [Google Scholar] [CrossRef]
- Koerner, G.R.; Koerner, R.M. Long-term temperature monitoring of geomembranes at dry and wet landfills. Geotext. Geomembr. 2006, 24, 72–77. [Google Scholar] [CrossRef]
- ASTM D2216-19; Standard Test Methods for Laboratory Determination of Water (Moisture) Content of Soil and Rock by Mass. American Society for Testing and Materials: West Conshohocken, PA, USA, 2019.
- ASTM D854-14; Standard Test Methods for Specific Gravity of Soil Solids by Water Pycnometer. American Society for Testing and Materials: West Conshohocken, PA, USA, 2016.
- ASTM D4318-17e1; Standard Test Methods for Liquid Limit, Plastic Limit, and Plasticity Index of Soils. American Society for Testing and Materials: West Conshohocken, PA, USA, 2018.
- ASTM D7382-20; Standard Test Methods for Determination of Maximum Dry Unit Weight of Granular Soils Using a Vibrating Hammer. American Society for Testing and Materials: West Conshohocken, PA, USA, 2020.
- ASTM D4253-16e1; Standard Test Methods for Maximum Index Density and Unit Weight of Soils Using a Vibratory Table. American Society for Testing and Materials: West Conshohocken, PA, USA, 2019.
- Koerner, G.R.; Koerner, R.M. In-Situ Temperature Monitoring of Geomembranes. In Proceedings of the GRI-18 Conference on Geosynthetics Research and Development In-Progress, GeoFrontiers, ASCE, Austin, TX, USA, 24–26 January 2005; pp. 318–323. [Google Scholar]
- Dantas, G.H.S.; Furlan, A.P.; Fabbri, G.T.P. On gyratory compaction of a clayey soil. EJGE 2016, 21, 5725–5733. [Google Scholar]
- ASTM D2974-20e1; Standard Test Methods for Determining the Water (Moisture) Content, Ash Content, and Organic Material of Peat and Other Organic Soils. American Society for Testing and Materials: West Conshohocken, PA, USA, 2020.
- Seright, R.S.; Campbell, A.R.; Mozley, P.S.; Han, P. Stability of partially hydrolyzed polyacrylamides at elevated temperatures in the absence of divalent cations. SPE J. 2010, 15, 341–348. [Google Scholar] [CrossRef]
- Shackelford, C.D.; Benson, C.H.; Katsumi, T.; Edil, T.B.; Lin, L. Evaluating the hydraulic conductivity of GCLs permeated with non-standard liquids. Geotext. Geomembr. 2000, 18, 133–161. [Google Scholar] [CrossRef]
- Moldrup, P.; Poulsen, T.G.; Schjønning, P.; Olesen, T.; Yamaguchi, T. Gas Permeability in Undisturbed Soils: Measurements and Predictive Models. Soil Sci. 1998, 163, 180–189. [Google Scholar] [CrossRef]
- McCarthy, K.P.; Brown, K.W. Soil gas permeability as influenced by soil gas-filled porosity. Soil Sci. Soc. Am. J. 1992, 56, 997–1003. [Google Scholar] [CrossRef]
- Taylor, S.A. Oxygen diffusion in porous media as a measure of soil aeration. Proc. Soil Sci. Soc. Am. 1949, 14, 55–61. [Google Scholar] [CrossRef]
- Su, Z. Using Gradient Method to Estimate Soil Surface CO2 Flux in Crop and Grass Field; China Agricultural University: Beijing, China, 2016. (In Chinese) [Google Scholar]
- Zhang, J. Comparative Analysis of Basic Properties to 11 Kind of Super-Absorbent-Polymers; Northwest Sci-Tech University of Agricultural and Forestry: Xianyang, China, 2009. (In Chinese) [Google Scholar]
- Republic of China. Specification of Soil Test; SL237-1999; China Architecture and Building Press: Beijing, China, 1999. [Google Scholar]
- Scalia, I.V.J.; Benson, C.H.; Bohnhoff, G.L. Long-term hydraulic conductivity of a bentonite-polymer composite permeated with aggressive inorganic solutions. J. Geotech. Geoenviron. Eng. 2014, 140, 04013025. [Google Scholar] [CrossRef]
- Lee, J.M.; Shackelford, C.D.; Benson, C.H.; Jo, H. Correlating index properties and hydraulic conductivity of geosynthetic clay liners. J. Geotech. Geoenviron. Eng. 2005, 131, 1319–1329. [Google Scholar] [CrossRef]
- Bouazza, A.; Rahman, F. Oxygen diffusion through partially hydrated geosynthetic clay liners. Civ. Eng. 2007, 57, 767–772. [Google Scholar] [CrossRef]
- Yang, C.D.; Lu, S.G. Effects of five different biochars on aggregation, water retention and mechanical properties of paddy soil: A field experiment of three-season crops. Soil Tillage Res. 2021, 205, 104798. [Google Scholar] [CrossRef]
- Soltani-Jigheh, H.; Bagheri, M.; Amani-Ghadim, A.R. Use of hydrophilic polymeric stabilizer to improve strength and durability of fine-grained soils. Cold Regions Sci. Technol. 2019, 157, 187–195. [Google Scholar] [CrossRef]
- Vangpaisal, T. Gas Permeability of Geosynthetic Clay Liners under Various Conditions. Ph.D. Thesis, Monash University, Melbourne, Australia, 2002. [Google Scholar]


| Parameters | Number of Samples | Mean | Test Method |
|---|---|---|---|
| Natural moisture content (%) | 40 | 22.5 | ASTM D2216 [31,39] |
| Specific gravity, Gs | 40 | 2.72 | ASTM D854 [40] |
| Liquid limit, LL (%) | 40 | 38.14 | ASTM D4318 [41] |
| Plastic limit, PI (%) | 40 | 14.90 | |
| Optimal moisture content (%) | 6 | 25.6 | ASTM D7382 [42] |
| Maximum dry density (g/cm3) | 6 | 1.78 | ASTM D4253 [43] |
| Organic content, Coc (%) | 6 | 4.2 | ASTM D2974 [44] |
| Testing Parameters | Values |
|---|---|
| Polymer type | Zwitterionic polyacrylamide (ZP) |
| Molecular weight | 12 million |
| Admixture amount (%) | 0, 0.2, 0.4, 0.6, 0.8, 1.0 |
| Drying time (h) | 0, 3.5, 7, 10.5, 14, 17.5 |
| Testing program | Moisture content, water loss, gas permeability, gas diffusion, liquid limit |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, Y.-Z.; Wen, J.-M.; Wu, H.-L.; Du, Y.-J. Evaluation of Performance of Polyacrylamide-Modified Compacted Clay as a Gas Barrier: Water Retention and Gas Permeability and Diffusion Characteristics. Appl. Sci. 2022, 12, 8379. https://doi.org/10.3390/app12168379
Bi Y-Z, Wen J-M, Wu H-L, Du Y-J. Evaluation of Performance of Polyacrylamide-Modified Compacted Clay as a Gas Barrier: Water Retention and Gas Permeability and Diffusion Characteristics. Applied Sciences. 2022; 12(16):8379. https://doi.org/10.3390/app12168379
Chicago/Turabian StyleBi, Yu-Zhang, Jia-Ming Wen, Hao-Liang Wu, and Yan-Jun Du. 2022. "Evaluation of Performance of Polyacrylamide-Modified Compacted Clay as a Gas Barrier: Water Retention and Gas Permeability and Diffusion Characteristics" Applied Sciences 12, no. 16: 8379. https://doi.org/10.3390/app12168379
APA StyleBi, Y.-Z., Wen, J.-M., Wu, H.-L., & Du, Y.-J. (2022). Evaluation of Performance of Polyacrylamide-Modified Compacted Clay as a Gas Barrier: Water Retention and Gas Permeability and Diffusion Characteristics. Applied Sciences, 12(16), 8379. https://doi.org/10.3390/app12168379








