Characterization, Luminescence and Optical Resonant Modes of Eu-Li Co-Doped ZnO Nano- and Microstructures
Abstract
1. Introduction
2. Experimental Method
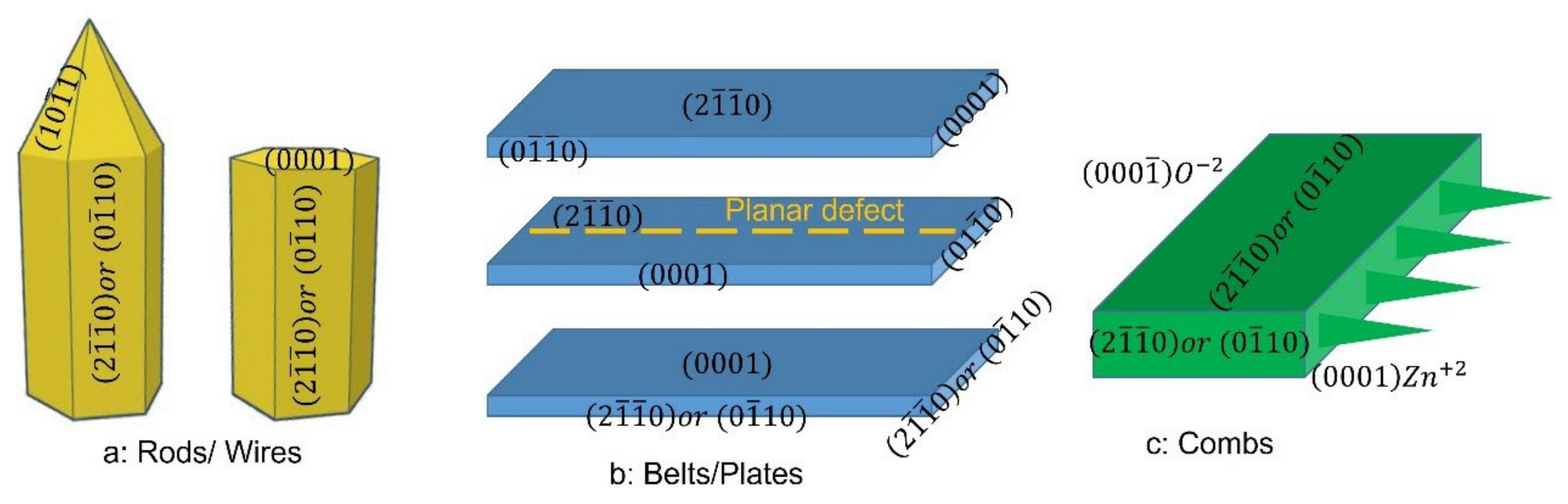
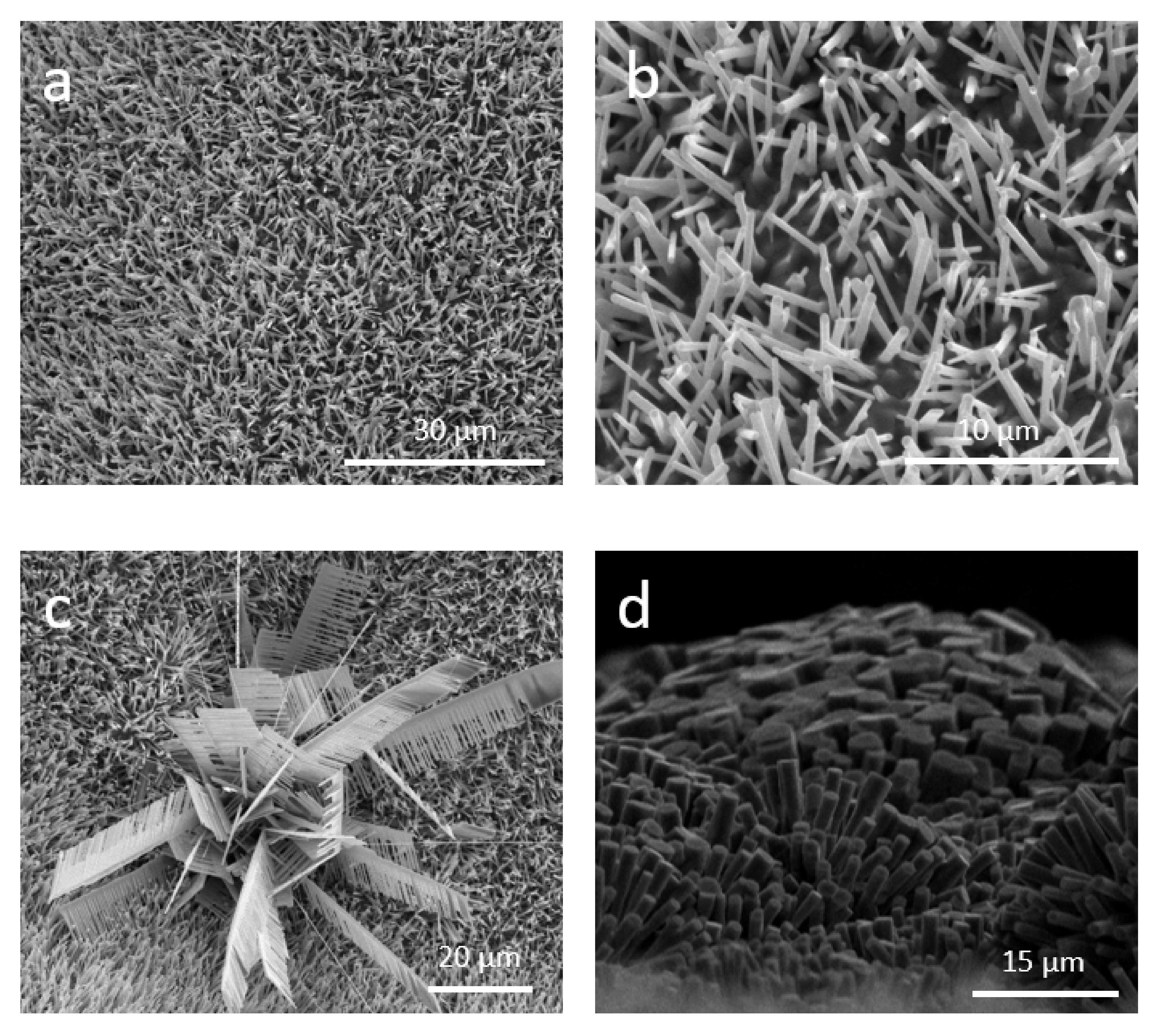
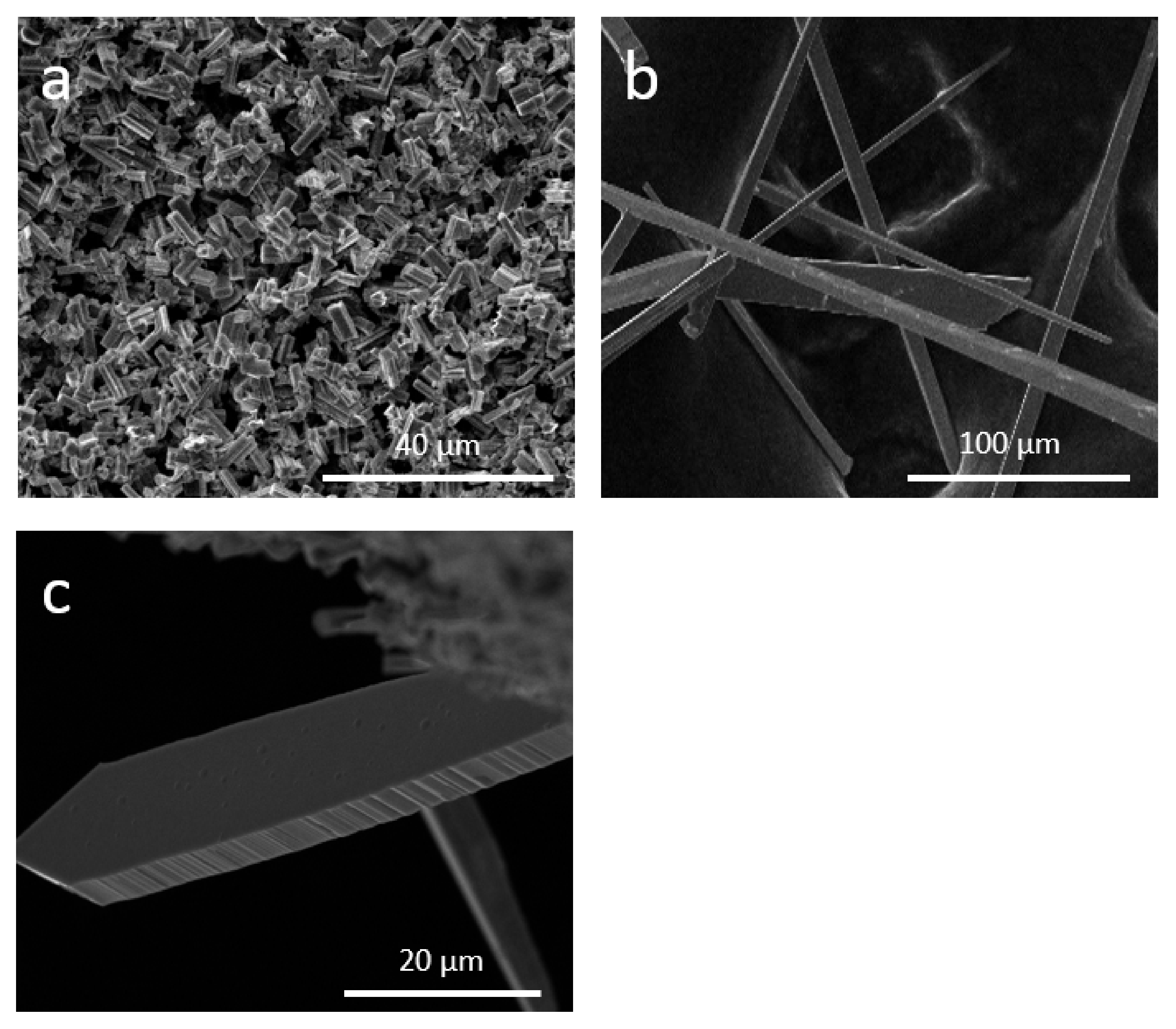
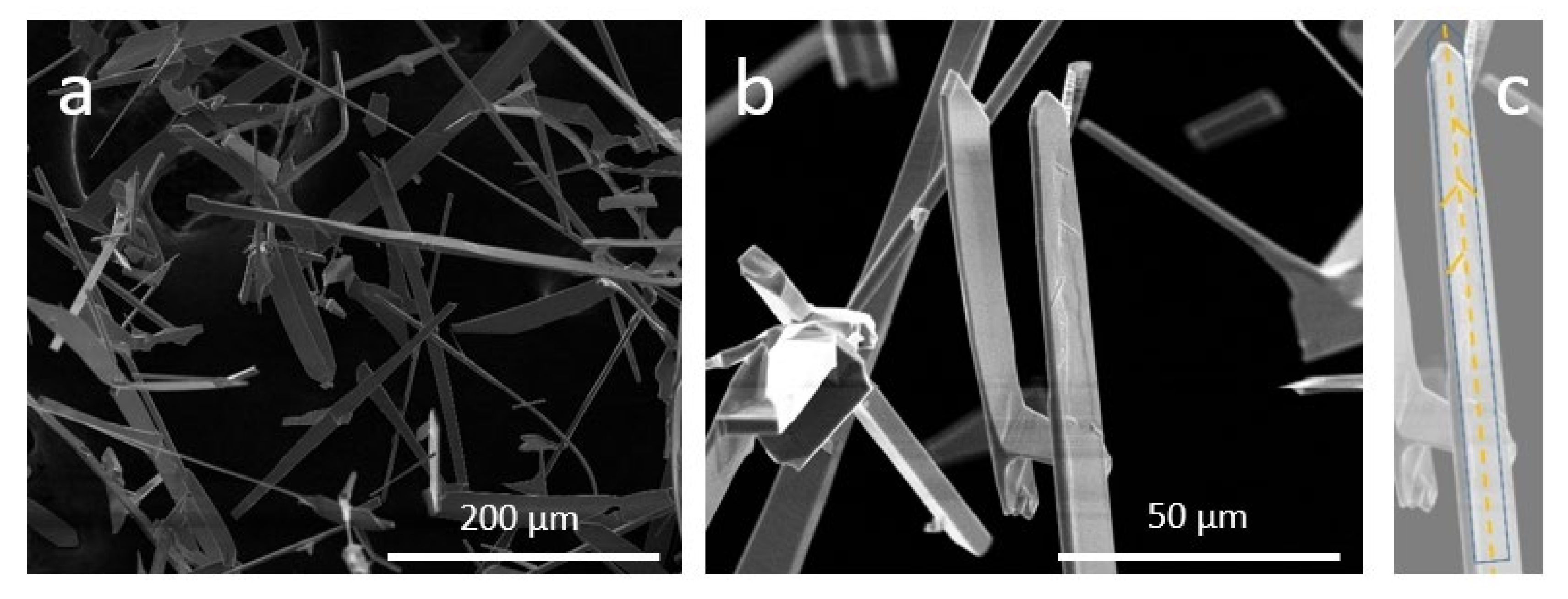
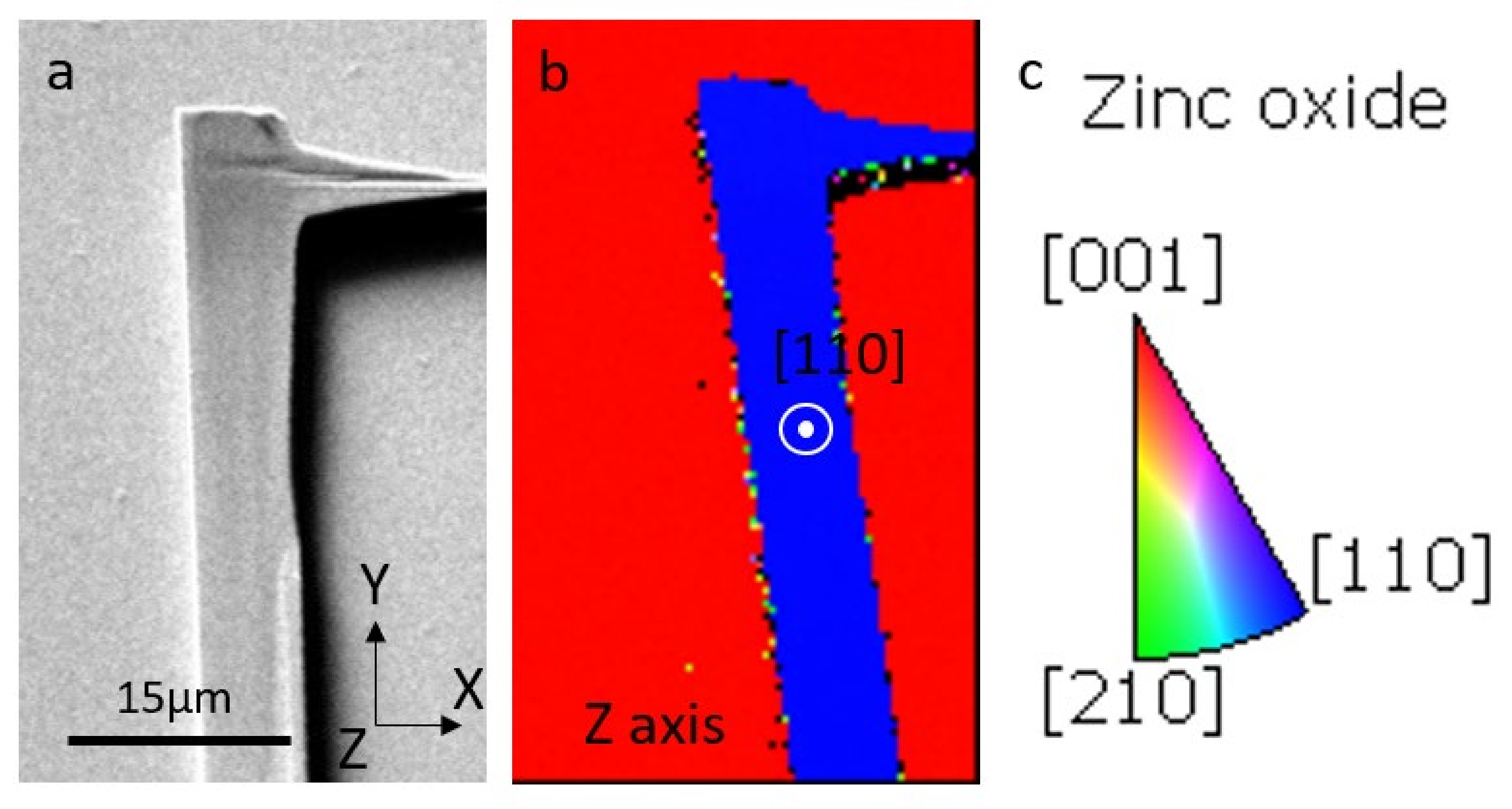
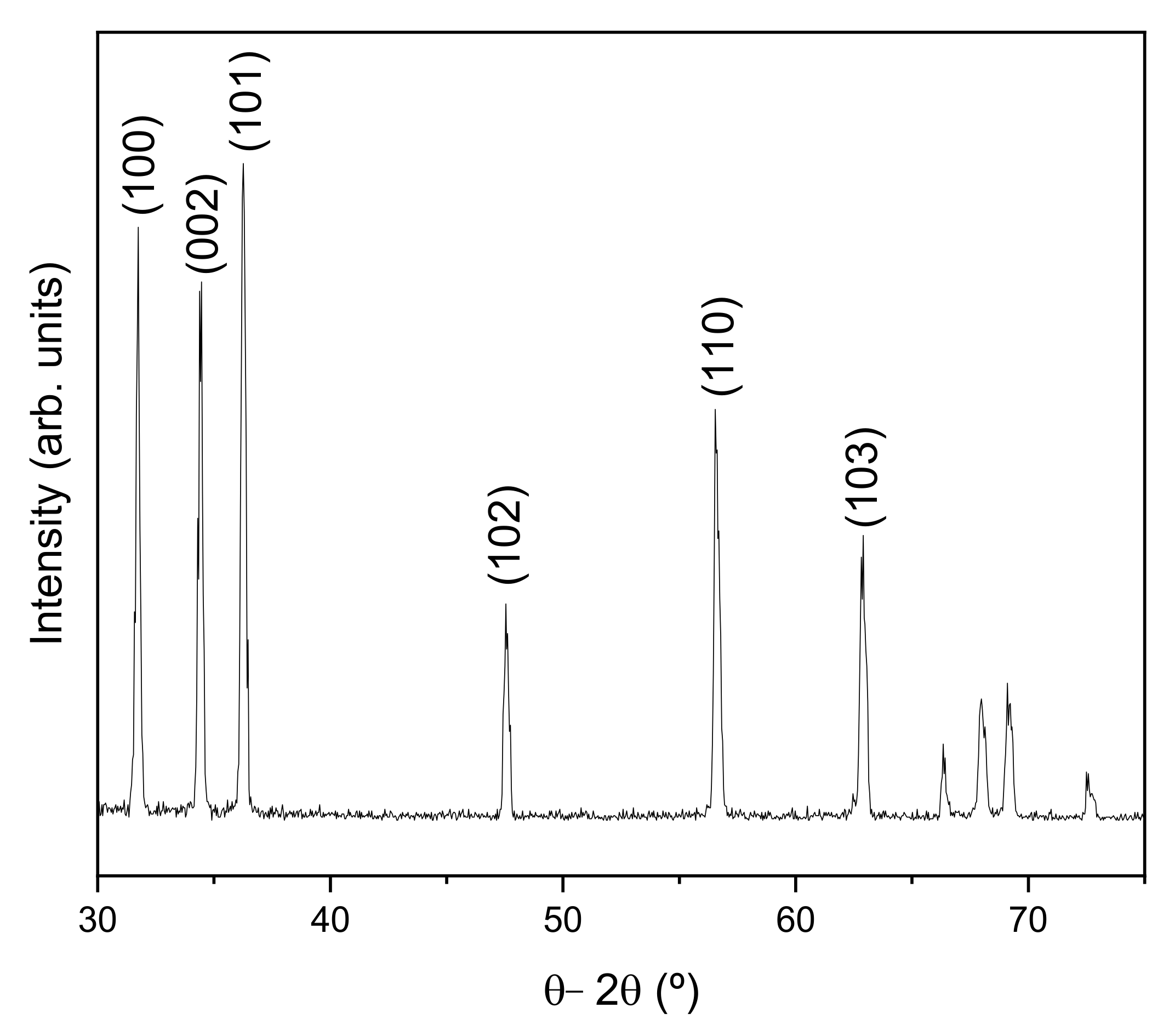
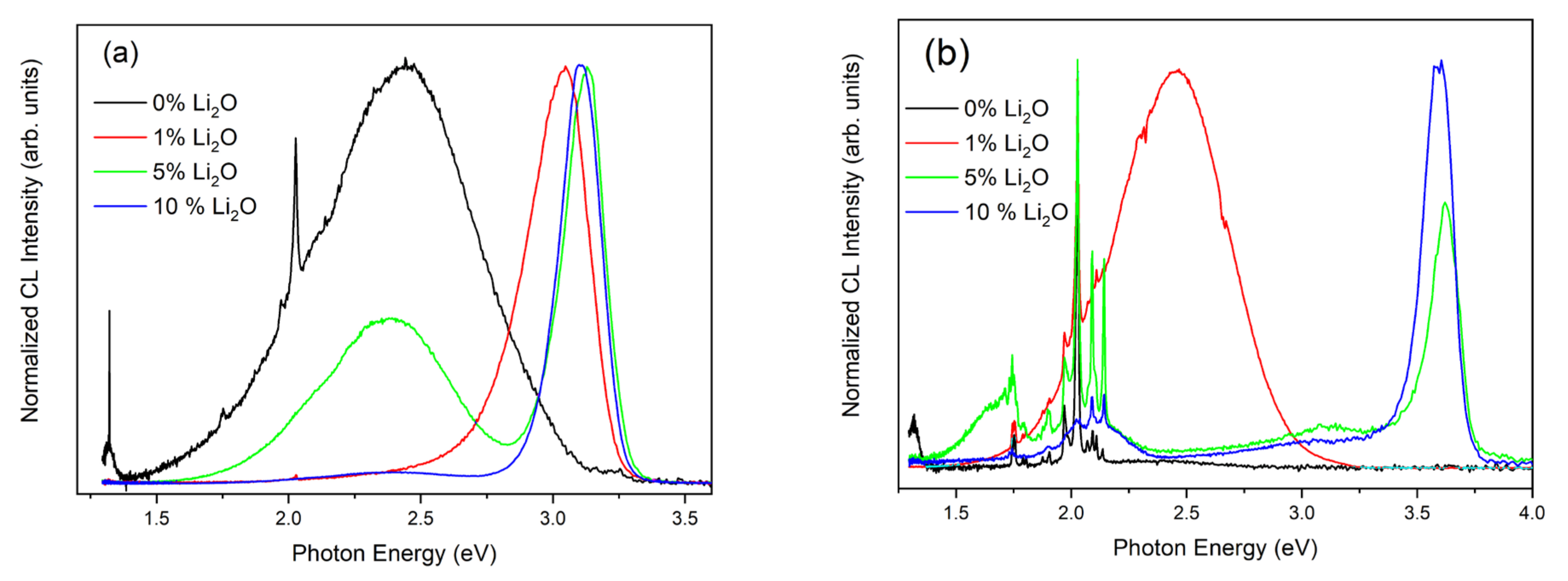
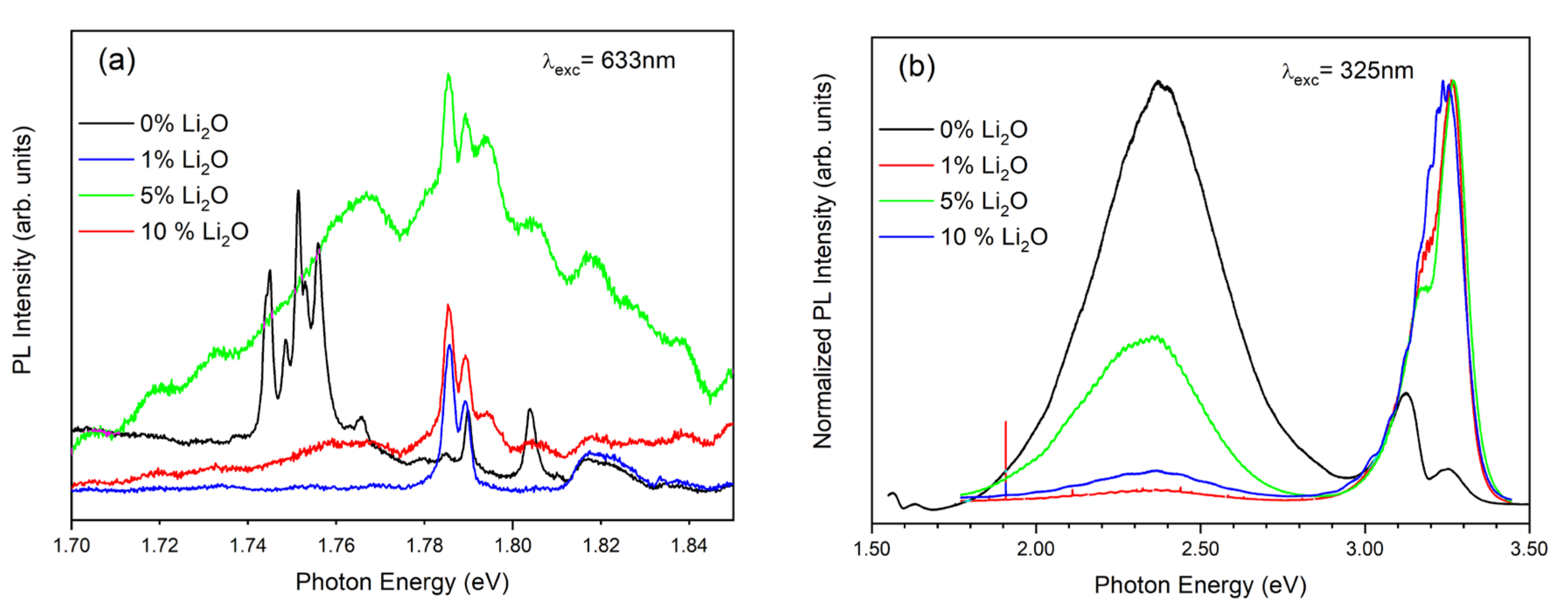
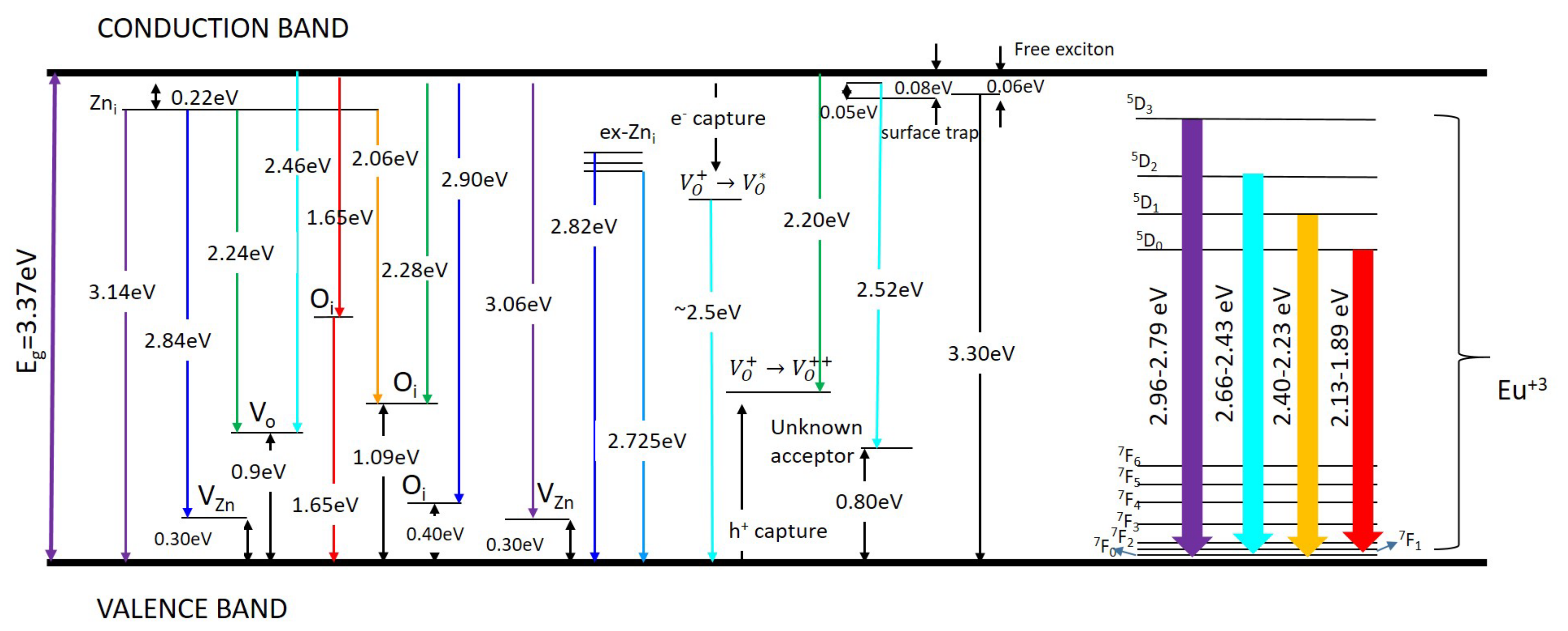
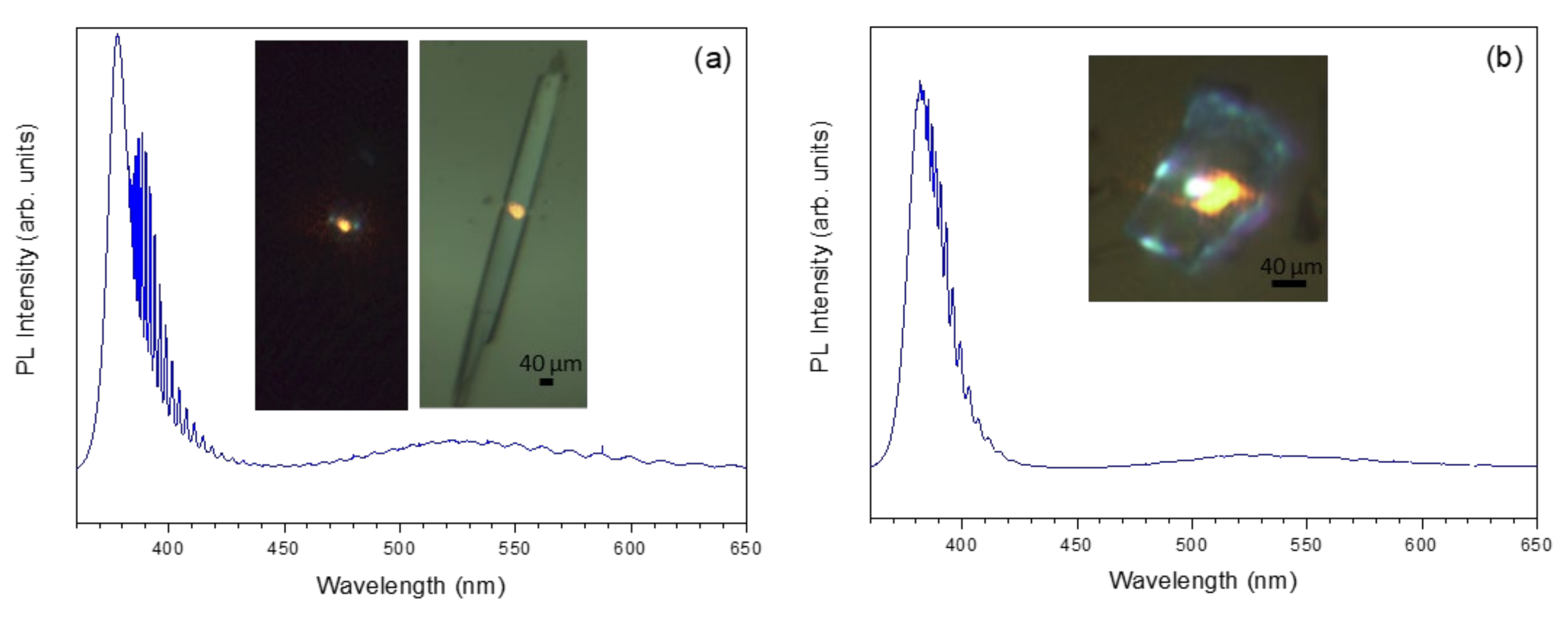
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wang, Z.L. Novel nanostructures of ZnO for nanoscale photonics, optoelectronics, piezoelectricity, and sensing. Appl. Phys. A Mater. Sci. Process. 2007, 88, 7–15. [Google Scholar] [CrossRef]
- Kolodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide-from synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [PubMed]
- Skompska, M.; Zarȩbska, K. Electrodeposition of ZnO nanorod arrays on transparent conducting substrates-a review. Electrochim. Acta 2014, 127, 467–488. [Google Scholar] [CrossRef]
- Wang, Z.L. ZnO nanowire and nanobelt platform for nanotechnology. Mater. Sci. Eng. R Rep. 2009, 64, 33–71. [Google Scholar] [CrossRef]
- Ronning, C.; Gao, P.X.; Ding, Y. Manganese-doped ZnO nanobelts for spintronics. Appl. Phys. Lett. 2004, 84, 783. [Google Scholar] [CrossRef]
- Wadeasa, A.; Nur, O.; Willander, M. Zinc oxide nanorod-based heterostructures on solid and soft substrates for white-light-emitting diode applications, (n.d.). New J. Phys. 2009, 11, 125020. [Google Scholar] [CrossRef]
- Willander, M.; Nur, O.; Sadaf, J.R.; Qadir, M.I.; Zaman, S.; Zainelabdin, A.; Bano, N.; Hussain, I. Luminescence from zinc oxide nanostructures and polymers and their hybrid devices. Materials 2010, 3, 2643–2667. [Google Scholar] [CrossRef]
- Daksh, D.; Agrawal, Y.K. Rare Earth-Doped Zinc Oxide Nanostructures: A Review. Rev. Nanosci. Nanotechnol. 2016, 5, 1–27. [Google Scholar] [CrossRef]
- Ariza, R.; Pavón, F.; Urbieta, A.; Fernández, P. Study of the influence of dopant precursor on the growth and properties of Li-doped ZnO. J. Phys. Chem. Solids 2020, 139, 109354. [Google Scholar] [CrossRef]
- Urbieta, A.; Fernández, P.; Piqueras, J. Nanowires and stacks of nanoplates of Mn doped ZnO synthesized by thermal evaporation-deposition. Mater. Chem. Phys. 2012, 132, 1119–1124. [Google Scholar] [CrossRef]
- Wang, Z.L.; Kong, X.Y.; Zuo, J.M. Induced growth of asymmetric nanocantilever arrays on polar surfaces. Phys. Rev. Lett. 2003, 91, 185502. [Google Scholar] [CrossRef]
- Wander, A.; Schedin, F.; Steadman, P.; Norris, A.; McGrath, R.; Turner, T.S.; Thornton, G.; Harrison, N.M. Stability of polar oxide surfaces. Phys. Rev. Lett. 2001, 86, 3811–3814. [Google Scholar] [CrossRef]
- Li, W.J.; Shi, E.W.; Zhong, W.Z.; Yin, Z.W. Growth mechanism and growth habit of oxide crystals. J. Cryst. Growth 1999, 203, 186–196. [Google Scholar] [CrossRef]
- Ching, K.L.; Li, G.; Ho, Y.L.; Kwok, H.S. The role of polarity and surface energy in the growth mechanism of ZnO from nanorods to nanotubes. Cryst. Eng. Comm. 2016, 18, 779–786. [Google Scholar] [CrossRef]
- Galli, G. Solid-state physics: Doping the undopable. Nature 2005, 436, 32–33. [Google Scholar] [CrossRef]
- Zhang, F.; Wei, S.H.; Zunger, A. Microscopic origin of the phenomenological equilibrium “doping limit rule” inn-Type III-V semiconductors. Phys. Rev. Lett. 2000, 84, 1232–1235. [Google Scholar] [CrossRef]
- Tang, K.; Gu, S.L.; Ye, J.D.; Zhu, S.M.; Zhang, R.; Zheng, Y.D. Recent progress of the native defects and p-type doping of zinc oxide. Chin. Phys. B. 2017, 26, 047702. [Google Scholar] [CrossRef]
- Avrutin, V.; Silversmith, D.J.; Morkoç, H. Doping asymmetry problem in ZnO: Current status and outlook. Proc. IEEE. 2010, 98, 1269–1280. [Google Scholar] [CrossRef]
- Yan, Y.; Wei, S.H. Doping asymmetry in wide-bandgap semiconductors: Origins and solutions. Phys. Status Solidi Basic Res. 2008, 245, 641–652. [Google Scholar] [CrossRef]
- Pal, P.P.; Manam, J. Enhanced luminescence of ZnO: RE3+ (RE = Eu, Tb) nanorods by Li+ doping and calculations of kinetic parameters. J. Lumin. 2014, 145, 340–350. [Google Scholar] [CrossRef]
- Kenyon, J. Recent developments in rare-earthdoped materials for optoelectronics. Prog. Quantum Electron. 2002, 26, 225–284. [Google Scholar] [CrossRef]
- Gaggero, E.; Calza, P.; Cerrato, E.; Paganini, M.C. Cerium-, Europium- and Erbium-Modified ZnO and ZrO2 for Photocatalytic Water Treatment Applications: A Review. Catalysts 2021, 11, 1520. [Google Scholar] [CrossRef]
- Kumar, V.; Ntwaeaborwa, O.M.; Soga, T.; Dutta, V.; Swart, H.C. Rare Earth Doped Zinc Oxide Nanophosphor Powder: A Future Material for Solid State Lighting and Solar Cells. ACS Photonics 2017, 4, 2613–2637. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, S.K.; Rai, S.B. Role of Li+ ion in the luminescence enhancement of lanthanide ions: Favorable modifications in host matrices. RSC Adv. 2014, 4, 27039–27061. [Google Scholar] [CrossRef]
- Eliseeva, S.V.; Bünzli, J.-C.G.; Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. Chemical Society Reviews Critical Review. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef]
- Krishna, R.; Haranath, D.; Singh, S.P.; Chander, H.; Pandey, A.C.; Kanjilal, D. Synthesis and improved photoluminescence of Eu:ZnO phosphor. J. Mater. Sci. 2007, 42, 10047–10051. [Google Scholar] [CrossRef]
- Khanum, R.; Das, N.M.; Moirangthem, R.S. Defect engineered ZnO whispering gallery modes via doping with alkali metal ions for label-free optical sensors. J. Appl. Phys. 2019, 125, 173107. [Google Scholar] [CrossRef]
- Ariza, R.; Sotillo, B.; Pavón, F.; Urbieta, A.; Fernández, P. Evolution of whispering gallery modes in Li-doped ZnO hexagonal micro-and nanostructures. Appl. Sci. 2020, 10, 8602. [Google Scholar] [CrossRef]
- Pavón, F.; Urbieta, A.; Fernández, P. Luminescence and light guiding properties of Er and Li codoped ZnO nanostructures. J. Lumin. 2018, 195, 396–401. [Google Scholar] [CrossRef]
- Pavón, F.; Ariza, R.; Urbieta, A.; Fernández, P. Morphology Luminescence, and Optical Properties of Tb- and Li-Codoped ZnO Elongated Nano- and Microstructures. Phys. Status Solidi Appl. Mater. Sci. 2022, 219, 2100805. [Google Scholar] [CrossRef]
- Yang, P.; Yan, H.; Mao, S.; Russo, R.; Johnson, J.; Saykally, R.; Morris, N.; Pham, J.; He, R.; Choi, H.J. Controlled growth of ZnO nanowires and their optical properties. Adv. Funct. Mater. 2002, 12, 323–331. [Google Scholar] [CrossRef]
- Johnson, J.C.; Yan, H.Q.; Yang, P.D.; Saykally, R.J. Optical cavity effects in ZnO nanowire lasers and waveguides. J. Phys. Chem. B. 2003, 107, 8816–8828. [Google Scholar] [CrossRef]
- Dong, H.; Liu, Y.; Sun, S.; Li, J.; Zhan, J.; Chen, Z.; Zhang, L. Geometry dependent evolution of the resonant mode in Zno elongated hexagonal Microcavity. Sci. Rep. 2016, 6, 19273. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.M.; Wei, X.L.; Dai, L.; Liu, S.F.; Chen, T.; Yue, S.; Li, Z.; Chen, Q.; Qin, G.G. Light coupling and modulation in coupled nanowire ring-fabry-pérot cavity. Nano Lett. 2009, 9, 2697–2703. [Google Scholar] [CrossRef]
- Cuscó, R.; Alarcón-Lladó, E.; Ibáñez, J.; Artús, L.; Jiménez, J.; Wang, B.; Callahan, M.J. Temperature dependence of Raman scattering in ZnO. Phys. Rev. B. 2007, 75, 165202. [Google Scholar] [CrossRef]
- Kurgan, N.; Karbivskyy, V.; Kasyanenko, V. Morphology and electronic structure of nanoscale powders of calcium hydroxyapatite. Nanoscale Res. Lett. 2015, 10, 41. [Google Scholar] [CrossRef][Green Version]
- Djurisic, A.B.; Leung, Y.H.H.; Tam, K.H.H.; Ding, L.; Ge, W.K.K.; Chen, H.Y.Y.; Gwo, S.; Djurišić, A.B.; Leung, Y.H.H.; Tam, K.H.H.; et al. Green, yellow, and orange defect emission from ZnO nanostructures Influence of excitation wavelength. Appl. Phys. Lett. 2006, 88, 103107. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H.; Tam, K.H.; Hsu, Y.F.; Ding, L.; Ge, W.K.; Zhong, Y.C.; Wong, K.S.; Chan, W.K.; Tam, H.L.; et al. Defect emissions in ZnO nanostructures. Nanotechnology 2007, 18, 095702. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.; Doǧan, S.; Avrutin, V.; Cho, S.J.; Morko, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 1–103. [Google Scholar] [CrossRef]
- Bruno, L.; Strano, V.; Scuderi, M.; Franzò, G.; Priolo, F.; Mirabella, S. Localized energy band bending in zno nanorods decorated with au nanoparticles. Nanomaterials 2021, 11, 2718. [Google Scholar] [CrossRef]
- Fabbri, F.; Villani, M.; Catellani, A.; Calzolari, A.; Cicero, G.; Calestani, D.; Calestani, G.; Zappettini, A.; Dierre, B.; Sekiguchi, T.; et al. Zn vacancy induced green luminescence on non-polar surfaces in ZnO nanostructures. Sci. Rep. 2014, 4, 5158. [Google Scholar] [CrossRef]
- Peres, M.; Cruz, A.; Pereira, S.; Correia, M.R.; Soares, M.J.; Neves, A.; Carmo, M.C.; Monteiro, T.; Pereira, A.S.; Martins, M.A.; et al. Optical studies of ZnO nanocrystals doped with Eu3+ ions. Appl. Phys. A Mater. Sci. Process. 2007, 88, 129–133. [Google Scholar] [CrossRef]
- Assadi, M.H.N.; Zhang, Y.; Zheng, R.K.; Ringer, S.P.; Li, S. Structural and electronic properties of Eu- and Pd-doped ZnO. Nanoscale Res. Lett. 2011, 6, 357. [Google Scholar] [CrossRef]
- Sotillo, B.; Fernández, P.; Piqueras, J. Growth by thermal evaporation of Al doped ZnS elongated micro- and nanostructures and their cathodoluminescence properties. J. Alloys Compd. 2014, 603, 57–64. [Google Scholar] [CrossRef]
- Vempati, S.; Mitra, J.; Dawson, P. One-step synthesis of ZnO nanosheets: A blue-white fluorophore. Nanoscale Res. Lett. 2012, 7, 470. [Google Scholar] [CrossRef]
- Hooda, A.; Khatkar, S.P.; Khatkar, A.; Malik, R.K.; Devi, S.; Dalal, J.; Taxak, V.B. Combustion synthesis, Judd–Ofelt parameters and optical properties of color tunable Ba3Y4O9: Eu3+ nanophosphor for near-UV based WLEDs. J. Mater. Sci. Mater. Electron. 2019, 30, 8751–8762. [Google Scholar] [CrossRef]



| Sample | ZnS | Eu2O3 | Li2O |
|---|---|---|---|
| Eu5 | 95 | 5 | 0 |
| Eu5_Li1 | 94 | 5 | 1 |
| Eu5_Li5 | 90 | 5 | 5 |
| Eu5_Li10 | 85 | 5 | 10 |
| Wavelength (nm) | Quality Factor (Q) | Fineness (F) |
|---|---|---|
| 384.5 | 1022.88 | 3.09 |
| 385.66 | 959.78 | 3.26 |
| 386.97 | 860.70 | 3.16 |
| 388.39 | 788.93 | 3.39 |
| 390.06 | 723.67 | 3.41 |
| 391.9 | 663.90 | 3.47 |
| 393.95 | 602.74 | 3.44 |
| 396.2 | 555.52 | 3.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavón, F.; Urbieta, A.; Fernández, P. Characterization, Luminescence and Optical Resonant Modes of Eu-Li Co-Doped ZnO Nano- and Microstructures. Appl. Sci. 2022, 12, 6948. https://doi.org/10.3390/app12146948
Pavón F, Urbieta A, Fernández P. Characterization, Luminescence and Optical Resonant Modes of Eu-Li Co-Doped ZnO Nano- and Microstructures. Applied Sciences. 2022; 12(14):6948. https://doi.org/10.3390/app12146948
Chicago/Turabian StylePavón, Fernanado, Ana Urbieta, and Paloma Fernández. 2022. "Characterization, Luminescence and Optical Resonant Modes of Eu-Li Co-Doped ZnO Nano- and Microstructures" Applied Sciences 12, no. 14: 6948. https://doi.org/10.3390/app12146948
APA StylePavón, F., Urbieta, A., & Fernández, P. (2022). Characterization, Luminescence and Optical Resonant Modes of Eu-Li Co-Doped ZnO Nano- and Microstructures. Applied Sciences, 12(14), 6948. https://doi.org/10.3390/app12146948








