The Potential of Trigona spp. Propolis as an Antioxidant Agent to Reduce Residual Peroxide after Intra-Coronal Bleaching Treatments
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Extraction
2.2. Tooth Staining Process
2.3. Intra-Coronal Bleaching Treatment and Dissolved Oxygen Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Setzer, F. Bleaching procedures. In Cohen’s Pathways of the Pulp, 11th ed.; Hargreaves, K.M., Berman, L.H., Eds.; Elsevier: St. Louis, MO, USA, 2016; pp. e96–e109. [Google Scholar]
- Ertürk-Avunduk, A.e.T.b.; Aksu, S.; Delikan, E. The Effects of Mouthwashes on the Color Stability of Resin-Based Restorative Materials. Odovtos Int. J. Dent. Sci. 2021, 23, 91–102. [Google Scholar] [CrossRef]
- Alazmah, A. Primary Teeth Stains and Discoloration: A Review. J. Child Sci. 2021, 11, 8. [Google Scholar] [CrossRef]
- Behl, M.; Patnana, A.K.; Khanna, V.; Chaudhry, K. Evaluation of Three Different Bleaching Agents in Permanent and Primary Teeth: An In Vitro Study. Int. J. Clin. Pediatr. Dent. 2020, 13, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Grossman, L.I. Bleaching of discolored teeth. In Grossman’s Endodontic Practice, 13th ed.; Chandra, S.B., Gopikrishna, V., Eds.; Wolters Kluwer: Gurgaon, India, 2014; pp. 499–519. [Google Scholar]
- Rotstein, I.; Li, Y. Tooth discoloration and bleaching. In Ingle’s Endodontics, 6th ed.; Ingle, J.I., Bakland, L.K., Baumgartner, J.G., Eds.; BC Decker Inc: Hamilton, New Zealand, 2008; pp. 1383–1399. [Google Scholar]
- Rotstein, I.; Walton, R.E. Bleaching discoloroed teeth. In Endodontics Principles and Practice, 5th ed.; Totabinejad, M., Walton, R.E., Fouad, A.F., Eds.; Elsevier: St. Louis, MO, USA, 2015; pp. 428–440. [Google Scholar]
- Greenwall, L.; Freedman, G.A.; Gordan, V.V.; Haywood, V.B.; kelleher, M.; McLaughlin, G.; Rotstein, I. Bleaching Techniques in Restorative Dentistry; Martin Dunitz: London, UK, 2001. [Google Scholar]
- Kansal, S.; Jindal, L.; Garg, K.; Thakur, K.; Mehta, S.; Pachori, H. Discoloration of Teeth: A Literature Review. Int. J. Health Clin. Res. 2020, 3, 58–62. [Google Scholar]
- Plotino, G.; Buono, L.; Grande, N.M.; Pameijer, C.H.; Somma, F. Nonvital tooth bleaching: A review of the literature and clinical procedures. J. Endod. 2008, 34, 394–407. [Google Scholar] [CrossRef]
- Mazilu Moldovan, A.; Popescu, V.; Ionescu, C.V.; Cuc, S.; Craciun, A.; Moldovan, M.; Dudea, D.; Mesaros, A.S. Various Aspects Involved in the Study of Tooth Bleaching Procedure: A Questionnaire-Based Study. Int. J. Environ. Res. Public Health 2022, 19, 3977. [Google Scholar] [CrossRef]
- Deshmukh, M.; Ahmed, N. Evaluation of etiology of discoloration, site of discoloured tooth and outcomes of different techniques used for vital tooth bleaching- a retrospective analysis. J. Contemp. Issues Bus. Gov. 2020, 26, 8. [Google Scholar] [CrossRef]
- Goulart, M.d.A.; Condessa, A.M.; Hilgert, J.B.; Hugo, F.N.; Celeste, R.K. Concerns about dental aesthetics are associated with oral health related quality of life in Southern Brazilian adults. Ciência Saúde Coletiva 2018, 23, 8. [Google Scholar] [CrossRef]
- Kahler, B. Present status and future directions—Managing discoloured teeth. Int. Endod. J. 2022, 1–29. [Google Scholar] [CrossRef]
- Şişmanoğlu, S. Bleaching of Nonvital Teeth: A Review. AJ Inst. Allied Health Sci. 2020, 2, 91–114. [Google Scholar]
- Okroj, N.; Michalska, K.; Jakusz, B. Effect of vibration and stirring on 90% and 98% hydrogen peroxide. Mater. Wysokoenergetyczne High Energy Mater. 2018, 10, 88–96. [Google Scholar] [CrossRef]
- Ozelin, A.A.; Guiraldo, R.D.; Carvalho, R.V.; Lopes, M.B.; Berger, S.B. Effects of green tea application time on bond strength after enamel bleaching. Braz. Dent. J. 2014, 25, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Baia, J.C.P.; Oliveira, R.P.; Ribeiro, M.E.S.; Lima, R.R.; Loretto, S.C.; Silva, E.S.J.M.H. Influence of Prolonged Dental Bleaching on the Adhesive Bond Strength to Enamel Surfaces. Int. J. Dent. 2020, 2020, 2609359. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.C.; Tay, F.R.; Cheung, G.S.; Mak, Y.F.; Carvalho, R.M.; Wei, S.H.; Toledano, M.; Osorio, R.; Pashley, D.H. Reversal of compromised bonding in bleached enamel. J. Dent. Res. 2002, 81, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Percival, M. Antioxidants. Clin. Nutr. Insights 1998, 4, 1–4. [Google Scholar]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.; Sulaiman Rahman, H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef]
- Mason, S.A.; Trewin, A.J.; Parker, L.; Wadley, G.D. Antioxidant supplements and endurance exercise: Current evidence and mechanistic insights. Redox Biol. 2020, 35, 101471. [Google Scholar] [CrossRef]
- Varesi, A.; Chirumbolo, S.; Campagnoli, L.I.M.; Pierella, E.; Piccini, G.B.; Carrara, A.; Ricevuti, G.; Scassellati, C.; Bonvicini, C.; Pascale, A. The Role of Antioxidants in the Interplay between Oxidative Stress and Senescence. Antioxidants 2022, 11, 1224. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxid. Med. Cell. Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef]
- Galeotti, F.; Maccari, F.; Fachini, A.; Volpi, N. Chemical Composition and Antioxidant Activity of Propolis Prepared in Different Forms and in Different Solvents Useful for Finished Products. Foods 2018, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Avula, B.; Sagi, S.; Masoodi, M.; Bae, J.-Y.; Wali, A.; Khan, I. Quantification and characterization of phenolic compounds from northern indian propolis extracts and dietary supplements. J. AOAC Int. 2020, 103, 16. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Rzepecka-Stojko, A.; Górecki, M.; Stojko, J.; Sosada, M.; Swierczek-Zieba, G. Structure and antioxidant activity of polyphenols derived from propolis. Molecules 2013, 19, 78–101. [Google Scholar] [CrossRef] [PubMed]
- Martinello, M.; Mutinelli, F. Antioxidant Activity in Bee Products: A Review. Antioxidants 2021, 10, 71. [Google Scholar] [CrossRef]
- de Oliveira, M.T.; de Andrade, M.A.C.; Michels, M. Oxygen release, microleakage and shear bond strength of composite restorations after home dental bleaching. Rev. Odonto Cienc. 2011, 26, 5. [Google Scholar] [CrossRef]
- El-Seoud, H.K.A.; Ibrahim, M.A.; Hafez, R.M. Proper timing of bonding composite resin to bleached enamel. CDJ 2008, 3, 10. [Google Scholar]
- Soares, D.; Marcomini, N.; Duque, C.; Bordini, E.; Zuta, U.; Basso, F.; Hebling, J.; Costa, C. Increased whitening efficacy and reduced cytotoxicity are achieved by the chemical activation of a highly concentrated hydrogen peroxide bleaching gel. J. Appl. Oral Sci. 2019, 27, 10. [Google Scholar] [CrossRef]
- Attia, R. Reversal of deleterious effect of extracoronal in- office bleaching on shear bond strength of composite resin to bleached enamel: Effect of delayed restoration and different antioxidants application—In vitro study. Egyption Dent. J. 2021, 67, 14. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Nesamani, R.; Sanjeev, K.; Sekar, M. Effect of Bleaching with Strawberry Extract and Hydrogen Peroxide on Colour Change, Surface Morphology and Micro-shear Bond Strength of Resin Composite to Enamel Surface. JCDR 2021, 15, 6. [Google Scholar] [CrossRef]
- Değirmenci, A.; Kara, E.; Ünalan değirmenci, B.; Özcan, M. Evaluation the Effect of Different Antioxidants Applied After Bleaching on Teeth Color Stability. Braz. Dent. Sci. 2020, 23, 1–9. [Google Scholar] [CrossRef]
- Ozakar-Ilday, N.; Karatas, O.; Alt1nok-Uygun, L.; Gul, P.n. The effects of different antioxidant agents on the microtensile bond strength of composite resin to bleached Enamel. Odovtos Int. J. Dent. Sci. 2022, 24, 87–98. [Google Scholar] [CrossRef]
- Ghaleb, M.; Orsini, G.; Putignano, A.; Dabbagh, S.; Haber, G.; Hardan, L. The Effect of Different Bleaching Protocols, Used with and without Sodium Ascorbate, on Bond Strength between Composite and Enamel. Materials 2020, 13, 2710. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.M.G.; Botta, A.C.; Barcellos, D.C.; Pagani, C.; Torres, C.R.G. Effect of antioxidant agents on bond strength of composite to bleached enamel with 38% hydrogen peroxide. Mat. Res. 2011, 14, 8. [Google Scholar] [CrossRef]
- Braz, R.; Patrício, C.E.; Ribeiro, A.I.; Guênes, G.M.; Dantas, D.C.; Montes, M.A.; Feitosa, D.A. Influence of antioxidants on stress of bonding agents in recently whitened teeth. Acta Odontol. Latinoam. 2011, 24, 252–257. [Google Scholar] [PubMed]
- Olmedo, D.; Kury, M.; Resende, B.A.; Cavalli, V. Use of antioxidants to restore bond strength after tooth bleaching with peroxides. Eur. J. Oral Sci. 2021, 129, e12773. [Google Scholar] [CrossRef]
- Silva, A.M.; Jordão-Basso, K.C.F.; Alencar, C.d.M.; Zaniboni, J.F.; Alves-de-Campos, E.; Kuga, M.C. Effect of Sodium Ascorbate or Alpha-Tocopherol on the Resin-Dentin Interface and Bond Strength after Endodontic Treatment and Bleaching. Int. J. Odontostomatol. 2021, 15, 586–594. [Google Scholar] [CrossRef]
- Moosavi, H.; Moghaddas, M.J.; Ghoddusi, J.; Rajabi, O. Effects of two antioxidants on the microleakage of resin-based composite restorations after nonvital bleaching. J. Contemp. Dent. Pract. 2010, 11, E033–E040. [Google Scholar] [CrossRef]
- Hasan, A.E.Z.; Mangunwidjaja, D.; Sunarti, T.C.; Suparno, O.; Setiyono, A. Optimization of propolis extraction using maceration with 70% ethanol solvent with microwave heating and characterization of its properties as antibreast cancer agent. J. Teknol. Ind. Pertan. 2013, 23, 7. [Google Scholar]
- Liben, T.; Atlabachew, M.; Abebe, A. Total phenolic, flavonoids and some selected metal content in honey and propolis samples from South Wolo zone, Amhara region, Ethiopia. Cogent Food Agric. 2018, 4, 1475925. [Google Scholar] [CrossRef]
- Jibril, F.I.; Hilmi, A.B.M.; Manivannan, L. Isolation and characterization of polyphenols in natural honey for the treatment of human diseases. Bull. Natl. Res. Cent. 2019, 43, 4. [Google Scholar] [CrossRef]
- Montoro, A.; Barquinero, J.-F.; Almonacid, M.; Sebastià, N.; Verdú, G.; Sahuquillo, V.; Serrano, J.; Saiz, M.; Villaescusa, I.; Soriano, J.M. Concentration-Dependent Protection by Ethanol Extract of Propolis against γ-Ray-Induced Chromosome Damage in Human Blood Lymphocytes. Evid. -Based Complement. Altern. Med. eCAM 2011, 2011, 174853. [Google Scholar] [CrossRef]
- El Adham, E.K.; Hassan, A.I.; Dawoud, M.M.A. Evaluating the role of propolis and bee venom on the oxidative stress induced by gamma rays in rats. Sci. Rep. 2022, 12, 2656. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, P.; Mahanta, D.; Kaul, A.; Ishida, Y.; Terao, K.; Wadhwa, R.; Kaul, S.C. Experimental Evidence for Therapeutic Potentials of Propolis. Nutrients 2021, 13, 2528. [Google Scholar] [CrossRef] [PubMed]
- Shruthi, E.; Suma, B.S. Health from the hive: Potential uses of propolis in general health. IJCM 2012, 3, 4. [Google Scholar] [CrossRef]
- Yusri, Y.; Trilaksana, A.C.; Rovani, C.A. Antioxidant effectivity to decrease coronal microleakage of composite resin restoration after intra-coronal bleaching. JDMFS 2016, 1, 5. [Google Scholar] [CrossRef][Green Version]
- Karadas, M.; Demirbuga, S. Influence of a short-time antioxidant application on the dentin bond strength after intracoronal bleaching. Microsc. Res. Tech. 2019, 82, 1720–1727. [Google Scholar] [CrossRef]
- Anil, A.; George, L.; Dhanapal, P.; Thomas, P.; Paul, S. Evaluation and comparison of the effect of 10% sodium ascorbate and propolis solution on the microtensile bond strength and resin tag penetration depth of composite after using 35% carbamide peroxide bleaching agent—An in vitro study. Endodontology 2021, 33, 11. [Google Scholar] [CrossRef]
- Parolia, A.; Thomas, M.; Mala, K.; Mohan, M. Propolis and its potential uses in oral health. IJMMS 2010, 2, 210–215. [Google Scholar]
- El-Sohaimy, S.; Masry, S. Phenolic Content, Antioxidant and Antimicrobial Activities of Egyptian and Chinese Propolis. Am. -Eurasian J. Agric. Environ. Sci. 2014, 14, 1116–1124. [Google Scholar] [CrossRef]
- Fidoski, J.; Benedetti, A.; Kirkov, A.; Iliev, A.; Stamatoski, A.; Baftijari, D. Nano-emulsion complex (propolis and vitamin C) promotes wound healing in the oral mucosa. Oral Maxillofac. Pathol. J. 2020, 11, 5. [Google Scholar]
- Ahmed, R.; Tanvir, E.M.; Hossen, M.S.; Afroz, R.; Ahmmed, I.; Rumpa, N.-E.N.; Paul, S.; Gan, S.H.; Sulaiman, S.A.; Khalil, M.I. Antioxidant Properties and Cardioprotective Mechanism of Malaysian Propolis in Rats. Evid. -Based Complement. Altern. Med. 2017, 2017, 5370545. [Google Scholar] [CrossRef] [PubMed]
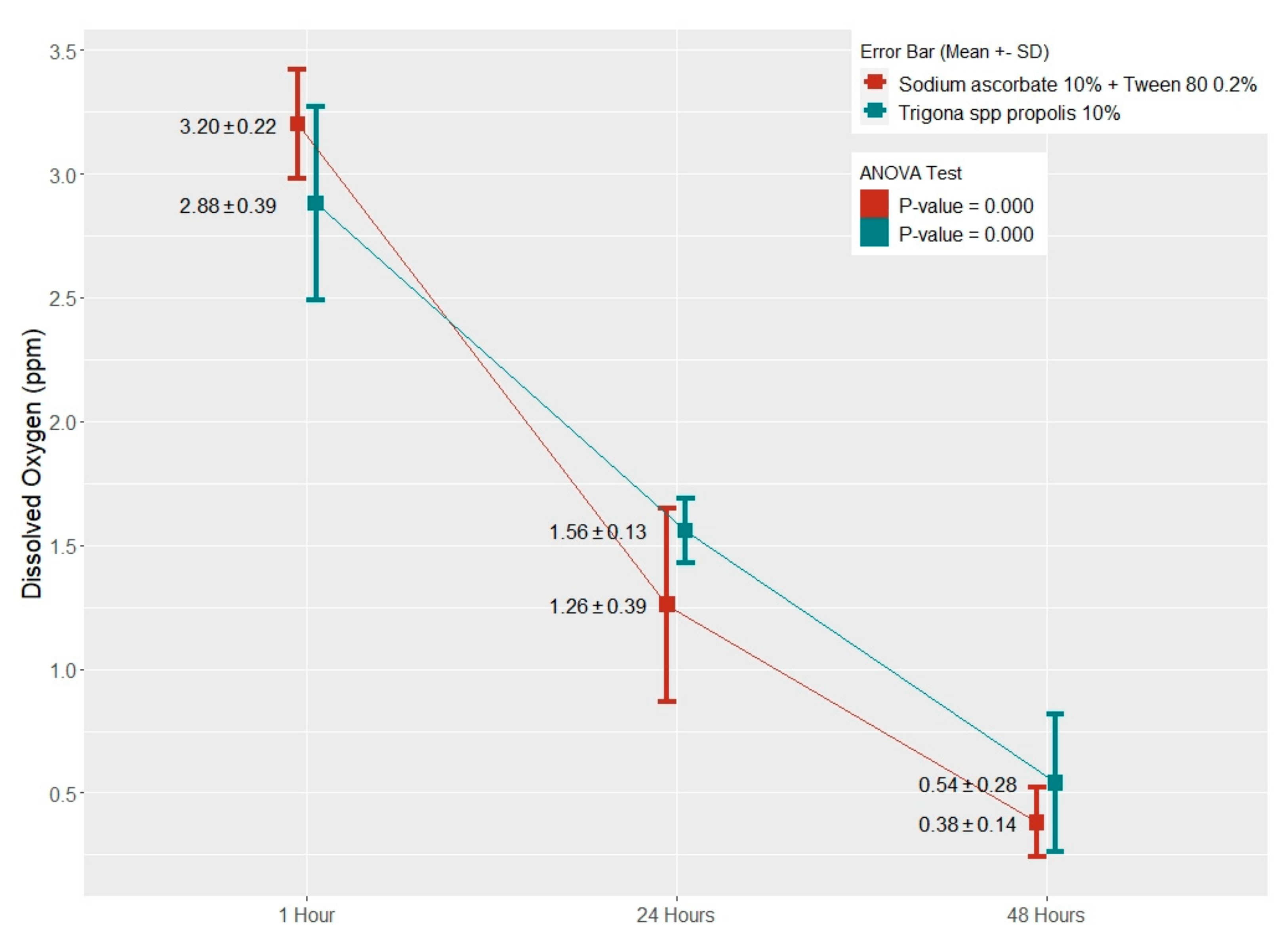
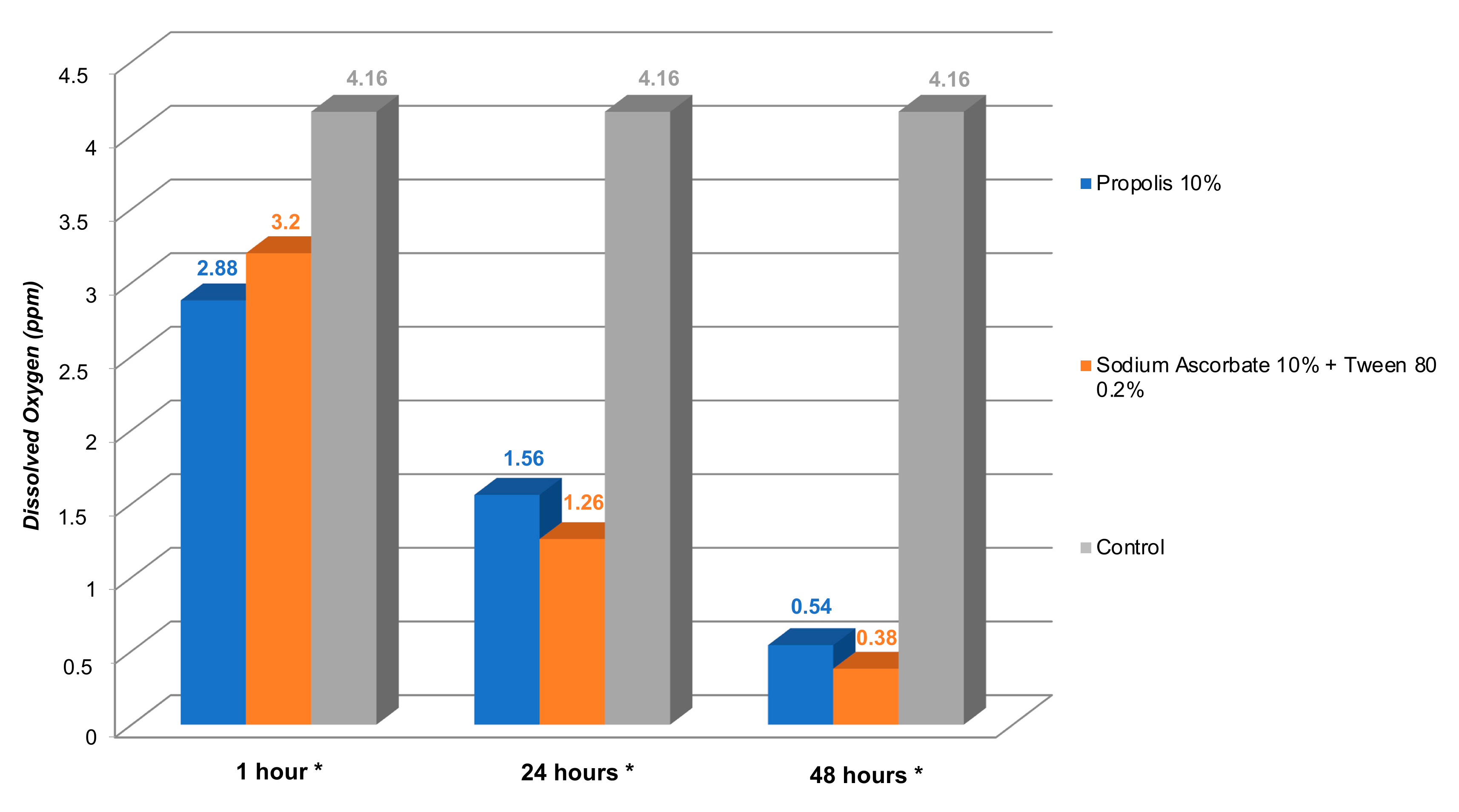
| Antioxidant | The Length of Application Time | Comparison | Mean Difference | 95% CI (Min–Max) | p-Value |
|---|---|---|---|---|---|
| Trigona spp. propolis 10% | 1 h | 24 h | 1.320 | 0.832–1.807 | 0.000 * |
| 48 h | 2.340 | 1.852–2.827 | 0.000 * | ||
| 24 h | 48 h | 1.020 | 0.532–1.507 | 0.000 * | |
| Sodium Ascorbate 10% + Tween 80 0.2% | 1 h | 24 h | 1.940 | 1.478–2.401 | 0.000 * |
| 48 h | 2.816 | 2.354–3.277 | 0.000 * | ||
| 24 h | 48 h | 0.876 | 0.414–1.337 | 0.001 * |
| The Length of Application Time | Antioxidant | Comparison | Mean Difference | 95% CI (Min–Max) | p-Value |
|---|---|---|---|---|---|
| 1 h | Trigona spp. propolis 10% | Sodium Ascorbate 10% + Tween 80 0.2% | −0.320 | −0.98–0.34 | 0.426 |
| Control | −1.280 | −1.94–−0.61 | 0.001 * | ||
| Sodium Ascorbate 10% + Tween 80 0.2% | Control | −0.960 | −1.62–−0.29 | 0.006 * | |
| 24 h | Trigona spp. propolis 10% | Sodium Ascorbate 10% + Tween 80 0.2% | 0.300 | −0.33–0.93 | 0.444 |
| Control | −2.600 | −3.23–−1.96 | 0.000 * | ||
| Sodium Ascorbate 10% + Tween 80 0.2% | Control | −2.900 | −3.53–−2.26 | 0.000 * | |
| 48 h | Trigona spp. propolis 10% | Sodium Ascorbate 10% + Tween 80 0.2% | 0.156 | −0.42–0.73 | 0.758 |
| Control | −3.620 | −4.20–−3.03 | 0.000 * | ||
| Sodium Ascorbate 10% + Tween 80 0.2% | Control | −3.776 | −4.35–−3.19 | 0.000 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trilaksana, A.C.; Syam, S.; Ruslin, M.; Shen, Y.-K. The Potential of Trigona spp. Propolis as an Antioxidant Agent to Reduce Residual Peroxide after Intra-Coronal Bleaching Treatments. Appl. Sci. 2022, 12, 6996. https://doi.org/10.3390/app12146996
Trilaksana AC, Syam S, Ruslin M, Shen Y-K. The Potential of Trigona spp. Propolis as an Antioxidant Agent to Reduce Residual Peroxide after Intra-Coronal Bleaching Treatments. Applied Sciences. 2022; 12(14):6996. https://doi.org/10.3390/app12146996
Chicago/Turabian StyleTrilaksana, Aries Chandra, Syamsiah Syam, Muhammad Ruslin, and Yung-Kang Shen. 2022. "The Potential of Trigona spp. Propolis as an Antioxidant Agent to Reduce Residual Peroxide after Intra-Coronal Bleaching Treatments" Applied Sciences 12, no. 14: 6996. https://doi.org/10.3390/app12146996
APA StyleTrilaksana, A. C., Syam, S., Ruslin, M., & Shen, Y.-K. (2022). The Potential of Trigona spp. Propolis as an Antioxidant Agent to Reduce Residual Peroxide after Intra-Coronal Bleaching Treatments. Applied Sciences, 12(14), 6996. https://doi.org/10.3390/app12146996







