Featured Application
Potential use in flap surgery, towards growing flap viability, diminishing marginal, or partial flap necrosis, as well as shortening the waiting period for angiogenesis in pedicled or tubulised flaps before second stage reconstruction.
Abstract
The evolution of reconstructive methods for defects of the human body cannot yet replace the use of flap surgery. Research is still preoccupied with the ideal techniques for offering the best chances of survival of the flaps. In our study, we investigated the effects of cold atmospheric plasma (CAP), N-nitro-L-arginine methyl ester (L-NAME), and platelet-rich plasma (PRP) injectable solutions on flap survival using an in vivo model. Twenty-four Wistar rats (four groups) had the McFarlane flap raised and CAP, L-NAME, and PRP substances tested through a single dose subcutaneous injection. The control group had only a saline solution injected. To the best of our knowledge, this is the first study that evaluated a CAP activated solution through injection on flaps. The flap survival rate was determined by clinical examination (photography documented), hematology, thermography, and anatomopathological tests. The image digital analysis performed on the flaps showed that the necrosis area (control—49.64%) was significantly lower for the groups with the three investigated solutions: CAP (14.47%), L-NAME (18.2%), and PRP (23.85%). Thermography exploration revealed less ischemia than the control group on the CAP, L-NAME, and PRP groups as well. Anatomopathological data noted the best degree of angiogenesis on the CAP group, with similar findings on the L-NAME and PRP treated flaps. The blood work did not indicate infection or a strong inflammatory process in any of the subjects. Overall, the study shows that the CAP activated solution has a similar (better) impact on the necrosis rate (compared with other solutions with known effects) when injected on the modified dorsal rat skin flap, and on top of that it can be obtained fast, in unlimited quantities, non-invasively, and through a standardized process.
1. Introduction
Until an ideal wound dressing device with synergetic effects is designed, skin flaps cannot be totally replaced, especially when a defect with vital structures exposed, such as arteries, nerves, bones, or joints, needs covering. Needless to say, idyllic wound treatment equals a better quality of life, a heavily investigated area for any pathology, considering the growth of life expectancy even in cancer patients [1].
On the threshold of treating acute or chronic wounds with stem cell techniques and 3D bioprinting devices, wound care companies already provide doctors with a huge variety of dressing choices. However, there is still solid interest for research regarding dermal microcirculation and endothelial response mechanisms [2,3,4]. Many of the research products with an already proven benefic angiogenetic effect have in common the treatment with drugs or solutions containing nitric oxide [5].
There have been comprehensive studies of a lot of substances that can reshape the vasculature, or they can increase survival of the modified McFarlane skin flap in rats [6,7,8,9,10,11,12,13]. This type of flap provides a very well described arterial anatomy, being a so called rando−axial flap, with a consistent vascular pedicle when caudally based and a predictable area of skin necrosis cranially [8,14,15,16].
The substances that have been injected or topically applied on the flap can influence the caudal pedicles or the random vascular part of the flap. Platelet rich plasma (PRP) has been investigated through subcutaneous injection in the flaps at a certain distance from the pedicles. It has been proven that it increases the viability of the flap, decreasing the area of necrosis, having pro-angiogenetic properties due to the release of factors such as vascular endothelial growth factor (VEGF) or platelet-derived angiogenesis factor (PDAF) [17,18,19]. Nitric oxide synthase (NOS) inhibitor N-nitro-L-arginine methyl ester (L-NAME) has also been studied to discover its effects in our model. Depending on the dose administrated, the duration of the treatment, or the route of administration, it might improve or decrease the viability of the flap [20,21,22].
Furthermore, plasma is the fourth aggregate state of matter, following solid, liquid, and gaseous. The production of plasma requires external energy input (e.g., thermal, electrical, and laser radiation) to create a gaseous mixture of charged particles (electrons and ions), excited atoms, molecules, or free radicals. In our study, there was no electrical field applied to the solution. The mixture was activated and administered shortly after the exposure on the plasma’s action, as described below. In human patients, the use of plasma is related to its thermal effects on the surface under treatment for some application such as electrocauterization, or to its pulsed chemistry, able to generate and deliver a mixture of reactive species to tissues and liquids. Its benefits consist of supporting wound healing, having anti-bacterial and anti-inflammatory effects, and deactivating multi-resistant pathogens, which explains its usage in wound therapy nowadays. There are studies that suggest that treatment with cold atmospheric plasma (CAP) activated therapy should be well balanced to avoid a delayed healing process and tissue damage. This balance refers to the time of exposure to CAP treatment, with several medical devices already on the market for clinical use [23,24,25].
To our knowledge, there are no studies yet regarding the investigation of the cold plasma activated solution through injectable administration in the McFarlane skin flap on rats. The aim of our study was to test the effects of a CAP activated solution on this type of flap and to compare the results with the PRP and L-NAME effects, which are already described in the literature.
2. Materials and Methods
2.1. Animals
The experimental study was approved by the Ethics Committee of Grigore T. Popa University of Medicine and Pharmacy of Iasi (no.11.05.2018) and took place at the Advanced Research and Development Centre for Experimental Medicine (CEMEX) unit of the same university. We used 28 adults male Wistar rats, accommodated individually in special cages and having conditions of constant temperature, day/night cycle from 07:00 a.m. to 19:00 p.m., and with access to water and food ad libitum. The experimental groups comprised of six animals, with a similar weight and age, that were randomly assigned into four groups (24 animals). Three of the groups were submitted to surgical procedures, tailoring the flap, combined with the action of different types of treatment (CAP, PRP, and L-NAME); one group remained treatment-free, after the flap was raised and sutured back, and was used as a control group. Four animals were used as blood-donors for PRP obtainment, as detailed bellow.
2.2. Surgical Procedure
Before surgery, the animals were anesthetized using Isoflurane (5% induction and 2% maintenance) through an inhalation mask. Then, they were shaved, and the limits of the flap were marked. The flap was drawn on the dorsal part, caudally based, and the dimensions of the flap were 8 × 3 cm. After that, the animals were moved to the operation post, for surgery. The flaps were raised at the level of the deep fascia, including the skin, subcutaneous tissue, panniculus carnosus, and superficial fascia.
2.3. Treatment Procedure
CAP activated solution and PRP were administered by injecting a single dose, 1 mL volume of substance into the flap, at a distance of 2 cm from the apparent entrance of the pedicles into the flap, with a mean distance on a bidimensional axis (x, y) between two administrations, of approximative 0.5 cm, into every point being administered approximately with 20 μL. Between the raised flaps and the subjacent tissue, a sterile mesh impregnated with Vaseline was interposed in order to prevent angiogenesis and neovascularization, and the flaps were sutured back. The L-NAME was administered subcutaneously (30 mg/kg), 30 min before the elevation of the flap.
The viability of the flaps was assessed on the fifth postoperative day, by clinical examination and photographic documentation (Nikon D3200 device—Nikon Inc., Long Island, New York City, NY, USA). The follow-up included an evaluation of the integrity of the flap, color, suture state, suppuration, turgor, temperature, fixation of the adjacent tissue, and smell. Captured images were manually analyzed by Fiji software (Eliceiri/LOCI group—University of Wisconsin-Madison, Madison, WI, USA, Jug and Tomancak labs—MPI-CBG, Dresden, Germany), the selection of normal and pathological areas of the flaps being acquired with a polygon selection tool, following the contour of the normal and pathological areas, first being acquired by the entire area of the flap and thereafter the pathological regions. For every individual, the ratio between the necrosis area (in pixels) and the entire flap area (in pixels) was determined and multiplied by 100 to calculate the relative necrosis surface (%).
2.4. PRP Obtainment
For platelet enriched plasma, 6–8 mL of fresh blood was collected from four donors, anesthetized (isoflurane 3%) rats, into special anticoagulant containing tubes, through cardiac puncture, and was immediately submitted to procedure for PRP production. Briefly, after the collection of blood, the tubes were centrifuged at a speed of 3100 rpm for 10 min. The supernatant was transferred into new tubes and centrifuged at 3600 rpm for 15 min. The PRP solution (the supernatant of the second centrifugation) was transferred into new tubes [19]. After the blood collecting procedure was finalized, the animals were euthanized through anesthetic overdose. The solution was kept in tubes immersed in ice in a thermo-insulating box and utilized fresh, with a maximum of 30 min from production until injection.
2.5. CAP Activated Solution Preparation
For obtaining the plasma solution, an air dielectric barrier discharge (DBD) plasma source was designed to produce the pulsed plasma directly into 24 well plates, for a quick and safe manipulation of activated liquids in biology and medicine laboratories [26]. One column of the well plate can be exposed at a time, using an array of four stainless steel electrodes, inserted into each well, leaving an open space between them and the walls of the wells.
A gap of 3 mm was imposed between the electrode array and the well plate bottom. The plate itself acts as a dielectric layer, and then the circuit can contain supplementary dielectric layers to prevent the breakdown of well plates. A planar back electrode, made of a 1.5 mm thick copper plate, also served as a mount for the entire well plate (Figure 1).
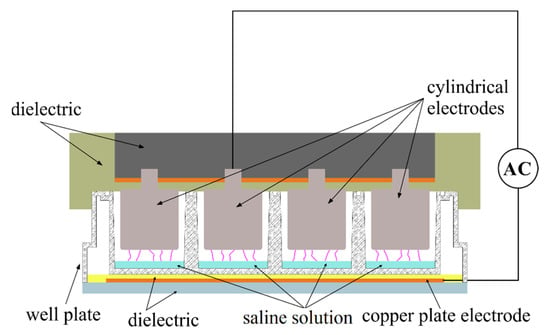
Figure 1.
The air DBD plasma experimental setup for saline solution activation.
The electrodes were connected using high voltage cables to a laboratory assembled power supply (neon transformer and Variac), which excited the plasma using the following electrical parameters: 5 kV surges of high voltage pulses, 28 kHz, and a maximum current of 20 mA. During operation, the electrical and optical parameters of the discharge were monitored using current probes and a fixed grating monochromator.
Before any experiment activation, fresh saline solution (0.90% w/v of NaCl) was transferred into all four wells of a selected column, using 150 μL volume for each well (0.6 mL). Then, the discharge was applied for 2 min, and the liquid was collected into sterile plastic containers. During the exposure time, the evaporation rate was negligible. The authors observed no significant variations of the macroscopic discharge global parameters (e.g., discharge current and total emission intensity). The temperature of the liquid measured immediately after the exposure was a maximum of 40 °C. The procedure was repeated using different columns, to avoid the thermal effects, until a desired volume of plasma activated saline solution (total of 10 mL) was produced for our in vivo experiments performed in the same day. The time between the plasma activation of the saline solution and the administration of the activated media was 2 h. Meanwhile, the plasma activated media was kept refrigerated in tubes that were tightly sealed.
2.6. Thermographic Exploration
Infrared thermograms were collected using an OPTRIS, PI160 IR (Optris GmbH, Berlin, Germany) medical camera with a measurable temperature range of −20 °C to 900 °C, spectrum 7.5–13 μm, recording rate of the 120 Hz image, and an optical resolution of 160 × 120 pixels. Infrared thermography is a non-invasive technique gaining ground as a monitoring and diagnostic tool. Monitoring flaps using infrared thermography involve the use of an infrared camera that generates a color map based on the amount of heat emitted by the tissues. The use of thermography for flaps can differentiate between arterial and venous flow problems. Changes in tissue perfusion often result in a modified tissue temperature.
Working protocol: the thermographic camera was positioned at a fixed distance of 54 cm from the subject, and the thermographic images were individually recorded on the fifth day after the flap was performed. After each recording, the thermal camera was recalibrated to eliminate any errors. Real-time images were sent to a laptop. Further processing included filtering and color conversion.
2.7. Hematological Tableau
As the investigators chose to document an eventual inflammatory response and exclude any infection bias about the flap necrosis, on the seventh postoperative day, all the animals were euthanized, after the blood samples were collected through major abdominal vessel puncture for the complete blood count test. This service was externalized at a private laboratory.
2.8. Anatomopathological Analysis
Briefly after euthanasia (day 7 of the experiment, as mentioned above), all flaps were harvested and fixed for 48 h in 10% buffered formalin and were embedded in paraffin with a tissue processor Leica TP1020 (Leica Microsystems GmbH, Wetzlar, Germany). Sections of 5 μm thickness were obtained with a Microtome SLEE CUT 6062 (SLEE Medical GmbH, Nieder-Olm, Germany), and were deparaffinized and stained by the Masson trichrome techniques. The qualitative histology was performed from stained sections using a light microscope Leica DM750 (Leica Microsystems GmbH, Germany) with an attached digital camera Leica ICC50 HD (Leica Microsystems GmbH, Germany). The photographs were done with Leica Application Suit Software (LAS) version 4.2.
For histological evaluation, we considered the following aspects: neutrophil exudation (presence of the inflammatory cells), angiogenesis status as evaluated on a 10,000 µm area localized near the contact zone between the flaps and subjacent tissue, the morphology of the new generated conjunctive tissue and the presence of the fibroblasts, and the presence of the necrosis into the skin flap’s structures.
2.9. Statistical Analysis
The paraclinical data were analyzed with Jamovi computer software (The jamovi project, 2019, Jamovi (Version 1.0), retrieved from https://www.jamovi.org (accessed on 6 December 2020). ANOVA test and Tukey post-hoc test for multiple comparisons were used.
3. Results
3.1. Effects of CAP Activated Solution, PRP and L-NAME on the Necrosis
The area of the necrosis of the flap, after digital image analysis, for the control group was 49.64%. The lowest area of necrosis was observed in the CAP group (14.47%), while for the PRP group there was a percentage of necrosis of 23.85% (Figure 2—* p < 0.05; ** p < 0.01). The L-NAME treated group had an 18.2% necrosis segment.
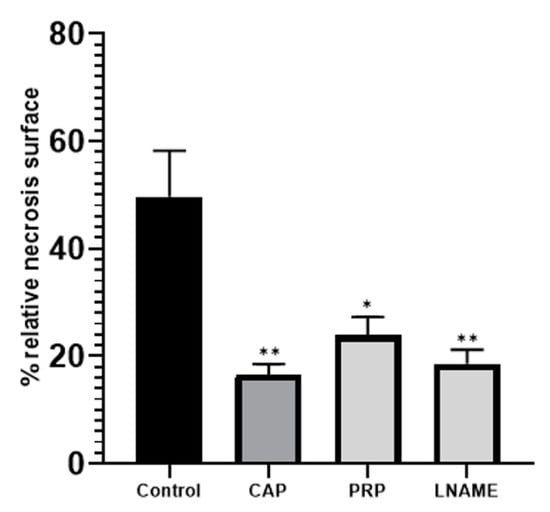
Figure 2.
Relative necrosis surface (%) from the entire flap surface as determined by photo analysis. Dehiscent and prenecrotic surfaces were included into the necrosis area. * p < 0.05; ** p < 0.01.
3.2. Thermographic Exploration
In the control group, after the thermographic analysis, it was noticeable that the maximum average temperature was 35.12 °C; the temperature of the flap was stable. It had maximum differentiation points and there were no visible hot spots. Diffusion of the flap temperature was homogenous both at the center and at the edges of the flap (Figure 3a).
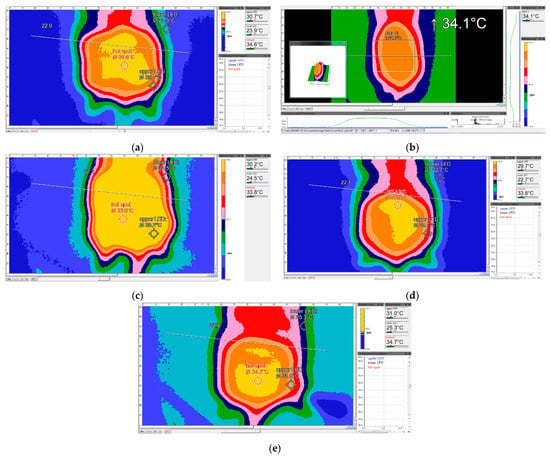
Figure 3.
Thermographic images of the groups of rats included in the study. (a) Thermographic image of a control-group rat above the flap—print screen (PS). (b) Thermographic image of a CAP-group rat above the flap—PS. (c) Thermographic image of a L-NAME-group rat above the flap—PS. (d) Thermographic image of a PRP-group rat above the flap—PS. (e) Thermographic image of a CAP-group rat above the flap—PS.
In the CAP group, the temperature of the flap did not increase and there were no visible hot spots (Figure 3b). For the cases where the infusion was normal, a slight vasodilation at the periphery of the flap was observed, with no arterial flow problems. Furthermore, in the CAP group, less ischemia was noticed, which, from a thermographic point of view, was observed as an average maximum temperature at the flap level of 35.95 °C, with a large revascularization area that included the entire flap. In addition, this group did not show any signs of ischemia through thermography (Figure 3e).
In the L-NAME group, as Figure 3c shows, the maximum mean flap temperature was 33.6 °C, much lower than the control group, but it was homogeneously distributed, with a visible deep vasodilation by thermographic imaging, covering a large area of the flap (extended yellow zone). The analyzed tissue was well perfused, with no visible ischemic signs, except for one subject.
In the PRP group, the average maximum flap temperature was 34.5 °C; for three subjects, it was noted that the flap temperature increased and there were visible hot spots, from which it could be deduced that there was a problem with the arterial flow. Hence, there was a higher necrosis rate than for the CAP and L-NAME groups. Due to the administered substance, there was a clearly visible thermodynamic vasodilation that covered a large area of the flap, but with signs of hypoperfusion (Figure 3d).
From the thermographic point of view, the best-perfused group without ischemic problems at the flap was the L-NAME group. There were no temperature variations at the flap. In addition, it had the lowest value of the coefficient of variation and standard deviation (Table 1).

Table 1.
Statistical interpretation of the data obtained from the processing of thermographic images (ns—non-significant).
Statistically, the variation coefficient for each group was less than 5%. Therefore, the groups were homogeneous, without temperature fluctuations of the flaps in each group.
3.3. Anatomopathological Results
In the control group (Figure 4a), massive leucocyte infiltrates were found with neutrophils aggregated (as observed into hypodermis), significant leukocyte exudation into the contact zone (namely hypodermis and dermis), necrotic detritus (rare lymphocytes and histiocytes), low neoangiogenesis, reduced number of fibroblasts with newly formed capillary vessels in small number, and immature and thin connective tissue with inflammatory cells (monocytes). Additionally, a well-distinguished necrosis area with inflammatory exudates, and marked muscular necrosis of the small fibers, were noticed.
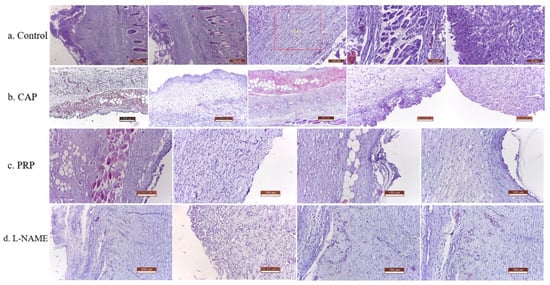
Figure 4.
Histopathological details of the flaps in the condition of different treatments; (a) Control group (1st line—5 pictures p.); (b) CAP group (2nd line—5 p.), (c) PRP group (3rd line—4 p.); (d) L-NAME group (4th line—4 p.).
In the CAP group (Figure 4b), reduced leucocyte infiltration could be observed, marked by a restricted area with small aggregates of neutrophils, connective scar tissue with numerous collagen fibers, and active fibroblasts, which were well vascularized and infiltrated with a few inflammatory cells. An intense angiogenesis with many newly formed capillaries and an extremely small or absent necrosis area was noticed, also with reduced denervation of the muscular fibers, with the predominance of the viable muscular fibers.
For the PRP group and L-NAME group (Figure 4c,d), the histological findings were similar and consisted of reduced leukocyte infiltration, connective scar tissue with numerous collagen fibers, and active fibroblasts, with good vascularization. Intense angiogenesis processes were noticed with reduced area of necrosis and low muscular fibers destruction. The overall scoring of the main modification into histological structures due to different types of treatment administered to flaps is presented in Table 2.

Table 2.
Scoring of the histological findings in control and treated groups at flaps’ level.
Analysis of the histological parameters in the case of the CAP activated solution treatment, compared with the control group, has revealed a significant improvement of the flap regeneration, and rewiring by a reduction in the inflammatory process and by intensification of angiogenesis and conjunctive tissue regeneration. In addition, the angiogenesis process was more intense in the case of the CAP group, as compared with PRP and L-NAME—with the consequence of such behavior being the smaller presence of necrosis.
3.4. Blood Work Parameters
In Table 3, we show the hematological parameters taken into consideration when these groups were studied. The number of the white blood cells and the number of the segmented neutrophils were lower in the CAP and PRP groups, compared with the control group. Regarding the values of the hemoglobin, hematocrit, and VEM, there were no significant differences between the groups. The lowest number of platelets was found in the CAP group. Overall, the hematology did not indicate any infections or a strong inflammatory process ongoing in any of the groups.

Table 3.
Hematological parameters of the groups (ns—non-significant).
4. Discussion
In this study, the effect of the CAP activated solution on the McFarlane skin flap was investigated and was compared with the effect of the PRP solution and a single dose of L-NAME. Reactive oxygen and nitrogen species (RONS) in an optimal dose are crucial players in the biological effects of plasmas [27]. The RONS are created by plasma treatment of aqueous solutions, thus forming the so-called short-lived species of nanoseconds to microseconds (e.g., hydroxyl (OH), peroxyl (HOO), superoxide anion (O2−), and singlet delta oxygen (1O2)) not the case for this study, as they are neutralized until the application, followed by their transition to long lived species. The CAP activated solution contains long chemical species like hydrogen peroxide (H2O2), nitrite ions (NO2−), and nitrate ions (NO3−), as persistent species, and peroxynitrites (ONOO−) as secondary species; the key components of the CAP activated solution used in this study are known to be involved as endogenous radicals in many intra- and inter-cellular processes, such us signaling and biological processes [27,28,29,30,31,32,33,34]. As denoted by Khlyustova et al. [27], a major challenge in plasma medicine remains the control of plasma generated reactive species formed in the liquid in order to have an indication of the therapeutical dose. Long-lived species such as H2O2 and NO2− are by far the species mostly characterized, followed by NO3−. The same authors [27] determined that the concentration of H2O2 (~1700 μmol/L) and NO2− (~1800 μmol/L) when using biocompatible liquids as the plasma activated media in similar conditions has positive effects on the indirect treatment. In addition, the NO3− concentration was found to be around 2600 μmol/L in comparable settings.
In this study, the CAP activated solution preserved a variable concentration of RONS as a function of the elapsed time (2 h later) from the direct exposure of saline to administration in the form of a single dose injection into the flap; therefore, the kinetics (depletion and transformation) of RONS in solution is strongly influenced by temperature (room temperature in this case), as also pointed out by a recent study by Tsoukou et al. [35]. It is expected that 2 h after activation, only the long-lived species such as H2O2, NO2−, and NO3− will remain in a mM concentration range [36,37].
Another hypothesis in such an experimental timescale (2 h) is that NO could have totally reacted with dioxygen and superoxide, and hence transformed into nitrate and nitrite in a nM concentration, resulting in the chemistry of NO being incompatible with the observed effects under the used conditions. Another observation of this study refers to the pH solution, but as no buffer was used in the CAP activation, it is reasonable to expect a significant pH drop in the range of 3 to 5, an effect reported in many papers [29,35,38].
Plasma activation of biocompatible liquids was studied previously so as to quantify the chemical transformations [35,39], the bacterial inactivation [40], and the anti-cancer capacity [31,36,41,42,43,44,45]. To the best of our knowledge, its injection on a rat skin flap has never been reported in previous studies. The results of this type of flap after subcutaneous injection of the CAP activated solution denote a positive potential.
Furthermore, apart from having the lowest necrosis rate, the histopathological examination showed a reduced inflammatory response and a strong angiogenetic effect. From the laboratory data, it could be observed that the CAP group had the lowest number of white cells. The CAP activated solution has been used in chronic human wounds, with good results regarding the stimulation of the wound skin cells, as well as with a strong antimicrobial effect. There is a possibility that the CAP activated solution stimulates angiogenesis in a strong manner and pushes the maximal vasodilation of the anastomotic skin vessels higher, as it does to keratinocytes, fibroblasts, or other cells [23,46,47]. Its effects on the skin microcirculation are related to the release of the nitric oxide (NO) and nitrogen dioxide (NO2). An efficient CAP activated solution should maximize the NO/NO2 levels and lower the reactive oxygen species level 1. Nowadays, the devices that use CAP for skin treatment are non-invasive. The model we depicted required injections in the flap, at a fixed distance from the pedicles.
A minor area of skin necrosis was also found in the L-NAME group. A single dose of this substance was injected, and as it is an inhibitor of NO synthesis, the resulted necrosis rate of 18.2% was not expected. It is known that L-NAME administration has a dose dependent effect on flap survival [20,21], and we showed that, therefore, a single administration of a dose of L-NAME (30 mg/kg) can increase flap survival. The triggers for this may be that the ischemia and the lesions produced by the surgical manipulation of the flap, as well as the single dose that blocks the synthesis of the NO, might be able to stimulate the compensatory production of NO [21], despite its use as an NOS antagonist. NO can be either cytotoxic or cytoprotective [48]. We assume that at this concentration of L-NAME, due to the local conditions of ischemia, the amount of NO that combines with superoxide free radical (O2−) to generate damaging hydroxyl free radical (OH) is lowered. In addition, L-NAME may have reduced the amount of inducible nitric oxide synthase (iNOS) in the distal part of the flap. NO produced from the inflammatory cells via iNOS may produce direct endothelial damage or influence the production of the OH [21]. There are several non-canonical actions of L-NAME, such as sympathetic activation [49], ROS generation, and of course an increase in NO production [50]. However, even if NO production via NOS activation by L-NAME is proposed, it is feasible that this increase occurs through a different mechanism [49]. According to Kopincova et al. [49] the regulation of NO production in vivo is precisely regulated, but not with a clear process. Disruption of this regulation may increase NOS expression or activity, differing on the condition of the experiment, duration of the treatment, dose of L-NAME, rat strain, tissue investigated, and NOS isoforms [49]. In a recent study by Liu et al. [50], L-NAME releases NO and potentiates subsequent nitroglycerin-mediated vasodilation in an in vivo model. In their experiment, NO was released from the nitro group of L-NAME via the Fenton reaction, a process that can co-found NOS inhibiting effects, possibly by contributing to a presumed intracellular NO store in the vasculature.
The impossibility to determine the vascular endothelial growth factor (VEGF) represents a limitation of this study, as the angiogenetic effect of this solution could not be documented.
This work opens the opportunity for investigating the CAP effects on other types of experimental flaps, and, after it passes safety and quality control, it could also be evaluated in a clinical setting. It would be of interest to test the CAP activated solution on an ischemic model, and to compare its results with PRP and L-NAME on the same flap. An important aspect to be considered for future experiments, considering the results obtained from this study, could involve the two-case scenario: one with a short CAP activated solution treatment period and the second with a longer period. This would verify if the 23.85% is the optimal reduction that can be achieved for the model using the CAP activated solution. Further consideration for upcoming studies could also represent testing an NO solution or an NO chemical donor solution. It might support the hypothesis of the study or aid in understanding the absence of vasodilation in certain cases.
5. Conclusions
The CAP activated solution had a particularly good impact on the necrosis rate of this type of flap in this study, as confirmed through the clinical follow-up, thermography, and histology, which was better than PRP, a well know solution with a regenerative potential. Further investigation should be performed to explain this effect at a biological level, in order to confirm a link between the CAP and NO release.
Author Contributions
Conceptualization, B.C. and I.L.S.; methodology, B.C., I.L.S., I.T., C.T.M. and I.G.; validation, I.L.S., I.T., C.T.M., C.L. and I.G.; formal analysis, G.D., C.L. and C.T.M.; investigation, B.C., C.L., C.T.M., I.C.C., I.M., S.P., G.D. and I.G.; data curation, C.T.M., G.D., B.C., C.L., S.P. and I.G.; writing—original draft preparation, B.C., I.L.S., I.T., I.M., C.L., I.C.C., S.P., G.D., B.H., C.T.M. and I.G.; writing—review and editing, I.L.S., I.T., I.C.C., C.T.M. and I.G.; visualization, C.T.M., I.C.C., C.L. and I.G.; supervision, I.L.S. and I.G.; project administration, B.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of Grigore T. Popa University of Medicine and Pharmacy of Iasi (no.11.05.2018).
Informed Consent Statement
Not applicable.
Data Availability Statement
The study did not report any data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gardikiotis, I.; Manole, A.; Azoicăi, D. Quality of life with mastectomy for breast cancer, in terms of patients’ responses of sf-36 questionnaire. Rev. Med. Chir. Soc. Med. Nat. 2015, 119, 529–535. [Google Scholar]
- Bauermeister, A.J.; Zuriarrain, A.; Newman, M.I. Three-Dimensional Printing in Plastic and Reconstructive Surgery: A Systematic Review. Ann. Plast. Surg. 2016, 77, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Chae, M.P.; Rozen, W.M.; McMenamin, P.G.; Findlay, M.W.; Spychal, R.T.; Hunter-Smith, D.J. Emerging Applications of Bedside 3D Printing in Plastic Surgery. Front. Surg. 2015, 2, 25. [Google Scholar] [CrossRef]
- Weum, S.; Mercer, J.B.; de Weerd, L. Evaluation of dynamic infrared thermography as an alternative to CT angiography for perforator mapping in breast reconstruction: A clinical study. BMC Med. Imaging 2016, 16, 43. [Google Scholar] [CrossRef]
- Suschek, C.V.; Opländer, C. The application of cold atmospheric plasma in medicine: The potential role of nitric oxide in plasma-induced effects. Clin. Plasma Med. 2016, 4, 1–8. [Google Scholar] [CrossRef]
- Fichter, A.M.; Ritschl, L.M.; Robitzky, L.K.; Wagenpfeil, S.; Mitchell, D.; Wolff, K.D.; Mucke, T. Impact of different antithrombotics on the microcirculation and viability of perforator-based ischaemic skin flaps in a small animal model. Sci. Rep. 2016, 6, 35833. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, M.J.; Forootan, K.S.; Jalali, S.Z.S.; Mousavi, S.J.; Pedram, M.S. The Effect of Enoxaparin and Clopidogrel on Survival of Random Skin Flap in Rat Animal Model. World J. Plast Surg. 2012, 1, 64–70. [Google Scholar]
- Caba, B.; Gardikiotis, I.; Strobescu-Ciobanu, C.; Strobescu-Ciobanu, C.; Mihai, C.T.; Dimitriu, G.; Huzum, B.; Serban, I.L. Clinical and echographic evaluation of the effects of epinephrine and platelet-rich plasma solution on modified mcfarlane skin flap in rats. Rev. Med. Chir. Soc. Med. Nat. 2019, 123, 319–327. [Google Scholar]
- Gazzalle, A.; Teixeira, L.F.; Pellizzari, A.C.; Cocolichio, F.; Zampieri, J.T.; Pezzin, L.S.; Zago, V.; Braga-Silva, L. Effect of Side-Stream Smoking on Random-Pattern Skin Flap Survival in Rats. Ann. Plast. Surg. 2014, 72, 463–466. [Google Scholar] [CrossRef]
- Gürsoy, K.; Teymur, H.; Koca, G.; Tanas Isikci, O.; Goktas, D.; Fethiye, B.; Kankaya, Y.; Ugur, K. The Effect of Astaxanthin on Random Pattern Skin Flaps. Ann. Plast. Surg. 2020, 84, 208–215. [Google Scholar] [CrossRef]
- Can, A.; Temel, M.; Dokuyucu, R.; Mutaf, M. The Effect of Coenzyme Q10 (Ubiquinone) on Random Pattern Skin Flap Survival in Rat Model. Ann. Plast. Surg. 2016, 77, e9–e14. [Google Scholar] [CrossRef]
- Choi, S.W.; Jeon, Y.R.; Baek, W.; Yun, C.-O.; Roh, T.S.; Lee, W.J. Dickkopf 2–Expressing Adenovirus Increases the Survival of Random-Pattern Flaps and Promotes Vasculogenesis in a Rat Model. Ann. Plast. Surg. 2020, 84, 588–594. [Google Scholar] [CrossRef]
- Şen, H.; Oruç, M.; Işik, V.M.; Sadic, M.; Sayar, H.; Citil, R.; Korkmaz, M.; Kocer, U. The Effect of Omeprazole Usage on the Viability of Random Pattern Skin Flaps in Rats. Ann. Plast. Surg. 2017, 78, e5–e9. [Google Scholar] [CrossRef]
- Schmauss, D.; Weinzierl, A.; Schmauss, V.; Harder, Y. Common Rodent Flap Models in Experimental Surgery. Eur. Surg. Res. 2018, 59, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Ohara, H.; Kishi, K.; Nakajima, T. Rat Dorsal Paired Island Skin Flaps: A Precise Model for Flap Survival Evaluation. Keio J. Med. 2008, 57, 211–216. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hosnuter, M.; Kargi, E.; Peksoy, İ.; Babucçu, O.; Payasli, C. An ameliorated skin flap model in rats for experimental research. J. Plast. Reconstr. Aesthet. Surg. 2006, 59, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Enomoto, M.; Ukegawa, M.; Hirai, T.; Sotome, S.; Wakabayashi, Y.; Shinomiya, K.; Okawa, A. Subcutaneous Injections of Platelet-Rich Plasma into Skin Flaps Modulate Proangiogenic Gene Expression and Improve Survival Rates. Plast. Reconstr. Surg. 2012, 129, 858–866. [Google Scholar] [CrossRef]
- Takikawa, M.; Sumi, Y.; Ishihara, M.; Kishimoto, S.; Nakamura, S.; Yanagibayashi, N.; Hattori, H.; Azuma, R.; Yamamoto, N.; Kiyosawa, T. PRP&F/P MPs Improved Survival of Dorsal Paired Pedicle Skin Flaps in Rats. J. Surg. Res. 2011, 170, e189–e196. [Google Scholar] [CrossRef]
- Orhan, E.; Uysal, A.Ç.; Başer, E.; Keskin, D.; Demiroğlu-Yakut, Ç. The effect of intradermal administration of inactive platelet-rich plasma on flap viability in rats. Acta Cir. Bras. 2017, 32, 280–286. [Google Scholar] [CrossRef]
- Knox, L.K.; Stewart, A.G.; Hayward, P.G.; Morrison, W.A. Nitric oxide synthase inhibitors improve skin flap survival in the rat. Microsurgery 1994, 15, 708–711. [Google Scholar] [CrossRef]
- Gribbe, Ö.; Samuelson, U.E.; Wiklund, N.P. Effects of nitric oxide synthase inhibition on blood flow and survival in experimental skin flaps. J. Plast. Reconstr. Aesthet. Surg. 2007, 60, 287–293. [Google Scholar] [CrossRef]
- Nezami, B.G.; Rahimpour, S.; Sadeghi, M.; Sianati, S.; Kalbasi Anaraki, D.; Ebrahimi, F.; Ghasemi, M.; Dehpour, A.R. Chronic Lithium Impairs Skin Tolerance to Ischemia in Random-Pattern Skin Flap of Rats. J. Surg. Res. 2011, 171, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Ermolaeva, S.A.; Petrov, O.F.; Naroditsky, B.S.; Fortov, V.E.; Morfill, G.E.; Gintsburg, A.L. Cold Plasma Therapy. In Comprehensive Biomedical Physics; Elsevier: Amsterdam, The Netherlands, 2014; pp. 343–367. [Google Scholar] [CrossRef]
- Balzer, J.; Demir, E.; Kogelheide, F.; Fuchs, P.C.; Stapelmann, K.; Opländer, C. Cold atmospheric plasma (CAP) differently affects migration and differentiation of keratinocytes via hydrogen peroxide and nitric oxide-related products. Clin. Plasma Med. 2019, 13, 1–8. [Google Scholar] [CrossRef]
- Hasse, S.; Duong Tran, T.; Hahn, O.; Kindler, S.; Metelman, H.R.; von Woedtke, T.; Masur, K. Induction of proliferation of basal epidermal keratinocytes by cold atmospheric-pressure plasma. Clin. Exp. Dermatol. 2015, 41, 202–209. [Google Scholar] [CrossRef]
- Gerber, I.C.; Mihai, C.T.; Gorgan, L.; Ciorpac, M.; Nita, A.; Pohoata, V.; Mihaila, I.; Topala, I. Viability and Cell Biology for HeLa and Vero Cells after Exposure to Low-Temperature Air Dielectric Barrier Discharge Plasma. Plasma Med. 2017, 7, 159–173. [Google Scholar] [CrossRef]
- Khlyustova, A.; Labay, C.; Machala, Z.; Ginebra, M.-P.; Canal, C. Important parameters in plasma jets for the production of RONS in liquids for plasma medicine: A brief review. Front. Chem. Sci. Eng. 2019, 13, 238–252. [Google Scholar] [CrossRef]
- Trizio, I.; Sardella, E.; Rizzi, V.; Dilecce, G.; Cosma, P.; Schmidt, M.; von Woedtke, T.; Gristina, R.; Favia, P. Characterization of Reactive Oxygen/Nitrogen Species Produced in PBS and DMEM by Air DBD Plasma Treatments. Plasma Med. 2016, 6, 13–19. [Google Scholar] [CrossRef]
- Tarabová, B.; Lukeš, P.; Hammer, M.U.; Jablonowski, H.; von Woedtke, T.; Reuter, S.; Machala, Z. Fluorescent measurement of peroxynitrite/peroxynitrous acid in cold air plasma treated aqueous solutions. Phys. Chem. Chem. Phys. 2019, 21, 8883–8896. [Google Scholar] [CrossRef]
- Yost, A.D.; Joshi, S.G. Atmospheric Nonthermal Plasma-Treated PBS Inactivates Escherichia coli by Oxidative DNA Damage. PLoS ONE 2015, 10, e0139903. [Google Scholar] [CrossRef]
- Van Boxem, W.; Van der Paal, J.; Gorbanev, Y.; Vanuytsel, S.; Smits, E.; Dewilde, S.; Bogaerts, A. Anti-cancer capacity of plasma-treated PBS: Effect of chemical composition on cancer cell cytotoxicity. Sci. Rep. 2017, 7, 16478. [Google Scholar] [CrossRef]
- Gorbanev, Y.; Privat-Maldonado, A.; Bogaerts, A. Analysis of short-lived reactive species in plasma–air–water systems: The dos and the do nots. Anal. Chem. 2018, 90, 13151–13158. [Google Scholar] [CrossRef]
- Bruno, G.; Wenske, S.; Lackmann, J.W.; Lalk, M.; von Woedtke, T.; Wende, K. On the Liquid Chemistry of the Reactive Nitrogen Species Peroxynitrite and Nitrogen Dioxide Generated by Physical Plasmas. Biomolecules 2020, 10, 1687. [Google Scholar] [CrossRef]
- Sklias, K.; Santos Sousa, J.; Girard, P.M. Role of Short-and Long-Lived Reactive Species on the Selectivity and Anti-Cancer Action of Plasma Treatment In Vitro. Cancers 2021, 13, 615. [Google Scholar] [CrossRef] [PubMed]
- Tsoukou, E.; Bourke, P.; Boehm, D. Temperature Stability and Effectiveness of Plasma-Activated Liquids over an 18 Months Period. Water 2020, 12, 3021. [Google Scholar] [CrossRef]
- Girard, P.M.; Arbabian, A.; Fleury, M.; Bauville, G.; Puech, V.; Dutreix, M.; Sousa, J.S. Synergistic Effect of H2O2 and NO2 in Cell Death Induced by Cold Atmospheric He Plasma. Sci. Rep. 2016, 6, 29098. [Google Scholar] [CrossRef]
- Kurake, N.; Tanaka, H.; Ishikawa, K.; Kondo, T.; Sekine, M.; Nakamura, K.; Kajiyama, H.; Kikkawa, F.; Mizuno, M.; Hori, M. Cell survival of glioblastoma grown in medium containing hydrogen peroxide and/or nitrite, or in plasma-activated medium. Arch. Biochem. Biophys. 2016, 605, 102–108. [Google Scholar] [CrossRef]
- Jirásek, V.; Koval’ová, Z.; Tarabová, B.; Lukeš, P. Leucine modifications by He/O2 plasma treatment in phosphate-buffered saline: Bactericidal effects and chemical characterization. J. Phys. D Appl. Phys. 2021, 54, 505206. [Google Scholar] [CrossRef]
- Takamatsu, T.; Kawate, A.; Uehara, K.; Oshita, T.; Miyahara, H.; Dobrynin, D.; Fridman, G.; Fridman, A.; Okino, A. Bacterial inactivation in liquids using multi-gas plasmas. Plasma Med. 2012, 2, 237–247. [Google Scholar] [CrossRef]
- Yang, L.; Niyazi, G.; Qi, Y.; Yao, Z.; Huang, L.; Wang, Z.; Guo, L.; Liu, D. Plasma-Activated Saline Promotes Antibiotic Treatment of Systemic Methicillin-Resistant Staphylococcus aureus Infection. Antibiotics 2021, 10, 1018. [Google Scholar] [CrossRef] [PubMed]
- Griseti, E.; Merbahi, N.; Golzio, M. Anti-cancer potential of two plasma-activated liquids: Implication of long-lived reactive oxygen and nitrogen species. Cancers 2020, 12, 721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, J.; Guo, B.; Chen, H.; Xu, D.; Kong, M.G. The Antitumor Effects of Plasma-Activated Saline on Muscle-Invasive Bladder Cancer Cells In Vitro and In Vivo Demonstrate Its Feasibility as a Potential Therapeutic Approach. Cancers 2021, 13, 1042. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, L.; Gjika, E.; Cheng, X.; Canady, J.; Keidar, M. Selective treatment of pancreatic cancer cells by plasma-activated saline solutions. IEEE TRPMS 2017, 2, 116–120. [Google Scholar] [CrossRef]
- Griseti, E.; Kolosnjaj-Tabi, J.; Gibot, L.; Fourquaux, I.; Rols, M.P.; Yousfi, M.; Merbahi, N.; Golzio, M. Pulsed Electric Field Treatment Enhances the Cytotoxicity of Plasma-Activated Liquids in a Three-Dimensional Human Colorectal Cancer Cell Model. Sci. Rep. 2019, 9, 7583. [Google Scholar] [CrossRef] [PubMed]
- Daeschlein, G.; Hillmann, A.; Gumbel, D.; Sicher, C.; von Podewils, S. Enhanced Anticancer Efficacy by Drug Chemotherapy and Cold Atmospheric Plasma Against Melanoma and Glioblastoma Cell Lines In Vitro. IEEE TRPMS 2018, 2, 153–159. [Google Scholar] [CrossRef]
- Mohd Nasir, N.; Lee, B.K.; Yap, S.S.; Thong, K.L.; Yap, S.L. Cold plasma inactivation of chronic wound bacteria. Arch. Biochem. Biophys. 2016, 605, 76–85. [Google Scholar] [CrossRef]
- Haertel, B.; Woedtke, T.; von Weltmann, K.-D.; Lindequist, U. Non-Thermal Atmospheric-Pressure Plasma Possible Application in Wound Healing. Biomol. Ther. 2014, 22, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Knox, L.K.; Angel, M.F.; Gamper, T.; Amiss, L.R.; Morgan, R.F. Secondary ischemic tolerance improved by administration of L-NAME in rat flaps. Microsurgery 1996, 17, 425–427. [Google Scholar] [CrossRef]
- Kopincová, J.; Púzserová, A.; Bernátová, I. L-NAME in the cardiovascular system—Nitric oxide synthase activator? Pharmacol. Rep. 2012, 64, 511–520. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, M.; Mukosera, G.T.; Borchardt, D.; Li, Q.; Tipple, T.E.; Ahmed, A.S.I.; Power, G.G.; Blood, A.B. L-NAME releases nitric oxide and potentiates subsequent nitroglycerin mediated vasodilation. Redox Biol. 2019, 26, 101238. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).