Assessing the Phytochemical Profile and Potential of Traditional Herbal Infusions against Aldose Reductase through In Silico Studies and LC-MS/MS Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Plant Material and Sample Preparation
2.3. In Silico Studies
2.3.1. Phenolic Compounds Collection and Protein Target Prediction
2.3.2. Molecular Docking Studies
2.4. LC-ESI(−)-MS/MS Analysis
3. Results and Discussion
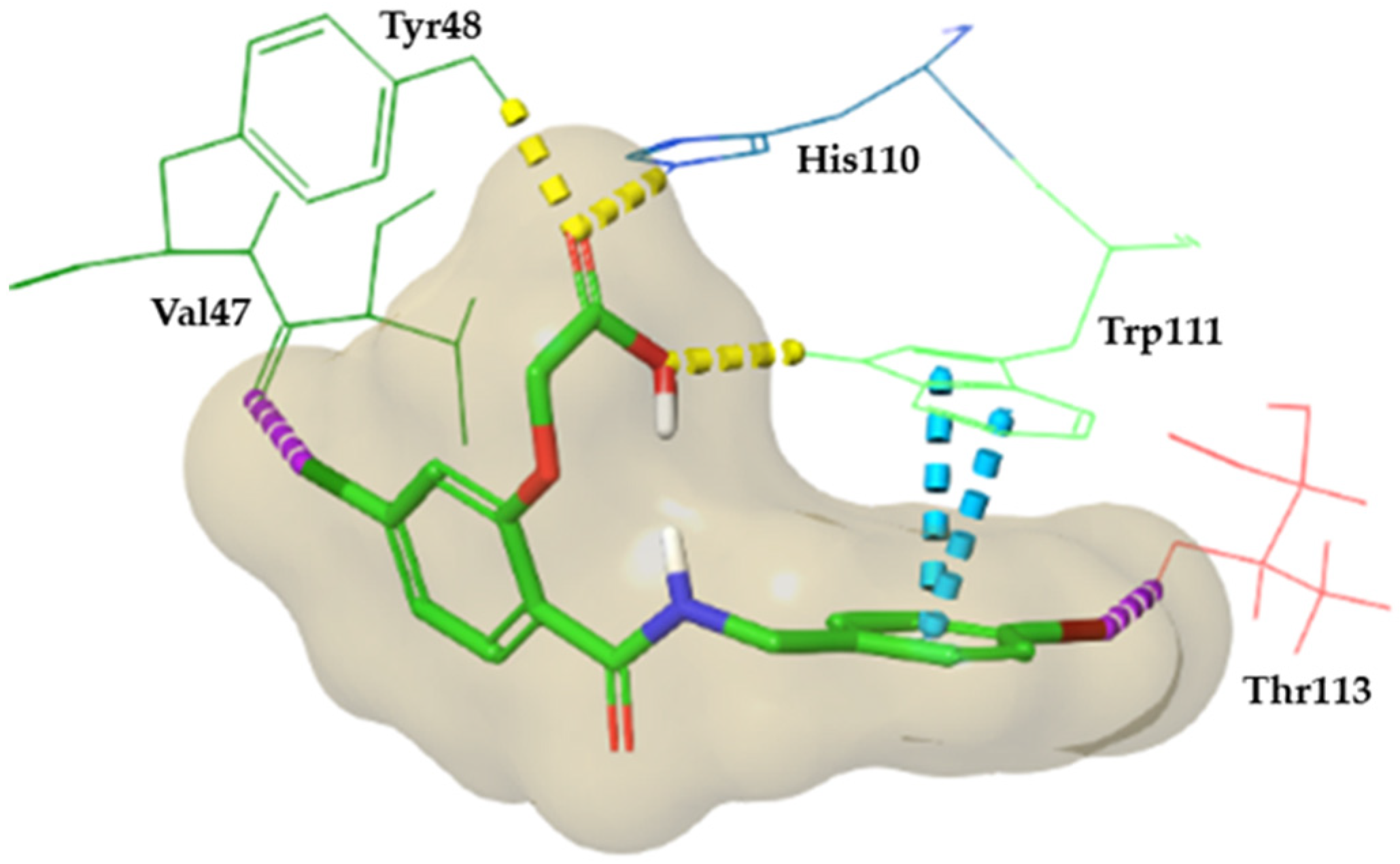
3.1. In silico Analysis
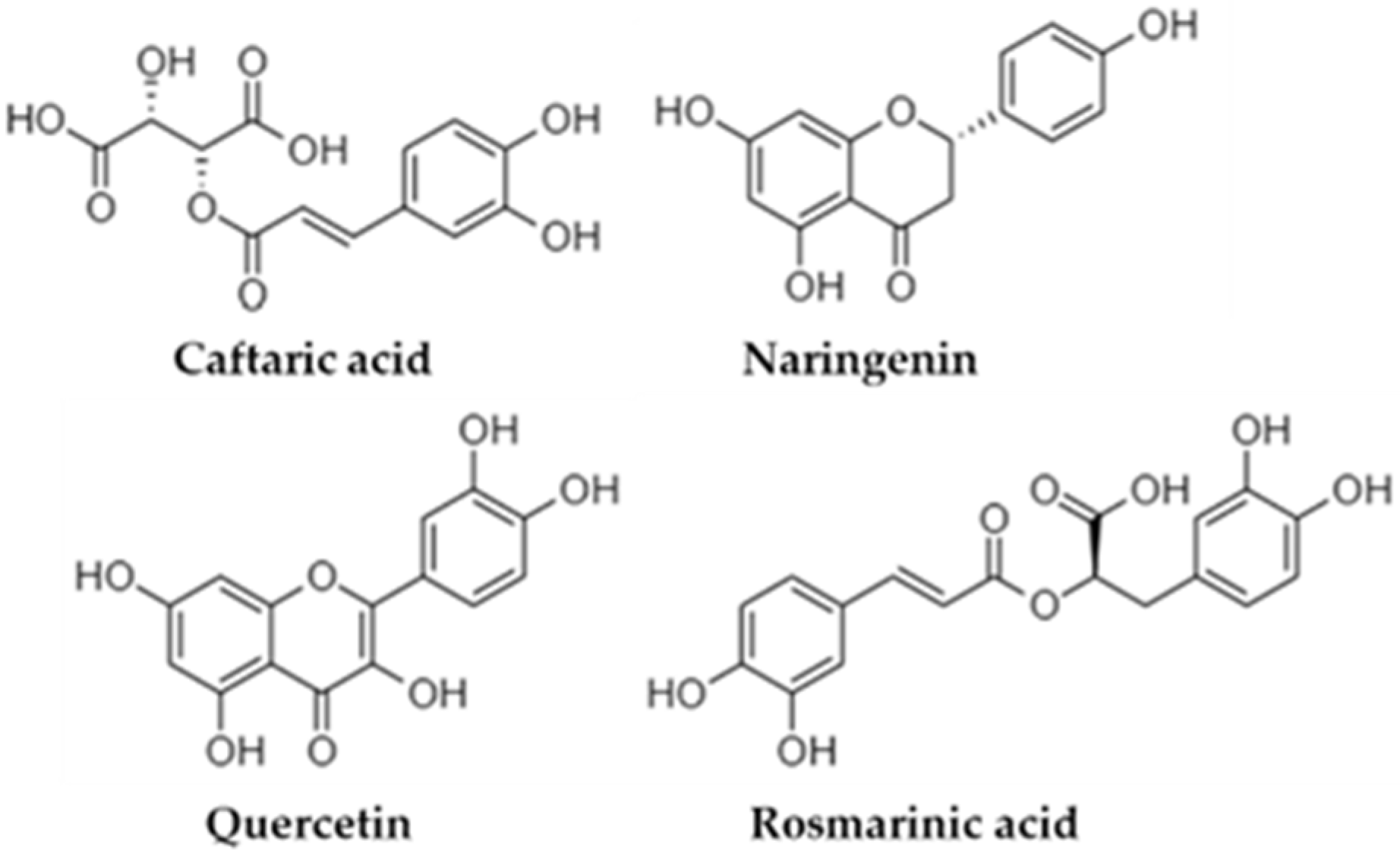
3.2. Phytochemical Phenolic Profile of the Examined Infusion by LC-MS/MS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tejera, E.; Pérez-Castillo, Y.; Toscano, G.; Noboa, A.L.; Ochoa-Herrera, V.; Giampieri, F.; Álvarez-Suarez, J.M. Computational Modeling Predicts Potential Effects of the Herbal Infusion “Horchata” against COVID-19. Food Chem. 2022, 366, 130589. [Google Scholar] [CrossRef] [PubMed]
- Krishnasamy, R.; T, A.; Baba, M.; Bharath, M.V.; Phuntsho, J.; Arunachalam, D.; K, V.; Natarajan, K.; Ramasamy, M. In Silico Analysis of Active Compounds from Siddha Herbal Infusion of Ammaiyar Koondhal Kudineer (Akk) against SARS-CoV-2 Spike Protein and Its ACE2 Receptor Complex. 2020. Available online: https://www.scienceopen.com/document?vid=51068cee-c7d2-49c5-9f1e-00592e601e74 (accessed on 17 July 2022).
- Hussain, S.A.; Panjagari, N.R.; Singh, R.R.B.; Patil, G.R. Potential Herbs and Herbal Nutraceuticals: Food Applications and Their Interactions with Food Components. Crit. Rev. Food Sci. Nutr. 2015, 55, 94–112. [Google Scholar] [CrossRef] [PubMed]
- Caldeirão, L.; Sousa, J.; Nunes, L.C.G.; Godoy, H.T.; Fernandes, J.O.; Cunha, S.C. Herbs and Herbal Infusions: Determination of Natural Contaminants (Mycotoxins and Trace Elements) and Evaluation of Their Exposure. Food Res. Int. 2021, 144, 110322. [Google Scholar] [CrossRef]
- Williamson, E.M.; Liu, X.; Izzo, A.A. Trends in Use, Pharmacology, and Clinical Applications of Emerging Herbal Nutraceuticals. Br. J. Pharmacol. 2020, 177, 1227–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, C.; Moura, A.P.; Cunha, L.M. Consumers’ Associations with Herbal Infusions and Home Preparation Practices. Food Qual. Prefer. 2020, 86, 104006. [Google Scholar] [CrossRef]
- Farzaneh, V.; Carvalho, I.S. A Review of the Health Benefit Potentials of Herbal Plant Infusions and Their Mechanism of Actions. Ind. Crops Prod. 2015, 65, 247–258. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Li, Y.; Zidorn, C. Seasonal Variations of Natural Products in European Herbs. Phytochem. Rev. 2022, 1–27. [Google Scholar] [CrossRef]
- Nwosu, O.K.; Ubaoji, K.I. Nutraceuticals: History, Classification and Market Demand. In Functional Foods and Nutraceuticals: Bioactive Components, Formulations and Innovations; Egbuna, C., Dable Tupas, G., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 13–22. ISBN 978-3-030-42319-3. [Google Scholar]
- Çelik, G.; Kılıç, G.; Kanbolat, Ş.; Özlem Şener, S.; Karaköse, M.; Yaylı, N.; Karaoğlu, Ş.A. Biological Activity, and Volatile and Phenolic Compounds from Five Lamiaceae Species. Flavour Fragr. J. 2021, 36, 223–232. [Google Scholar] [CrossRef]
- Fotakis, C.; Tsigrimani, D.; Tsiaka, T.; Lantzouraki, D.Z.; Strati, I.F.; Makris, C.; Tagkouli, D.; Proestos, C.; Sinanoglou, V.J.; Zoumpoulakis, P. Metabolic and Antioxidant Profiles of Herbal Infusions and Decoctions. Food Chem. 2016, 211, 963–971. [Google Scholar] [CrossRef]
- Barral-Martinez, M.; Garcia-Oliveira, P.; Nuñez-Estevez, B.; Silva, A.; Finimundy, T.C.; Calhelha, R.; Nenadic, M.; Sokovic, M.; Barroso, F.; Simal-Gandara, J.; et al. Plants of the Family Asteraceae: Evaluation of Biological Properties and Identification of Phenolic Compounds. Chem. Proc. 2021, 5, 51. [Google Scholar] [CrossRef]
- Rezaeifar, M.; Mehdizadeh, T.; Mojaddar Langroodi, A.; Rezaei, F. Effect of Chitosan Edible Coating Enriched with Lemon Verbena Extract and Essential Oil on the Shelf Life of Vacuum Rainbow Trout (Oncorhynchus mykiss). J. Food Saf. 2020, 40, e12781. [Google Scholar] [CrossRef]
- Silva, M.; Cueva, C.; Alba, C.; Rodriguez, J.M.; de Pascual-Teresa, S.; Jones, J.; Caturla, N.; Victoria Moreno-Arribas, M.; Bartolomé, B. Gut Microbiome-Modulating Properties of a Polyphenol-Enriched Dietary Supplement Comprised of Hibiscus and Lemon Verbena Extracts. Monitoring of Phenolic Metabolites. J. Funct. Foods 2022, 91, 105016. [Google Scholar] [CrossRef]
- Hematian Sourki, A.; Ghani, A.; Kiani, F.; Alipour, A. Phytochemical Profiles of Lemon Verbena (Lippia citriodora H.B.K.) and Its Potential Application to Cookie Enrichment. Food Sci. Nutr. 2021, 9, 3100–3113. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Marzo, N.; Lozano-Sánchez, J.; de la Luz Cádiz-Gurrea, M.; Herranz-López, M.; Micol, V.; Segura-Carretero, A. Relationships between Chemical Structure and Antioxidant Activity of Isolated Phytocompounds from Lemon Verbena. Antioxidants 2019, 8, 324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gani, A.; Jan, R.; Ashwar, B.A.; Ashraf, Z.U.; Shah, A.; Gani, A. Encapsulation of Saffron and Sea Buckthorn Bioactives: Its Utilization for Development of Low Glycemic Baked Product for Growing Diabetic Population of the World. LWT 2021, 142, 111035. [Google Scholar] [CrossRef]
- Gâtlan, A.-M.; Gutt, G. Sea Buckthorn in Plant Based Diets. An Analytical Approach of Sea Buckthorn Fruits Composition: Nutritional Value, Applications, and Health Benefits. Int. J. Environ. Res. Public Health 2021, 18, 8986. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, Y.; Xu, Q.; Shi, Z.; Xiang, M.; Li, H.; Wang, Y.; Qi, C.; Zhang, Y. (±)-Hyperpyran A: Terpenoid-Based Bicyclic Dihydropyran Enantiomers with Hypoglycemic Activity from Hypericum perforatum (St. John’s Wort). Fitoterapia 2022, 161, 105221. [Google Scholar] [CrossRef]
- Lou, H.; Ma, F.; Yi, P.; Hu, Z.; Gu, W.; Huang, L.; He, W.; Yuan, C.; Hao, X. Bioassay and UPLC-Q-Orbitrap-MS/MS Guided Isolation of Polycyclic Polyprenylated Acylphloroglucinols from St. John’s Wort and Their Neuroprotective Activity. Arab. J. Chem. 2022, 15, 104057. [Google Scholar] [CrossRef]
- Mullaicharam, A.R.; Halligudi, N. St John’s Wort (Hypericum perforatum L.): A Review of Its Chemistry, Pharmacology and Clinical Properties. Int. J. Res. Phytochem. Pharmacol. Sci. 2019, 1, 5–11. [Google Scholar] [CrossRef]
- Muyumba, N.W.; Mutombo, S.C.; Sheridan, H.; Nachtergael, A.; Duez, P. Quality Control of Herbal Drugs and Preparations: The Methods of Analysis, Their Relevance and Applications. Talanta Open 2021, 4, 100070. [Google Scholar] [CrossRef]
- Balekundri, A.; Mannur, V. Quality Control of the Traditional Herbs and Herbal Products: A Review. Future J. Pharm. Sci. 2020, 6, 67. [Google Scholar] [CrossRef]
- Tsiaka, T.; Kritsi, E.; Tsiantas, K.; Christodoulou, P.; Sinanoglou, V.J.; Zoumpoulakis, P. Design and Development of Novel Nutraceuticals: Current Trends and Methodologies. Nutraceuticals 2022, 2, 71–90. [Google Scholar] [CrossRef]
- Sharma, S.; Bhatia, V. Phytochemicals for Drug Discovery in Alzheimer’s Disease: In Silico Advances. Curr. Pharm. Des. 2021, 27, 2848–2860. [Google Scholar] [CrossRef]
- Sharanya, C.S.; Sabu, A.; Haridas, M. Potent Phytochemicals against COVID-19 Infection from Phyto-Materials Used as Antivirals in Complementary Medicines: A Review. Future J. Pharm. Sci. 2021, 7, 113. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Noboa, A.; Proaño-Ojeda, J.; Guevara, M.; Gallo, B.; Berrueta, L.A.; Giampieri, F.; Perez-Castillo, Y.; Battino, M.; Álvarez-Suarez, J.M.; Tejera, E. Metabolomic Profile and Computational Analysis for the Identification of the Potential Anti-Inflammatory Mechanisms of Action of the Traditional Medicinal Plants Ocimum basilicum and Ocimum tenuiflorum. Food Chem. Toxicol. 2022, 164, 113039. [Google Scholar] [CrossRef] [PubMed]
- Zainab, B.; Ayaz, Z.; Alwahibi, M.S.; Khan, S.; Rizwana, H.; Soliman, D.W.; Alawaad, A.; Mehmood Abbasi, A. In-Silico Elucidation of Moringa oleifera Phytochemicals against Diabetes Mellitus. Saudi J. Biol. Sci. 2020, 27, 2299–2307. [Google Scholar] [CrossRef] [PubMed]
- Taamalli, A.; Arráez-Román, D.; Abaza, L.; Iswaldi, I.; Fernández-Gutiérrez, A.; Zarrouk, M.; Segura-Carretero, A. LC-MS-Based Metabolite Profiling of Methanolic Extracts from the Medicinal and Aromatic Species Mentha pulegium and Origanum majorana. Phytochem. Anal. 2015, 26, 320–330. [Google Scholar] [CrossRef]
- Milevskaya, V.V.; Prasad, S.; Temerdashev, Z.A. Extraction and Chromatographic Determination of Phenolic Compounds from Medicinal Herbs in the Lamiaceae and Hypericaceae Families: A Review. Microchem. J. 2019, 145, 1036–1049. [Google Scholar] [CrossRef]
- Skendi, A.; Irakli, M.; Chatzopoulou, P. Analysis of Phenolic Compounds in Greek Plants of Lamiaceae Family by HPLC. J. Appl. Res. Med. Aromat. Plants 2017, 6, 62–69. [Google Scholar] [CrossRef]
- Jabri-Karoui, I.; Bettaie, I.; Msaada, K.; Hammami, M.; Marzouk, B. Research on the Phenolic Compounds and Antioxidant Activities of Tunisian Thymus capitatus. J. Funct. Foods 2012, 4, 661–669. [Google Scholar] [CrossRef]
- Cirlini, M.; Mena, P.; Tassotti, M.; Herrlinger, K.A.; Nieman, K.M.; Dall’Asta, C.; Del Rio, D. Phenolic and Volatile Composition of a Dry Spearmint (Mentha spicata L.) Extract. Molecules 2016, 21, 1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho, J.; Barros, L.; Dias, M.I.; Finimundy, T.C.; Amaral, J.S.; Alves, M.J.; Calhelha, R.C.; Santos, P.F.; Ferreira, I.C.F.R. Echinacea purpurea (L.) Moench: Chemical Characterization and Bioactivity of Its Extracts and Fractions. Pharmaceuticals 2020, 13, 125. [Google Scholar] [CrossRef] [PubMed]
- Haghi, G.; Hatami, A.; Safaei, A.; Mehran, M. Analysis of Phenolic Compounds in Matricaria chamomilla and Its Extracts by UPLC-UV. Res. Pharm. Sci. 2014, 9, 31–37. [Google Scholar]
- Villegas-Aguilar, M.D.C.; Leyva-Jiménez, F.J.; Cádiz-Gurrea, M.D.L.L.; Segura-Carretero, A.; Arráez-Román, D. Comprehensive Analysis of Antioxidant Compounds from Lippia citriodora and Hibiscus sabdariffa Green Extracts Attained by Response Surface Methodology. Antioxidants 2020, 9, 1175. [Google Scholar] [CrossRef]
- Ivanović, M.; Alañón, M.E.; Arráez-Román, D.; Segura-Carretero, A. Enhanced and Green Extraction of Bioactive Compounds from Lippia citriodora by Tailor-Made Natural Deep Eutectic Solvents. Food Res. Int. 2018, 111, 67–76. [Google Scholar] [CrossRef]
- Zadernowski, R.; Naczk, M.; Czaplicki, S.; Rubinskiene, M.; Szałkiewicz, M. Composition of Phenolic Acids in Sea Buckthorn (Hippophae rhamnoides L.) Berries. J. Am. Oil Chem. Soc. 2005, 82, 175–179. [Google Scholar] [CrossRef]
- Tusevski, O.; Krstikj, M.; Stanoeva, J.P.; Stefova, M.; Gadzovska-Simic, S. Phenolic Profile and Biological Activity of Hypericum perforatum L.: Can Roots Be Considered as a New Source of Natural Compounds? S. Afr. J. Bot. 2018, 177, 301–310. [Google Scholar] [CrossRef]
- Yao, Z.J.; Dong, J.; Che, Y.J.; Zhu, M.F.; Wen, M.; Wang, N.N.; Wang, S.; Lu, A.P.; Cao, D.S. TargetNet: A Web Service for Predicting Potential Drug-Target Interaction Profiling via Multi-Target SAR Models. J. Comput.-Aided Mol. Des. 2016, 30, 413–424. [Google Scholar] [CrossRef]
- Schrödinger Release 2020-3: Protein Preparation Wizard; Schrödinger, LLC: New York, NY, USA, 2020.
- Schrödinger Release 2020-3: LigPrep; Schrödinger, LLC: New York, NY, USA, 2020.
- Schrödinger Release 2020-3: Maestro; Schrödinger, LLC: New York, NY, USA, 2020.
- Schrödinger Release 2020-3: Glide; Schrödinger, LLC: New York, NY, USA, 2020.
- Antony, P.; Vijayan, R. Identification of Novel Aldose Reductase Inhibitors from Spices: A Molecular Docking and Simulation Study. PLoS ONE 2015, 10, e0138186. [Google Scholar] [CrossRef]
- Grewal, A.S.; Bhardwaj, S.; Pandita, D.; Lather, V.; Sekhon, B.S. Updates on Aldose Reductase Inhibitors for Management of Diabetic Complications and Non-Diabetic Diseases. Mini Rev. Med. Chem. 2016, 16, 120–162. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Hao, X.; Han, H.; Zhu, S.; Yang, Y.; Wu, B.; Hussain, S.; Parveen, S.; Jing, C.; Ma, B.; et al. Design and Synthesis of Potent and Multifunctional Aldose Reductase Inhibitors Based on Quinoxalinones. J. Med. Chem. 2015, 58, 1254–1267. [Google Scholar] [CrossRef] [PubMed]
- Digiacomo, M.; Sartini, S.; Nesi, G.; Sestito, S.; Coviello, V.; La Motta, C.; Rapposelli, S. Synthesis and Functional Evaluation of Novel Aldose Reductase Inhibitors Bearing a Spirobenzopyran Scaffold. Open Med. Chem. J. 2017, 11, 9–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imran, A.; Shehzad, M.T.; Shah, S.J.A.; Adhami, T.; Laws, A.; Rahman, K.M.; Alharthy, R.D.; Khan, I.A.; Shafiq, Z.; Iqbal, J. Development and Exploration of Novel Substituted Thiosemicarbazones as Inhibitors of Aldose Reductase via in Vitro Analysis and Computational Study. Sci. Rep. 2022, 12, 5734. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Lee, J.M.; Lee, K.H.; Ahn, Y.H.; Lee, S.H. Aldose Reductase Inhibition by Luteolin Derivatives from Parasenecio pseudotaimingasa. Nat. Prod. Sci. 2011, 17, 367–371. [Google Scholar]
- Veeresham, C.; Rama Rao, A.; Asres, K. Aldose Reductase Inhibitors of Plant Origin. Phytother. Res. 2014, 28, 317–333. [Google Scholar] [CrossRef]
- Koşar, M.; Dorman, H.J.D.; Can Başer, K.H.; Hiltunen, R. Screening of Free Radical Scavenging Compounds in Water Extracts of Mentha Samples Using a Postcolumn Derivatization Method. J. Agric. Food Chem. 2004, 52, 5004–5010. [Google Scholar] [CrossRef]
- Miron, T.L.; Plaza, M.; Bahrim, G.; Ibáñez, E.; Herrero, M. Chemical Composition of Bioactive Pressurized Extracts of Romanian Aromatic Plants. J. Chromatogr. A 2011, 1218, 4918–4927. [Google Scholar] [CrossRef] [Green Version]
- Raal, A.; Orav, A.; Püssa, T.; Valner, C.; Malmiste, B.; Arak, E. Content of Essential Oil, Terpenoids and Polyphenols in Commercial Chamomile (Chamomilla recutita L. Rauschert) Teas from Different Countries. Food Chem. 2012, 131, 632–638. [Google Scholar] [CrossRef]
- Pellati, F.; Orlandini, G.; Benvenuti, S. Simultaneous Metabolite Fingerprinting of Hydrophilic and Lipophilic Compounds in Echinacea Pallida by High-Performance Liquid Chromatography with Diode Array and Electrospray Ionization-Mass Spectrometry Detection. J. Chromatogr. A 2012, 1242, 43–58. [Google Scholar] [CrossRef]
- Rainha, N.; Koci, K.; Coelho, A.V.; Lima, E.; Baptista, J.; Fernandes-Ferreira, M. HPLC-UV-ESI-MS Analysis of Phenolic Compounds and Antioxidant Properties of Hypericum undulatum Shoot Cultures and Wild-Growing Plants. Phytochemistry 2013, 86, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Fecka, I.; Turek, S. Determination of Polyphenolic Compounds in Commercial Herbal Drugs and Spices from Lamiaceae: Thyme, Wild Thyme and Sweet Marjoram by Chromatographic Techniques. Food Chem. 2008, 108, 1039–1053. [Google Scholar] [CrossRef] [PubMed]
- Stashenko, E.E.; Martínez, J.R.; Cala, M.P.; Durán, D.C.; Caballero, D. Chromatographic and Mass Spectrometric Characterization of Essential Oils and Extracts from Lippia (Verbenaceae) Aromatic Plants. J. Sep. Sci. 2013, 36, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xu, X.-M.; Chen, Y.; Yu, M.-Y.; Wen, F.-Y.; Zhang, H. Identification, Quantification and Antioxidant Activity of Acylated Flavonol Glycosides from Sea Buckthorn (Hippophae rhamnoides Ssp. Sinensis). Food Chem. 2013, 141, 1573–1579. [Google Scholar] [CrossRef]
- Bouyahya, A.; Chamkhi, I.; Guaouguaou, F.-E.; Benali, T.; Balahbib, A.; El Omari, N.; Taha, D.; El-Shazly, M.; El Menyiy, N. Ethnomedicinal Use, Phytochemistry, Pharmacology, and Food Benefits of Thymus capitatus. J. Ethnopharmacol. 2020, 259, 112925. [Google Scholar] [CrossRef]
- Seyis, F.; Yurteri, E.; Özcan, A.; Cirak, C. Altitudinal Impacts on Chemical Content and Composition of Hypericum perforatum, a Prominent Medicinal Herb. S. Afr. J. Bot. 2020, 135, 391–403. [Google Scholar] [CrossRef]
- Jafari Khorsand, G.; Morshedloo, M.R.; Mumivand, H.; Emami Bistgani, Z.; Maggi, F.; Khademi, A. Natural Diversity in Phenolic Components and Antioxidant Properties of Oregano (Origanum vulgare L.) Accessions, Grown under the Same Conditions. Sci. Rep. 2022, 12, 5813. [Google Scholar] [CrossRef]
- Özer, Z. Investigation of Phenolic Compounds and Antioxidant Activity of Mentha spicata L. Subsp. Spicata and M. longifolia (L.) L. Subsp. Typhoides (Briq.) Harley Decoction and Infusion. J. Turk. Chem. Soc. Sect. A Chem. 2018, 5, 445–456. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Grijalva, E.P.; Picos-Salas, M.A.; Leyva-López, N.; Criollo-Mendoza, M.S.; Vazquez-Olivo, G.; Heredia, J.B. Flavonoids and Phenolic Acids from Oregano: Occurrence, Biological Activity and Health Benefits. Plants 2018, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Zuo, G.; Je, K.-H.; Guillen Quispe, Y.N.; Shin, K.-O.; Kim, H.Y.; Kim, K.H.; Arce, P.H.G.; Lim, S.S. Separation and Identification of Antioxidants and Aldose Reductase Inhibitors in Lepechinia meyenii (Walp.) Epling. Plants 2021, 10, 2773. [Google Scholar] [CrossRef]
- Duțu, L.E.; Popescu, M.L.; Purdel, C.N.; Ilie, E.I.; Luță, E.-A.; Costea, L.; Gîrd, C.E. Traditional Medicinal Plants—A Possible Source of Antibacterial Activity on Respiratory Diseases Induced by Chlamydia pneumoniae, Haemophilus influenzae, Klebsiella pneumoniae and Moraxella catarrhalis. Diversity 2022, 14, 145. [Google Scholar] [CrossRef]
- Marc, R.A.; Mureșan, V.; Mureșan, A.E.; Mureșan, C.C.; Tanislav, A.E.; Pușcaș, A.; Marţiș, G.S.; Ungur, R.A. Spicy and Aromatic Plants for Meat and Meat Analogues Applications. Plants 2022, 11, 960. [Google Scholar] [CrossRef] [PubMed]
- Mutlu-Ingok, A.; Devecioglu, D.; Dikmetas, D.N.; Karbancioglu-Guler, F.; Capanoglu, E. Antibacterial, Antifungal, Antimycotoxigenic, and Antioxidant Activities of Essential Oils: An Updated Review. Molecules 2020, 25, 4711. [Google Scholar] [CrossRef] [PubMed]
- Saija, A.; Speciale, A.; Trombetta, D.; Leto, C.; Tuttolomondo, T.; La Bella, S.; Licata, M.; Virga, G.; Bonsangue, G.; Gennero, M.C.; et al. Phytochemical, Ecological and Antioxidant Evaluation of Wild Sicilian Thyme: Thymbra capitata (L.) Cav. Chem. Biodivers. 2016, 13, 1641–1655. [Google Scholar] [CrossRef] [PubMed]
- Amaghnouje, A.; Mechchate, H.; Es-safi, I.; Boukhira, S.; Aliqahtani, A.S.; Noman, O.M.; Nasr, F.A.; Conte, R.; Calarco, A.; Bousta, D. Subacute Assessment of the Toxicity and Antidepressant-Like Effects of Origanum majorana L. Polyphenols in Swiss Albino Mice. Molecules 2020, 25, 5653. [Google Scholar] [CrossRef]
- Quilantang, N.G.; Ryu, S.H.; Park, S.H.; Byun, J.S.; Chun, J.S.; Lee, J.S.; Rodriguez, J.P.; Yun, Y.-S.; Jacinto, S.D.; Lee, S. Inhibitory Activity of Methanol Extracts from Different Colored Flowers on Aldose Reductase and HPLC-UV Analysis of Quercetin. Hortic. Environ. Biotechnol. 2018, 59, 899–907. [Google Scholar] [CrossRef]
- Waidyanatha, S.; Pierfelice, J.; Cristy, T.; Mutlu, E.; Burback, B.; Rider, C.V.; Ryan, K. A Strategy for Test Article Selection and Phytochemical Characterization of Echinacea purpurea Extract for Safety Testing. Food Chem. Toxicol. 2020, 137, 111125. [Google Scholar] [CrossRef]
- Senica, M.; Mlinsek, G.; Veberic, R.; Mikulic-Petkovsek, M. Which Plant Part of Purple Coneflower (Echinacea purpurea (L.) Moench) Should Be Used for Tea and Which for Tincture? J. Med. Food 2019, 22, 102–108. [Google Scholar] [CrossRef]
- Ajiboye, B.O.; Iwaloye, O.; Owolabi, O.V.; Ejeje, J.N.; Okerewa, A.; Johnson, O.O.; Udebor, A.E.; Oyinloye, B.E. Screening of Potential Antidiabetic Phytochemicals from Gongronema latifolium Leaf against Therapeutic Targets of Type 2 Diabetes Mellitus: Multi-Targets Drug Design. SN Appl. Sci. 2021, 4, 14. [Google Scholar] [CrossRef]
- Chiou, S.-Y.; Sung, J.-M.; Huang, P.-W.; Lin, S.-D. Antioxidant, Antidiabetic, and Antihypertensive Properties of Echinacea purpurea Flower Extract and Caffeic Acid Derivatives Using In Vitro Models. J. Med. Food 2017, 20, 171–179. [Google Scholar] [CrossRef]
- Wang, Z.; Hwang, S.H.; Guillen Quispe, Y.N.; Gonzales Arce, P.H.; Lim, S.S. Investigation of the Antioxidant and Aldose Reductase Inhibitory Activities of Extracts from Peruvian Tea Plant Infusions. Food Chem. 2017, 231, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, H.A.; Tehseen, Y.; Maryam, K.; Uroos, M.; Siddiqui, B.S.; Hameed, A.; Iqbal, J. Identification of New Potent Inhibitor of Aldose Reductase from Ocimum basilicum. Bioorg. Chem. 2017, 75, 62–70. [Google Scholar] [CrossRef] [PubMed]

| Compound | Retention Time (min) | Parent Ion [M–H] | Product Ion (MS/MS) |
|---|---|---|---|
| Gallic acid | 1.51 | 169.33 | 124 |
| Protocatechuic acid | 2.66 | 153.11 | 108.79 |
| Hydroxytyrosol | 2.67 | 153.17 | 122.84 |
| Gentisic acid | 3.65 | 153.02 | 108.84 |
| 4-Hydroxybenzoic acid | 4.75 | 136.94 | 92.87 |
| Chlorogenic acid | 4.91 | 353.03 | 191.02 |
| (+)-Catechin | 5.08 | 289.38 | 245.05 |
| 2-4-Dihydroxy benzoic acid | 3.16 | 153.01 | 108.88 |
| Vanillic acid | 5.81 | 166.99 | 151.96 |
| Caffeic acid | 6.16 | 179.21 | 134.86 |
| Syringic acid | 6.28 | 197.02 | 181.94 |
| (−)-Catechin | 6.40 | 289.38 | 245.06 |
| 4-Hydroxybenzaldehyde | 6.63 | 120.84 | 58.78 |
| Vanillin | 7.28 | 151.00 | 135.87 |
| Syringaldehyde | 7.45 | 181.07 | 165.94 |
| p-Coumaric acid | 7.52 | 163.00 | 118.84 |
| Ferulic acid | 7.73 | 193.06 | 148.86 |
| Rutin | 7.84 | 609.37 | 301.03 |
| Ellagic acid | 8.12 | 301.31 | 257.10 |
| Taxifolin | 8.12 | 303.38 | 285.04 |
| Sinapic acid | 8.14 | 223.10 | 207.88 |
| trans-3-Hydroxycinnamic acid | 8.27 | 163.18 | 118.90 |
| Acetosyringone | 8.70 | 195.14 | 179.98 |
| Salicylic acid | 9.09 | 136.91 | 92.89 |
| Benzoic acid | 9.17 | 121.03 | 58.78 |
| Naringin | 9.35 | 579.41 | 459.09 |
| Hesperidin | 9.66 | 609.27 | 301.02 |
| Rosmarinic acid | 9.68 | 359.09 | 160.88 |
| o-Coumaric acid | 9.82 | 163.10 | 118.87 |
| Oleuropein | 10.70 | 539.37 | 376.82 |
| Lariciresinol | 11.36 | 418.91 | 329.12 |
| Eriodictyol | 11.80 | 287.70 | 150.86 |
| Luteolin | 12.16 | 285.30 | 174.97 |
| Quercetin | 12.18 | 301.23 | 178.86 |
| Resveratrol | 12.22 | 227.40 | 184.95 |
| Cinnamic acid | 12.34 | 147.05 | 102.84 |
| Naringenin | 12.87 | 271.29 | 150.88 |
| Kaempferol | 12.94 | 285.28 | 285.04 |
| Isorhamnetin | 13.22 | 315.75 | 300.08 |
| Detected Compounds | M.Chamomilla | Ech.Purpurea | L.Citriodora | M.Pulegium | M.Spicata | O.Majorana | O.Vulgare | T.Capitatus | Hyp.Perforatum | Hip.Rhamnoides | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (−) Catechin (s) 1 | √ 2 | ||||||||||
| (+) Catechin (s) | √ | ||||||||||
| 4-Hydroxycinnamic acid (s) | √ | ||||||||||
| Caffeic acid (s) | √ | √ | √ | ||||||||
| Chlorogenic acid (s) | √ | ||||||||||
| p-Coumaric acid (s) | √ | ||||||||||
| Ellagic acid (s) | √ | √ | |||||||||
| Ferulic acid (s) | √ | √ | |||||||||
| Gentisic acid (s) | √ | ||||||||||
| Naringenin (s) | √ | ||||||||||
| Quercetin (s) | √ | ||||||||||
| Resveratrol (s) | √ | ||||||||||
| Rosmarinic acid (s) | √ | √ | √ | ||||||||
| Rutin (s) | √ | √ | √ | ||||||||
| Apigenin | √ | [53] | |||||||||
| Apigenin-c-hexoside-c-hexoside | √ | [54] | |||||||||
| Apιgenin-7-o-glucoside | √ | [55] | |||||||||
| Caftaric acid | √ | [56] | |||||||||
| Catechin dimer | √ | [57] | |||||||||
| Cichoric acid | √ | [56] | |||||||||
| Citric acid | √ | [58] | |||||||||
| Dihydrocaffeic acid | √ | [59] | |||||||||
| Ellagic acid (p-coumaryl) hexoside | √ | [60] | |||||||||
| Ellagic acid hexoside | √ | [60] | |||||||||
| Ellagic acid pentoside | √ | √ | [58,60] | ||||||||
| Eriodictyol-7-o-glucuronide | √ | [58] | |||||||||
| Ferulic acid glucoside | √ | [55] | |||||||||
| Ferulic acid hexoside dimer | √ | [55] | |||||||||
| Gallocatechin | √ | [58] | |||||||||
| Isoquercitrin | √ | [57] | |||||||||
| Kaempherol-3-o-rutinoside | √ | √ | √ | √ | [56,57,58] | ||||||
| Laricitrin-3-o-glucoside | √ | [55] | |||||||||
| Luteolin-7-o-glucoside | √ | √ | √ | √ | [53,58,59] | ||||||
| Medioresinol | √ | [54] | |||||||||
| Quercetin-3o-glucoside | √ | √ | [57,58] | ||||||||
| Rosmarinic acid-o-hexoside | √ | [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsiaka, T.; Kritsi, E.; Lantzouraki, D.Z.; Christodoulou, P.; Tsigrimani, D.; Strati, I.F.; Sinanoglou, V.J.; Zoumpoulakis, P. Assessing the Phytochemical Profile and Potential of Traditional Herbal Infusions against Aldose Reductase through In Silico Studies and LC-MS/MS Analysis. Appl. Sci. 2022, 12, 8361. https://doi.org/10.3390/app12168361
Tsiaka T, Kritsi E, Lantzouraki DZ, Christodoulou P, Tsigrimani D, Strati IF, Sinanoglou VJ, Zoumpoulakis P. Assessing the Phytochemical Profile and Potential of Traditional Herbal Infusions against Aldose Reductase through In Silico Studies and LC-MS/MS Analysis. Applied Sciences. 2022; 12(16):8361. https://doi.org/10.3390/app12168361
Chicago/Turabian StyleTsiaka, Thalia, Eftichia Kritsi, Dimitra Z. Lantzouraki, Paris Christodoulou, Diamantina Tsigrimani, Irini F. Strati, Vassilia J. Sinanoglou, and Panagiotis Zoumpoulakis. 2022. "Assessing the Phytochemical Profile and Potential of Traditional Herbal Infusions against Aldose Reductase through In Silico Studies and LC-MS/MS Analysis" Applied Sciences 12, no. 16: 8361. https://doi.org/10.3390/app12168361
APA StyleTsiaka, T., Kritsi, E., Lantzouraki, D. Z., Christodoulou, P., Tsigrimani, D., Strati, I. F., Sinanoglou, V. J., & Zoumpoulakis, P. (2022). Assessing the Phytochemical Profile and Potential of Traditional Herbal Infusions against Aldose Reductase through In Silico Studies and LC-MS/MS Analysis. Applied Sciences, 12(16), 8361. https://doi.org/10.3390/app12168361










