1. Introduction
Among the currently available materials, copper and copper alloys possess interesting physical and chemical properties, such as high conductivity, good strength, ductility, machinability, and durability, with good resistance to oxidation and corrosion. Adding other elements is possible in order to acquire other properties for special applications, such as aerospace, marine, automotive, and others [
1,
2].
Manganese bronze alloy has some designations normative as UNS C67600, CZ115, CW722R, and CuZn40Mn1Pb1FeSn provided in technical standards from copper alloys, for example, ASTM B138, DIN EN 12164-12165-12167, and BS 2872–2874. This alloy has average 60 wt.% of copper, 1 wt.% of lead, 1.5 wt.% of tin, 1.3 wt.% of iron, 0.5 wt.% of manganese, and zinc is the remainder [
1,
3,
4,
5,
6,
7,
8]. These types of copper alloys are applied when the characteristics of high mechanical strength and corrosion resistance are required particularly in under seawater conditions, such as the mechanical components in marine environments, for instance, gate valve stems [
9]. Stems are machined before they become gate valve stems; therefore, the addition of lead to improve the machinability level during the manufacturing process and other additional elements give the alloy special technical features; for instance, iron improves the tensile strength, tin increases the corrosion resistance, and manganese is used to obtain excellent elastic properties [
9,
10].
The increase in the mechanical properties observed after the forming process is because, when metals have their geometric shapes permanently deformed, there is an increase in the density of discordance in the microstructure, which is known as hardening or cold working [
11]. According to ASKELAND and PHULÉ [
12], in metal alloys, plastic deformation occurs by the movement of discordances, and the hardening mechanism is to increase the mechanical resistance of metals through the obstruction of the movement of discordances. During plastic deformation, the discordances change in quantity and make interactions with each other, obtaining high reliefs and groupings that hinder the movement of discordances. To overcome them, it is necessary to use the application of stresses [
11,
13].
Evaluations made by JIANG, BRITTON, and WILKINSON [
14] in a tensile test at room temperature, through high angular resolution–electron back scatter diffraction (HR-EBSD), showed clear increases in the density of the discordances in copper pure oxygen-free (10 ppm max) microstructure. This stress attribution can be observed in the engineering stress–strain or true stress–strain curves, which are generally acquired through the results generated in mechanical tests in laboratory environments to obtain predictions of the behaviors of metals in the manufacturing processes where plastic deformations occur through cold working, such as drawing, rolling, stamping, and others [
15].
Cold working process is typically combined with annealing sequentially. During the deformation process, a part of the applied mechanical energy is transformed into heat, however, the remainder is stored in the metal. Therefore, during annealing, these stored energies promote the processes of recovery and recrystallization. Through these procedures, it is possible to achieve the mechanical properties desired. This study aims to establish the hardening coefficient (n) and the plastic constant (k) using the Hollomon Model and to evaluate the behavior at different temperatures correlating with the results generated in the microstructure and in the mechanical properties of manganese bronze alloy.
Although the recrystallization behavior and texture evolution during annealing have been studied by many researchers in Brass and several Bronze alloy series [
16,
17], there is still a gap of research to determination of temperatures ranges to achieve the mechanical and microstructure properties foreseen in technical standard ASTM B138 due to heat treatment process, according to
Table 1 [
3]. Temperatures commonly used for annealing cold-worked to this alloy are not provided for in technical books, as is the case for other manganese bronze alloys [
1,
18,
19,
20,
21].
The results of this work provide new knowledge on the annealing influence on the tensile strength, yield strength, elongation, and microstructure of manganese bronze UNS C67600, which can be used as reference in literature and consequently in the heat treatment process in industries.
2. Materials and Methods
The material used in this work was manganese bronze produced in the foundry of the department of Research and Development of Termomecanica of São Paulo S.A. The alloy was melted in an induction furnace at 1100 °C, and the chemical composition of the alloys was determined using X-ray fluorescence spectrometry (XRF) (MAGIX FAST model Panalitycal) and is shown in
Table 2.
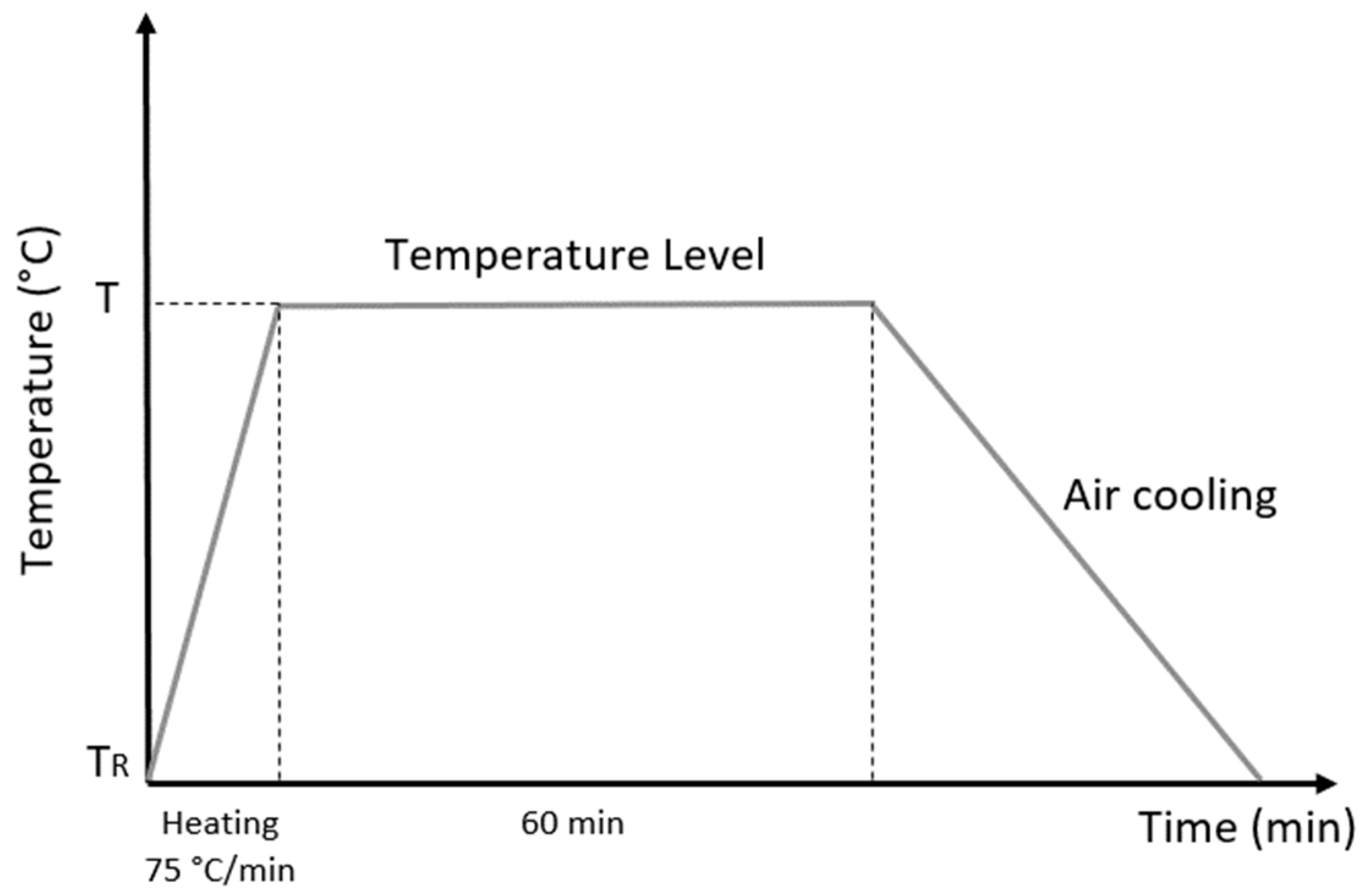
After casting, the sample roll-shaped samples with a diameter of 340 mm were reduced by hot and cold rolling processes to a diameter of 18.5 mm and cut into 200 mm lengths, with 34% cold work. The choice of this percentage was based on the average value used in industrial processes. Subsequently, thirteen specimens were heat-treated for one hour with different temperatures ranging from 200 °C to 750 °C, increasing by 50 °C for each sample in an electric furnace with an atmosphere environment and then cooled in air as shown in
Figure 1. This process was determined to enable reproduction on an industrial scale, since cooling is not normally carried out with a protective atmosphere. In research on the microstructure and mechanical properties, the reduction area for the cold work and order of magnitude of the heat treatment were the important features of the investigation.
Mechanical testing involved hardness and tensile tests. The hardness measurement using a Vickers method under an applied load of 10 kgf and the dwell time were set to 10 s on the Universal Hardness Tester Closed Loop durometer (Wolpert Group, Aachen, Germany), and an average value of five measurements was conducted on different areas of each specimen for mechanically polished surfaces. Tensile tests were carried out using an Instron 4482 (Instron, Norwood, MA, USA) with a crosshead speed of 20 mm/min to obtain stress–strain curves with the same strain rate as well as the tensile strengths, yield, and elongation of each specimen.
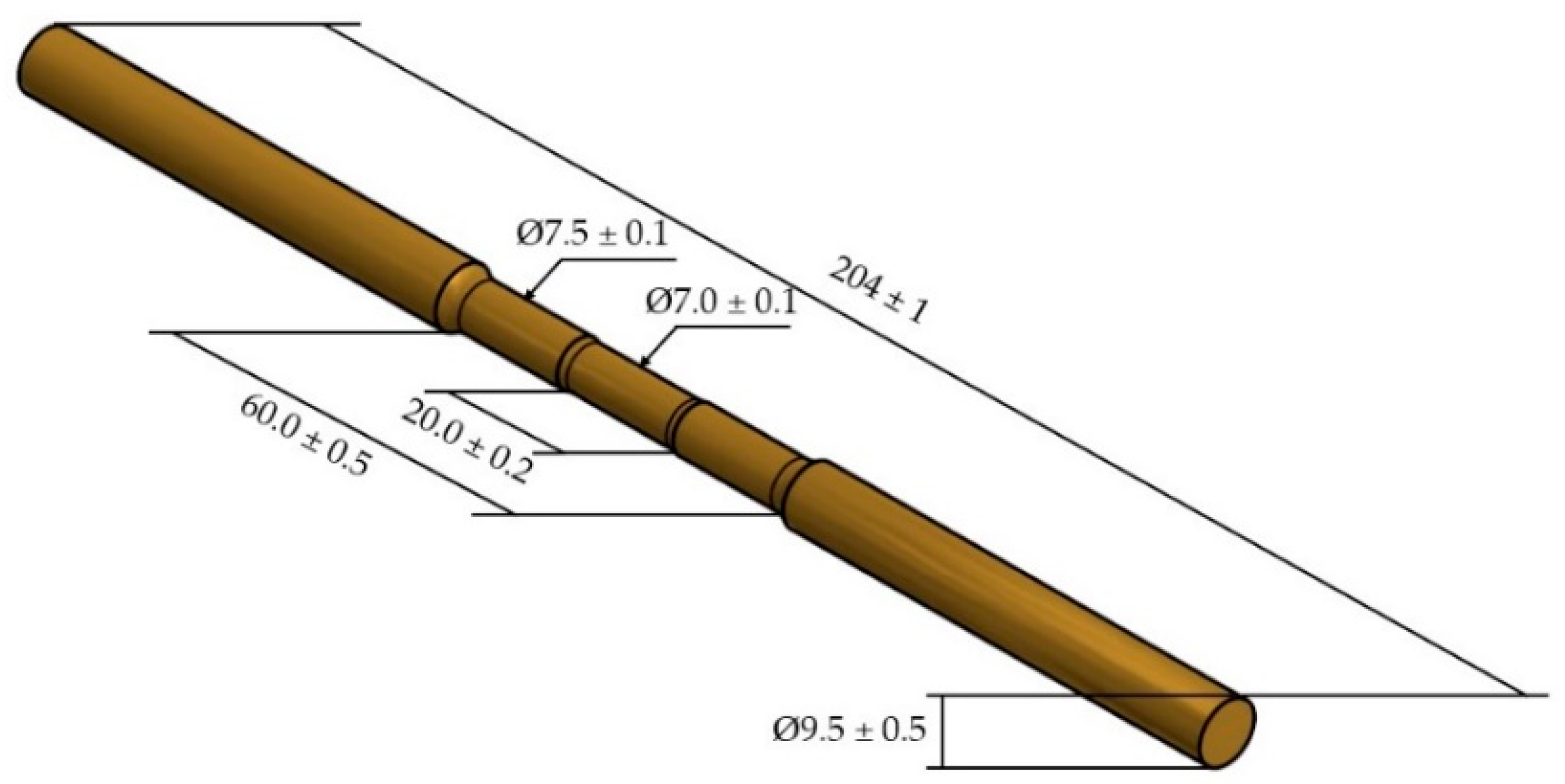
Thirteen specimens were prepared according to ASTM E-8 Plate-Type specifications, with gage lengths exceeding 60.00 mm and a gage diameter of 7.00 mm. As shown in
Figure 2, this format is used for fracture tendency in the proper region. The microstructure characterizations were performed using a Leica DM 2700 M (Leica Microsystems CMS GmbH, Wetzlar, Germany) light microscope. The specimens were prepared with mechanical polishing according to the ASTM E-3-11 specifications and the chemical etching to reveal the microstructure were performed using a solution of 5 g FeCl
3, 16 mL HCl, and 60 mL ethanol (95%) according to the ASTM E407-07. The scanning electron microscopy was performed using Phenom Pro-X equipment (Termofisher, Waltham, MA, USA) with objective study of the domain microstructure this alloy and the phase transformation relationship with different temperatures for annealing and area reduction.
The experiments were aimed at determining the relationship between the mechanical properties and microstructure with the annealing temperature and using the Hollomon model to relate these mechanical properties and microstructure with the strain-hardening coefficient (n) and the strength coefficient (K).
3. Results and Discussion
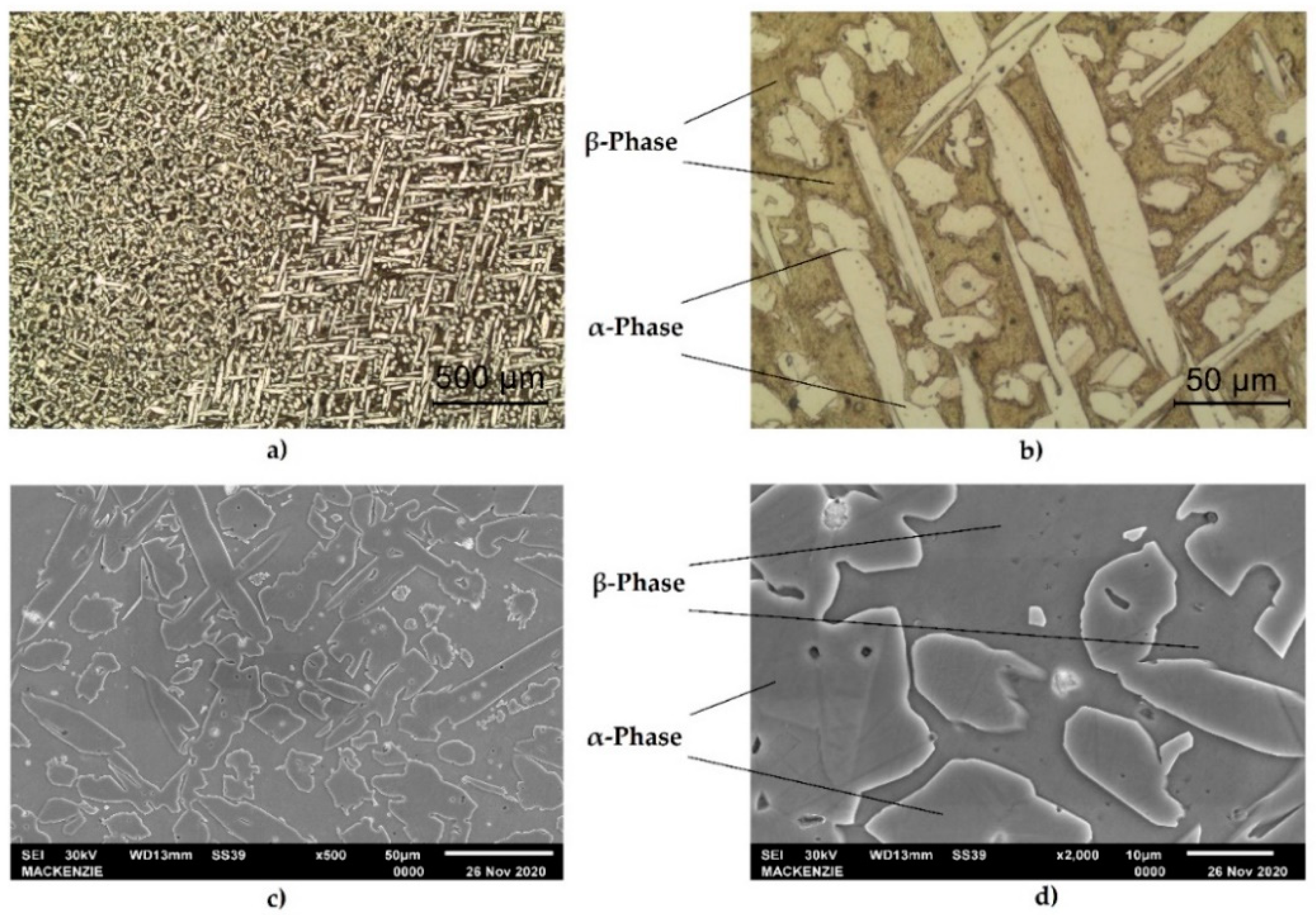
The optical micrographs of the materials shown in
Figure 3a,b indicate that the alloy consisted of α-phase and β- phase and that the α-phase had a long island shape that is typical for manganese bronze [
1]. In the microstructure, the light areas correspond to the α-phase, which possesses a face-centered cubic (fcc) lattice, while the dark areas correspond to the b-phase possessing a body-centered cubic (bcc) lattice.
Figure 3c,d show the SEM micrograph of the structure.
Figure 3d shows several types of non-dissolved small intermetallic precipitation that contain darker particles in the α-phase and lighter particles in the β-phase. Therefore, technical scanning electron microscopy and energy dispersive spectroscopy (SEM-EDS) mapping was used to investigate the qualitative distribution of the elements in the sample. It is important to highlight that the different magnifications used in tests (optical and SEM micrographs) and the crystallographic orientation of the grains produce variations in the microstructure analysis. Each micrograph in
Figure 3 was described in terms of these conditions.
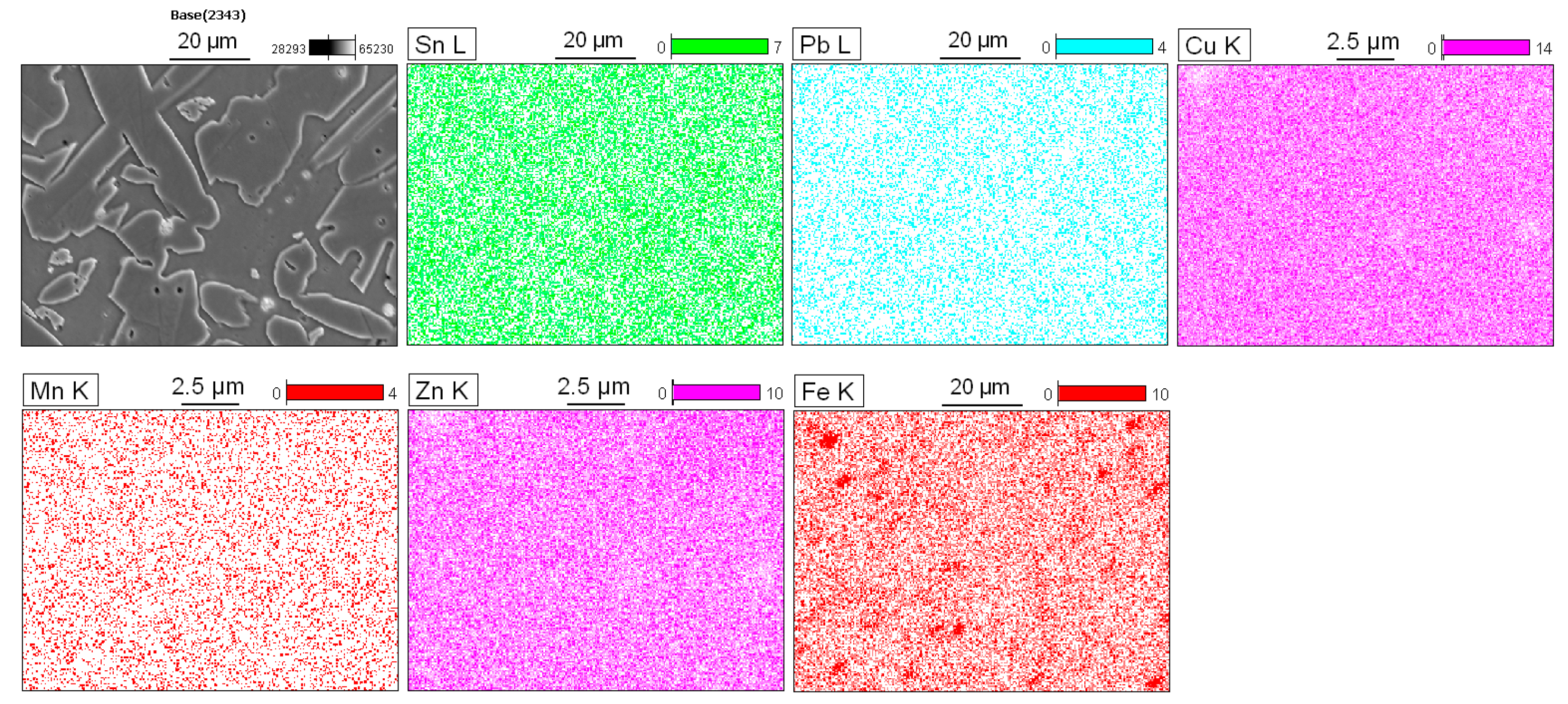
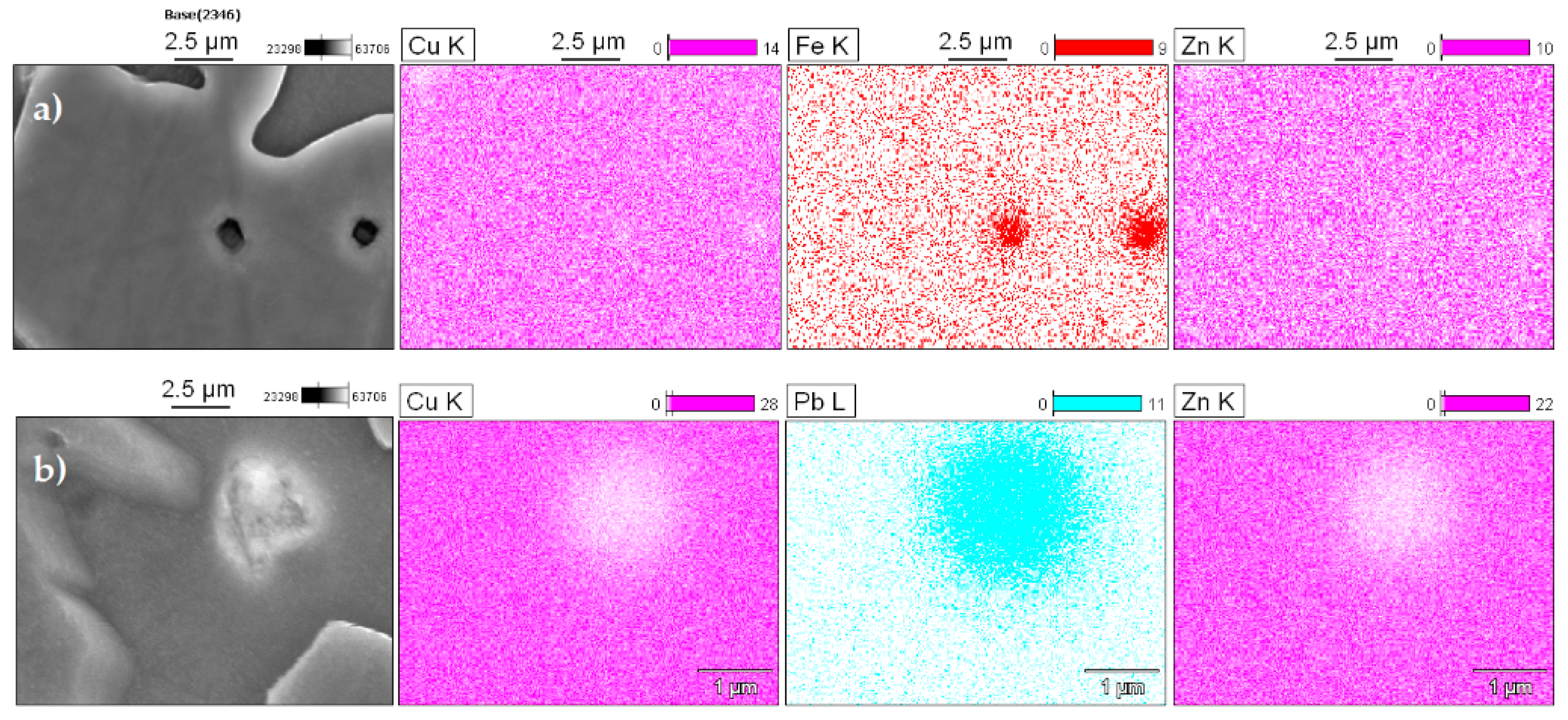
Figure 4 shows the results obtained using energy dispersive spectroscopy (EDS). The copper and zinc showed a partially uniform distribution with small variations in the α- and β-phase. In addition, the regions corresponding to the particles showed a significant decrease in the concentration of these elements. The manganese was uniformly distributed throughout the matrix, and the tin showed variations according to the region analyzed, which will be discussed in more detail later. The iron had a significant accumulation in determinate areas, indicating that specific particles of this element exist in the matrix. Lastly, the lead was homogeneously distributed; however, there were some areas that were less intense for this element.
The SEM-EDS maps identified several types of non-dissolved particles: Fe spherical shapes with a dark color and Pb agglomerates with a light color. In
Figure 5a, iron is predominantly concentrated at the α-phase, and this occurs because it has a low solubility in the molten copper alloys. According to Sun et al., these particles have a bcc structure and, based on their crystal structure and chemistry, can be identified as α-Fe [
22]. In 60/40 brass, the solubility of iron reduces from about 1.5 wt.% at 1020 °C to 0.04 wt.% at 950 °C [
23]. This promotes the fine precipitation of iron particles that act as nuclei for new α-phase grains [
24].
Lead has low solubility in solid copper, and so the lead particles appear as light and discrete globules at the α/β grain boundaries as high surface energy sites [
25]. To prove this, the samples were inspected with elemental microanalysis using energy dispersive spectroscopy (EDS), and
Figure 5b shows the results.
Figure 6a shows the microstructure observed in SEM micrograph indicating the phases and particles. The EDX point chemical analysis of the α- and β-phase are shown in
Figure 6b,c, respectively. The results are similar to those noted in the literature, in which the β-phase has approximately 55% copper, and the α-phase has 64% [
25].
According to the literature, first, the liquid metal transforms into an α-phase solid, and then the β-phase precipitates at the α grain boundaries. With continuous cooling, the disorder β-phase is transformed to the ordered β′. In addition, the tin content of the alloy was more stable in the β′-phase, and this information can be proven with the results presented in
Figure 5, which shows that, in the EDS spectrum, Sn was contained only in the β′-phase [
26].
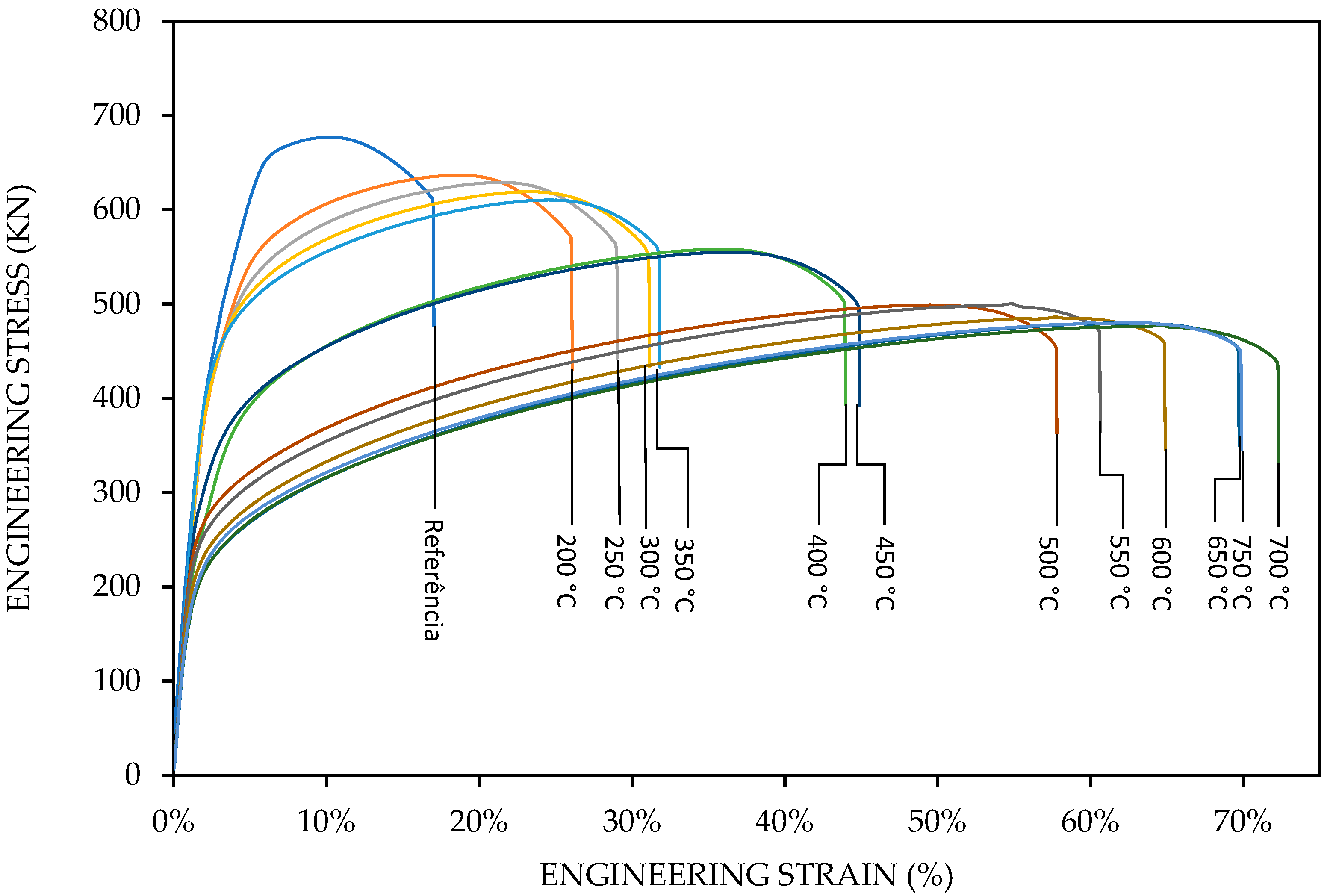
The stress–strain results of 13 samples, including the reference sample with 34% cold work, are shown in
Figure 7. All specimens fractured in the center of the gauge length. We observed, in
Figure 7, the effect of temperature on the shape of the stress–strain curves of manganese bronze.
Technologically, the stress–strain curves from 200 to 350 °C, shown in
Figure 7, represent the temperature range of warm working, where the recovery process takes place; therefore, the degree of hardening by deformation is considerably less than in cold working. Probably, the stress–strain curves from 350 to 750 °C represent the hot working, also called hot forming, that involves deformation at temperatures above the recrystallization temperature.
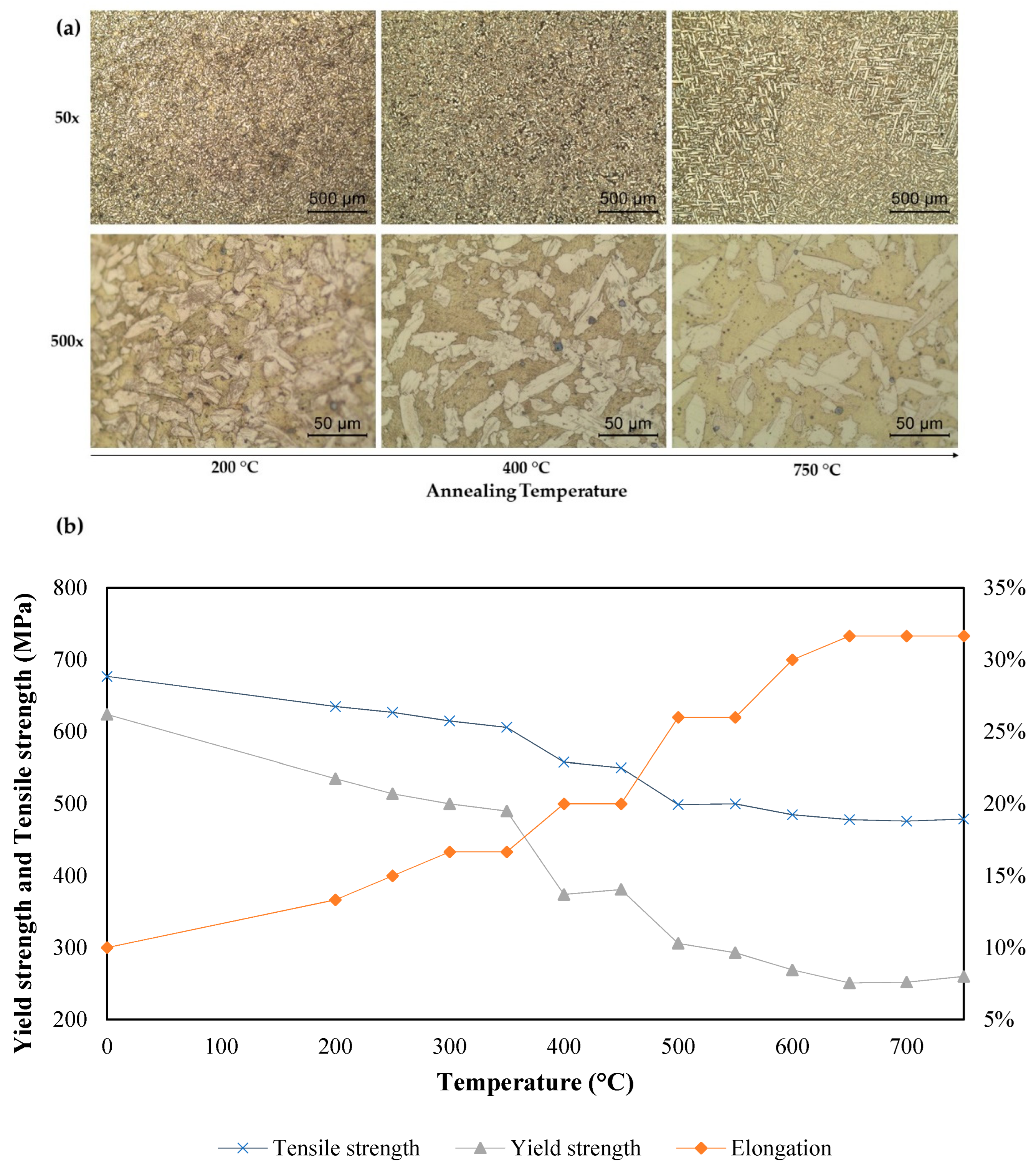
The microstructure changes and variation of the yield strength, tensile strength, and ductility with the temperature during the heat treatment are shown in
Figure 8a,b, respectively.
Figure 8 presents the increasing annealing temperature with the subsequent increased ductility and decrease in the ultimate tensile strength and yield strength. Studies found the annealing of metallic material with addition of plasticity [
27].
This effect occurred because, during the annealing, there was a decrease of the stored energy during plastic deformation due to the mechanisms of rearrangement and the annihilation of crystal defects. This process can be divided in three states: recovery, recrystallization, and grain growth. The first mechanism is characterized by the annihilation and rearrangement of dislocations occurring normally in low temperatures and involves thermal activation, with the formation of subgrains with low-angles within the as-deformed grains, and thus high-angle grain boundaries are usually unaffected.
The second stage occurs with the nucleation and formation of strain-free new grains, where the driving force is the stored energy in the form of crystalline defects. In this step, the material restores the ductility, hardness, and strength that it had prior to cold deformation. The lowest temperature that forms new grains in a plastically deformed metal is known as the recrystallization temperature and normally corresponds to 1/3 to 1/2 of the melting point on the Kelvin scale. The last stage is characterized by the migration of boundaries; however, in this case, the driving force is the energy of the high angle boundaries [
28,
29].
The heat treatment of the samples showed that the recovery may have occurred between 200 and 350 °C, with the gradual reduction of engineering stress when compared with the reference sample. The reference sample presented a solid β solution matrix structure with the presence of a tensioned circular α-phase and intermetallic constituents dispersed in the material structure. This tensioning was gradually reduced with the increasing temperature, as shown in
Figure 9. When reaching 350 °C, the material probably started its recrystallization with the formation of stress-free α-phase grains and ended at 500 °C. In this interval, there was an abrupt gap in the engineering stress and increase of elongation. In literature, for some manganese bronze alloys (UNS C67000, C67400, and C67500), the temperatures commonly used for annealing cold-worked are 425–600 °C, therefore the behavior found in
Figure 8 is in accordance with previous research [
1,
18,
19,
20,
21].
At 700 °C, the sample showed a structural discontinuity between the surface and the core of the material. On the surface, the material showed β granular formation with the presence of a widmanstatten α-phase and intermetallic microconstituents dispersed in the material structure. In the core, the material presented a solid β solution matrix structure and the presence of an α-phase in a circular and widmanstatten pattern.
The true stress–true strain curves were calculated from the engineering stress and engineering strain to represent the plastic deformation phenomenon. The true stress–strain curve is known as the flow curve and can be expressed by the power law, which was proposed by Hollomon [
30].
where
n is the exponent, which represents the strain-hardening coefficient; σ is the true stress, MPa; ε is the true strain; and
K is the strength coefficient, MPa [
31]. There is an approximate linear relationship between the true stress and true strain when plotted on a log–log scale. Therefore, through linear fitting of the lnσ-lnε curves, the strain-hardening exponent
n was obtained by the fitted slopes, while the material constant lnK was obtained by the fitted intercept.
Therefore, Equation (2) represents a linear system, the slope of the line gives the
n value
n = ∂lnσ/∂lnε, and the intercept at ε = 1 gives the
K value.
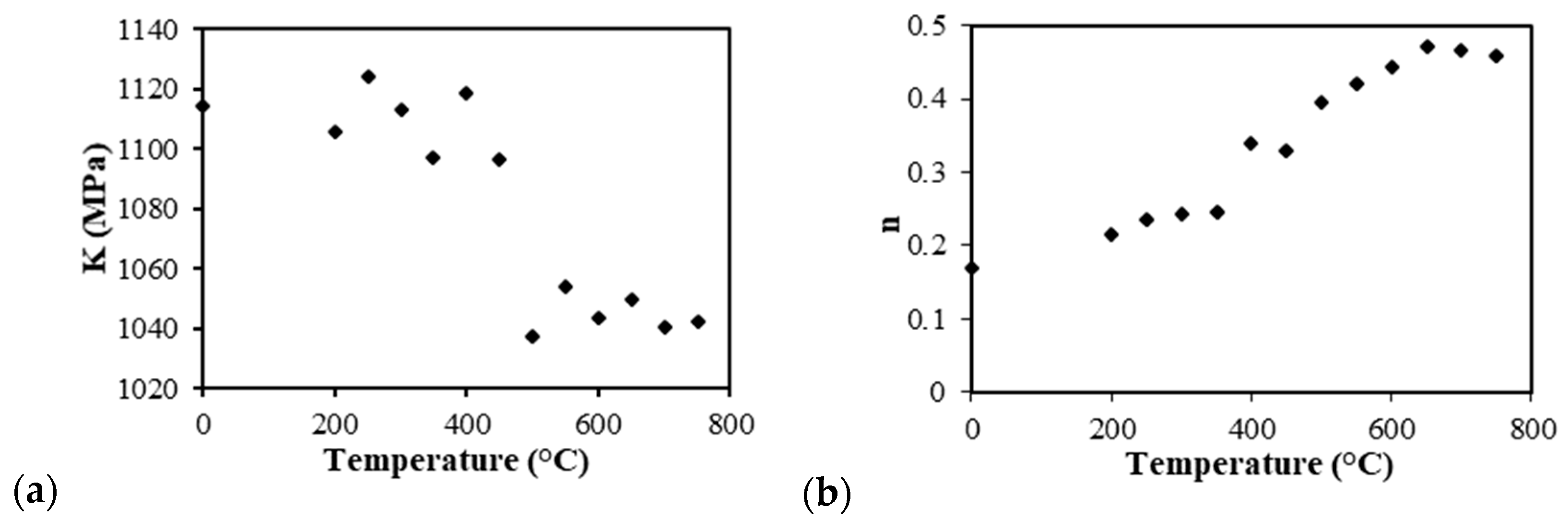
Figure 10 presents graphs of the strain-hardening exponent
n and strength coefficient
K with respect to the annealing temperatures with the experimental data fitted with the Hollomon equation. Comparing the values that were determined in
Figure 10b, when the annealing temperature was increased from 200 to 750 °C, the (
n) values were found enhanced together with the variation from 0.2 to 0.5. The
n value is related to the mechanical properties through the Hollomon equation, which describes that the value
n was small, and the hardening rate was high initially with the plastic deformation; however, the hardening rate decreased rapidly with the increased strain. On the other hand, with a high
n, the hardening rate was smaller at the initial plastic deformation; however, the hardening rate remained low until the high strains. In other words, the higher the hardening coefficient
n, the more the materials will be able to deform/elongate.
According to William, the strain hardening behavior described by n depends on the composition, microstructure, and dislocation population. In this case, the composition was not altered, and neither was the β-phase (on average 50%); therefore, the values of the strength and elongation changed according to the microstructure and dislocation, and the
n values corresponded to the annealing temperature each stage, as shown in
Figure 8 [
32].
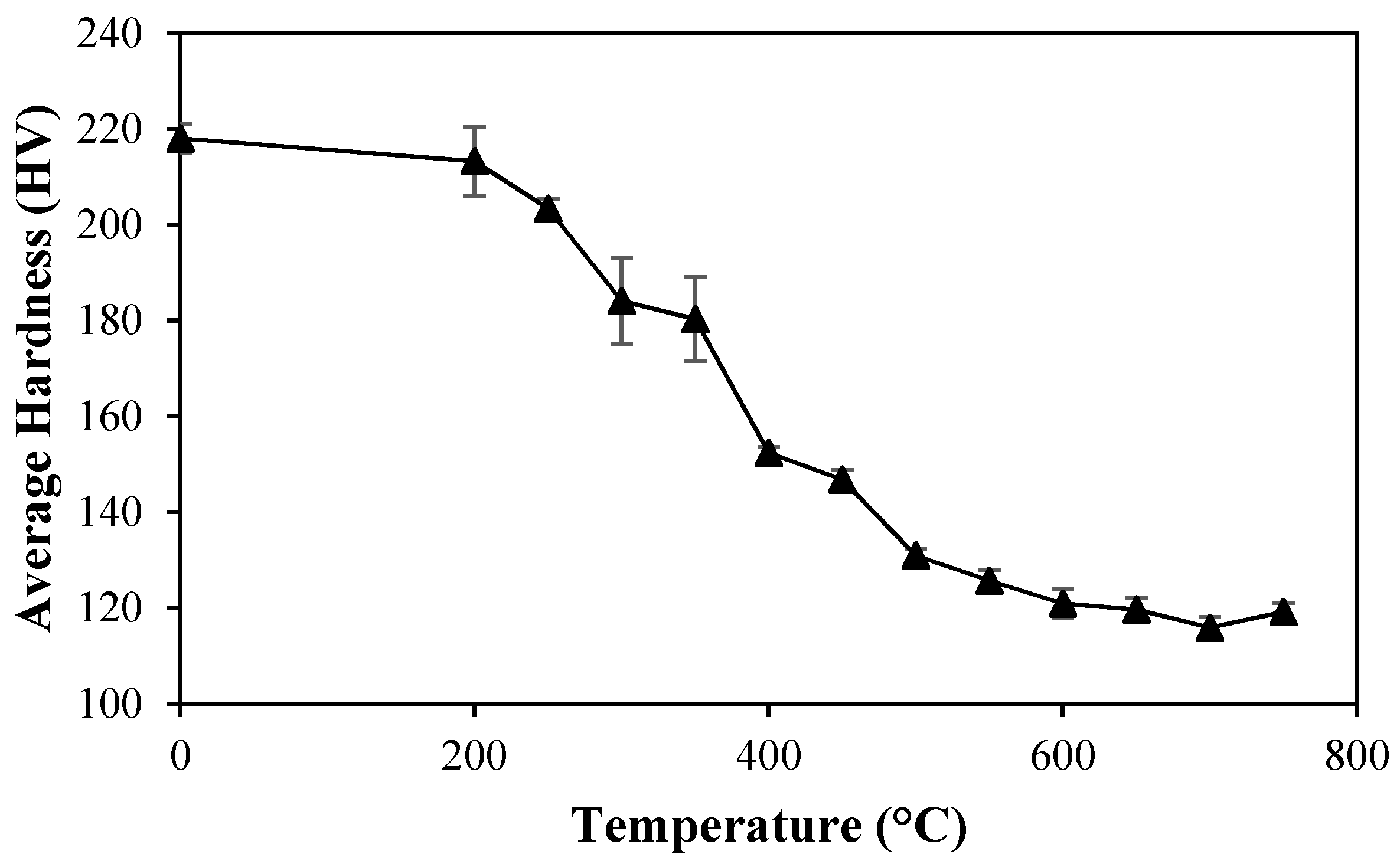
The effect of temperature on the Vickers hardness is shown in
Figure 11. Each point in the figure represents an average of five readings in the perpendicular plane of the samples.
At low temperatures, small and continuous changes in the hardness occurred, which was due the reduction in the dislocation density causing a gradual decrease in the elastic distortion due to the formation of low energy structures [
33]. When subjected to 400 °C, the sample showed a sharp drop in hardness, because, as previously discussed, the recrystallization begins at this temperature. Lastly, after 500 °C, few changes were noted, and they were only associated with grain growth at this stage.