Activation of Tissue Reparative Processes by Glow-Type Plasma Discharges as an Integral Part of the Therapy of Decubital Ulcers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
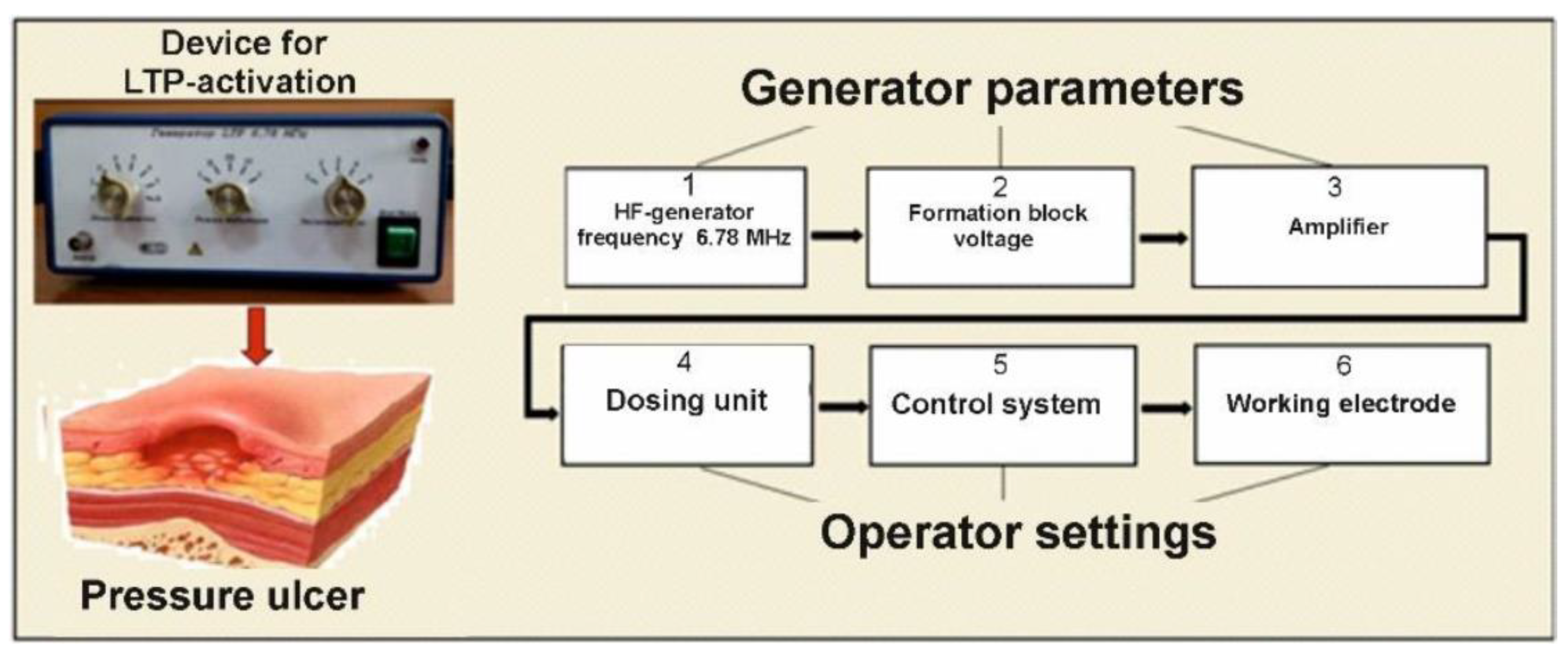
2.2. Device and Procedure for Activating the WP
2.3. Morphological Studies and Immunohistochemistry
2.4. Bacteriological Analysis
2.5. Statistical Analysis
3. Results
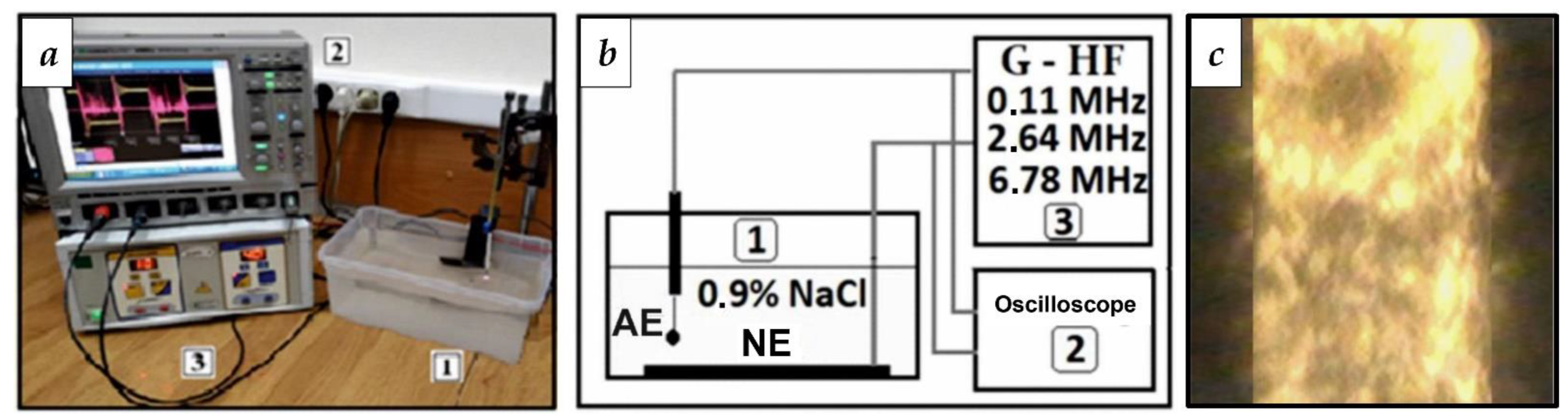
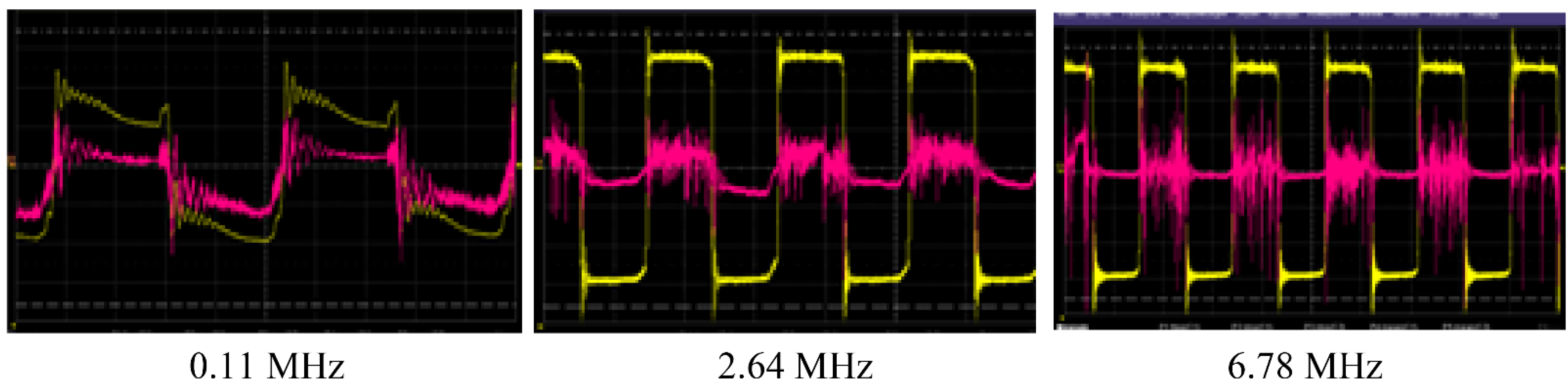
3.1. Selection of RF Current Parameters for WP Activation
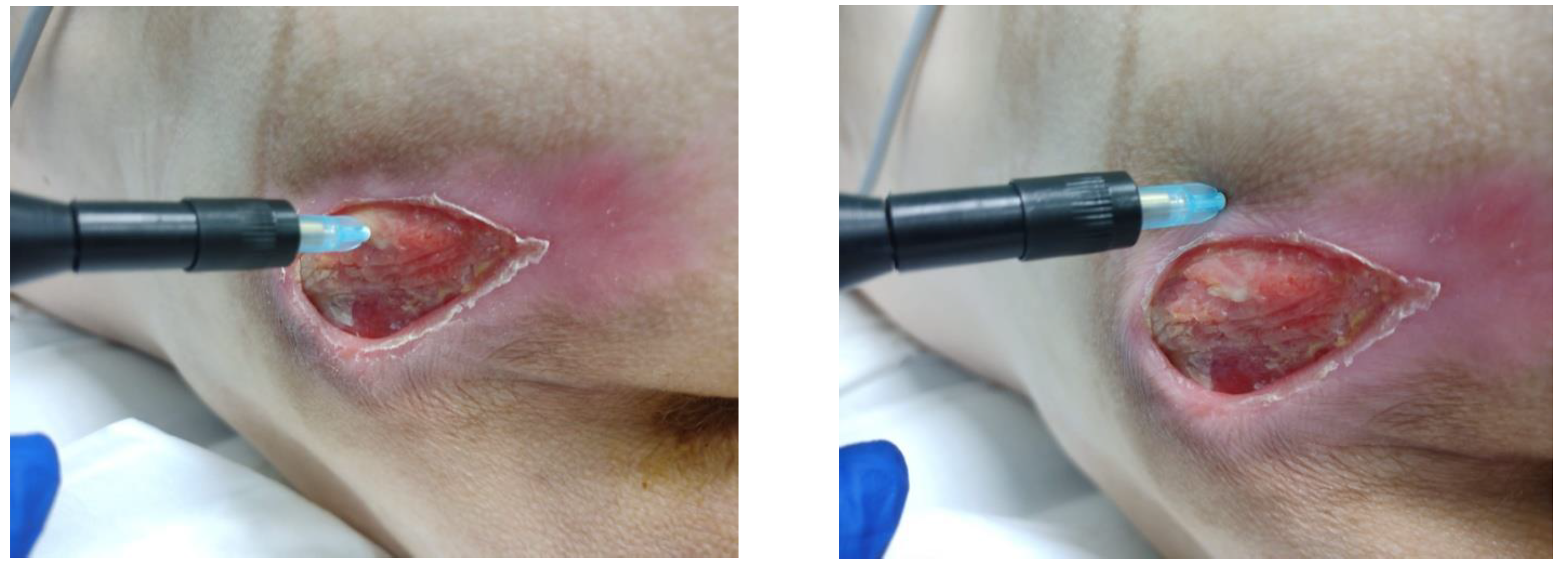
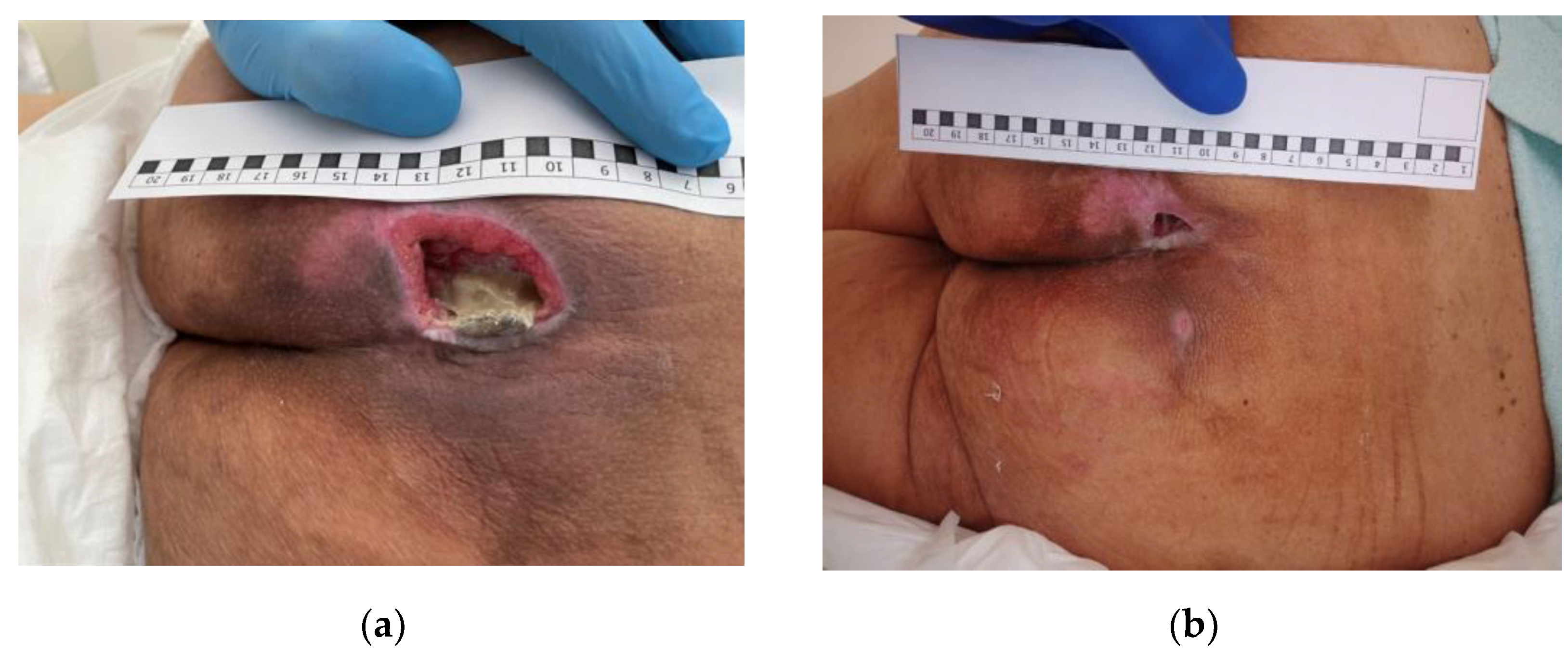
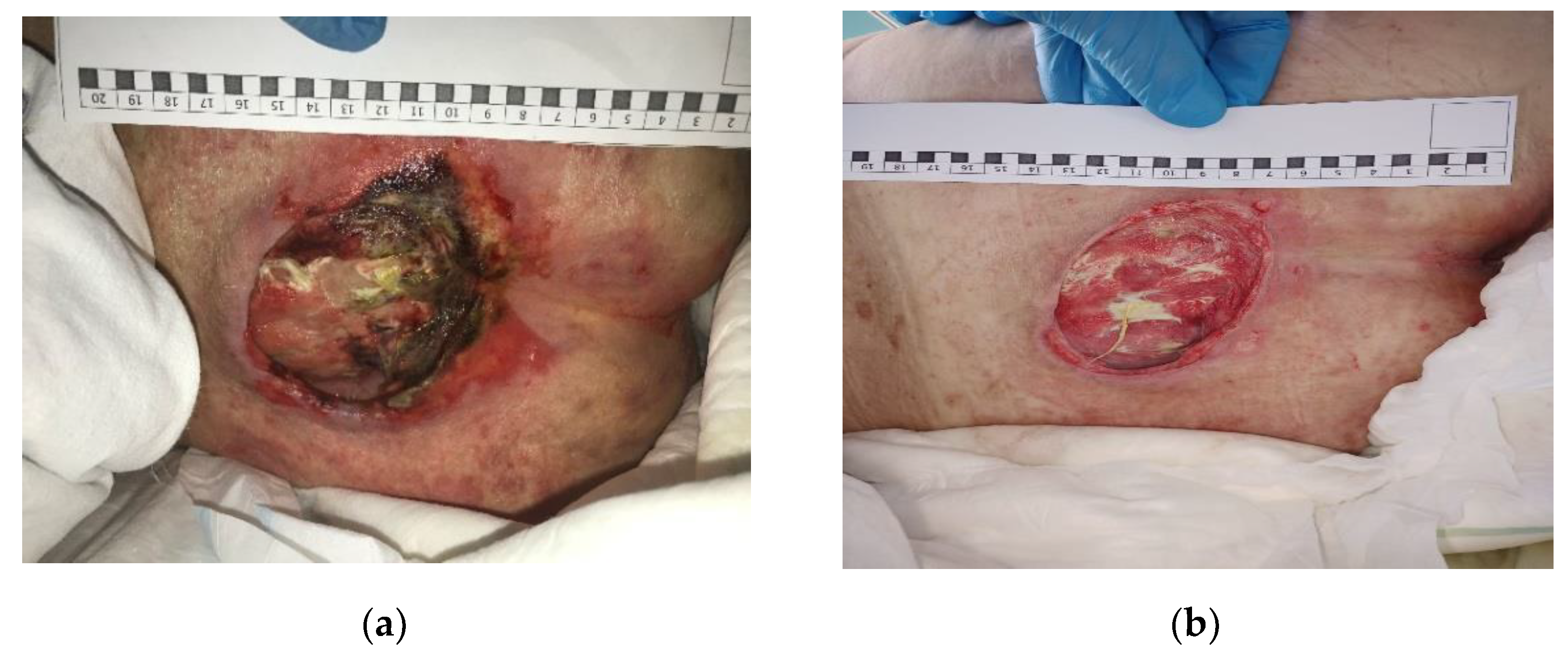
3.2. Clinical Research
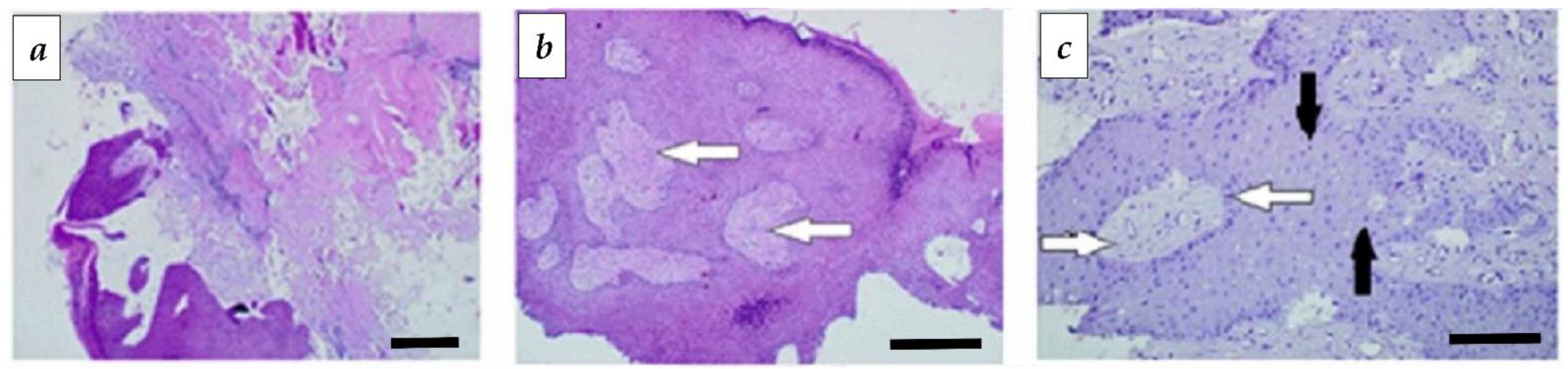
3.3. Morphological Studies
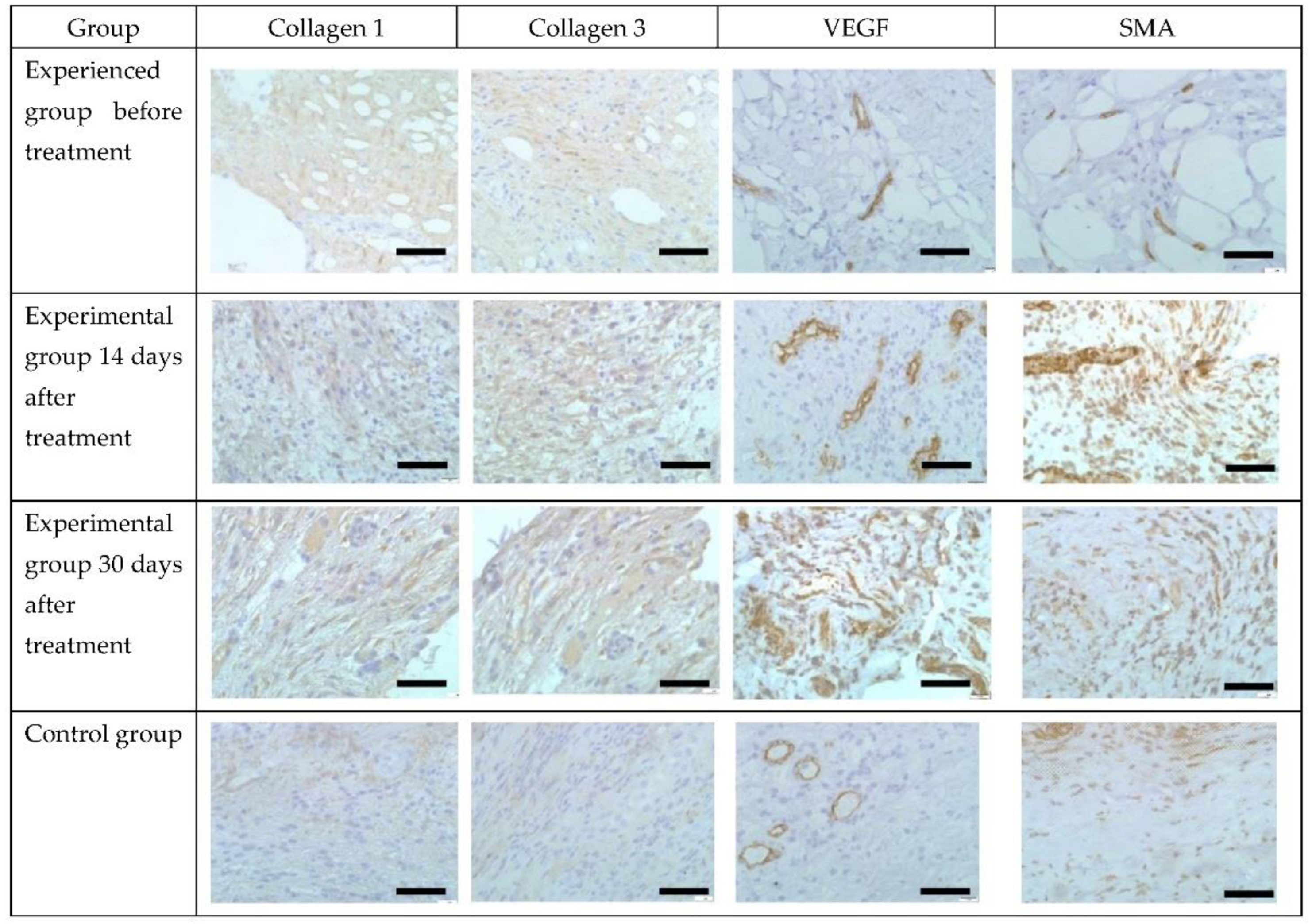
3.4. Immunohistochemical Analysis
3.5. Bacteriological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaul, E. Assessment and management of pressure ulcers in the elderly: Current strategies. Drugs Aging 2010, 27, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Mishra, R.K. Pressure ulcers: Current understanding and newer modalities of treatment. Indian J. Plast. Surg. 2015, 48, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.; Gorecki, C.; Nelson, E.A.; Closs, S.J.; Defloor, T.; Halfens, R.; Farrin, A.; Brown, J.; Schoonhoven, L.; Nixon, J. Patient risk factors for pressure ulcer development: Systematic review. Int. J. Nurs. Stud. 2013, 50, 974–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Krause, J.S.; DiDro, N. Risk factors for mortality after spinal cord injury in the USA. Spinal Cord 2013, 51, 413–821. [Google Scholar] [CrossRef] [Green Version]
- Dibirov, M.D. Decubitus: Prevention and treatment. Ambul. Surg. 2016, 1–2, 55–63. (In Russian) [Google Scholar]
- Ayello, E.; Sibbald, R. From Decubitus and Pressure Ulcers to Pressure Injuries. Adv. Skin Wound Care 2019, 32, 101. [Google Scholar] [CrossRef]
- Sumarno, A. Pressure ulcers: The core, care and cure approach. Br. J. Community Nurs. 2019, 1, 38–42. [Google Scholar] [CrossRef]
- Boyko, T.; Longaker, M.; Yang, G. Review of the Current Management of Pressure Ulcers. Adv. Wound Care 2018, 7, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Damert, H.; Meyer, F.; Altmann, S. Therapeutic options for pressure ulcers. Zentralbl. Chir. 2015, 140, 193–200. [Google Scholar]
- Supilnikov, A.A.; Devyatkin, A.A.; Pavlova, O.N.; Gulenko, O.N. Morphological and physiological aspects of the course of the wound process (literature review). Med. Bull. 2016, 23, 26–30. [Google Scholar]
- Westby, M.; Dumville, J.; Soares, M.; Stubbs, N.; Norman, G. Dressings and topical agents for treating pressure ulcers. Cochrane Database Syst. Rev. 2017, 22, CD011947. [Google Scholar]
- Demoly, P.; Adkinson, N.F.; Brockow, K.; Castells, M.; Chiriac, A.M.; Greenberger, P.A.; Khan, D.A.; Lang, D.M.; Park, H.-S.; Pichler, W.J.; et al. International Consensus on drug allergy. Allergy 2014, 69, 420–437. [Google Scholar] [CrossRef]
- Simons, F.E.; Ebisawa, M.; Sanchez-Borges, M.; Thong, B.Y.; Worm, M.; Tanno, L.K.; Lockey, R.F.; El-Gamal, Y.E.; Brown, S.G.; Park, H.; et al. 2015 update of the evidence base: World Allergy Organization anaphylaxis guidelines. World Allergy Organ. J. 2015, 8, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, S.F.; Phillips, E.J.; Wiese, M.; Heddle, R.J.; Brown, S.G. Immediate-type hypersensitivity drug reactions. Br. J. Clin. Pharmacol. 2014, 78, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Gómez, E.; Torres, V.J.; Mayorga, C.; Blanca, M. Immunologic evaluation of drug allergy. Allergy Asthma Immunol. Res. 2012, 4, 251–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marzano, A.V.; Borghi, A.; Cugno, M. Adverse drug reactions and organ damage: The skin. Eur. J. Intern. Med. 2016, 28, 17–24. [Google Scholar] [CrossRef]
- Zukiewicz-Sobczak, W.A.; Wróblewska, P.; Adamczuk, P.; Zwoliński, J.; Oniszczuk, A.; Wojtyła-Buciora, P.; Silny, W. Drugs as important factors causing allergies. Postepy Derm. Alergol. 2015, 32, 388–392. [Google Scholar] [CrossRef] [Green Version]
- Uetrecht, J.; Naisbitt, D.J. Idiosyncratic adverse drug reactions: Current concepts. Pharmacol. Rev. 2013, 65, 779–808. [Google Scholar] [CrossRef] [Green Version]
- Arndt, S.; Unger, P.; Wacker, E.; Shimizu, T.; Heinlin, J.; Li, Y.-F.; Thomas, H.M.; Morfill, G.E.; Zimmermann, J.L.; Bosserhoff, A.K.; et al. Cold atmospheric plasma (CAP) changes gene expression of key molecules of the wound healing machinery and improves wound healing in vitro and in vivo. PLoS ONE 2013, 8, e79325. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.S.; Joo, S.Y.; Cho, Y.S.; Park, J.H.; Kim, J.-B.; Seo, C.H. Effect of Combining Low Temperature Plasma, Negative Pressure Wound Therapy, and Bone Marrow Mesenchymal Stem Cells on an Acute Skin Wound Healing Mouse Model. Int. J. Mol. Sci. 2020, 21, 3675. [Google Scholar] [CrossRef]
- Ponte, G.D.; Sardella, E.; Fanelli, F.; d’Agostino, R.; Favia, P. Trends in surface engineering of biomaterials: Atmospheric pressure plasma deposition of coatings for biomedical applications. Eur. Phys. J. Appl. Phys. 2011, 56, 24023. [Google Scholar] [CrossRef] [Green Version]
- Canal, C.; Modic, M.; Cvelbar, U.; Ginebra, M.P. Regulating the antibiotic drug release from β-tricalcium phosphate ceramics by atmospheric plasma surface engineering. Biomater. Sci. 2016, 4, 1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, J.-S.; Kim, Y.H.; Choi, E.H.; Kim, K.-N. The effects of non-thermal atmospheric pressure plasma jet on attachment of osteoblast. Curr. Appl. Phys. 2013, 13, S42. [Google Scholar] [CrossRef]
- Xu, D.; Luo, X.; Xu, Y.; Cui, Q.; Yang, Y.; Liu, D.; Chen, H.; Kong, M.G. The effects of cold atmospheric plasma on cell adhesion, differentiation, migration, apoptosis and drug sensitivity of multiple myeloma. Biochem. Biophys. Res. Commun. 2016, 473, 1125. [Google Scholar] [CrossRef]
- Liu, J.; Xu, G.; Shi, X.; Zhang, G.-J. Low temperature plasma promoting fibroblast proliferation by activating the NF-κB pathway and increasing cyclinD1 expression. Sci. Rep. 2017, 7, 11698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tominami, K.; Kanetaka, H.; Sasaki, S.; Mokudai, T.; Kaneko, T.; Niwano, Y. Cold atmospheric plasma enhances osteoblast differentiation. PLoS ONE 2017, 12, e0180507. [Google Scholar] [CrossRef]
- Belov, S.V.; Danyleiko, Y.K.; Egorov, A.B.; Lukanin, V.I.; Tsvetkov, V.B.; Osmanov, E.G.; Shulutko, A.M.; Altukhov, E.L.; Yakovlev, A.A. Activation of Repair Processes in Patients with Bedsores Using Pulsed Radio Frequency Currents. Biomed. Eng. 2021, 56, 169–174. [Google Scholar] [CrossRef]
- Belov, S.V.; Gudkov, S.V.; Danileiko, Y.K.; Lukanin, V.I.; Egorov, A.B.; Altukhov, E.L.; Petrova, M.V.; Yakovlev, A.A.; Osmanov, E.G.; Shulutko, A.M.; et al. Device for activating reparative processes by low-temperature plasma discharges in patients with decubitus ulcers: Parameter optimization and efficiency assessment. Med. Tech. 2022, 3, 3–9. [Google Scholar]
- Cheng, H.; Xu, J.; Li, X.; Liu, D.; Lu, X. On the dose of plasma medicine: Equivalent total oxidation potential (ETOP). Phys. Plasmas 2020, 27, 063514. [Google Scholar] [CrossRef]
- Cheng, H.; Luo, J.; Song, K.; Zhao, F.; Liu, D.; Nie, L.; Lu, X. On the dose of plasma medicine: Plasma-activated medium (PAM) and its effect on cell viability. Phys. Plasmas 2022, 29, 063506. [Google Scholar] [CrossRef]
- Frolov, S.A.; Sushkov, O.I.; Maksimova, L.V.; Pshelenskaya, A.I.; Belov, S.V.; Danilejko, Y.K.; Osiko, V.V.; Salyuk, V.A. High-frequency electrical stimulation of the wound process in patients after surgical treatment of rectal fistulas and epithelial coccygeal tract. Coloproctology 2010, 3, 3–8. [Google Scholar]
- Belov, S.V.; Danileiko, Y.K.; Nefedov, S.M.; Osiko, V.V.; Salyuk, V.A.; Sidorov, V.A. Specific Features of Generation of Low-Temperature Plasma in High-Frequency Plasma Electrosurgical Apparatuses. Biomed. Eng. 2011, 45, 59–63. [Google Scholar] [CrossRef]
- Bates-Jensen, B.; Vredevoe, D.; Brecht, M. Validity and reliability of the Pressure Sore Status Tool. Decubitus 1992, 5, 20–28. [Google Scholar] [PubMed]
- Ashurov, M.K.; Belov, S.V.; Gudkov, S.V.; Danyleiko, Y.K.; Egorov, A.B.; Savranskii, V.V.; Temnov, A.A. Effects of Low Temperature Plasma Glow Discharge on the Proliferative Activity of Cells and the Repair Functions of Tissues in Animals and Plants. Biomed. Eng. 2020, 53, 407–412. [Google Scholar] [CrossRef]
- Morozov, A.; Tver State Medical University; Sergeyev, A.; Sergeev, N.; Dubatolov, G.; Ryzhova, T.; Pakhomov, M.; Peltikhina, O. Modern methods of stimulating process of postoperative wounds regeneration. Sib. Med. Rev. 2020, 3, 54–60. [Google Scholar] [CrossRef]
- Zelickson, B.D.; Kist, D.; Bernstein, E.; Brown, D.B.; Ksenzenko, S.; Burns, J.; Kilmer, S.; Mehregan, D.; Pope, K. Histological and ultrastructural evaluation of the effects of a radiofrequency based nonablative dermal remodeling device: A pilot study. Arch. Dermatol. 2004, 140, 204–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zavodnik, I.B. Mitochondria, calcium homeostasis and calcium signaling. Biomed. Chem. 2016, 62, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Weiger, T.M.; Hermann, A.; Levitan, I.B. Modulation of calciumactivated potassium channels. J. Comp. Physiol. 2002, 188, 79–87. [Google Scholar] [CrossRef]
- Weltmann, K.D.; Kindel, E.; Woedtke, T.; Hahnel, M.; Stieber, M.; Brandenburg, R. Atmospheric-pressure plasma sources: Prospective tools for plasma medicine. Pure Appl. Chem. 2010, 82, 1223–1237. [Google Scholar] [CrossRef]
- Yousfi, M.; Merbahi, N.; Pathak, A.; Eichwald, O. Low-temperature plasmas at atmospheric pressure: Towards new pharmaceutical treatments in medicine. fundam. Clin. Pharmacol. 2014, 28, 123–135. [Google Scholar] [CrossRef]
- Machala, Z.; Tarabova, B.; Hensel, K.; Spetlikova, E.; Sikurova, L.; Lukes, P. Formation of ROS and RNS in water electro-sprayed through transient spark discharge in air and their bactericidal effects. Plasma Process. Polym. 2013, 10, 649–659. [Google Scholar] [CrossRef]
- Girard, F.; Peret, M.; Dumont, N.; Badets, V.; Blanc, S.; Gazeli, K.; Noël, C.; Belmonte, T.; Marlin, L.; Cambus, J.-P.; et al. Correlations between gaseous and liquid phase chemistries induced by cold atmospheric plasmas in a physiological buffer. Phys. Chem. Chem. Phys. 2018, 20, 9198–9210. [Google Scholar] [CrossRef] [PubMed]
- Belov, S.V.; Lobachevsky, Y.P.; Danilejko, Y.K.; Egorov, A.B.; Simakin, A.V.; Maleki, A.; Temnov, A.A.; Dubinin, M.V.; Gudkov, S.V. The Role of Mitochondria in the Dual Effect of Low-Temperature Plasma on Human Bone Marrow Stem Cells: From Apoptosis to Activation of Cell Proliferation. Appl. Sci. 2020, 10, 8971. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.-Y. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid. Med. Cell Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [Green Version]
- Gomes, L.C.; Scorrano, L. High levels of Fis1, a pro-fission mitochondrial protein, trigger autophagy. Biochim. Biophys. Acta 2008, 1777, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Wittkampf, F.H.; van Es, R.; Neven, K. Electroporation and its Relevance for Cardiac Catheter Ablation. JACC Clin. Electrophysiol. 2018, 4, 777–986. [Google Scholar] [CrossRef]
- Rems, L.; Miklavcic, D. Textbook: Electroporation of cells in complex materials and tissues. J. Appendix. Phys. 2016, 119, 201101. [Google Scholar] [CrossRef]
- Chang, D.C.; Reese, T.S. Changes in membrane structure induced by electroporation as revealed by rapid-freezing electron microscopy. Biophys. J. 1990, 58, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Danilejko, Y.K.; Belov, S.V.; Egorov, A.B.; Lukanin, V.I.; Sidorov, V.A.; Apasheva, L.M.; Dushkov, V.Y.; Budnik, M.I.; Belyakov, A.M.; Kulik, K.N.; et al. Increase of Productivity and Neutralization of Pathological Processes in Plants of Grain and Fruit Crops with the Help of Aqueous Solutions Activated by Plasma of High-Frequency Glow Discharge. Plants 2021, 10, 2161. [Google Scholar] [CrossRef]
- Belov, S.V.; Danyleiko, Y.K.; Glinushkin, A.P.; Kalinitchenko, V.P.; Egorov, A.V.; Sidorov, V.A.; Konchekov, E.M.; Gudkov, S.V.; Dorokhov, A.S.; Lobachevsky, Y.P.; et al. An Activated Potassium Phosphate Fertilizer Solution for Stimulating the Growth of Agricultural Plants. Front. Phys. 2021, 8, 618320. [Google Scholar] [CrossRef]

| Index | Main Group (n = 38) | Control (n = 29) | p-Value |
|---|---|---|---|
| Terms of complete cleansing of DU (M ± m), days | 11.2 ± 0.5 | 19.8 ± 0.01 | <0.05 |
| Time of appearance of the first granulations in the wound (M ± m), days | 12.4 ± 0.2 | 19.0 ± 0.4 | <0.05 |
| Filling of DU with granulation tissue by 100% (M ± m), days | 32.4 ± 1.0 | 39.4 ± 0.1 | <0.05 |
| Beginning of epithelialization of DU (M ± m), days | 27.4 ± 0.8 | 34.0 ± 0.6 | <0.05 |
| Relief of paravulnar inflammation (M ± m), days | 28.9 ± 0.3 | 34.7 ± 0.1 | <0.05 |
| The rate of epithelialization of the DU | 2.8 ± 0.2% | 2.0 ± 0.5% | <0.05 |
| DU scores on the Bates-Jensen wound assessment tool in days 14/21/28, M | 31/26/24 | 32/30/27 | - |
| Terms of treatment, Me [C25; C75] | 36 [30; 53] | 44 [37; 63] | <0.05 |
| Collagen 1 (In Points) | Collagen 3 (In Points) | VEGF (% Positive Wound Stromal Cells) | SMA (% Positive Wound Stromal Cells) | |
|---|---|---|---|---|
| Experienced group before treatment | 0 | 0 | 15.0 ± 0.5 | 10.0 ± 1.0 |
| Experimental group 14 days after treatment | 4.0 ± 0.3 * | 4.0 ± 0.3 * | 30.0 ± 1.5 * | 60 ± 2.0 * |
| Experimental group 30 days after treatment | 6.0 ± 0.5 * | 6.0 ± 0.5 * | 70 ± 2.0 * | 30.0 ± 1.2 * |
| Control | 0 | 0 | 12 ± 0.5 | 15.0 ± 1.0 * |
| Type of Microorganism in DU | Isolation Frequency from DU, % | ||
|---|---|---|---|
| Before Treatment | 14th Day | 28th Day | |
| Acinetobacter baumannii | 5.1% | 4.8% | 5.0% |
| Acinetobacter baumannii/calcoaceticus complex | 2.5% | 2.0% | 2.0% |
| Citrobacter farmeri | 2.5% | 3.5% | 3.1% |
| Citrobacter freundii | 2.5% | 2.4% | 2.8% |
| Corynebacterium amycolatum/striatum | 2.6% | 2.1% | 2.9% |
| Enterococcus faecalis | 6.8% | 7.1% | 5.6% |
| Enterococcus faecium | 3.4% | 4.3% | 4.2% |
| Escherichia coli | 5.1% | 1.9% | 6.8% |
| Klebsiella ozaenae | 2.6% | 3% | 2.0% |
| Klebsiella pneumoniae | 15.3% | 14.8% | 16% |
| Proteus mirabillis | 18.8% | 17.6% | 18.0% |
| Providencia stuartii | 3.4% | 2.9% | 3.1% |
| Pseudomonas aeruginosa | 17% | 16% | 17% |
| Serratia marcescens | 1.7% | 2.0% | 2.1% |
| Staphylococcus aureus | 4.2% | 7.4% | 5.1% |
| Staphylococcus epidermidis | 3% | 3.5% | 1.9% |
| Staphylococcus haemolyticus | 2.6% | 2.5% | 2% |
| Candida | 0.9% | 2.5% | 0.4% |
| CFU/mL (Microbial Bodies per 1 g of Tissue or 1 mL of Wound Exudate) | ||||||
|---|---|---|---|---|---|---|
| 1 Day | 14 Day | 28 Day | ||||
| Main Group | Control Group | Main Group | Control Group | Main Group | Control Group | |
| Klebsiella pneumoniae | Me 12 × 105 [9 ×105; 9.8 × 107] | Me 2 × 106 [11 × 105; 9.3 × 107] | Me 7 × 105 [1.6 × 105; 11 × 105] | Me 4 × 106 [3.8 × 105; 16 × 106] | Me 7.5 × 104 [6.25 × 104; 8 × 104] | Me 5.3 × 105 [2.6 × 104; 5.8 × 106] |
| Proteus mirabillis | Me 17 × 106 [5 × 106; 56 × 106] | Me 10 × 106 [5 × 106; 44 × 106] | Me 3.6 × 106 [1.05 × 106; 9 × 106] | Me 8.8 × 106 [5 × 106; 24 × 106] | Me 5.5 × 105 [1.9 × 105; 6 × 105] | Me 18 × 105 [12 × 105; 22 × 106] |
| Pseudomonas aeruginosa | Me 6.5 × 105 [2.3 × 105; 13.8 × 105] | Me 7.1 105 [2.0 × 105; 14.2 × 105] | Me 3.9 × 105 [2.5 × 105; 7.5 × 105] | Me 7.0 × 105 [3.7 × 105; 12.5 × 105] | Me 6.7 × 104 [5.3 × 104; 33 × 104] | Me 6.8 × 105 [2.8 × 105; 10.8 × 105] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belov, S.V.; Danilejko, Y.K.; Gudkov, S.V.; Egorov, A.B.; Lukanin, V.I.; Tsvetkov, V.B.; Altukhov, E.L.; Petrova, M.V.; Yakovlev, A.A.; Osmanov, E.G.; et al. Activation of Tissue Reparative Processes by Glow-Type Plasma Discharges as an Integral Part of the Therapy of Decubital Ulcers. Appl. Sci. 2022, 12, 8354. https://doi.org/10.3390/app12168354
Belov SV, Danilejko YK, Gudkov SV, Egorov AB, Lukanin VI, Tsvetkov VB, Altukhov EL, Petrova MV, Yakovlev AA, Osmanov EG, et al. Activation of Tissue Reparative Processes by Glow-Type Plasma Discharges as an Integral Part of the Therapy of Decubital Ulcers. Applied Sciences. 2022; 12(16):8354. https://doi.org/10.3390/app12168354
Chicago/Turabian StyleBelov, Sergej V., Yurij K. Danilejko, Sergey V. Gudkov, Aleksej B. Egorov, Vladimir I. Lukanin, Vladimir B. Tsvetkov, Evgeny L. Altukhov, Marina V. Petrova, Alexey A. Yakovlev, Elkhan G. Osmanov, and et al. 2022. "Activation of Tissue Reparative Processes by Glow-Type Plasma Discharges as an Integral Part of the Therapy of Decubital Ulcers" Applied Sciences 12, no. 16: 8354. https://doi.org/10.3390/app12168354
APA StyleBelov, S. V., Danilejko, Y. K., Gudkov, S. V., Egorov, A. B., Lukanin, V. I., Tsvetkov, V. B., Altukhov, E. L., Petrova, M. V., Yakovlev, A. A., Osmanov, E. G., Dubinin, M. V., Kogan, E. A., Seredin, V. P., & Shulutko, A. M. (2022). Activation of Tissue Reparative Processes by Glow-Type Plasma Discharges as an Integral Part of the Therapy of Decubital Ulcers. Applied Sciences, 12(16), 8354. https://doi.org/10.3390/app12168354








