Computer-Aided Surgical Simulation through Digital Dynamic 3D Skeletal Segments for Correcting Torsional Deformities of the Lower Limbs in Children with Cerebral Palsy
Abstract
1. Introduction
2. Materials and Methods
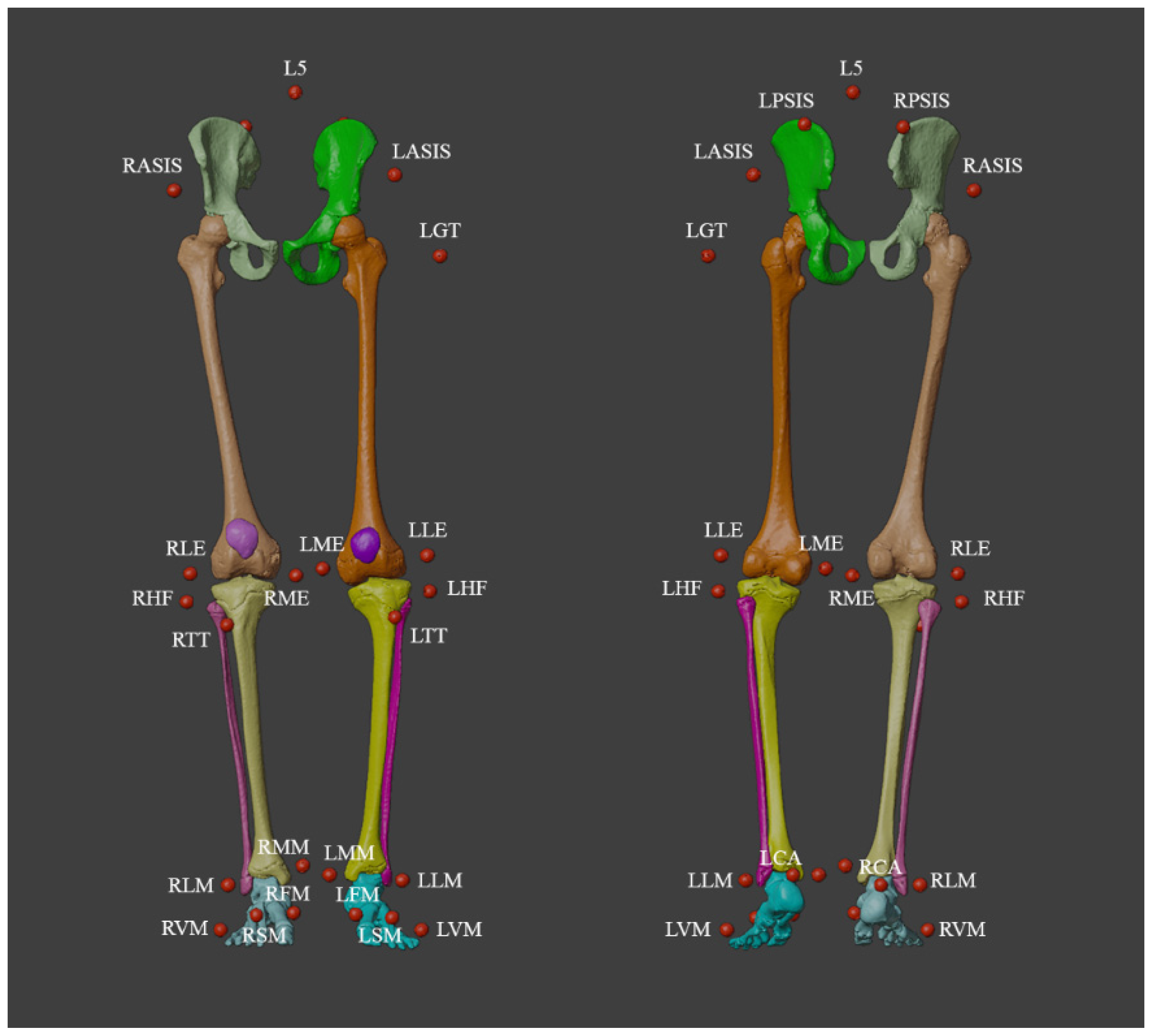
2.1. Gait Analysis Acquisition
2.2. CT Scan Protocol
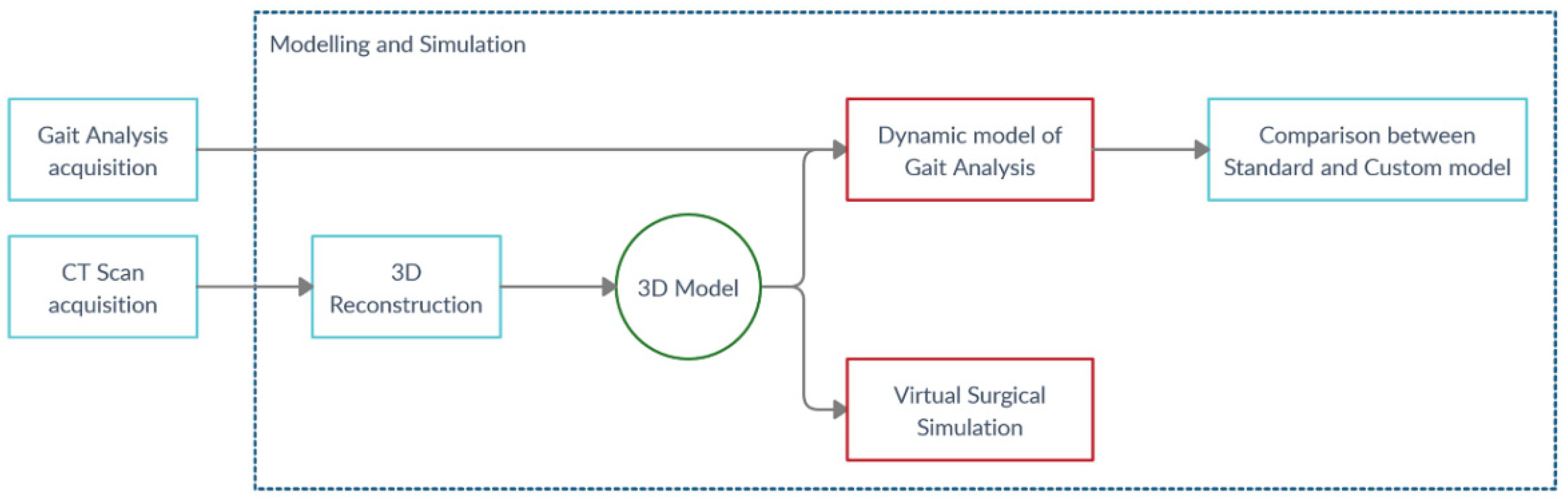
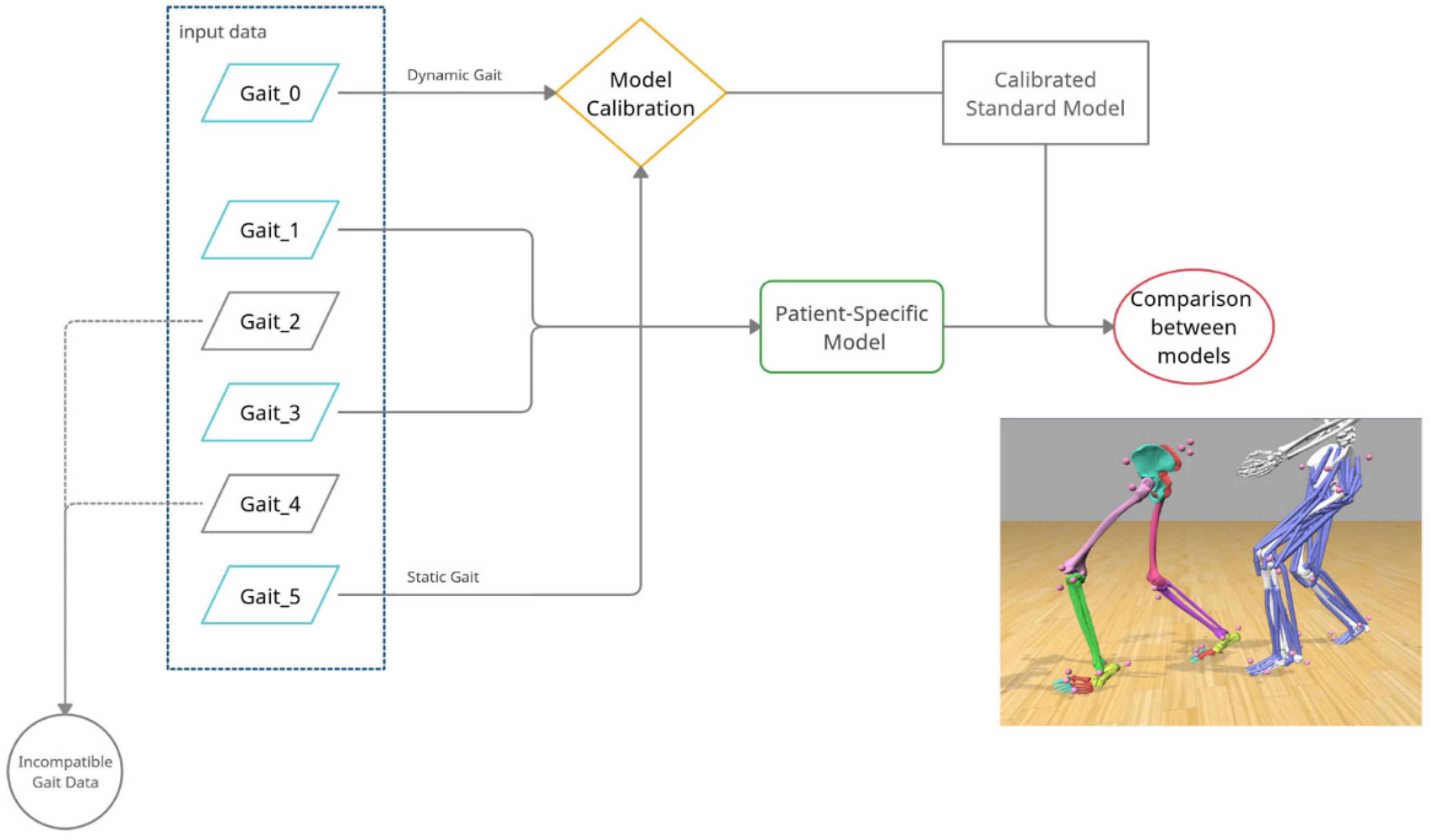
2.3. Modelling and Simulation Workflow
3. Results
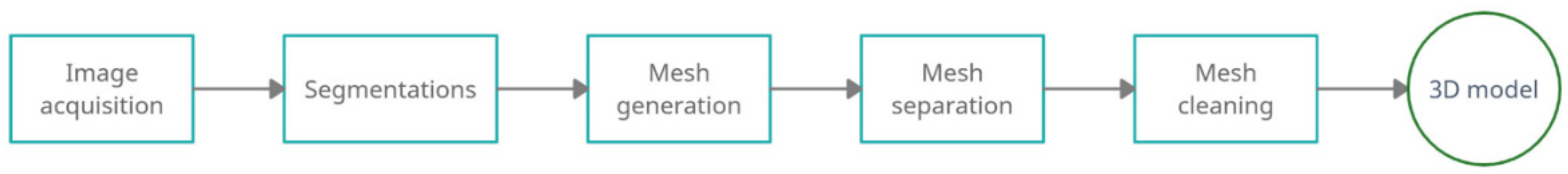
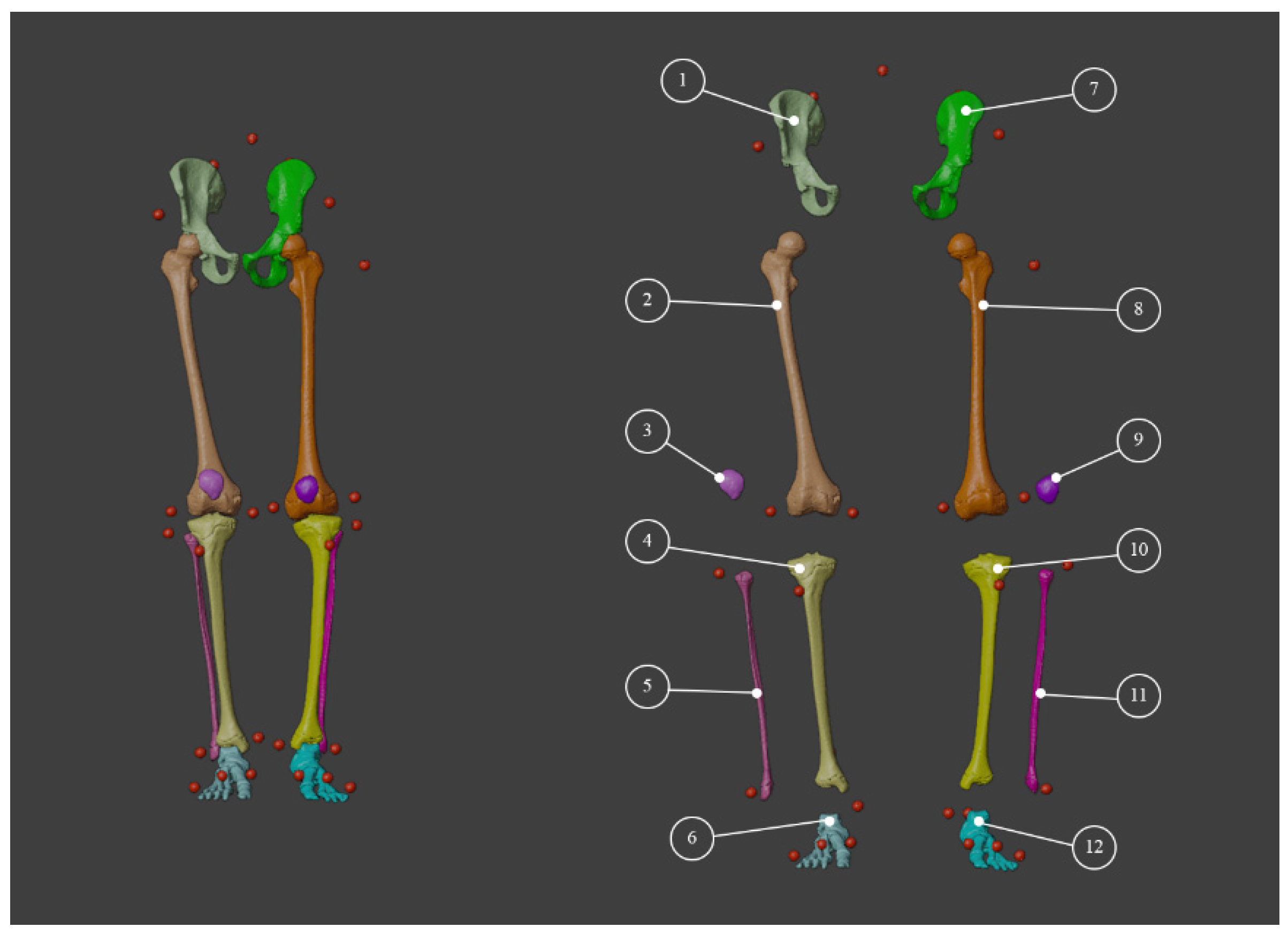
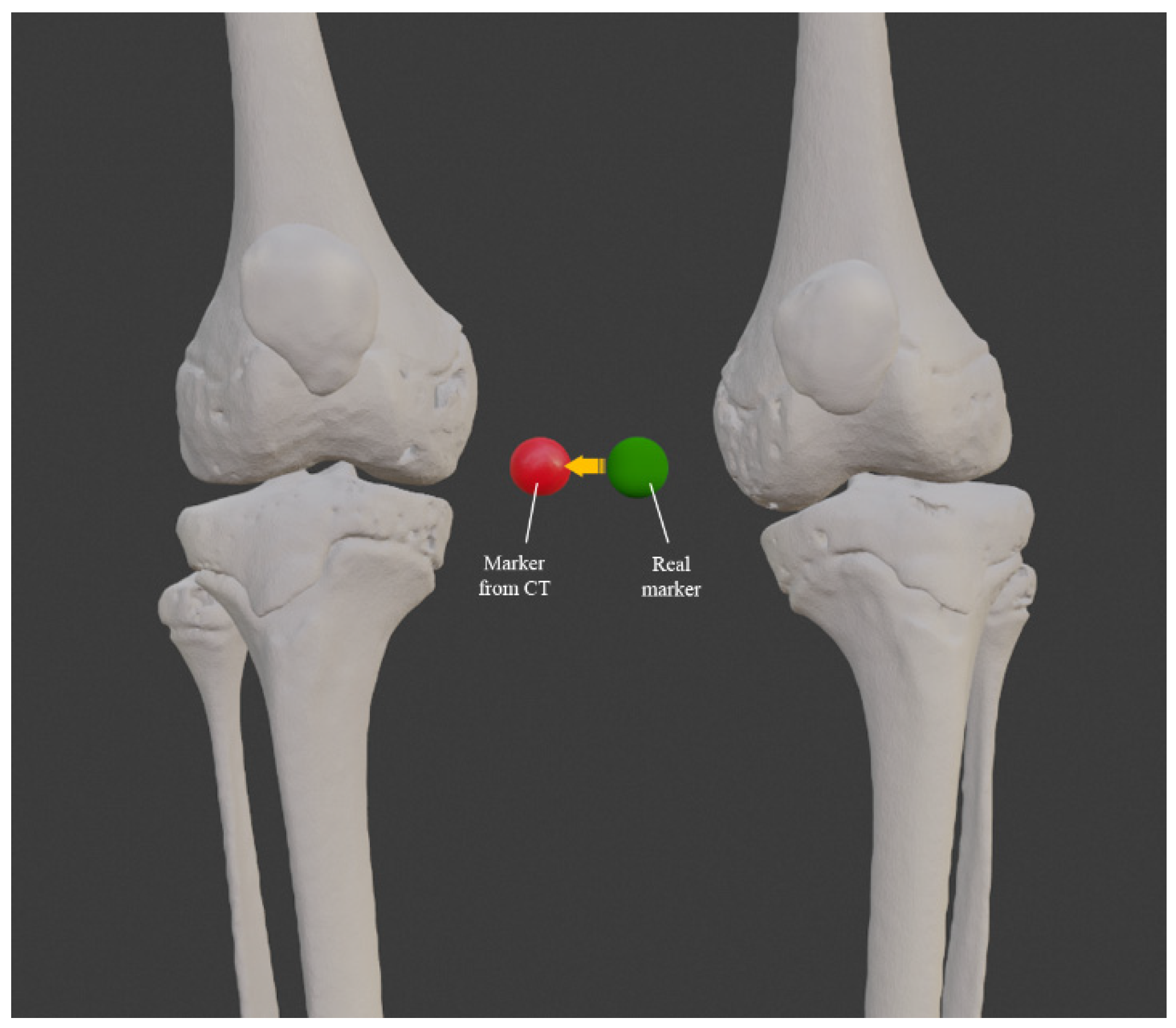
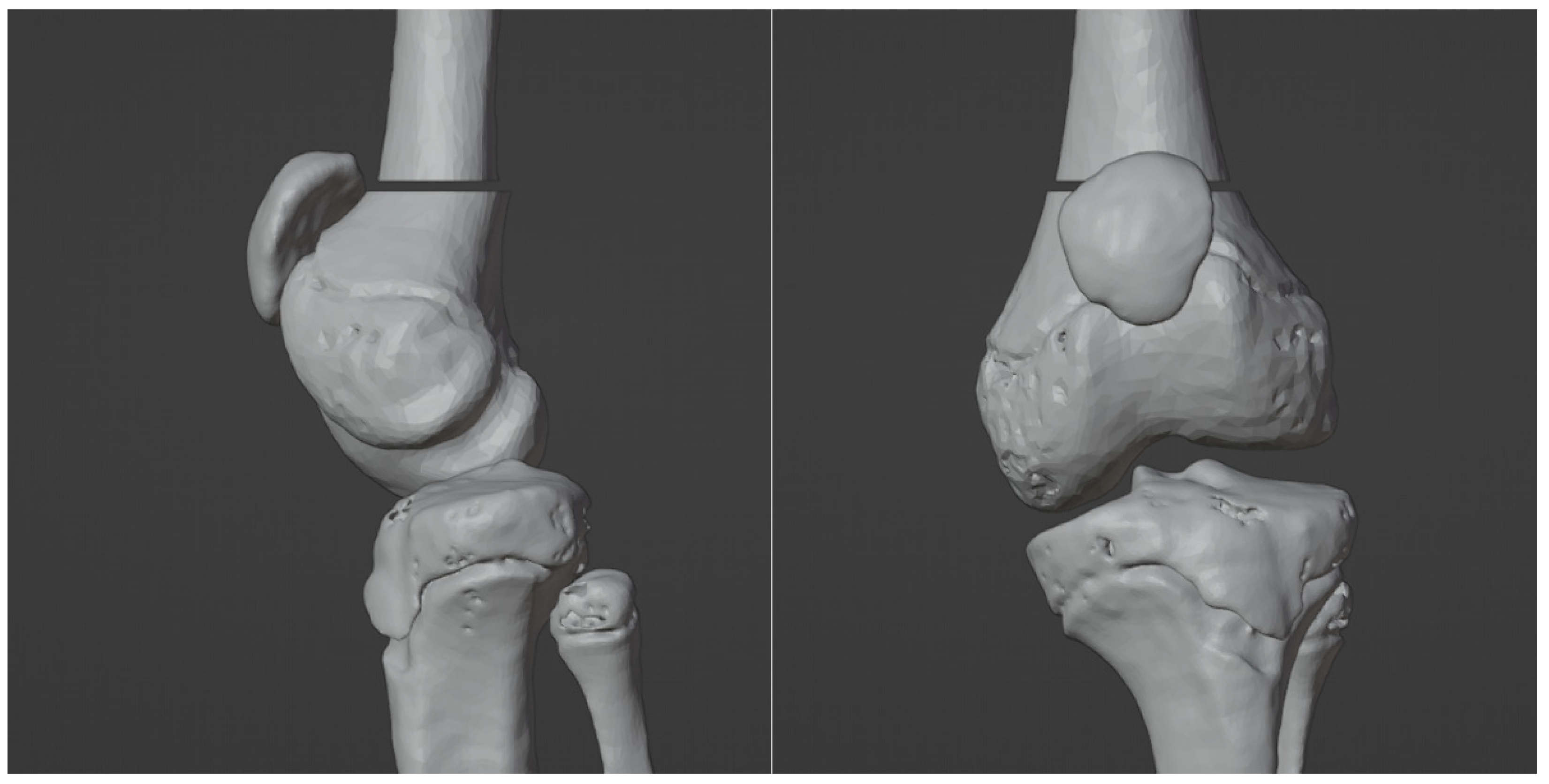
3.1. 3D Reconstruction of Patient-Specific CT Scan
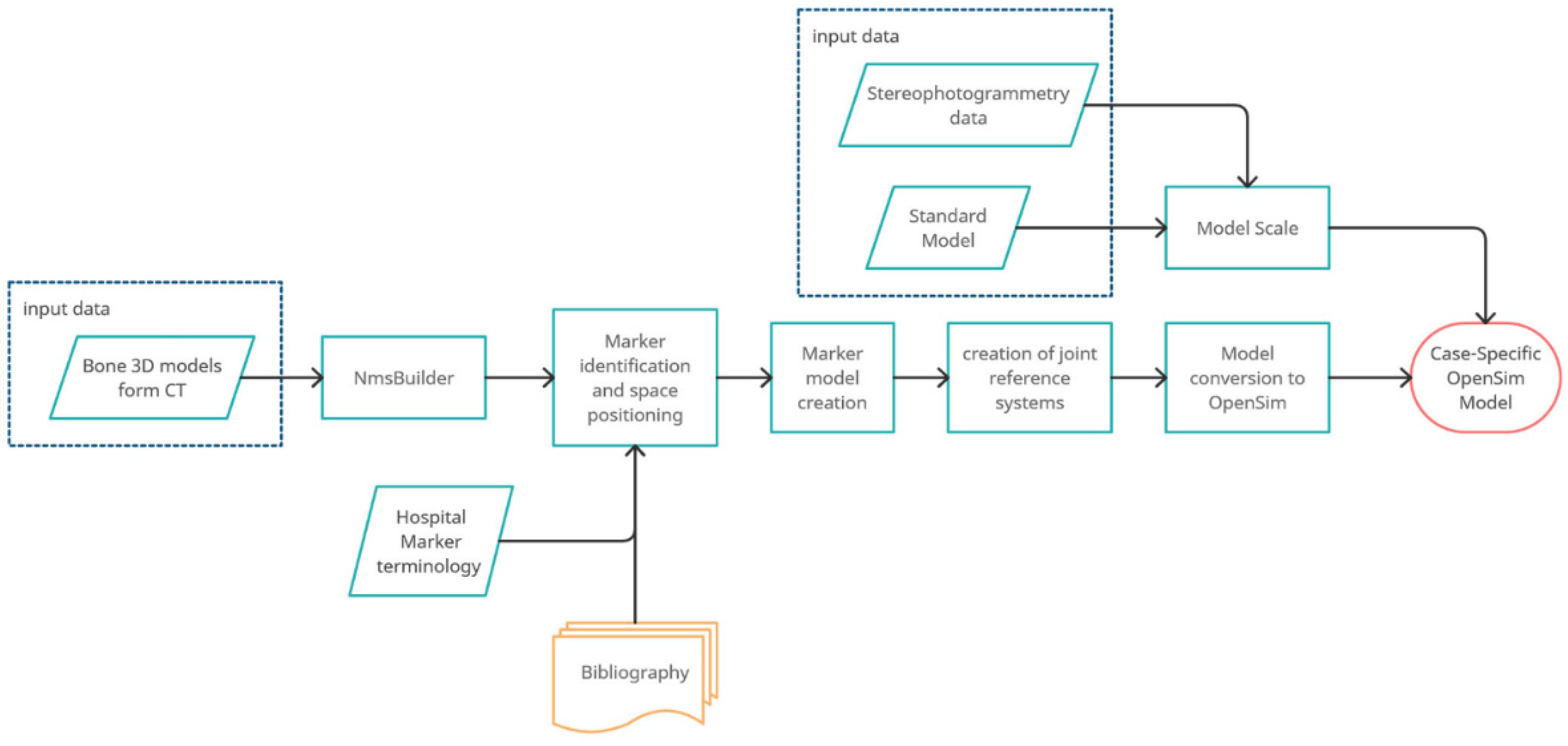
3.2. Dynamic Model of Patient-Specific Gait Analysis
- Importation of the model in “.stl” format (including the markers) into the NmsBuilder;
- Identification of the names of the markers associated with the stereophotogrammetry files and their position in the model;
- Creation of the necessary anatomical markers directly suggested by the software;
- Use of the newly created markers to create the reference systems for the joints:
- Creation of the OpenSim model.
3.3. Comparison between Standard and Custom Model
- Total Square Error (TSE)
- Root Mean Square Error (RMSE)
- Maximum Error (ME).
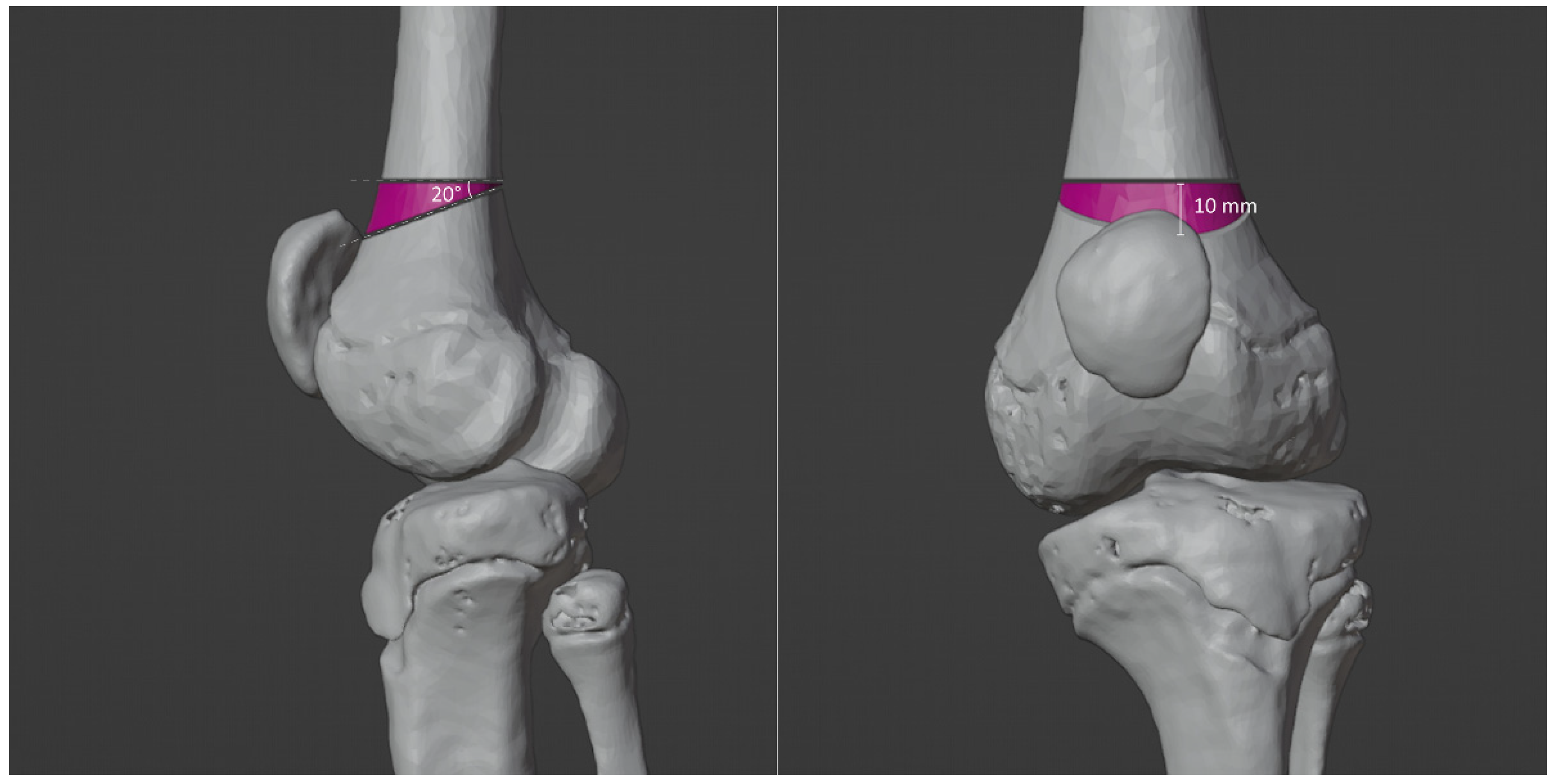
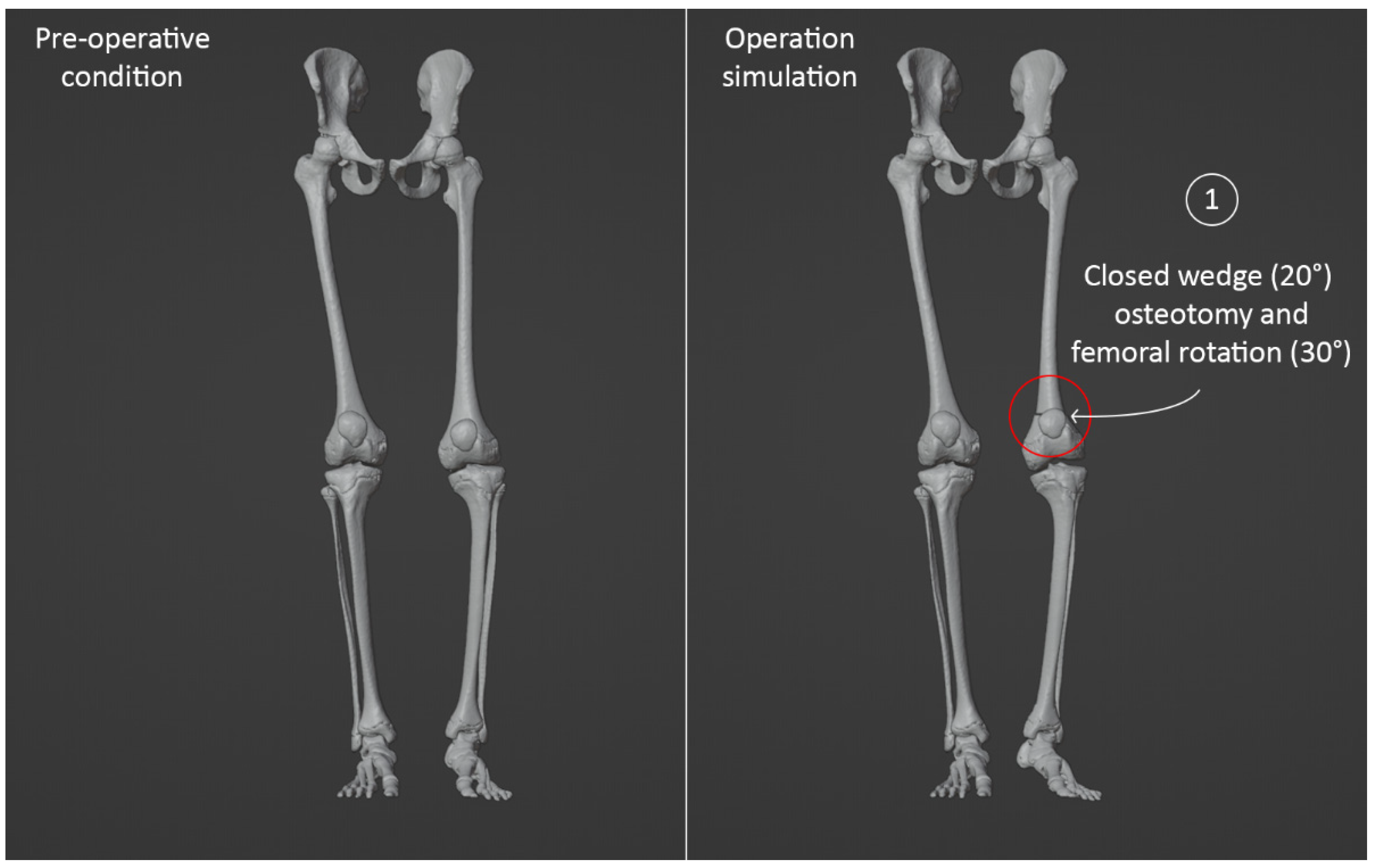
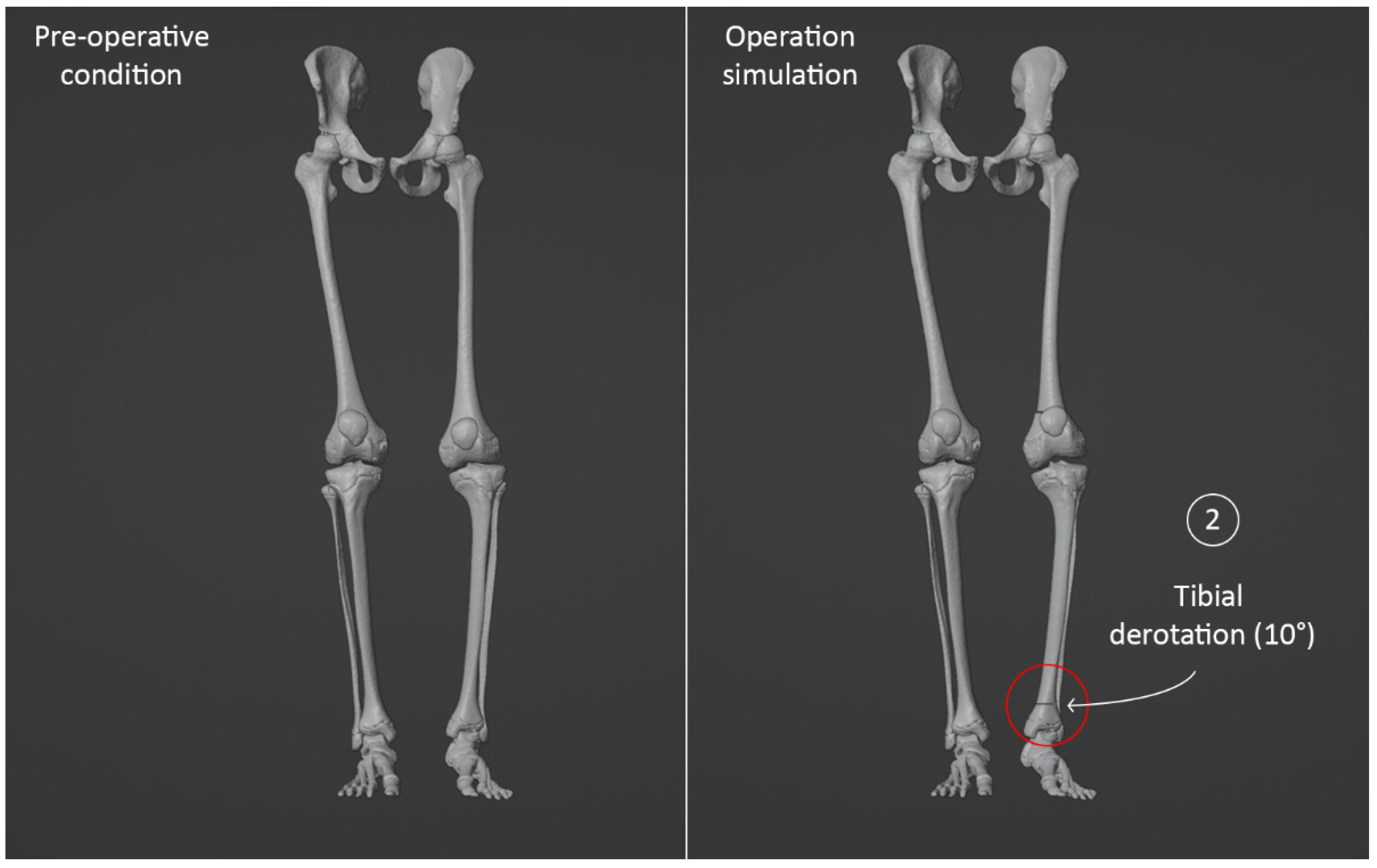
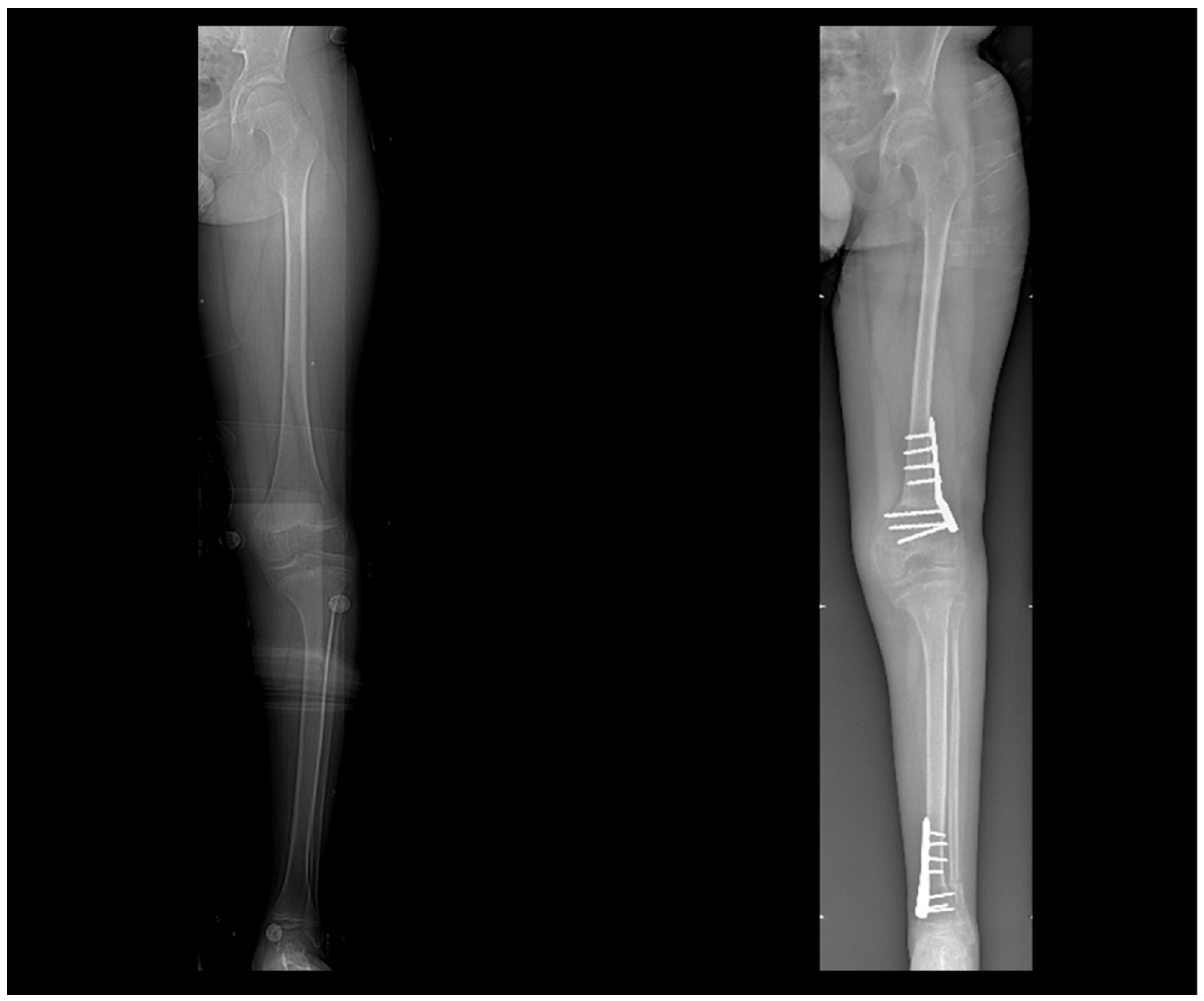
3.4. Virtual Surgical Simulation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, H.Y.; Cha, Y.H.; Chun, Y.S.; Shin, H.S. Correlation of the Torsion Values Measured by Rotational Profile, Kinematics, and CT Study in CP Patients. Gait Posture 2017, 57, 241–245. [Google Scholar] [CrossRef]
- O’Sullivan, R.; Walsh, M.; Jenkinson, A.; O’Brien, T. Factors Associated with Pelvic Retraction during Gait in Cerebral Palsy. Gait Posture 2007, 25, 425–431. [Google Scholar] [CrossRef]
- Chang, W.N.; Tsirikos, A.I.; Miller, F.; Schuyler, J.; Glutting, J. Impact of Changing Foot Progression Angle on Foot Pressure Measurement in Children with Neuromuscular Diseases. Gait Posture 2004, 20, 14–19. [Google Scholar] [CrossRef]
- Thielen, M.; Wolf, S.I.; Klotz, M.C.M.; Geisbüsch, A.; Putz, C.; Krautwurst, B.; Dreher, T. Supracondylar Femoral Rotation Osteotomy Affects Frontal Hip Kinetics in Children with Bilateral Cerebral Palsy. Dev. Med. Child Neurol. 2019, 61, 322–328. [Google Scholar] [CrossRef]
- Amirmudin, N.A.; Lavelle, G.; Theologis, T.; Thompson, N.; Ryan, J.M. Multilevel Surgery for Children With Cerebral Palsy: A Meta-analysis. Pediatrics 2019, 143, e20183390. [Google Scholar] [CrossRef]
- Lamberts, R.P.; Burger, M.; Du Toit, J.; Langerak, N.G. A Systematic Review of the Effects of Single-Event Multilevel Surgery on Gait Parameters in Children with Spastic Cerebral Palsy. PLoS ONE 2016, 11, e0164686. [Google Scholar] [CrossRef]
- McGinley, J.L.; Dobson, F.; Ganeshalingam, R.; Shore, B.J.; Rutz, E.; Graham, H.K. Single-Event Multilevel Surgery for Children with Cerebral Palsy: A Systematic Review. Dev. Med. Child Neurol. 2012, 54, 117–128. [Google Scholar] [CrossRef]
- Novak, I.; Mcintyre, S.; Morgan, C.; Campbell, L.; Dark, L.; Morton, N.; Stumbles, E.; Wilson, S.A.; Goldsmith, S. A Systematic Review of Interventions for Children with Cerebral Palsy: State of the Evidence. Dev. Med. Child Neurol. 2013, 55, 885–910. [Google Scholar] [CrossRef]
- Sung, K.H.; Kwon, S.S.; Chung, C.Y.; Lee, K.M.; Cho, G.H.; Park, M.S. Long-Term Outcomes over 10 Years after Femoral Derotation Osteotomy in Ambulatory Children with Cerebral Palsy. Gait Posture 2018, 64, 119–125. [Google Scholar] [CrossRef]
- McCarthy, J.; Shrader, M.W.; Graham, K.; Veerkamp, M.; Brower, L.; Chambers, H.; Davids, J.R.; Kay, R.M.; Narayanan, U.; Novacheck, T.F.; et al. Establishing Surgical Indications for Hamstring Lengthening and Femoral Derotational Osteotomy in Ambulatory Children with Cerebral Palsy. J. Child. Orthop. 2020, 14, 50–57. [Google Scholar] [CrossRef]
- Wren, T.A.L.; Lening, C.; Rethlefsen, S.A.; Kay, R.M. Impact of Gait Analysis on Correction of Excessive Hip Internal Rotation in Ambulatory Children with Cerebral Palsy: A Randomized Controlled Trial. Dev. Med. Child Neurol. 2013, 55, 919–925. [Google Scholar] [CrossRef]
- Dugan, E.L.; Shilt, J.S. The Role of Motion Analysis in Surgical Planning for Gait Abnormalities in Cerebral Palsy. Phys. Med. Rehabil. Clin. N. Am. 2020, 31, 107–115. [Google Scholar] [CrossRef]
- Benedetti, M.G.; Beghi, E.; De Tanti, A.; Cappozzo, A.; Basaglia, N.; Cutti, A.G.; Cereatti, A.; Stagni, R.; Verdini, F.; Manca, M.; et al. SIAMOC Position Paper on Gait Analysis in Clinical Practice: General Requirements, Methods and Appropriateness. Results of an Italian Consensus Conference. Gait Posture 2017, 58, 252–260. [Google Scholar] [CrossRef]
- Baker, R.; Esquenazi, A.; Benedetti, M.G.; Desloovere, K. Gait Analysis: Clinical Facts. Eur. J. Phys. Rehabil. Med. 2016, 52, 560–574. [Google Scholar]
- Klöpfer-Krämer, I.; Brand, A.; Wackerle, H.; Müßig, J.; Kröger, I.; Augat, P. Gait Analysis—Available Platforms for Outcome Assessment. Injury 2020, 51, S90–S96. [Google Scholar] [CrossRef]
- Massaad, A.; Assi, A.; Bakouny, Z.; Sauret, C.; Khalil, N.; Skalli, W.; Ghanem, I. Three-Dimensional Evaluation of Skeletal Deformities of the Pelvis and Lower Limbs in Ambulant Children with Cerebral Palsy. Gait Posture 2016, 49, 102–107. [Google Scholar] [CrossRef]
- Lofterød, B.; Terjesen, T.; Skaaret, I.; Huse, A.B.; Jahnsen, R. Preoperative Gait Analysis Has a Substantial Effect on Orthopedic Decision Making in Children with Cerebral Palsy: Comparison between Clinical Evaluation and Gait Analysis in 60 Patients. Acta Orthop. 2007, 78, 74–80. [Google Scholar] [CrossRef]
- Ferrari, A.; Brunner, R.; Faccioli, S.; Reverberi, S.; Benedetti, M.G. Gait Analysis Contribution to Problems Identification and Surgical Planning in CP Patients: An Agreement Study. Eur. J. Phys. Rehabil. Med. 2015, 51, 39–48. [Google Scholar]
- Sees, J.P.; Truong, W.H.; Novacheck, T.F.; Miller, F.; Georgiadis, A.G. What’s New in the Orthopaedic Treatment of Ambulatory Children with Cerebral Palsy Using Gait Analysis. J. Pediatr. Orthop. 2020, 40, E498–E503. [Google Scholar] [CrossRef]
- Khouri, N.; Desailly, E. Contribution of Clinical Gait Analysis to Single-Event Multi-Level Surgery in Children with Cerebral Palsy. Orthop. Traumatol. Surg. Res. 2017, 103, S105–S111. [Google Scholar] [CrossRef]
- Carty, C.P.; Walsh, H.P.J.; Gillett, J.G.; Phillips, T.; Edwards, J.M.; deLacy, M.; Boyd, R.N. The Effect of Femoral Derotation Osteotomy on Transverse Plane Hip and Pelvic Kinematics in Children with Cerebral Palsy: A Systematic Review and Meta-Analysis. Gait Posture 2014, 40, 333–340. [Google Scholar] [CrossRef]
- Westberry, D.E.; Wack, L.I.; Davis, R.B.; Hardin, J.W. Femoral Anteversion Assessment: Comparison of Physical Examination, Gait Analysis, and EOS Biplanar Radiography. Gait Posture 2018, 62, 285–290. [Google Scholar] [CrossRef]
- Bosmans, L.; Jansen, K.; Wesseling, M.; Molenaers, G.; Scheys, L.; Jonkers, I. The Role of Altered Proximal Femoral Geometry in Impaired Pelvis Stability and Hip Control during CP Gait: A Simulation Study. Gait Posture 2016, 44, 61–67. [Google Scholar] [CrossRef]
- Scheys, L.; Desloovere, K.; Suetens, P.; Jonkers, I. Level of Subject-Specific Detail in Musculoskeletal Models Affects Hip Moment Arm Length Calculation during Gait in Pediatric Subjects with Increased Femoral Anteversion. J. Biomech. 2011, 44, 1346–1353. [Google Scholar] [CrossRef]
- Scheys, L.; Desloovere, K.; Spaepen, A.; Suetens, P.; Jonkers, I. Calculating Gait Kinematics Using MR-Based Kinematic Models. Gait Posture 2011, 33, 158–164. [Google Scholar] [CrossRef]
- Scheys, L.; Van Campenhout, A.; Spaepen, A.; Suetens, P.; Jonkers, I. Personalized MR-Based Musculoskeletal Models Compared to Rescaled Generic Models in the Presence of Increased Femoral Anteversion: Effect on Hip Moment Arm Lengths. Gait Posture 2008, 28, 358–365. [Google Scholar] [CrossRef]
- Pitto, L.; Kainz, H.; Falisse, A.; Wesseling, M.; Van Rossom, S.; Hoang, H.; Papageorgiou, E.; Hallemans, A.; Desloovere, K.; Molenaers, G.; et al. SimCP: A Simulation Platform to Predict Gait Performance Following Orthopedic Intervention in Children With Cerebral Palsy. Front. Neurorobot. 2019, 13, 54. [Google Scholar] [CrossRef]
- Galarraga, O.C.; Vigneron, V.; Khouri, N.; Dorizzi, B.; Desailly, E. Predictive Simulation of Surgery Effect on Cerebral Palsy Gait. Comput. Methods Biomech. Biomed. Engin. 2017, 20, 85–86. [Google Scholar] [CrossRef][Green Version]
- Frizziero, L.; Santi, G.M.; Liverani, A.; Napolitano, F.; Papaleo, P.; Maredi, E.; Di Gennaro, G.L.; Zarantonello, P.; Stallone, S.; Stilli, S.; et al. Computer-Aided Surgical Simulation for Correcting Complex Limb Deformities in Children. Appl. Sci. 2020, 10, 5181. [Google Scholar] [CrossRef]
- Rajagopal, A.; Dembia, C.L.; DeMers, M.S.; Delp, D.D.; Hicks, J.L.; Delp, S.L. Full-Body Musculoskeletal Model for Muscle-Driven Simulation of Human Gait. IEEE Trans. Biomed. Eng. 2016, 63, 2068–2079. [Google Scholar] [CrossRef]
- Leardini, A.; Sawacha, Z.; Paolini, G.; Ingrosso, S.; Nativo, R.; Benedetti, M.G. A New Anatomically Based Protocol for Gait Analysis in Children. Gait Posture 2007, 26, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Viceconti, M.; Taddei, F.; Montanari, L.; Testi, D.; Leardini, A.; Clapworthy, G.; Van Sint Jan, S. Multimod Data Manager: A Tool for Data Fusion. Comput. Methods Programs Biomed. 2007, 87, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Sholukha, V.; Leardini, A.; Salvia, P.; Rooze, M.; Van Sint Jan, S. Double-Step Registration of in Vivo Stereophotogrammetry with Both in Vitro 6-DOFs Electrogoniometry and CT Medical Imaging. J. Biomech. 2006, 39, 2087–2095. [Google Scholar] [CrossRef] [PubMed]
- Thibault, J.B. Veo CT model-based iterative reconstruction. GE Healthcare Chalfont St. Giles. 2010, 1–10. [Google Scholar]
- Chiesa, A.M.; Spinnato, P.; Miceli, M.; Facchini, G. Radiologic Assessment of Osteosarcoma Lung Metastases: State of the Art and Recent Advances. Cells 2021, 10, 553. [Google Scholar] [CrossRef]
- Osti, F.; Santi, G.M.; Neri, M.; Liverani, A.; Frizziero, L.; Stilli, S.; Maredi, E.; Zarantonello, P.; Gallone, G.; Stallone, S.; et al. CT Conversion Workflow for Intraoperative Usage of Bony Models: From DICOM Data to 3D Printed Models. Appl. Sci. 2019, 9, 708. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an Image Computing Platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef]
- Blender.com Blender.Org—Home of the Blender Project—Free and Open 3D Creation Software. Available online: https://www.blender.org/ (accessed on 2 August 2022).
- MeshLab. Available online: https://www.meshlab.net/ (accessed on 2 August 2022).
- Napolitano, F.; Frizziero, L.; Santi, G.M.; Donnici, G.; Liverani, A.; Papaleo, P.; Giuseppetti, V. Description of the Cad-Am Process for 3d Bone Printing: The Case Study of a Flat Foot. In Proceedings of the 5th NA International Conference on Industrial Engineering and Operations, Detroit, MI, USA, 10–14 August 2020. [Google Scholar]
- Valente, G.; Crimi, G.; Vanella, N.; Schileo, E.; Taddei, F. NMSBUILDER: Freeware to Create Subject-Specific Musculoskeletal Models for OpenSim. Comput. Methods Programs Biomed. 2017, 152, 85–92. [Google Scholar] [CrossRef]
- Bakhshayesh, P.; Sandberg, O.; Kumar, V.; Ali, A.; Enocson, A. Volume Fusion of CT Images to Measure Femoral Symmetricity. Surg. Radiol. Anat. 2020, 42, 635–639. [Google Scholar] [CrossRef]
- Zeng, C.; Xing, W.; Wu, Z.; Huang, H.; Huang, W. A Combination of Three-Dimensional Printing and Computer-Assisted Virtual Surgical Procedure for Preoperative Planning of Acetabular Fracture Reduction. Injury 2016, 47, 2223–2227. [Google Scholar] [CrossRef]
- Shen, S.; Wang, P.Z.; Li, X.Y.; Han, X.; Tan, H.L. Pre-Operative Simulation Using a Three-Dimensional Printing Model for Surgical Treatment of Old and Complex Tibial Plateau Fractures. Sci. Rep. 2020, 10, 6044. [Google Scholar] [CrossRef]
- Frizziero, L.; Santi, G.M.; Liverani, A.; Giuseppetti, V.; Trisolino, G.; Maredi, E.; Stilli, S. Paediatric Orthopaedic Surgery with 3D Printing: Improvements and Cost Reduction. Symmetry 2019, 11, 1317. [Google Scholar] [CrossRef]
- Ravera, E.P.; Crespo, M.J.; Catalfamo Formento, P.A. A Subject-Specific Integrative Biomechanical Framework of the Pelvis for Gait Analysis. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2018, 232, 1083–1097. [Google Scholar] [CrossRef]
- Alrehily, F.; Hogg, P.; Twiste, M.; Johansen, S.; Tootell, A. Scoliosis Imaging: An Analysis of Radiation Risk in the CT Scan Projection Radiograph and a Comparison with Projection Radiography and EOS. Radiography 2019, 25, e68–e74. [Google Scholar] [CrossRef]
- Rosskopf, A.B.; Ramseier, L.E.; Sutter, R.; Pfirrmann, C.W.A.; Buck, F.M. Femoral and Tibial Torsion Measurement in Children and Adolescents: Comparison of 3D Models Based on Low-Dose Biplanar Radiography and Low-Dose CT. Am. J. Roentgenol. 2014, 202, W285–W291. [Google Scholar] [CrossRef]
- Escott, B.G.; Ravi, B.; Weathermon, A.C.; Acharya, J.; Gordon, C.L.; Babyn, P.S.; Kelley, S.P.; Narayanan, U. EOS Low-Dose Radiography: A Reliable and Accurate Upright Assessment of Lower-Limb Lengths. J. Bone Jt. Surg. 2013, 95, e183. [Google Scholar] [CrossRef]
- Altahawi, F.; Pierce, J.; Aslan, M.; Li, X.; Winalski, C.S.; Subhas, N. 3D MRI of the Knee. Semin. Musculoskelet. Radiol. 2021, 25, 455–467. [Google Scholar] [CrossRef]
- MSMS Software Downloads—USC Viterbi|Department of Biomedical Engineering. Available online: https://bme.usc.edu/msms-software-downloads/ (accessed on 2 August 2022).
- Cleather, D.J.; Bull, A.M.J. The Development of a Segment-Based Musculoskeletal Model of the Lower Limb: Introducing FREEBODY. R. Soc. Open Sci. 2015, 2, 140449. [Google Scholar] [CrossRef]

| Gait 1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Standard Model | Patient-Specific Model | Comparison | ||||||
| AVERAGE (mm) | MAX (mm) | STANDARD DEV (mm) | AVERAGE (mm) | MAX (mm) | STANDARD DEV (mm) | VARIATION MAX ERROR (%) | VARIATION STD DEV (%) | |
| TSE | 5.2 | 9.7 | 1.6 | 5.6 | 7.1 | 0.6 | 26.6 | 59.2 |
| RMSE | 14.0 | 19.3 | 2.1 | 15.0 | 16.9 | 0.8 | 12.6 | 58.9 |
| Max Error | 30.6 | 41.8 | 5.4 | 27.8 | 33.7 | 2.1 | 19.3 | 61.1 |
| Gait 3 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Standard Model | Patient-Specific Model | Comparison | ||||||
| AVERAGE (mm) | MAX (mm) | STANDARD DEV (mm) | AVERAGE (mm) | MAX (mm) | STANDARD DEV (mm) | VARIATION MAX ERROR (%) | VARIATION STD DEV (%) | |
| TSE | 4.9 | 9.1 | 1.4 | 5.9 | 7.6 | 0.8 | 16.9 | 43.4 |
| RMS | 13.6 | 18.7 | 1.8 | 15.3 | 17.4 | 1.0 | 7.0 | 45.3 |
| Max Error | 28.4 | 40.3 | 5.6 | 28.6 | 34.0 | 2.0 | 15.4 | 64.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frizziero, L.; Trisolino, G.; Santi, G.M.; Alessandri, G.; Agazzani, S.; Liverani, A.; Menozzi, G.C.; Di Gennaro, G.L.; Farella, G.M.G.; Abbruzzese, A.; et al. Computer-Aided Surgical Simulation through Digital Dynamic 3D Skeletal Segments for Correcting Torsional Deformities of the Lower Limbs in Children with Cerebral Palsy. Appl. Sci. 2022, 12, 7918. https://doi.org/10.3390/app12157918
Frizziero L, Trisolino G, Santi GM, Alessandri G, Agazzani S, Liverani A, Menozzi GC, Di Gennaro GL, Farella GMG, Abbruzzese A, et al. Computer-Aided Surgical Simulation through Digital Dynamic 3D Skeletal Segments for Correcting Torsional Deformities of the Lower Limbs in Children with Cerebral Palsy. Applied Sciences. 2022; 12(15):7918. https://doi.org/10.3390/app12157918
Chicago/Turabian StyleFrizziero, Leonardo, Giovanni Trisolino, Gian Maria Santi, Giulia Alessandri, Simone Agazzani, Alfredo Liverani, Grazia Chiara Menozzi, Giovanni Luigi Di Gennaro, Giuseppina Maria Grazia Farella, Alida Abbruzzese, and et al. 2022. "Computer-Aided Surgical Simulation through Digital Dynamic 3D Skeletal Segments for Correcting Torsional Deformities of the Lower Limbs in Children with Cerebral Palsy" Applied Sciences 12, no. 15: 7918. https://doi.org/10.3390/app12157918
APA StyleFrizziero, L., Trisolino, G., Santi, G. M., Alessandri, G., Agazzani, S., Liverani, A., Menozzi, G. C., Di Gennaro, G. L., Farella, G. M. G., Abbruzzese, A., Spinnato, P., Berti, L., & Benedetti, M. G. (2022). Computer-Aided Surgical Simulation through Digital Dynamic 3D Skeletal Segments for Correcting Torsional Deformities of the Lower Limbs in Children with Cerebral Palsy. Applied Sciences, 12(15), 7918. https://doi.org/10.3390/app12157918










