Abstract
Obesity is arising as a global pandemic throughout the world. Over the past few decades, obesity has tripled worldwide, creating an alarming situation. The majority of people nowadays are suffering from obesity and overweight. It affects health of people of all age groups, ethnicity, gender, and sex, and is linked to a sedentary lifestyle of people, poor eating habits, and disturbed sleeping patterns. It causes several diseases such as diabetes mellitus type 2, hypertension, cardiovascular diseases, asthma, gallstones, and colon cancer. Many synthetic anti-obesity drugs such as orlistat, lorcaserin, phentermine, bupropion, and liraglutide are already available on the market. However, these drugs have side effects, including dry mouth and sleeping disorders, dizziness, blood pressure, heart rate elevation, constipation, and headache. Humans have a long and ancient history of dependency on traditional medicinal plants and their major bioactive antioxidant components, such as quercetin, anthocyanins, and ellagic acid, for treating such diseases and disorders. This review discusses the herbal approach, bioactive compounds, and their mechanism for treating obesity.
1. Introduction
Obesity is defined as an elevation of the fat tissue mass in the body, which is not limited to any age group. It is characterized by increased body and fat mass, hormonal disturbances, food intake (eating pattern), and genetic factors [1]. Obesity is a significant contributor to the global burden of several chronic diseases such as diabetes mellitus type 2, cardiovascular diseases, asthma, etc., which affect the human body. It has been proclaimed a global pandemic with a death rate of approximately 2.8 million people annually [2,3]. Since fat is present all over the body, it is not possible to measure it directly. Therefore, body mass index (BMI) is used to observe the relationship between weight and height. Whereas waist circumference, waist/hip ratio, skinfold thickness, and bioimpedance help to assess obesity and overweight [4].
Suppose the BMI of the person lies in the range of overweight, the chances of getting affected by other diseases such as diabetes mellitus type 2, hypertension, cardiovascular diseases, gallstones, etc., increase. For obesity class 1, the chances are moderate, while in the case of obesity class 2, the chances are severe. In the case of extreme obesity, the chances are very high, particularly if the person is affected by other obesity-related diseases [5,6]. Figure 1 summarizes the BMI classification recommended by WHO and the National Institute of Health in the United States.
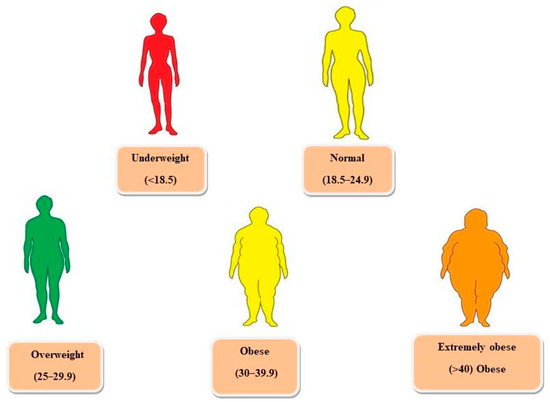
Figure 1.
Classification of weight according to body mass index (BMI).
There are two types of fats, visceral and subcutaneous. The fat deposited over visceral organs such as the kidney, abdomen, liver, intestines, heart, and pancreas is known as visceral fat. The other name for visceral fat is active fat because research has proved that it significantly affects our hormonal activity, resulting in metabolic syndrome and insulin resistance. The hormonal activity influences metabolism, body fat distribution, and appetite [7]. Moreover, the fat underneath the skin is known as subcutaneous fat and can be felt underarm and legs [8]. The body attains two types of shapes, apple, and pear, due to fat deposition in certain body areas [9]. In the case of an apple shape (Android), fat accumulation occurs in the upper area of the waist and abdomen. Some deposition also appears on the neck, arms, and shoulders. The main reason behind this body shape is visceral fat, which is associated with the health risk of type 2 diabetes [10]. In the pear shape (Gynoid), fat deposition occurs on the thighs and buttocks. The amount of visceral fat is low in this type of body shape, resulting in low chances of weight-related diseases [11].
Obesity also causes several complications such as reproductive (women and men), respiratory, cardiovascular diseases, pancreas, and gastrointestinal, as shown in Figure 2.
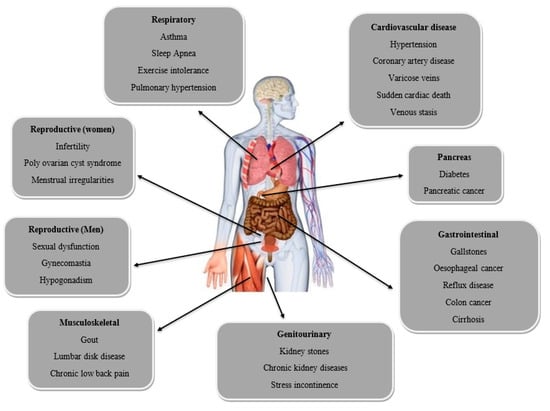
Figure 2.
Several complications as a result of obesity.
According to the survey performed by the Organisation for Economic Co-operation and Development (OECD) in 2017, the United States of America is ranked first, followed by China and India in obesity. From 1999 to 2000 and 2015 to 2016, there has been a remarkable increase in obesity [12]. In 2016, the World Health Organization (WHO) reported that over 1.9 billion adults who were either 18 years old or above were overweight, and among these 1.9 billion, 650 million were suffering from obesity [2]. According to a survey, 25 million people die annually because of obesity or being overweight [12]. According to another study by WHO (World Health Organization) in 2019, 38.2 million children under the age of 5 were either suffering from obesity or overweight [13].
Even in India, the prevalence of obesity varies from urban to rural and state-wise. It was reported that the prevalence of obesity in males was higher in urban areas (37.5%) compared to rural areas (20.78%) [14]. Some of the states of India affected by obesity are Jharkhand, Kerala, Pondicherry, Chhattisgarh, Madhya Pradesh, Bihar, and Andhra Pradesh [14,15].
The vital factor that plays a headway role in the case of obesity is the person’s lifestyle and eating habits [16]. Most food items with high fat and sugar are responsible for increasing body weight, and such food materials have low micronutrients [17,18]. Consumption of excessive refined grains, junk foods, and soft drinks can lead to a big waist:hip ratio, and the person’s fat mass also increases [19].
2. Hormones Related to Obesity
2.1. Leptin
Leptin is a significant hormone that is related to an increase in body weight [20]. Leptin, the result of the ob (obese) gene product, is the first adipose hormone that links the central regulation of metabolism with peripheral adipose fat mass. In the arcuate nucleus of the hypothalamus, leptin suppresses appetite by either decreasing the activity of orexigenic neuropeptides (NPY and AgRP) or boosting the activity of anorexigenic neuropeptides (α-MSH and CART) [21,22]. As adiposity increases, serum leptin concentrations increases along with it. Higher circulating levels of leptin reduce energy intake and increase energy expenditure in a homeostatic system, but this is not the case in those who are suffering from obesity or overweight, thus, indicating a condition of leptin resistance. Circulating soluble leptin receptor (SLR) levels decline with obesity. These receptors are blood-circulating proteins that directly support leptin activity. This combination of high levels of leptin and low SLR may contribute to the leptin resistance seen in obese people [7]. Clinical investigations have demonstrated that the administration of leptin has minimal effect on the body weight of obese participants, despite enthusiastic research on the use of leptin in the treatment of obesity [21,22]. If there is a deficiency of leptin, it can lead to extreme obesity [23].
2.2. Ghrelin
A 28-amino acid peptide hormone called ghrelin is mainly produced in the stomach. Ghrelin increases the growth hormone release by directly interacting with the GH secretagogue receptor at the pituitary level [24,25]. Ghrelin seems to have a role in neuroendocrine and metabolic responses to food intake. Its circulating levels are elevated in anorexia and cachexia and lowered in obesity [26,27,28]. Plasma ghrelin levels are inversely related to body fat mass, body mass index, plasma leptin levels, insulin, and glucose levels [29]. Various studies imply that ghrelin may be crucial in controlling appetite and weight. GH secretion, food intake, and obesity are all controlled by the GH secretagogue receptor (GHS-R1a) in the arcuate nucleus [30,31].
2.3. Insulin
Excess body weight is frequently linked to insulin resistance, a condition in which tissues, particularly skeletal muscle, the liver, and adipose tissue, are less receptive to the physiological effects of insulin [32,33]. Plasma insulin levels are persistently increased in insulin-resistant conditions, even while fasting. Increased plasma concentrations of free fatty acids, continually produced from adipose tissue, are the leading cause of insulin resistance by excess weight. Increased hepatic and muscle fatty acid absorption and oxidation due to increased free fatty acid concentrations result in metabolic changes that reduce these tissues’ ability to absorb and utilize glucose for energy metabolism. Reduced insulin receptor levels and post-receptor abnormalities in insulin signaling are included in these adaptations. Increases in intra-abdominal body fat storage, which release free fatty acids into the circulation more quickly than other adipose tissue compartments, are most significantly associated with insulin resistance [34,35,36]. Weight reduction often increases insulin sensitivity and normalizes plasma insulin concentrations in obese patients [37].
2.4. Adiponectin
Mature adipocytes release adiponectin, and its circulating levels are lower in obese and diabetic people. The plasma level of adiponectin is increased by anti-diabetic and anti-obesity medications such PPARagonists (thiazolidinediones) and CB1 antagonists (rimonabant). Through the activation of AMP kinases, which have been linked with the adiponectin receptors R1 and R2 in animal models and human studies, adiponectin increases insulin sensitivity [38,39]. In humans, atherosclerosis, dyslipidemia, and insulin resistance have been linked to lower adiponectin levels in obesity. With an increase in insulin sensitivity, plasma adiponectin levels considerably rise with weight reduction [7]. As a result, treating insulin resistance associated with obesity and type 2 diabetes may include targeting adiponectin and adiponectin receptors.
2.5. Omentin
In visceral fat tissue, omentin is released predominantly by stromal-vascular cells rather than adipocytes [40]. Omentin functions as an insulin sensitizer and has favorable effects on glucose absorption. Although adipose tissues produce a significant amount of omentin, obese individuals have lower plasma levels of omentin [41]. Its expression is lowered in type 2 diabetes and insulin resistance. Adiponectin and high-density lipoprotein levels correlate positively with omentin, but body mass index, waist circumference, triglycerides, insulin resistance, and leptin levels are negatively correlated [7]. Omentin’s mode of action, relevant receptors, and target tissues must be clarified before it can be used in anti-obesity therapeutics.
2.6. Peptide YY (PYY)
Peptide YY (PYY) is a 36-amino acid peptide produced and released into the bloodstream by specialized enteroendocrine cells termed L cells, mainly found in the distal gastrointestinal tract. PYY1-36 and PYY3-36 are the two primary forms of PYY that have been reported. Circulating PYY levels rise in response to nutrient intake and food consistency, caloric load, and nutrient composition influencing its circulating levels. PYY levels usually peak 1–2 h after intake, followed by a period during which levels are steady [42,43]. Increased appetite and food consumption are linked to low peptide YY concentrations. Low levels of peptide YY are observed in obesity and before the beginning of type 2 diabetes and may contribute to acquiring weight in these conditions [44].
2.7. Acylation-Stimulating Protein (ASP)
Acylation-stimulating protein, a 76-amino acid peptide produced by adipocytes, stimulates the synthesis of triglycerides (TG) in adipose tissue by acting on its C5L2 receptor [45]. Patients with obesity, type 2 diabetes, and cardiovascular disease have higher plasma ASP levels than healthy people, whereas exercise or weight loss lowers the ASP levels. A disrupted adipose tissue metabolism and dyslipidemia, frequent in diabetes and cardiovascular disease, have also been linked to an ASP-resistant condition. It has been proposed that complement C3, an ASP precursor in adipose tissue, is activated to generate ASP [46]. Therefore, ASP could offer a target for regulating fat accumulation.
3. Synthetic Drugs
Many anti-obesity synthetic drugs such as orlistat, lorcaserin, phentermine/topiramate, bupropion/naltrexone, and liraglutide are already available on the market. These drugs target obesity by increasing noradrenaline, dopamine, and serotonin. However, they have many side effects related to cardiovascular health, increased blood pressure, and affect the central nervous system by causing changes in sleep patterns and affecting hormonal secretion [47,48].
3.1. Orlistat
Orlistat, approved in 1998, is a synthetic derivative of lipstatin [49], a potent and reversible inhibitor of gastrointestinal lipase that inhibits pancreatic and stomach lipase to reduce dietary fat absorption by 30% [50]. Orlistat was produced as one of the lipstatin analogs and is known as an anti-obesity medication due to its ability to suppress pancreatic lipase activity selectively. Orlistat works by reducing fat absorption by permanently inhibiting lipase activity in the gastrointestinal tract, which reduces energy intake [49]. The most common side effects include diarrhea, abdominal pain, bloating, flatulence, oily stools, dyspepsia, reduced absorption of fat-soluble vitamins, and in some rare cases, it can cause severe liver and kidney injury [50].
3.2. Lorcaserin
Lorcaserin, approved in 2012 in the USA, is a selective serotonin 2C (5-HT2C) receptor agonist that decreases body weight by reducing food intake [50]. Serotonin (5-HT) is released when lorcaserin interacts with the 5-hydroxytryptamine 2C (5-HT2C) receptor, and serotonin uptake is subsequently inhibited [51]. Because lorcaserin has a low specificity for the 5-HT2B receptor (about 100 times lower than that of the 5-HT2C receptor), there is a limited chance that long-term use of lorcaserin may result in heart-valve abnormalities [6,52]. The side effects of lorcaserin include serotonin syndrome or neuroleptic malignant syndrome-like reactions: headache, dizziness, nausea, fatigue, dry mouth, constipation, low blood sugar (hypoglycemia), headache, cough, back pain, and fatigue in diabetic patients [53].
3.3. Phentermine/Extended-Release Topiramate (Qnexa)
A combination treatment of phentermine and extended-release (ER) topiramate, developed by Vivus, is known as Qnexa. Although the exact mode of action is unknown, phentermine is believed to stimulate the release of catecholamines (including dopamine and noradrenaline) in the hypothalamus. In contrast, topiramate enhances the activity of the neurotransmitter gamma-aminobutyrate, regulates voltage-gated ion channels, and inhibits carbonic anhydrase and AMPA/kainite excitatory glutamate receptors [54,55]. The side effects reported include dizziness, paresthesia (tingling in the hands and feet), changed taste perception, dry mouth, dysgeusia, insomnia, and constipation, without increased risk of serious cardiovascular problems [55].
3.4. Bupropion/Naltrexone (Contrave)
The combination of bupropion and naltrexone synergistically enhances neuronal activity and decreases food uptake by stimulating satiety [49]. Although bupropion is a relatively poor inhibitor of dopamine and norepinephrine uptake, it stimulates the hypothalamic pro-opiomelanocortin (POMC) system, which activates melanocortin receptors and induces weight reduction through appetite suppression and increased energy expenditure. Naltrexone functions as an antagonist of the opioid receptor that generally causes a negative feedback-mediated inhibition of POMC activation and thus acts synergistically to prolong the bupropion’s action on metabolism [56]. The most common adverse effects (AEs) reported are nausea, headache, dizziness, vomiting, insomnia, constipation, and dry mouth [57].
3.5. Liraglutide
An analog of glucagon-like peptide (GLP)-1, liraglutide has a half-life of 13 h, which significantly prolongs its activation of the GLP-1 receptor (the half-life of native GLP-1 is about 1.5 min), increasing stimulation of glucose-dependent insulin secretion and exerting a more substantial effect on glucagon suppression [58]. Native GLP-1 has central effects that control appetite and feeding centers in the brain [59]; hence, liraglutide may potentially have the ability to influence energy intake. A 5-week partial crossover trial with liraglutide demonstrated that liraglutide caused weight reduction most likely mediated by lowering appetite and decreasing food consumption. It did not enhance energy expenditure, as measured by appetite scores, post-prandial satiety, and fullness ratings [60]. The most common side effects reported include nausea, diarrhea, vomiting, constipation, decreased appetite, and low blood sugar (hypoglycemia) [61].
4. Herbs That Control Obesity
Herbs have always proved to be an essential and functional source for many chronic diseases, and obesity is one of them. Other than a few allergic reactions in susceptible individuals, herbs have fewer adverse effects than single-compound drugs. Medicinal herbs such as Nigella sativa, Hibiscus sabdariffa, Ilex paraguariensis, Coffea arabica, Caralluma fimbriata, Panax ginseng, and many others have shown positive effects on obesity by different mechanisms such as suppressing appetite; reducing triglyceride levels; increasing the metabolic rate; inhibiting pancreatic lipase, etc. Hence, they facilitate the process of weight loss [62,63].
4.1. Nigella sativa
The herb is a member of the Ranunculaceae family, also known as black seed. The origin of the herb belongs to south European countries, including Albania, Bosnia, Bulgaria, and Cyprus, as well as some southwest Asian countries such as Indonesia, Thailand, and Singapore. It is also grown in India, Pakistan, Turkey, and Syria [64,65]. The herb contains vitamins and minerals such as copper, potassium, zinc, and iron [66]. It treats various diseases and disorders related to liver, cardiovascular health, inflammation, gastrointestinal health, and diabetes. It has antioxidant, anti-inflammatory, anti-bacterial, and anti-fungal properties [67,68]. The herb is a potent anti-obesity agent. The main bioactive compound, Thymoquinone (TQ), is mainly responsible for anti-obesity activity. It targets obesity by suppressing the appetite and reducing triglyceride levels. It also plays a significant role in adiponectin hormone level, which plays a vital role in protecting against insulin obstinacy in the body [69,70].
4.2. Hibiscus sabdariffa
The other name for the herb is roselle; it is a sub-shrub that can grow up to 8 ft in height. It is a native plant of India and Malaysia but is grown in other parts of the world, such as China, Thailand, Sudan, Nigeria, Jamaica, etc. [71,72]. It is a vital source of vitamins and minerals such as calcium, iron, phosphorus, riboflavin, and vitamin C. Hibiscus sabdariffa helps to fight against many diseases related to kidney stones, gastrointestinal disorders, and liver damage. It has antioxidant, antimicrobial, anti-inflammatory, anti-carcinogenic, anti-obesity, and anti-diabetic properties [73]. The main bioactive compounds, such as anthocyanins, flavonoids, and organic acids, are responsible for the anti-obesity activity of the herb [74]. The extract of Hibiscus sabdariffa has the potential to enhance insulin sensitivity and resistance while lowering lipid accumulation and oxidative stress. An increase in lipoprotein lipase activity and a decrease in the expression of the adipogenesis genes are two ways that anthocyanin suppresses lipid accumulation and decreases fat mass in high-fat diet-induced obese rats. The loss of visceral adipose tissue reduces inflammatory cytokines and oxidative state, which reduces insulin resistance and enhances insulin sensitivity in obese rats [75].
4.3. Ilex paraguariensis
The herb’s common name is yerba mate; it belongs to the Aquifoliaceae family. It is a native plant of South America (Brazil, Argentina, etc.) [76]. The height of the plant can be up to 20 m. It is one of the richest sources of flavonoids (quercetin and rutin) and phenolic acids such as chlorogenic and caffeic acid. It also contains saponins and caffeine. It has many pharmacological properties such as antioxidant activity, anti-obesity, anti-diabetic, anti-tumor, neuroprotective, and anti-inflammatory [77]. It helps in the regulation of insulin as well as targets obesity by inhibiting the expression of genes that regulate adipogenesis, improve the lipid profile, and act as an appetite suppressant. It also inhibits the inflammatory action in adipose tissue caused by obesity. It enhances peripheral insulin sensitivity [78] and modulates the leptin release by adipose tissue [79].
4.4. Rosmarinus officinalis
The other name of the herb is rosemary; it belongs to Lamiaceae Mint family. The herb is native to the Mediterranean region. The leaves are a vital part of the herb used for medicinal purposes [80]. Anti-bacterial, antioxidant, anti-cancer, anti-diabetic, anti-inflammatory, anti-thrombotic, neuroprotective, and hepatoprotective are some of the herb’s uses [81]. It also has an anti-viral effect and helps to regulate blood pressure [82,83]. Certain studies revealed a significant anti-obesity impact with the help of carnosic acids that alter the activity of 3T3-L1 preadipocytes differentiation [84,85]. Evidence of the inhibitory effect of rosemary extract on pancreatic lipase activity, a crucial enzyme in the digestion and absorption of fat, as well as on gastric lipase activity, suggests that limiting lipid absorption is the primary mechanism by which rosemary extract lowered weight gain and adiposity index [86].
4.5. Coffea arabica
The herb’s common name is green coffee beans; it belongs to the Rubiaceae family. It is an upright tropical evergreen shrub. It is the native plant of Ethiopia, now commercially grown in tropical and subtropical countries throughout the world. The herb possesses various medicinal properties such as anti-diabetic, antimicrobial, anti-cancerous, anti-inflammatory, antioxidant, anti-obesity, etc. [87]. The major bioactive compounds present in the herb include chlorogenic acids, caffeic, vanillic, trigonelline, p-coumaric, feruloyl acids, tannins, and anthraquinones [88]. The active compound 3-CQA is mainly responsible for the anti-obesity activity of the herb. It dramatically reduces the body weight gain and white adipose tissue (WAT) weights with the regulation of adipose tissue lipolysis hormones such as leptin and adiponectin. It reduces the mRNA expression levels of genes associated with adipogenesis and adipocyte metabolism and the corresponding protein expression. Thus, the herb shows a potential activity that prevents obesity [89].
4.6. Aframomum melegueta
The common name of the herb is grains of paradise or Guinea grains. It is a perennial, herbaceous plant that belongs to the ginger family, Zingiberaceae. It is commonly found along the West African coast of Nigeria. The different parts of the herb possess specific bioactive compounds such as flavonoids, alkaloids, phenolic compounds, tannins, saponins, terpenoids, and cardiac glycosides that shows healing potential and therapeutic purposes [90,91]. These compounds possess various biological activities such as antimicrobial, anti-inflammatory, anti-allergic, antioxidant, anti-clotting, anti-cancer, anti-obesity, anti-diabetic, and hepatoprotective effects [91]. The anti-obesity effect of the herb is mainly due to the presence of vanilloids such as 6-paradol and 6-gingerol. Specific to the liver and adipose tissue, 6-paradol and 6-gingerol controlled several gene expressions and AMPK phosphorylation. It appears that 6-paradol may affect adipose differentiation by preventing free fatty acid translocation, and 6-gingerol may, in particular, enhance hepatic metabolism by improving fatty acid β-oxidation. These components inhibit lipid accumulation and fatty acid consumption during adipogenic differentiation by regulating genes involved in biosynthesis. The enhancement of lipolysis, fatty acid β-oxidation, and the decrease in TG accumulation contribute to the decreasing adipocyte size, inhibiting the growth of WAT [92].
4.7. Panax ginseng
The other name of the herb is ginseng; it belongs to the Araliaceae family. It is a herbal treatment used for thousands of years in East Asia (Korea, China, and Japan) to improve health, vitality, and longevity [93]. It has been demonstrated that Panax ginseng has many physiological and pharmacological benefits such as anti-diabetic, anti-cancer, anti-inflammatory, anti-obesity, neuroregulation, antimicrobial, wound healing, etc. In addition to claimed benefits in chronic fatigue, these include advantageous effects against cancer, diabetes, hypertension, nociception, and stroke [94]. The main active components of this herb are ginseng saponins and polysaccharides [95]. The primary active constituent responsible for its anti-obesity effect is ginsenosides. Ginseng is reported to have an impact on the levels of hormones such as leptin, ghrelin, and adiponectin, as well as appetite. It reduces the chronic inflammation of the hypothalamus brought on by HFD, enhancing leptin resistance and decreasing neuropeptide Y release. Additionally, it is suggested that ginseng inhibits pancreatic lipase activity that prevents the digestion and absorption of fat and carbohydrates, lowers blood glucose, and increases fecal weight. By controlling PAR-γ/C/EBP-α, PPAR-α, and AMPK, ginseng may also have an antiadipogenic impact and improve fat oxidation and energy expenditure [94].
4.8. Caralluma fimbriata
This herb belongs to the Asclepiadaceae family; it is a native herb of the countries India, Pakistan, and Afghanistan [96]. The major phytochemical compounds of the herb are pregnane glycosides, megastigmane glycosides, flavone glycosides, and saponins. These phytochemicals contribute to its biological activities such as antimicrobial, anti-inflammatory, anti-obesity, anti-diabetic, antinociceptive, antipyretic, antioxidant, anti-helminthic, etc. [97]. The active component, pregnane glycosides, is known for its appetite-suppressing effects in the hypothalamus. It suppresses appetite by inhibiting ghrelin production in the stomach and neuropeptide Y (NPY) release in the hypothalamus [96]. It increases the fat-burning capacity and inhibits the synthesis of fat inside the body [98]. The pregnancy glycosides are suggested to reduce fat accumulation by inhibiting citrate lyase. It also inhibits malonyl coenzyme A, further preventing fat synthesis in the metabolic pathway. By acting on malonyl coenzyme, it prevents the formation of new fat cells [99].
4.9. Capsicum annum
The herb’s common name is chili pepper; it belongs to the Solanaceae family. The herb is a native plant of Mexico [100]. The major bioactive compounds of this herb are flavonoids, phenolic acids, ascorbic acid, and carotenoids [101]. These compounds possess various pharmacological activities such as antioxidant, antimicrobial, anti-obesity, anti-cancer, anti-neoplastic, anti-ulcer, and anti-inflammatory [102]. The main active component responsible for its anti-obesity activity is capsaicin. It triggers the activation of the TRPV1 mechanism that can reduce abnormal glucose homeostasis by promoting the release of insulin and increasing the levels of glucagon-like peptide-1 (GLP-1). It promotes lipid oxidation, inhibits adipogenesis, activates thermogenesis, decreases appetite, and increases satiety, controlled by neuronal circuits in the hypothalamus [103]. In addition to inhibiting the expression of PPAR-γ, C/EBP-α, and leptin, capsaicin dramatically reduced the glycerol-3-phosphate dehydrogenase (GDPH) activity and intracellular triglyceride in 3T3-L1 adipocytes [104].
4.10. Zingiber officinale
Commonly known as ginger, it is a herbaceous perennial plant that grows up to 1 m in height [105]. The herb is native to Southeast Asia, the Indian subcontinent, China, and New Guinea. This herb is consumed all over the world for culinary and medicinal uses. The main bioactive compounds for herb’s pharmacological properties such as anti-inflammatory, anti-diabetic, anti-cancer, anti-obesity, anti-arthritis, anti-bacterial, anti-fungal, etc. [106] are zingerone, gingerols, shogaols, and paradols [107]. The primary active compounds responsible for its anti-obesity effects are 6-gingerol and 6-shogaol. It is reported that 6-gingerol and 6-shogaol can effectively suppress the differentiation of 3T3-L1 preadipocytes into adipocytes and decrease the levels of triglycerides. They inhibit lipid accumulation and reduce glycerol-3-phosphate dehydrogenase (GPDH) activity. They also decrease the mRNA expression levels of adipogenesis-related transcription factors such as PPAR-γ, C/EBP-α, and their main lipogenic enzymes such as fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC). Additionally, they inhibit the activity of pancreatic lipase and amylase, which leads to a decrease in plasma and tissue lipids [108,109,110].
Several clinical trials have been conducted on different herbs for treating obesity in humans and to observe the efficacy of herbs such as Nigella sativa, Hibiscus sabdariffa, Ilex paraguariensis, Coffea arabica, Caralluma fimbriata, Panax ginseng, etc., as shown in Table 1.

Table 1.
Clinical trials on herbs for the treatment of obesity in humans.
5. Plant-Based Perspective
Plant-based diets have lower all-cause mortality and a lower risk of obesity, type 2 diabetes, and coronary heart disease [139]. Plants have been used as traditional and natural pharmacological remedies from time immemorial. Phytomedicines are effective alternatives to synthetic pharmaceuticals in today’s pharmacological assistance. Plant-derived secondary metabolites that have pharmacological or toxicological effects on humans and animals but play little or no role in plant growth and development are called phytomolecules. A wide range of plant products, including crude extracts and isolated pure natural components, can counteract diet-induced obesity and cause weight loss [140]. Obesity’s rising danger to world health has prompted scientists and researchers to invest more effort into developing an effective anti-obesity component. Numerous natural-source promising materials, as well as their active constituents, have been explored. Most of these natural elements are produced from plants, such as fruits, vegetables, cereals, and herbs. The presence of an abundance of phytochemicals, fiber, and unsaturated fatty acids contributes to the biological advantages of these natural components.
The anti-obesity products in the market can be classified into three categories: (1) food ingredients, (2) herbal ingredients, and (3) other functional supplements. The most popular section of the functional supplement market is arguably the development of functional items from what people usually eat in their everyday lives. Products derived from fruits (citrus and berries), grains (soybean), vegetables, or drinking liquids (tea leaves) are considered safer and more acceptable by customers. To treat patients with obesity, traditional Chinese medical practitioners utilize herbal medicines that are generally mixtures of several herbs, such as turmeric (Curcuma longa) and mulberry leaf (Morus alba). Herbal remedies have recently been popular not just in Asia but also in the Western world. That is why herbal materials might be a significant category of anti-obesity therapies. Other substances, such as probiotics and calcium supplements, have also shown anti-obesity properties. Citrus fruits are one of the essential categories for developing and commercializing novel anti-obesity therapies. Phytochemicals such as triterpenoids, flavonoids, and alkaloids have been plentiful in citrus fruits’ peel and pulp. Citrus fruit extracts have anti-obesity properties in cell and animal experiments, helping to reduce body weight increase and white adipose tissue weight [141]. Leptin, a crucial hormone generated by adipocytes that regulates food intake and energy expenditure, was lowered by citrus fruit consumption. This alteration in hormonal activity is desired for creating an anti-obesity treatment based on citrus. The main bioactive flavonoid components in citrus fruits that might affect plasma leptin levels are methoxylated flavones and flavanone glycosides. Green tea (Camellia sinensis)-derived anti-obesity products are also popular in the functional food sector. Polyphenols, which comprise flavonols, flavones, and flavan-3-ols, are the most abundant bioactive compounds in green tea, accounting for up to 35% of the dry weight. Clinical investigations have shown that catechins (270 to 1200 mg/day) have positive benefits, such as reduced body weight, lower blood leptin levels, and reduced fatty acid absorption. Caffeine, another bioactive component of tea leaves, modulates somatic nervous system activity and works synergistically with catechins to promote energy expenditure and fat burning [142].
6. Conclusions
The use of herbal plants to treat obesity is now attaining much attention. Only a small portion of the active components found in herbs have been discovered; however, as the composition of the herbs is better understood, the target and precise mechanism of action can be established. As was already said, herbal therapy has several advantages over pharmaceutical treatments for treating individuals with obesity. It also has fewer or no side effects. According to extensive preclinical and clinical studies, the medicinal benefits of herbal plants include being anti-obesity, anti-diabetic, antioxidant, anti-hyperlipidemic, and anti-inflammatory. Nigella Sativa, Hibiscus sabdariffa, Ilex paraguariensis, Rosemary officinalis, Coffea arabica, and Afamomum melegueta are a few of the herbs that have been known for reducing obesity. Modern pharmacological science has made recognizing the active agents in herbal medicine compounds simpler, making it easier to conduct scientific research on their efficacy. To further support the safety and anti-obesity efficacy of herbal medicine and ultimately prevent obesity by use of herbal treatment in people, more and more clinical trials and a standardized technique for producing herbal medicine are required.
7. Future Perspective
Timeously, herbal medicines have proven effective against obesity and other disorders. Benefits of herbal products include weight loss without any adverse side effects. It is readily available and is organic, making it safe and fit for consumption [143]. Developed as well as developing countries have accepted it. The review provides information about many herbs and their positive approach to weight loss. In some cases, it alters the appetite; in others, it manages the triglyceride levels; in this way, it helps to maintain weight. Although there are available data on the practical approach of herbal medicines in controlling obesity, more research is required to understand the mode of action.
Author Contributions
M.K. conceived, designed, and edited the manuscript. D.K., J.K. and A.K. wrote the first draft of the manuscript. C.P., F.O. and E.O. edited the manuscript. P.G. and P.K. revised and edited the manuscript. A.A. and R.R. made the figure and aligned the table. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
In this article, no ethical review and approval were required due to already-published data.
Informed Consent Statement
No requirement of consent as already-published data were used.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Apovian, C.M.; Aronne, L.J.; Bessesen, D.H.; McDonnell, M.E.; Murad, M.H.; Pagotto, U.; Still, C.D. Pharmacological management of obesity: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2015, 100, 342–362. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Noncommunicable Diseases Country Profiles 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Forse, R.A.; Betancourt-Garcia, M.M.; Kissee, M.C. Epidemiology and Discrimination in Obesity. In The ASMBS Textbook of Bariatric Surgery; Springer: Cham, Switzerland, 2020; pp. 3–14. [Google Scholar]
- Smith, K.B.; Smith, M.S. Obesity Statistics. Prim. Care 2016, 43, 121–135, ix. [Google Scholar] [CrossRef] [PubMed]
- James, P.T.; Leach, R.; Kalamara, E.; Shayeghi, M. The worldwide obesity epidemic. Obes. Res. 2001, 9, 228S–233S. [Google Scholar] [CrossRef]
- Smith, S.R.; Weissman, N.J.; Anderson, C.M.; Sanchez, M.; Chuang, E.; Stubbe, S. Behavioral Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) Study Group. Multicenter, placebo-controlled trial of lorcaserin for weight management. N. Engl. J. Med. 2010, 363, 245–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidhu, S.; Parikh, T.; Burman, K.D. Endocrine Changes in Obesity. In Endotext; Feingold, K.R., Ed.; MDText.com, Inc.: South Dartmouth, MA, USA, 2017. [Google Scholar]
- Zhang, Y.; Yu, L.; Cai, W.; Fan, S.; Feng, L.; Ji, G.; Huang, C. Protopanaxatriol, a novel PPARγ antagonist from Panax ginseng, alleviates steatosis in mice. Sci. Rep. 2014, 4, 7375. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.; Vázquez-Polo, M.; Pérez-Junkera, G.; Fernández-Gil, M.D.P.; Bustamante, M.Á.; Navarro, V.; Martínez, O. FODMAP intake in Spanish population: Open approach for risk assessment. Int. J. Environ. Res. Public Health 2020, 17, 5882. [Google Scholar] [CrossRef]
- Pollex, R.L.; Hanley, A.J.; Zinman, B.; Harris, S.B.; Khan, H.M.; Hegele, R.A. Metabolic syndrome in aboriginal Canadians: Prevalence and genetic associations. Atherosclerosis 2006, 184, 121–129. [Google Scholar] [CrossRef]
- Kumar, M.; Guleria, S.; Chawla, P.; Khan, A.; Modi, V.K.; Kumar, N.; Kaushik, R. Anti-obesity efficacy of the selected high altitude Himalayan herbs: In vitro studies. J. Food Sci. Technol. 2020, 57, 3081–3090. [Google Scholar] [CrossRef]
- Ogden, C.L.; Carroll, M.D.; Curtin, L.R.; McDowell, M.A.; Tabak, C.J.; Flegal, K.M. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 2006, 295, 1549–1555. [Google Scholar] [CrossRef]
- Pimpley, V.; Patil, S.; Srinivasan, K.; Desai, N.; Murthy, P.S. The chemistry of chlorogenic acid from green coffee and its role in attenuation of obesity and diabetes. Prep. Biochem. Biotechnol. 2020, 50, 969–978. [Google Scholar] [CrossRef]
- Mishra, D.; Naorem, K.; Saraswathy, K.N. Angiotensin-Converting Enzyme Gene Insertion/Deletion Polymorphism and Cardiometabolic Risk Factors: A Study Among Bhil Tribal Population from Two Environmental Settings. Biochem. Genet. 2018, 56, 295–314. [Google Scholar] [CrossRef] [PubMed]
- Kandpal, V.; Sachdeva, M.P.; Saraswathy, K.N. An assessment study of CVD-related risk factors in a tribal population of India. BMC Public Health 2016, 16, 434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cameron, A.J.; Zimmet, P.Z.; Dunstan, D.W.; Dalton, M.; Shaw, J.E.; Welborn, T.A.; Jolley, D. Overweight and obesity in Australia: The 1999–2000 Australian diabetes, obesity, and lifestyle study (AusDiab). Med. J. Aust. 2003, 178, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Ruxton, C.H.S. Nutritional implications of obesity and dieting. Nutr. Bull. 2011, 36, 199–211. [Google Scholar] [CrossRef]
- Mendez, M.A.; Popkin, B.M.; Buckland, G.; Schroder, H.; Amiano, P.; Barricarte, A.; González, C.A. Alternative methods of accounting for underreporting and overreporting when measuring dietary intake-obesity relations. Am. J. Epidemiol. 2011, 173, 448–458. [Google Scholar] [CrossRef] [Green Version]
- Paradis, A.M.; Godin, G.; Pérusse, L.; Vohl, M.C. Associations between dietary patterns and obesity phenotypes. Int. J. Obes. 2009, 33, 1419–1426. [Google Scholar] [CrossRef] [Green Version]
- Paz-Filho, G.; Mastronardi, C.A.; Licinio, J. Leptin treatment: Facts and expectations. Metabolism 2015, 64, 146–156. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Greenberg, A.S.; Fujioka, K.; Dixon, R.M.; Kushner, R.; Hunt, T.; Lubina, J.A.; Patane, J.; Self, B.; Hunt, P.; et al. Recombinant leptin for weight loss in obese and lean adults: A randomized, controlled, dose-escalation trial. JAMA 1999, 282, 1568–1575. [Google Scholar] [CrossRef]
- Fogteloo, A.J.; Pijl, H.; Frölich, M.; McCamish, M.; Meinders, A.E. Effects of recombinant human leptin treatment as an adjunct of moderate energy restriction on body weight, resting energy expenditure and energy intake in obese humans. Diabetes Nutr. Metab. 2003, 16, 109–114. [Google Scholar]
- Ahima, R.S.; Flier, J.S. Leptin. Annu. Rev. Physiol. 2000, 62, 413–437. [Google Scholar] [CrossRef] [Green Version]
- Cordido, F.; Penalva, A.; Dieguez, C.; Casanueva, F.F. Massive growth hormone (GH) discharge in obese subjects after the combined administration of GH-releasing hormone and GHRP-6: Evidence for a marked somatotroph secretory capability in obesity. J. Clin. Endocrinol. Metab. 1993, 76, 819–823. [Google Scholar] [PubMed]
- Alvarez-Castro, P.; Isidro, M.L.; Garcia-Buela, J.; Leal-Cerro, A.; Broglio, F.; Tassone, F. Marked GH secretion after ghrelin alone or combined with GH-releasing hormone (GHRH) in obese patients. Clin. Endocrinol. 2004, 61, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Hosoda, H.; Matsuo, H.; Kangawa, K. Ghrelin: Discovery of the natural endogenous ligand for the growth hormone secretagogue receptor. Trends Endocrinol. Metab. 2001, 12, 118–122. [Google Scholar] [CrossRef]
- Tschop, M.; Weyer, C.; Tataranni, P.A.; Devanarayan, V.; Ravussin, E.; Heiman, M.L. Circulating ghrelin levels are decreased in human obesity. Diabetes 2001, 50, 707–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otto, B.; Cuntz, U.; Fruehauf, E.; Wawarta, R.; Folwaczny, C.; Riepl, R.L. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur. J. Endocrinol. 2001, 145, 669–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ukkola, O. Ghrelin and insulin metabolism. Eur. J. Clin. Investig. 2003, 33, 183–185. [Google Scholar] [CrossRef]
- Shuto, Y.; Shibasaki, T.; Otagiri, A.; Kuriyama, H.; Ohata, H.; Tamura, H. Hypothalamic growth hormone secretagogue receptor regulates growth hormone secretion, feeding, and adiposity. J. Clin. Investig. 2002, 109, 1429–1436. [Google Scholar] [CrossRef]
- Cummings, D.E.; Frayo, R.S.; Marmonier, C.; Aubert, R.; Chapelot, D. Plasma ghrelin levels and hunger scores in humans initiating meals voluntarily without time- and food-related cues. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E297–E304. [Google Scholar] [CrossRef]
- Abate, N. Insulin resistance and obesity. The role of fat distribution pattern. Diabetes Care 1996, 19, 292–294. [Google Scholar] [CrossRef]
- Bjorntorp, P. Metabolic abnormalities in visceral obesity. Ann. Med. 1992, 24, 3–5. [Google Scholar] [CrossRef]
- Flier, J.S. Insulin receptors and insulin resistance. Annu. Rev. Med. 1983, 34, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Kahn, C.R. Role of insulin receptors in insulin-resistant states. Metabolism 1980, 29, 455–466. [Google Scholar] [CrossRef]
- Moller, D.E.; Flier, J.S. Insulin resistance-mechanisms, syndromes, and implications. N. Engl. J. Med. 1991, 325, 938–948. [Google Scholar] [PubMed]
- Kaaks, R.; Lukanova, A.; Kurzer, M.S. Obesity, endogenous hormones, and endometrial cancer risk: A synthetic review. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1531–1543. [Google Scholar]
- Rabe, K.; Lehrke, M.; Parhofer, K.G.; Broedl, U.C. Adipokines and insulin resistance. Mol. Med. 2008, 14, 741–751. [Google Scholar] [CrossRef]
- Guerre-Millo, M. Adiponectin: An update. Diabetes Metab. 2008, 34, 12–18. [Google Scholar] [CrossRef]
- Yang, R.Z.; Lee, M.J.; Hu, H.; Pray, J.; Wu, H.B.; Hansen, B.C.; Shuldiner, A.R.; Fried, S.K.; McLenithan, J.C.; Gong, D.W. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: Possible role in modulating insulin action. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1253–E1261. [Google Scholar] [CrossRef]
- de Souza Batista, C.M.; Yang, R.Z.; Lee, M.J.; Glynn, N.M.; Yu, D.Z.; Pray, J.; Ndubuizu, K.; Patil, S.; McLenithan, J.C. Omentin plasma levels and gene expression are decreased in obesity. Diabetes 2007, 56, 1655–1661. [Google Scholar] [CrossRef] [Green Version]
- Adrian, T.E.; Ferri, G.L.; Bacarese-Hamilton, A.J.; Fuessl, H.S.; Polak, J.M.; Bloom, S.R. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology 1985, 89, 1070–1077. [Google Scholar] [CrossRef]
- Batterham, R.L.; Cowley, M.A.; Small, C.J.; Herzog, H.; Cohen, M.A.; Dakin, C.L.; Wren, A.M.; Brynes, A.E.; Low, M.J.; Ghatei, M.A.; et al. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature 2002, 418, 650–654. [Google Scholar] [CrossRef]
- Karra, E.; Batterham, R.L. The role of gut hormones in the regulation of body weight and energy homeostasis. Mol. Cell Endocrinol. 2010, 316, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Paglialunga, S.; Schrauwen, P.; Roy, C.; Moonen-Kornips, E.; Lu, H.; Hesselink, M.K.C.; Deshaies, Y.; Richard, D.; Cianflone, K. Reduced adipose tissue triglyceride synthesis and increased muscle fatty acid oxidation in C5L2 knockout mice. J. Endocrinol. 2007, 194, 293–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, I.; Havel, P.J.; Sniderman, A.D.; Cianflone, K. Reduced body weight, adipose tissue, and leptin levels despite increased energy intake in female mice lacking acylation-stimulating protein. Endocrinology 2000, 141, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Cruickshank, C.C.; Dyer, K.R. A review of the clinical pharmacology of methamphetamine. Addiction 2009, 104, 1085–1099. [Google Scholar] [CrossRef]
- Frohmader, K.S.; Pitchers, K.K.; Balfour, M.E.; Coolen, L.M. Mixing pleasures: Review of the effects of drugs on sex behavior in humans and animal models. Horm. Behav. 2010, 58, 149–162. [Google Scholar] [CrossRef]
- Oh, S.; Kim, K.S.; Chung, Y.S.; Shong, M.; Park, S.B. Anti-obesity agents: A focused review on the structural classification of therapeutic entities. Curr. Top Med. Chem. 2009, 9, 466–481. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.G.; Park, C.Y. Anti-Obesity Drugs: A Review about Their Effects and Safety. Diabetes Metab. J. 2012, 36, 13–25. [Google Scholar] [CrossRef] [Green Version]
- United States Food and Drug Administration. Lorcaserin Briefing Information: Endocrinologic and Metabolic Drugs Advisory Committee. 2012. Available online: http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/ucm225628.htm (accessed on 28 October 2015).
- Weissman, N.J.; Sanchez, M.; Koch, G.G.; Smith, S.R.; Shanahan, W.R.; Anderson, C.M. Echocardiographic assessment of cardiac valvular regurgitation with lorcaserin from analysis of 3 phase 3 clinical trials. Circ. Cardiovasc. Imaging 2013, 6, 560–567. [Google Scholar] [CrossRef]
- Fidler, M.C.; Sanchez, M.; Raether, B.; Weissman, N.J.; Smith, S.R.; Shanahan, W.R.; Anderson, C.M. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: The BLOSSOM trial. J. Clin. Endocrinol. Metab. 2011, 96, 3067–3077. [Google Scholar] [CrossRef] [Green Version]
- Scozzafava, A.; Supuran, C.T.; Carta, F. Antiobesity carbonic anhydrase inhibitors: A literature and patent review. Expert. Opin. Ther. Pat. 2013, 23, 725–735. [Google Scholar] [CrossRef]
- Gadde, K.M.; Allison, D.B.; Ryan, D.H.; Peterson, C.A.; Troupin, B.; Schwiers, M.L.; Day, W.W. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): A randomised, placebo-controlled, phase 3 trial. Lancet 2011, 377, 1341–1352. [Google Scholar] [CrossRef]
- Unites States Food and Drug Administration. Contrave (Naltrexone 4 mg, 8 mg/Bupropion HCL 90 mg Extended Release Tablet) Briefing Document, NDA 200063. 2010. Available online: http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/endocrinologicandmetabolicdrugsadvisorycommitee/ucm235671.pdf (accessed on 28 October 2015).
- Wadden, T.A.; Foreyt, J.P.; Foster, G.D. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: The COR-BMOD trial. Obesity 2011, 19, 110–120. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency. Victoza Summary of Product Characteristics. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001026/WC500050017.pdf (accessed on 28 October 2015).
- Turton, M.D.; O’Shea, D.; Gunn, I. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 1996, 379, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Van Can, J.; Sloth, B.; Jensen, C.B. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int. J. Obes. 2014, 38, 784–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United States Food and Drug Administration. FDA Approves Weight-Management Drug Saxenda. 2014. Available online: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427913.htm (accessed on 28 October 2015).
- Bahmani, M.; Eftekhari, Z.; Saki, K.; Fazeli-Moghadam, E.; Jelodari, M.; Rafieian-Kopaei, M. Obesity phytotherapy: Review of native herbs used in traditional medicine for obesity. J. Evid.-Based Complement. Altern. Med. 2016, 21, 228–234. [Google Scholar] [CrossRef]
- Yun, J.W. Possible anti-obesity therapeutics from nature—A review. Phytochemistry 2010, 71, 1625–1641. [Google Scholar] [CrossRef]
- Dessie, A.B.; Abate, T.M.; Adane, B.T.; Tesfa, T.; Getu, S. Estimation of technical efficiency of black cumin (Nigella sativa L.) farming in northwest Ethiopia: A stochastic frontier approach. J. Econ. Struct. 2020, 9, 18. [Google Scholar] [CrossRef]
- Goreja, W.G. Black Seed: Nature’s Miracle Remedy; Karger Publishers: Basel, Switzerland, 2003. [Google Scholar]
- Cheikh-Rouhou, S.; Besbes, S.; Lognay, G.; Blecker, C.; Deroanne, C.; Attia, H. Sterol composition of black cumin (Nigella sativa L.) and Aleppo pine (Pinus halepensis Mill.) seed oils. J. Food Compos. Anal. 2008, 21, 162–168. [Google Scholar] [CrossRef]
- Mehta, B.K.; Verma, M.; Gupta, M. Novel lipid constituents identified in seeds of Nigella sativa (Linn). J. Braz. Chem. Soc. 2008, 19, 458–462. [Google Scholar] [CrossRef]
- Nickavar, B.; Mojab, F.; Javidnia, K.; Amoli, M.A.R. Chemical composition of the fixed and volatile oils of Nigella sativa L. from Iran. Z. Nat. C 2003, 58, 629–631. [Google Scholar] [CrossRef] [Green Version]
- Morikawa, T.; Xu, F.; Ninomiya, K.; Matsuda, H.; Yoshikawa, M. Nigellamines A3, A4, A5, and C, new dolabellane-type diterpene alkaloids, with lipid metabolism-promoting activities from the Egyptian medicinal food black cumin. Chem. Pharm. Bull. 2004, 52, 494–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datau, E.A.; Surachmanto, E.E.; Pandelaki, K.; Langi, J.A. Efficacy of Nigella sativa on serum free testosterone and metabolic disturbances in central obese male. Acta Med. Indones. 2010, 42, 130–134. [Google Scholar]
- Adebayo-Tayo, B.C.; Samuel, U.A. Microbial Quality and Proximate Composition of Dried Hibiscus sabdariffa Calyxes in Uyo, Eastern Nigeria. Malays. J. Microbiol. 2009, 5, 13–18. [Google Scholar] [CrossRef]
- Hirunpanich, V.; Utaipat, A.; Morales, N.P.; Bunyapraphatsara, N.; Sato, H.; Herunsale, A.; Suthisisang, C. Hypocholesterolemic and antioxidant effects of aqueous extracts from the dried calyx of Hibiscus sabdariffa L. in hypercholesterolemic rats. J. Ethnopharmacol. 2006, 103, 252–260. [Google Scholar] [CrossRef]
- Sheba, A.L.; Ilakkia, A. Anti-obesity effect of hibiscus Sabdariffa L.—A review. Int. J. Pharma Bio Sci. 2016, 7, 13. [Google Scholar] [CrossRef]
- Ojulari, O.V.; Lee, S.G.; Nam, J.O. Beneficial Effects of Natural Bioactive Compounds from Hibiscus sabdariffa L. on Obesity. Molecules 2019, 24, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janson, B.; Prasomthong, J.; Malakul, W.; Boonsong, T.; Tunsophon, S. Hibiscus sabdariffa L. calyx extract prevents the adipogenesis of 3T3-L1 adipocytes, and obesity-related insulin resistance in high-fat diet-induced obese rats. Biomed. Pharmacother. 2021, 138, 111438. [Google Scholar] [CrossRef]
- Mosimann, A.L.P.; Wilhelm-Filho, D.; Da Silva, E.L. Aqueous extract of Ilex paraguariensis attenuates the progression of atherosclerosis in cholesterol-fed rabbits. Biofactors 2006, 26, 59–70. [Google Scholar] [CrossRef]
- Kim, S.Y.; Oh, M.R.; Kim, M.G.; Chae, H.J.; Chae, S.W. Anti-obesity effects of Yerba Mate (Ilex paraguariensis): A randomized, double-blind, placebo-controlled clinical trial. BMC Complement. Altern. Med. 2015, 15, 338. [Google Scholar] [CrossRef] [Green Version]
- Hussein, G.M.; Matsuda, H.; Nakamura, S.; Akiyama, T.; Tamura, K.; Yoshikawa, M. Protective and ameliorative effects of maté (Ilex paraguariensis) on metabolic syndrome in TSOD mice. Phytomedicine 2011, 19, 88–97. [Google Scholar] [CrossRef]
- Kang, Y.R.; Lee, H.Y.; Kim, J.H.; Moon, D.I.; Seo, M.Y.; Park, S.H.; Cho, S.W. Anti-obesity and anti-diabetic effects of Yerba Mate (Ilex paraguariensis) in C57BL/6J mice fed a high-fat diet. Lab. Anim. Res. 2012, 28, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Mukhtar, E.; Selman, S.; Sahib, Z.; Naji, H. Antidepressant-Like Effect of Rosmarinus officinalis Extract in Male Mice. Med. J. Babylon 2013, 10, 803–808. [Google Scholar]
- Hussain, A.; Anwar, F.; Chatha, S.A.S.; Jabbar, A.; Mahboob, S.; Nigam, P. Rosmarinus officinalis essential oil: Antiproliferative, antioxidant and anti-bacterial activities. Braz. J. Microbiol. 2010, 41, 1070–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karthik, D.; Viswanathan, P.; Anuradha, C.V. Administration of rosmarinic acid reduces cardiopathology and blood pressure through inhibition of p22phox NADPH oxidase in fructose-fed hypertensive rats. J. Cardiovasc. Pharmacol. 2011, 58, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Amaral, G.P.; de Carvalho, N.R.; Barcelos, R.P.; Dobrachinski, F.; de Lima Portella, R.; da Silva, M.H.; Athayde, M.L. Protective action of ethanolic extract of Rosmarinus officinalis L. in gastric ulcer prevention induced by ethanol in rats. Food Chem. Toxicol. 2013, 55, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Gaya, M.; Repetto, V.; Toneatto, J.; Anesini, C.; Piwien-Pilipuk, G.; Moreno, S. Antiadipogenic effect of carnosic acid, a natural compound present in Rosmarinus officinalis, is exerted through the C/EBPs and PPARγ pathways at the onset of the differentiation program. Biochim. Biophys. Acta—Gen. Subj. 2013, 1830, 3796–3806. [Google Scholar] [CrossRef] [PubMed]
- Bustanji, Y.; Issa, A.; Mohammad, M.; Hudaib, M.; Tawah, K.; Alkhatib, H.; Al-Khalidi, B. Inhibition of hormone sensitive lipase and pancreatic lipase by Rosmarinus officinalis extract and selected phenolic constituents. J. Med. Plant Res. 2010, 4, 2235–2242. [Google Scholar]
- Shatla, I.M.; Abdel-Hamid, A.M.; Metwally, M. The Effect of Rosmarinus officinalis L. Extract on High Fat Diet-Induced Obesity in Adult Male Albino Rats. Al-Azhar Med. J. 2017, 46, 749–764. [Google Scholar] [CrossRef]
- AL-Asmari, K.M.; Abu Zeid, I.M.; Al-Attar, A.M. Medicinal Properties of Arabica coffee (Coffea arabica) Oil: An Overview. Adv. Life Sci. 2020, 8, 20–29. [Google Scholar]
- Sudeep, H.V.; Shyam Prasad, K. Supplementation of green coffee bean extract in healthy overweight subjects increases lean mass/fat mass ratio: A randomized, double-blind clinical study. SAGE Open Med. 2021, 9, 20503121211002590. [Google Scholar] [CrossRef]
- Choi, B.K.; Park, S.B.; Lee, D.R.; Lee, H.J.; Jin, Y.Y.; Yang, S.H.; Suh, J.W. Green coffee bean extract improves obesity by decreasing body fat in high-fat diet-induced obese mice. Asian Pac. J. Trop. Med. 2016, 9, 635–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osuntokun, O.T. Aframomum Melegueta (Grains of Paradise). Ann. Microbiol. Infect. Dis. 2020, 3, 1–6. [Google Scholar]
- Yu Sheng Toh, E.; Lim, C.L.; Pick Kiong Ling, A.; Chye, S.M.; Koh, R.Y. Overview of the Pharmacological Activities of Aframomum melegueta. Pertanika J. Trop. Agric. Sc. 2019, 42, 1–13. [Google Scholar]
- Hattori, H.; Mori, T.; Shibata, T.; Kita, M.; Mitsunaga, T. 6-Paradol Acts as a Potential Anti-obesity Vanilloid from Grains of Paradise. Mol. Nutr. Food Res. 2021, 65, 2100185. [Google Scholar] [CrossRef] [PubMed]
- Amerikanou, C.; Kaliora, A.C.; Gioxari, A. The efficacy of Panax ginseng in obesity and the related metabolic disorders. Pharmacol. Res. Modern Chin. Med. 2021, 1, 100013. [Google Scholar] [CrossRef]
- Park, H.S.; Cho, J.H.; Kim, K.W.; Chung, W.S.; Song, M.Y. Effects of Panax ginseng on Obesity in Animal Models: A Systematic Review and Meta-Analysis. Evid.-Based Complement. Altern. Med. 2018, 2018, 2719794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Sun, M.; Yao, H.; Liu, Y.; Gao, R. Herbal Medicine for the Treatment of Obesity: An Overview of Scientific Evidence from 2007 to 2017. Evid.-Based Complement. Altern. Med. 2017, 2017, 8943059. [Google Scholar] [CrossRef]
- Rao, A.; Briskey, D.; Dos Reis, C.; Mallard, A.R. The effect of an orally-dosed Caralluma fimbriata extract on appetite control and body composition in overweight adults. Sci. Rep. 2021, 11, 6791. [Google Scholar] [CrossRef]
- Devi, S.G.; Dhamotharan, R. Caralluma fimbriata—An Important Medicinal Plant: A Review of Its Traditional Uses, Phytochemistry and Pharmacological Properties. Int. J. PharmTech Res. 2016, 9, 223–230. [Google Scholar]
- Ambadasu, B.; Dange, S.; Wali, R. Effect of Caralluma fimbriata extract on appetite, body weight lipid profile in cafeteria diet-induced obesity in rats. Int. J. Pharm. Pharm. Sci. 2013, 5, 536–539. [Google Scholar]
- Saboo, B.; Zaveri, H. Recent update in management of obesity and overweight patients: Standardized extract of Caralluma fimbriata safe and effective therapy. IJCCI 2011, 2, 5–9. [Google Scholar]
- García-Gaytán, V.; Gómez-Merino, F.C.; Trejo-Téllez, L.I.; Baca-Castillo, G.A.; García-Morales, S. The Chilhuacle Chili (Capsicum annuum L.) in Mexico: Description of the Variety, Its Cultivation, and Uses. Int. J. Agron. 2017, 2017, 5641680. [Google Scholar] [CrossRef] [Green Version]
- Azlan, A.; Sultana, S.; Huei, C.S.; Razman, M.R. Antioxidant, Anti-Obesity, Nutritional and Other Beneficial Effects of Different Chili Pepper: A Review. Molecules 2022, 27, 898. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.; Alqahtani, A.; Ojo, O.A.; Shaheen, H.M.; Wasef, L.; Elzeiny, M.; Ismail, M.; Shalaby, M.; Murata, T.; Zaragoza-Bastida, A.; et al. Biological Properties, Bioactive Constituents, and Pharmacokinetics of Some Capsicum spp. and Capsaicinoids. Int. J. Mol. Sci. 2020, 21, 5179. [Google Scholar] [CrossRef]
- Zheng, J.; Zheng, S.; Feng, Q.; Zhang, Q.; Xiao, X. Dietary capsaicin and its anti-obesity potency: From mechanism to clinical implications. Biosci. Rep. 2017, 37, BSR20170286. [Google Scholar] [CrossRef]
- Mehmet, B.; Metin, Y.; Gulhan, A.; Omer, T.; Oruc, A. Effect of capsaicin on transcription factor in 3T3-L1 cell line. East. J. Med. 2015, 20, 34–45. [Google Scholar]
- Khan, S.; Pandotra, P.; Qazi, A.K.; Lone, S.A.; Muzafar, M.; Gupta, A.P.; Gupta, S. Medicinal and Nutritional Qualities of Zingiber officinale. In Fruits, Vegetables, and Herbs; Academic Press: Cambridge, MA, USA, 2016; pp. 525–550. [Google Scholar]
- Mbaveng, A.T.; Kuete, V. Zingiber officinale. In Medicinal Spices and Vegetables from Africa; Academic Press: Cambridge, MA, USA, 2017; pp. 627–639. [Google Scholar]
- Ortega, A.M.M.; Campos, M.R.S. Medicinal Plants and Their Bioactive Metabolites in Cancer Prevention and Treatment. In Bioactive Compounds; Woodhead Publishing: Cambridge, UK, 2019; pp. 85–109. [Google Scholar]
- Tzeng, T.F.; Liu, I.M. 6-Gingerol prevents adipogenesis and the accumulation of cytoplasmic lipid droplets in 3T3-L1 cells. Phytomedicine 2013, 20, 481–487. [Google Scholar] [CrossRef]
- Li, C.; Zhou, L. Inhibitory effect 6-gingerol on adipogenesis through activation of the Wnt/β-catenin signaling pathway in 3T3-L1 adipocytes. Toxicol. In Vitro 2015, 30, 394–401. [Google Scholar] [CrossRef]
- Suk, S.; Seo, S.G.; Yu, J.G.; Yang, H.; Jeong, E.; Jang, Y.J.; Lee, K.W. A bioactive constituent of ginger, 6-shogaol, prevents adipogenesis and stimulates lipolysis in 3T3-L1 adipocytes. J. Food Biochem. 2016, 40, 84–90. [Google Scholar] [CrossRef]
- Latiff, L.A.; Parhizkar, S.; Dollah, M.A.; Hassan, S.T. Alternative supplement for enhancement of reproductive health and metabolic profile among perimenopausal women: A novel role of Nigella sativa. Iran. J. Basic Med. Sci. 2014, 17, 980–985. [Google Scholar]
- Mahdavi, R.; Namazi, N.; Alizadeh, M.; Farajnia, S. Effects of Nigella sativa oil with a low-calorie diet on cardiometabolic risk factors in obese women: A randomized controlled clinical trial. Food Funct. 2015, 6, 2041–2048. [Google Scholar] [CrossRef] [PubMed]
- Mohtashami, A.; Entezari, M.H. Effects of Nigella sativa supplementation on blood parameters and anthropometric indices in adults: A systematic review on clinical trials. J. Res. Med. Sci. 2016, 21, 3. [Google Scholar] [PubMed]
- Namazi, N.; Larijani, B.; Ayati, M.H.; Abdollahi, M. The effects of Nigella sativa L. on obesity: A systematic review and meta-analysis. J. Ethnopharmacol. 2018, 219, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Soltani, R.; Zolghadr, M.; Keshvari, M.; Sarrafzadegan, N. Evaluation of the effects of roselle (Hibiscus sabdariffa L.) on oxidative stress and serum levels of lipids, insulin and hs-CRP in adult patients with metabolic syndrome: A double-blind placebo-controlled clinical trial. J. Complement. Integr. Med. 2016, 13, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Peng, C.H.; Yeh, D.M.; Kao, E.S.; Wang, C.J. Hibiscus sabdariffa extract inhibits obesity and fat accumulation, and improves liver steatosis in humans. Food Funct. 2014, 5, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Kuriyan, R.; Kumar, D.R.; Rajendran, R.; Kurpad, A.V. An evaluation of the hypolipidemic effect of an extract of Hibiscus sabdariffa leaves in hyperlipidemic Indians: A double-blind, placebo-controlled trial. BMC Complement. Altern. Med. 2010, 10, 27. [Google Scholar] [CrossRef] [Green Version]
- Labban, L.; Mustafa, U.E.S.; Ibrahim, Y.M. The effects of rosemary (Rosmarinus officinalis) leaves powder on glucose level, lipid profile and lipid peroxidation. Int. J. Clin. Med. 2014, 5, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Haidari, F.; Samadi, M.; Mohammadshahi, M.; Jalali, M.T.; Engali, K.A. Energy restriction combined with green coffee bean extract affects serum adipocytokines and the body composition in obese women. Asia Pac. J. Clin. Nutr. 2017, 26, 1048–1054. [Google Scholar]
- Shahmohammadi, H.A.; Hosseini, S.A.; Hajiani, E.; Malehi, A.S.; Alipour, M. Effects of green coffee bean extract supplementation on patients with non-alcoholic fatty liver disease: A randomized clinical trial. Hepat. Mon. 2017, 17, e12299. [Google Scholar] [CrossRef] [Green Version]
- Hosseinabadi, S.; Rafraf, M.; Asghari, S.; Asghari-Jafarabadi, M.; Vojouhi, S. Effect of green coffee extract supplementation on serum adiponectin concentration and lipid profile in patients with non-alcoholic fatty liver disease: A randomized, controlled trial. Complement. Ther. Med. 2020, 49, 102290. [Google Scholar] [CrossRef]
- Balsan, G.; Pellanda, L.C.; Sausen, G.; Galarraga, T.; Zaffari, D.; Pontin, B.; Portal, V.L. Effect of yerba mate and green tea on paraoxonase and leptin levels in patients affected by overweight or obesity and dyslipidemia: A randomized clinical trial. Nutr. J. 2019, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Sanchez, K.; Leyva, M.J.; Wu, M.; Betts, N.M.; Aston, C.E.; Lyons, T.J. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J. Am. Coll. Nutr. 2010, 29, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.J.; Liu, C.Y.; Chiu, J.P.; Hsu, C.H. Therapeutic effect of high-dose green tea extract on weight reduction: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. 2016, 35, 592–599. [Google Scholar] [CrossRef]
- Hsu, C.H.; Tsai, T.H.; Kao, Y.H.; Hwang, K.C.; Tseng, T.Y.; Chou, P. Effect of green tea extract on obese women: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. 2008, 27, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Hursel, R.; Viechtbauer, W.; Westerterp-Plantenga, M.S. The effects of green tea on weight loss and weight maintenance: A meta-analysis. Int. J. Obes. 2009, 33, 956–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssens, P.L.; Hursel, R.; Westerterp-Plantenga, M.S. Long-term green tea extract supplementation does not affect fat absorption, resting energy expenditure, and body composition in adults. J. Nutr. 2015, 145, 864–870. [Google Scholar] [CrossRef] [Green Version]
- Vieira Senger, A.E.; Schwanke, C.H.; Gomes, I.; Valle Gottlieb, M.G. Effect of green tea (Camellia sinensis) consumption on the components of metabolic syndrome in elderly. J. Nutr. Health Aging 2012, 16, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wen, Y.; Du, Y.; Yan, X.; Guo, H.; Rycroft, J.A.; Mela, D.J. Effects of catechin enriched green tea on body composition. Obesity 2010, 18, 773–779. [Google Scholar] [CrossRef]
- Song, M.Y.; Kim, B.S.; Kim, H. Influence of Panax ginseng on obesity and gut microbiota in obese middle-aged Korean women. J. Ginseng Res. 2014, 38, 106–115. [Google Scholar] [CrossRef] [Green Version]
- Arora, E.; Khajuria, V.; Tandon, V.R.; Sharma, A.; Mahajan, A.; Gillani, Z.H.; Choudhary, N. To evaluate efficacy and safety of Caralluma fimbriata in overweight and obese patients: A randomized, single blinded, placebo control trial. Perspect. Clin. Res. 2015, 6, 39–44. [Google Scholar]
- Astell, K.J.; Mathai, M.L.; Mcainch, A.J.; Stathis, C.G.; Su, X.Q. A pilot study investigating the effect of Caralluma fimbriata extract on the risk factors of metabolic syndrome in overweight and obese subjects: A randomised controlled clinical trial. Complement. Ther. Med. 2013, 21, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Kuriyan, R.; Raj, T.; Srinivas, S.K.; Vaz, M.; Rajendran, R.; Kurpad, A.V. Effect of Caralluma fimbriata extract on appetite, food intake and anthropometry in adult Indian men and women. Appetite 2007, 48, 338–344. [Google Scholar] [CrossRef]
- Seo, S.H.; Fang, F.; Kang, I. Ginger (Zingiber officinale) Attenuates Obesity and Adipose Tissue Remodeling in High-Fat Diet-Fed C57BL/6 Mice. Int. J. Environ. Res. Public Health 2021, 18, 631. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimzadeh Attari, V.; Malek Mahdavi, A.; Javadivala, Z.; Mahluji, S.; Zununi Vahed, S.; Ostadrahimi, A. A systematic review of the anti-obesity and weight lowering effect of ginger (Zingiber officinale Roscoe) and its mechanisms of action. Phytother. Res. 2018, 32, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ke, W.; Bao, R.; Hu, X.; Chen, F. Beneficial effects of ginger Zingiber officinale Roscoe on obesity and metabolic syndrome: A review. Ann. N. Y. Acad. Sci. 2017, 1398, 83–98. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Rahmani, J.; Kord-Varkaneh, H.; Sheikhi, A.; Larijani, B.; Esmaillzadeh, A. Cinnamon supplementation positively affects obesity: A systematic review and dose-response meta-analysis of randomized controlled trials. Clin. Nutr. 2020, 39, 123–133. [Google Scholar] [CrossRef]
- Medagama, A.B. The glycaemic outcomes of Cinnamon, a review of the experimental evidence and clinical trials. Nutr. J. 2015, 14, 108. [Google Scholar] [CrossRef] [Green Version]
- Trigueros, L.; Peña, S.; Ugidos, A.V.; Sayas-Barberá, E.; Pérez-Álvarez, J.A.; Sendra, E. Food ingredients as anti-obesity agents: A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 929–942. [Google Scholar] [CrossRef]
- Sharma, T.; Kanwar, S.S. Phytomolecules for obesity and body weight management. J. Biochem. Cell Biol. 2018, 1, 1–8. [Google Scholar]
- Paccosi, S.; Cresci, B.; Pala, L.; Rotella, C.M.; Parenti, A. Obesity therapy: How and why? Curr. Med. Chem. 2020, 27, 174–186. [Google Scholar] [CrossRef]
- Sun, N.N.; Wu, T.Y.; Chau, C.F. Natural dietary and herbal products in anti-obesity treatment. Molecules 2016, 21, 1351. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimpour, S.; Zakeri, M.; Esmaeili, A. Crosstalk between obesity, diabetes, and Alzheimer’s disease: Introducing quercetin as an effective triple herbal medicine. Ageing Res. Rev. 2020, 62, 101095. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).