α-MnO2 Nanowire Structure Obtained at Low Temperature with Aspects in Environmental Remediation and Sustainable Energy Applications
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of α-MnO2 Materials
2.2. Preparation of the Modified Electrodes
2.3. Characterization Techniques
3. Results and Discussions
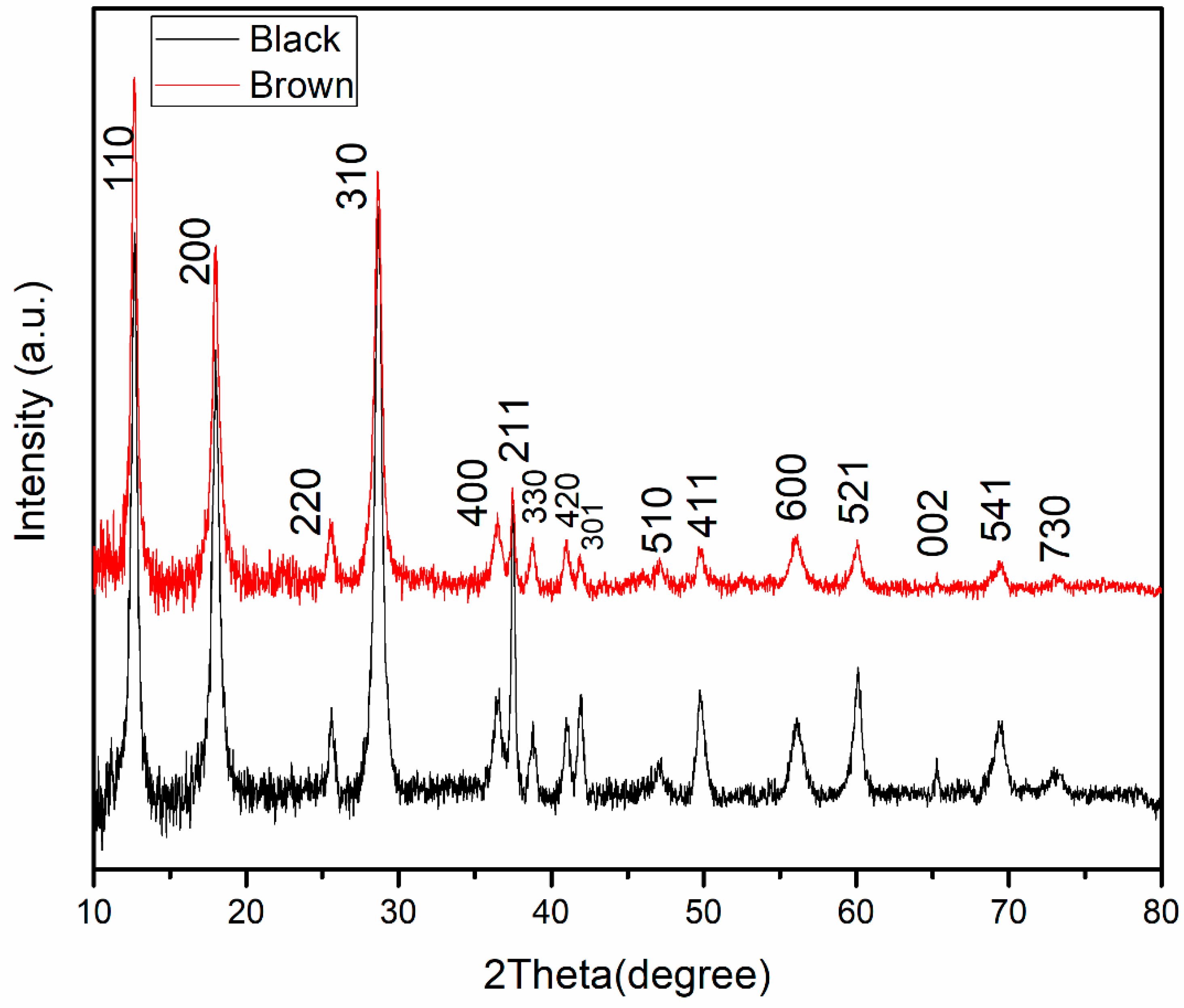
3.1. X-ray Diffraction
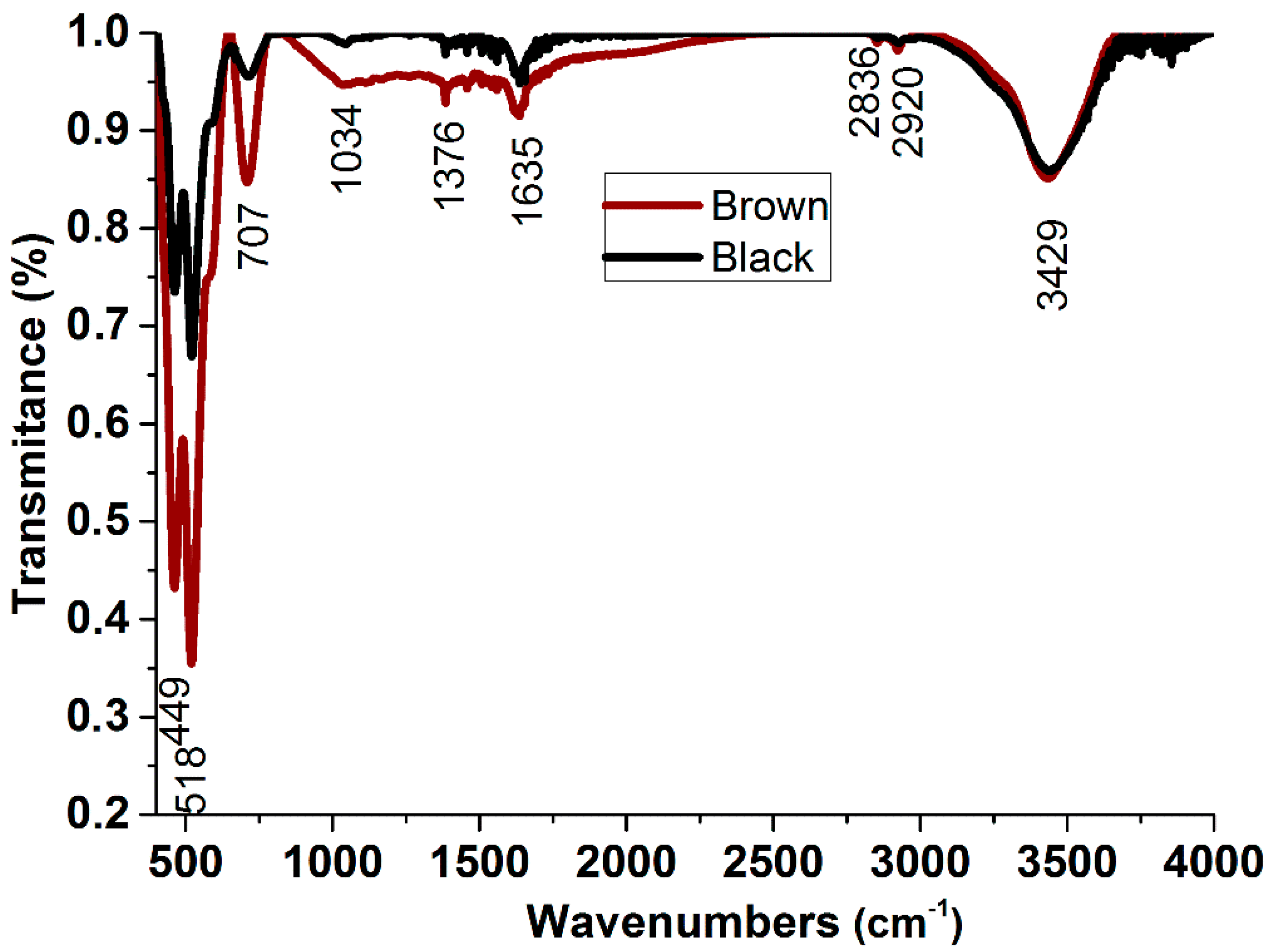
3.2. FT-IR Analysis
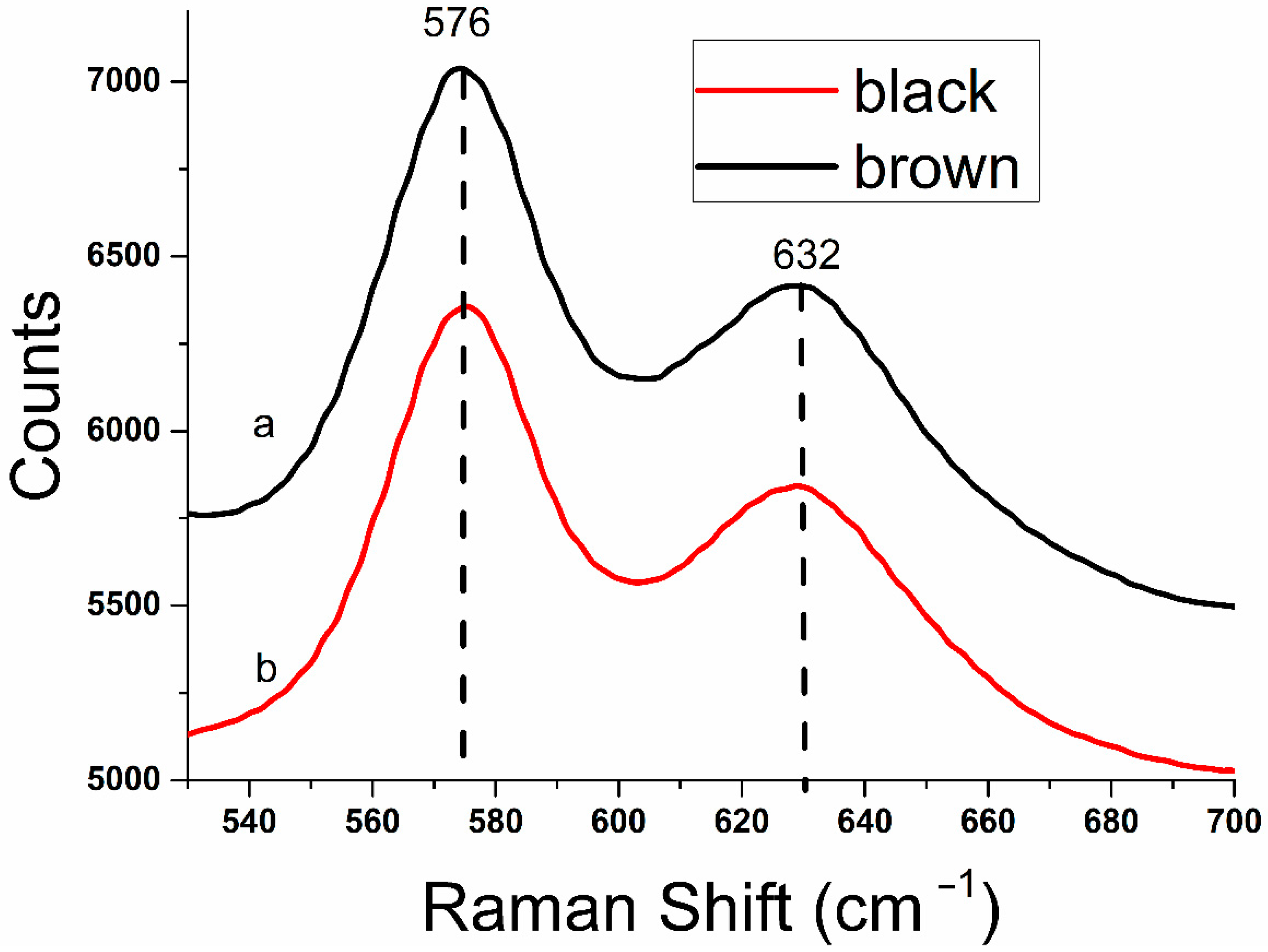
3.3. Raman Shift Spectroscopy
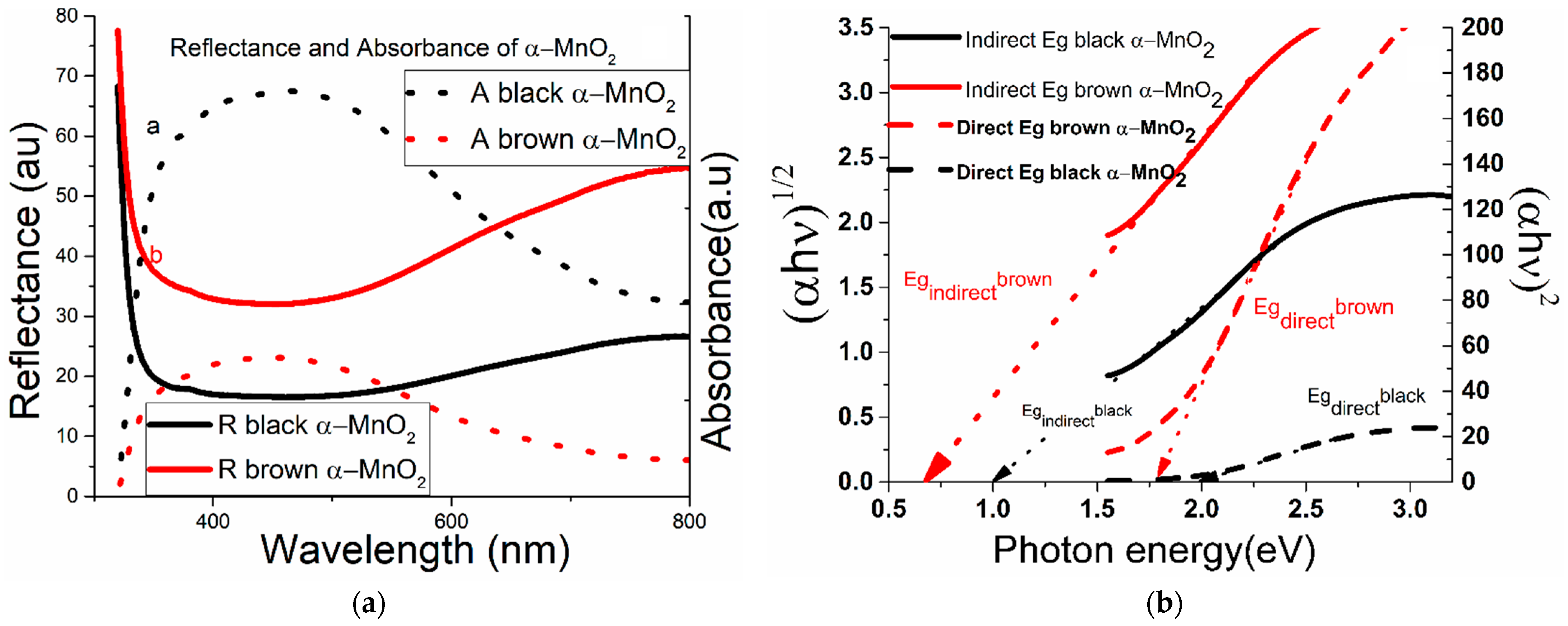
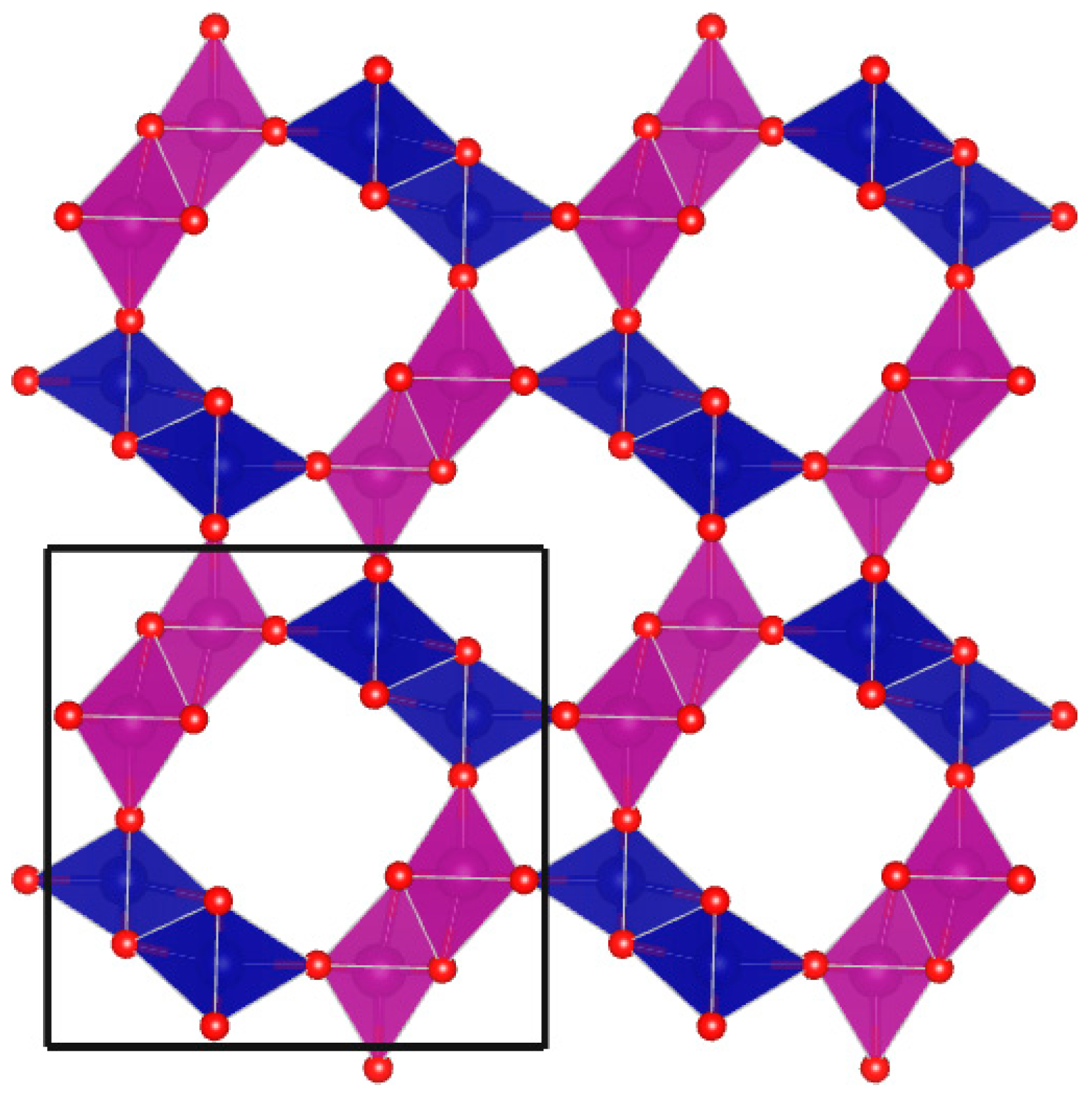
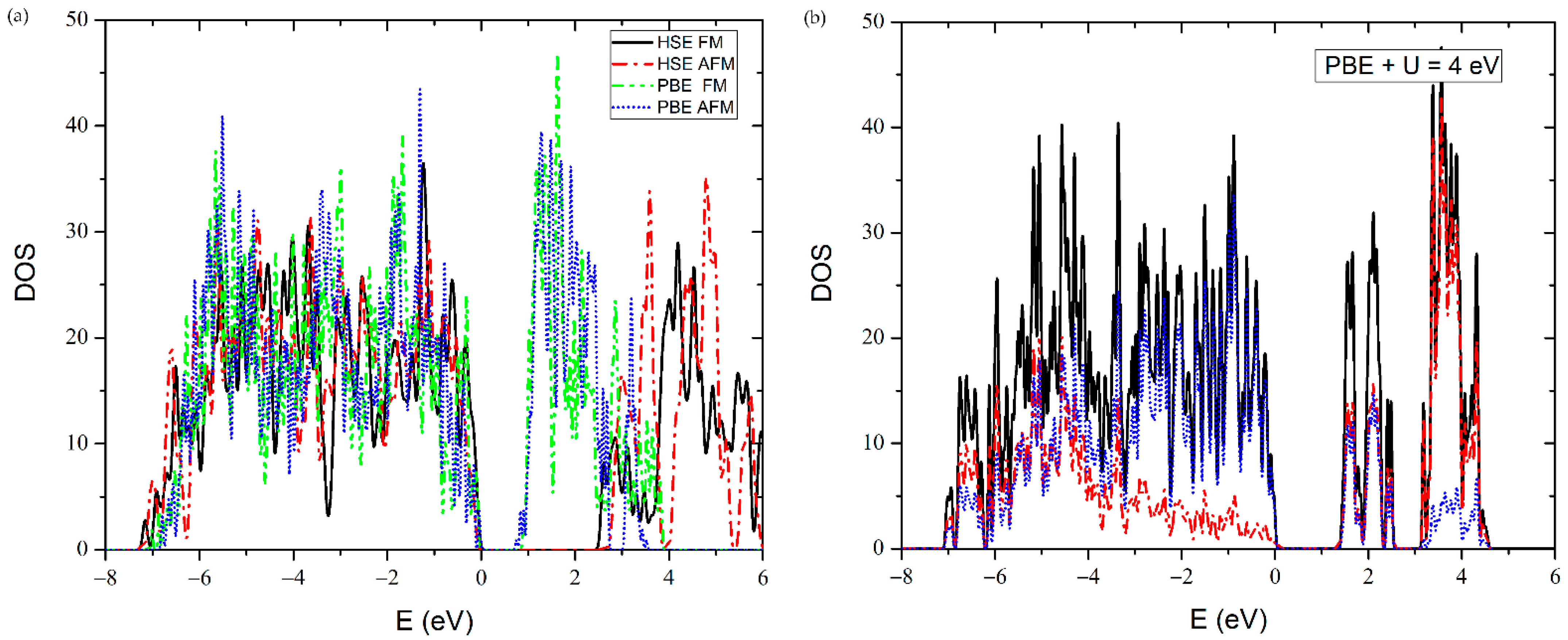
3.4. Ultraviolet-Visible Spectroscopy and Ab Initio Calculations
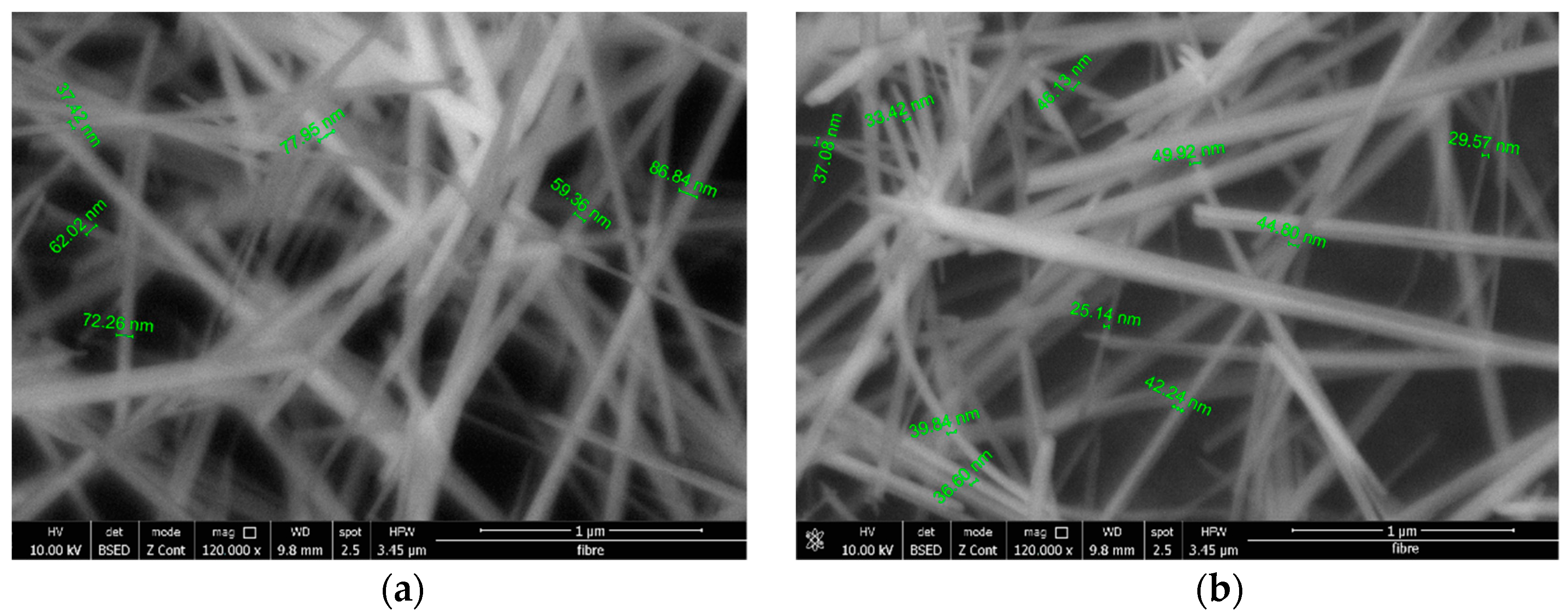
3.5. Scanning Electron Microscopy
3.6. Thermal Analysis
3.7. Electrochemical Measurements
3.8. Catalytic Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duffy, P.; Fitzpatrick, C.; Conway, T.; Lynch, R.P. Energy sources and supply grids—The growing need for storage. In Energy Storage Options and Their Environmental Impact; Royal Society of Chemistry: London, UK, 2019; pp. 1–41. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, C.; Chen, P.; Yuan, D.; Guo, K. MnO2-decorated hierarchical porous carbon composites for high-performance asymmetric supercapacitors. J. Power Sources 2019, 425, 1–9. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Wang, J.; Xu, C.; Jiang, H.; Li, C.; Zhang, L.; Lin, J.; Shen, Z.X. Advanced energy storage devices: Basic principles, analytical methods, and rational materials design. Adv. Sci. 2018, 5, 1700322. [Google Scholar] [CrossRef]
- Shao, H.; Wu, Y.C.; Lin, Z.; Taberna, P.L.; Simon, P. Nanoporous carbon for electrochemical capacitive energy storage. Chem. Soc. Rev. 2020, 49, 3005–3039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Xie, X.; Zhang, Y.; Zhang, D.; Du, W.; Zhang, X.; Wang, B. MnO2/carbon composites for supercapacitor: Synthesis and electrochemical performance. Front. Mater. 2020, 7, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.; Wang, Z.; Li, H.; Xiong, Y.; Liu, Z.; Tang, Z.; Huang, Y.; Rogach, A.L.; Zhi, C. Light-permeable, photoluminescent microbatteries embedded in the color filter of a screen. Energy Environ. Sci. 2018, 11, 2414–2422. [Google Scholar] [CrossRef]
- Zeng, Y.; Yu, M.; Meng, Y.; Fang, P.; Lu, X.; Tong, Y. Iron-based supercapacitor electrodes: Advances and challenges. Adv. Energy Mater. 2016, 6, 1–17. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Hu, L.; Vosgueritchian, M.; Wang, H.; Xie, X.; McDonough, J.R.; Cui, X.; Cui, Y.; Bao, Z. Solution-processed graphene/MnO2 nanostructured textiles for high-performance electrochemical capacitors. Nano Lett. 2011, 11, 2905–2911. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A. Nanostructured MnO2 as electrode materials for energy storage. Nanomaterials 2017, 7, 396. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.K. Diversity in the family of manganese oxides at the nanoscale: From fundamentals to applications. ACS Omega 2020, 5, 25493–25504. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, E.; Komanoya, T.; Kamata, K.; Hara, M. Heterogeneously-catalyzed aerobic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with MnO2. ChemSusChem 2017, 10, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Gao, P.; Wang, L.; Zhang, Y.; Deng, Y.; Huang, R.; Zhao, S.; Yu, Z.; Wei, Y.; Wang, G.; et al. Regulating crystal facets of MnO2 for enhancing peroxymonosulfate activation to degrade pollutants: Performance and mechanism. Catalysts 2022, 12, 342. [Google Scholar] [CrossRef]
- Song, H.; Xu, L.; Chen, M.; Cui, Y.; Wu, C.-E.; Qiu, J.; Xu, L.; Cheng, G.; Hu, X. Recent progresses in the synthesis of MnO2 nanowire and its application in environmental catalysis. RSC Adv. 2021, 11, 35494–35513. [Google Scholar] [CrossRef]
- Ferraz, C.P.; Da Silva, A.G.M.; Rodrigues, T.S.; Camargo, P.H.C.; Paul, S.; Wojcieszak, R. Furfural oxidation on gold supported on MnO2: Influence of the support structure on the catalytic performances. Appl. Sci. 2018, 8, 1246. [Google Scholar] [CrossRef] [Green Version]
- Kuźniarska-Biernacka, I.; Garbarz-Glos, B.; Skiba, E.; Maniukiewicz, W.; Bąk, W.; Antonova, M.; Rebelo, S.; Freire, C. Evaluation of Rhodamine B photocatalytic degradation over BaTiO3-MnO2 ceramic materials. Materials 2021, 14, 3152. [Google Scholar] [CrossRef]
- Ji, X.; Guo, Y.; Hua, S.; Li, H.; Zhang, S. Interaction-determined sensitization photodegradation of dye complexes by boron nitride under visible light irradiation: Experimental and theoretical studies. New J. Chem. 2020, 44, 9238–9247. [Google Scholar] [CrossRef]
- Chan, Y.-L.; Pung, S.-Y.; Sreekantan, S.; Yeoh, F.-Y. Photocatalytic activity of β-MnO2 nanotubes grown on PET fibre under visible light irradiation. J. Exp. Nanosci. 2016, 11, 603–618. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.-J.; Huang, H.-Z.; Yuan, B.; Fu, M.-L. Decolorization of RhB dye by manganese oxides: Effect of crystal type and solution pH. Geochem. Trans. 2015, 16, 10. [Google Scholar] [CrossRef] [Green Version]
- Sabna, V.; Thampi, S.G.; Chandrakaran, S. Degradation of Rhodamine B with manganese dioxide nanorods. J. Water Health 2018, 16, 846–856. [Google Scholar] [CrossRef]
- Periyasamy, G.; Patil, I.M.; Kakade, B.; Veluswamy, P.; Archana, J.; Ikeda, H.; Annamalai, K. Reduced graphene oxide-wrapped α-Mn2O3/α-MnO2 nanowires for electrocatalytic oxygen reduction in alkaline medium. J. Mater. Sci. Mater. Electron. 2022, 33, 8644–8654. [Google Scholar] [CrossRef]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.W.A.; Idris, A. Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Revathi, B.; Balakrishnan, L.; Pichaimuthu, S.; Grace, A.N.; Chandar, N.K. Photocatalytic degradation of Rhodamine B using BiMnO3 nanoparticles under UV and visible light irradiation. J. Mater. Sci. Mater. Electron. 2020, 31, 22487–22497. [Google Scholar] [CrossRef]
- Sundararajan, M.; Sailaja, V.; Kennedy, L.J.; Vijaya, J.J. Photocatalytic degradation of Rhodamine B under visible light using nanostructured zinc doped cobalt ferrite: Kinetics and mechanism. Ceram. Int. 2017, 43, 540–548. [Google Scholar] [CrossRef]
- Liu, C.; Pan, D.; Tang, X.; Hou, M.; Zhou, Q.; Zhou, J. Degradation of Rhodamine B by the α-MnO2/peroxymonosulfate system. Water Air Soil Pollut. 2016, 227, 92. [Google Scholar] [CrossRef]
- Wang, S.; Guan, A.; Wang, J.; Fu, X.; Guo, X.; Tian, Y.; Wang, K.; Cao, W.; Zhao, C. Highly efficient degradation of Rhodamine B by α-MnO2 nanorods. Bull. Mater. Sci. 2022, 45, 35. [Google Scholar] [CrossRef]
- Wang, S.; Guan, A.; Wang, J.; Fu, X.; Guo, X.; Tian, Y.; Wang, K.; Cao, W. Facile Synthesis of a High Purity α-MnO2 Nanorod for Rapid Degradation of Rhodamine B. 2021. Available online: https://www.researchsquare.com/article/rs-679600/v1 (accessed on 16 June 2022).
- Sui, N.; Duan, Y.; Jiao, X.; Chen, D. Large-scale preparation and catalytic properties of one-dimensional α/β-MnO2 nanostructures. J. Phys. Chem. C 2009, 113, 8560–8565. [Google Scholar] [CrossRef]
- Chandiran, K.; Murugesan, R.A.; Balaji, R.; Andrews, N.G.; Pitchaimuthu, S.; Raja, K.C.N. Long single crystalline α-Mn2O3 nanorods: Facile synthesis and photocatalytic application. Mater. Res. Express 2020, 7, 074001. [Google Scholar] [CrossRef]
- Pokhrel, R.; Goetz, M.K.; Shaner, S.E.; Wu, X.; Stahl, S.S. The “best catalyst” for water oxidation depends on the oxidation method employed: A case study of manganese oxides. J. Am. Chem. Soc. 2015, 137, 8384–8387. [Google Scholar] [CrossRef]
- Cheng, J.-H.; Shao, G.; Yu, H.-J.; Xu, J.-J. Excellent catalytic and electrochemical properties of the mesoporous MnO2 nanospheres/nanosheets. J. Alloys Compd. 2010, 505, 163–167. [Google Scholar] [CrossRef]
- Bing, Y.; Zhang, L.; Mu, S.; Zhang, J. Facile synthesis of α-MnO2 with a 3D staghorn coral-like micro-structure assembled by nano-rods and its application in electrochemical supercapacitors. Appl. Sci. 2017, 7, 511. [Google Scholar] [CrossRef] [Green Version]
- Kanha, P.; Saengkwamsawang, P. Effect of stirring time on morphology and crystalline features of MnO2 nanoparticles synthesized by co-precipitation method. Inorg. Nano-Met. Chem. 2017, 47, 1129–1133. [Google Scholar] [CrossRef]
- Sanchez-Botero, L.; Herrera, A.P.; Hinestroza, J.P. Oriented growth of α-MnO2 nanorods using natural extracts from grape stems and apple peels. Nanomaterials 2017, 7, 117. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Zhang, Y.; Banis, M.N.; Liu, J.; Geng, D.; Li, R.; Sun, X. Facile controlled synthesis and growth mechanisms of flower-like and tubular MnO2 nanostructures by microwave-assisted hydrothermal method. J. Colloid Interface Sci. 2011, 369, 123–128. [Google Scholar] [CrossRef]
- Shah, S.I.; Zulfiqar; Khan, T.; Khan, R.; Khan, S.A.; Khattak, S.A.; Khan, G. Study of structural, optical and dielectric properties of α-MnO2 nanotubes (NTS). J. Mater. Sci. Mater. Electron. 2019, 30, 19199–19205. [Google Scholar] [CrossRef]
- Saratovsky, I.; Wightman, P.G.; Pastén, P.A.; Gaillard, J.-F.; Poeppelmeier, K.R. Manganese oxides: Parallels between abiotic and biotic structures. J. Am. Chem. Soc. 2006, 128, 11188–11198. [Google Scholar] [CrossRef]
- Gao, T.; Glerup, M.; Krumeich, F.; Nesper, R.; Fjellvåg, H.; Norby, P. Microstructures and spectroscopic properties of cryptomelane-type manganese dioxide nanofibers. J. Phys. Chem. C 2008, 112, 13134–13140. [Google Scholar] [CrossRef]
- Wu, T.-H.; Hesp, D.; Dhanak, V.; Collins, C.; Braga, F.; Hardwick, L.J.; Hu, C.-C. Charge storage mechanism of activated manganese oxide composites for pseudocapacitors. J. Mater. Chem. A 2015, 3, 12786–12795. [Google Scholar] [CrossRef]
- Polverejan, M.; Villegas, A.J.C.; Suib, S.L. Higher valency ion substitution into the manganese oxide framework. J. Am. Chem. Soc. 2004, 126, 7774–7775. [Google Scholar] [CrossRef]
- Gao, T.; Fjellvåg, H.; Norby, P. A comparison study on Raman scattering properties of α- and β-MnO2. Anal. Chim. Acta 2009, 648, 235–239. [Google Scholar] [CrossRef]
- Li, S.; Ma, Z.; Wang, L.; Liu, J. Influence of MnO2 on the photocatalytic activity of P-25 TiO2 in the degradation of methyl orange. Sci. China Ser. B Chem. 2008, 51, 179–185. [Google Scholar] [CrossRef]
- Xie, Y.; Yu, Y.; Gong, X.; Guo, Y.; Guo, Y.; Wang, Y.; Lu, G. Effect of the crystal plane figure on the catalytic performance of MnO2 for the total oxidation of propane. CrystEngComm 2015, 17, 3005–3014. [Google Scholar] [CrossRef]
- Hernández, W.; Centeno, M.A.; Sarria, F.R.; Ivanova, S.; Montes, M.; Odriozola, J.A. Modified cryptomelane-type manganese dioxide nanomaterials for preferential oxidation of CO in the presence of hydrogen. Catal. Today 2010, 157, 160–165. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV–Vis spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Cui, X.; Zeng, R.; Du, G.; Sun, Z.; Zheng, R.; Ringer, S.; Dou, S.X. Performance modulation of α-MnO2 nanowires by crystal facet engineering. Sci. Rep. 2015, 5, srep08987. [Google Scholar] [CrossRef] [PubMed]
- Toufiq, A.M.; Wang, F.; Javed, Q.-U. Synthesis, characterization and optical property of shrimps-like nanostructures of MnO2 by hydrothermal route. J. Nanosci. Nanotechnol. 2013, 13, 2948–2952. [Google Scholar] [CrossRef] [PubMed]
- Zahan, M.; Podder, J. Structural, optical and electrical properties of Cu:MnO2 nanostructured thin films for glucose sensitivity measurements. SN Appl. Sci. 2020, 2, 385. [Google Scholar] [CrossRef] [Green Version]
- Cockayne, E. Thermodynamics of the flexible metal–organic framework material MIL-53(Cr) from first-principles. J. Phys. Chem. C 2017, 121, 4312–4317. [Google Scholar] [CrossRef] [Green Version]
- Hamann, D.R. Optimized norm-conserving Vanderbilt pseudopotentials. Phys. Rev. B 2013, 88, 085117. [Google Scholar] [CrossRef] [Green Version]
- Krukau, A.V.; Vydrov, O.A.; Izmaylov, A.F.; Scuseria, G.E. Influence of the exchange screening parameter on the performance of screened hybrid functionals. J. Chem. Phys. 2006, 125, 224106. [Google Scholar] [CrossRef]
- Van Setten, M.; Giantomassi, M.; Bousquet, E.; Verstraete, M.; Hamann, D.; Gonze, X.; Rignanese, G.-M. The PseudoDojo: Training and grading a 85 element optimized norm-conserving pseudopotential table. Comput. Phys. Commun. 2018, 226, 39–54. [Google Scholar] [CrossRef] [Green Version]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef] [PubMed]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Nardelli, M.B.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys. Condens. Matter 2017, 29, 465901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudarev, S.L.; Botton, G.A.; Savrasov, S.Y.; Humphreys, C.J.; Sutton, A.P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys. Rev. B 1998, 57, 1505–1509. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Bruno, M.; Palummo, M.; Marini, A.; Del Sole, R.; Ossicini, S. From Si nanowires to porous silicon: The role of excitonic effects. Phys. Rev. Lett. 2007, 98, 036807. [Google Scholar] [CrossRef] [Green Version]
- Corpuz, R.D.; De Juan-Corpuz, L.M.; Nguyen, M.T.; Yonezawa, T.; Wu, H.-L.; Somwangthanaroj, A.; Kheawhom, S. Binder-free α-MnO2 nanowires on carbon cloth as cathode material for Zinc-Ion batteries. Int. J. Mol. Sci. 2020, 21, 3113. [Google Scholar] [CrossRef]
- Song, J.-X.; Guo, T.; Ding, W.; Yao, M.; Yang, L.; Zhang, X.-N.; Yu, Z.-S.; Wu, J.-X.; Zhang, J.; Fang, X. The effect of al particles size on the thermal behavior and kinetics of Al-MnO2 thermite system. Adv. Mater. Sci. Eng. 2020, 2020, 3097404. [Google Scholar] [CrossRef]
- Ramli, N.I.; Ismail, N.A.B.; Abd-Wahab, F.; Salim, W.W.A.W. Cyclic voltammetry and electrical impedance spectroscopy of electrodes modified with PEDOT:PSS-reduced graphene oxide composite. In Transparent Conducting Films; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Zaman, W.Q.; Sun, W.; Cao, L.-M.; Tariq, M.; Yang, J. Cultivating crystal lattice distortion in IrO2 via coupling with MnO2 to boost the oxygen evolution reaction with high intrinsic activity. Chem. Commun. 2018, 54, 4959–4962. [Google Scholar] [CrossRef]
- Taranu, B.O.; Fagadar-Cosma, E.; Popa, I.; Plesu, N.; Taranu, I. Adsorbed functionalized porphyrins on polyaniline modified platinum electrodes. Comparative electrochemical properties. Dig. J. Nanomat. Biostruct. 2014, 9, 667–679. [Google Scholar]
- Kellenberger, A.; Ambros, D.; Plesu, N. Scan rate dependent morphology of polyaniline films electrochemically deposited on nickel. Int. J. Electrochem. Sci. 2014, 9, 6821–6833. [Google Scholar]
- Gira, M.J.; Tkacz, K.P.; Hampton, J.R. Physical and electrochemical area determination of electrodeposited Ni, Co, and NiCo thin films. Nano Converg. 2016, 3, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebarchievici, I.; Taranu, B.; Rus, S.F.; Vlazan, P.; Poienar, M.; Sfirloaga, P. Electro-oxidation of ascorbic acid on perovskite-modified electrodes. In Proceedings of the 25th International Symposium on Analytical and Environmental Problems, Szeged, Hungary, 7–8 October 2019; pp. 273–275. [Google Scholar]
- Kölbach, M.; Fiechter, S.; van de Krol, R.; Bogdanoff, P. Evaluation of electrodeposited α-Mn2O3 as a catalyst for the oxygen evolution reaction. Catal. Today 2017, 290, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Sebarchievici, I.; Taranu, B.; Rus, S.F.; Fagadar-Cosma, E. Electrochemical behaviour and analytical applications of a manganese porphyrin-silica hybrid film prepared by pulsed laser deposition. J. Electroanal. Chem. 2020, 865, 114127. [Google Scholar] [CrossRef]
- Ciríaco, M.L.F.; Silva-Pereira, M.I.; Nunes, M.R.; Costa, F.M. Electrochemical behaviour of BaSn0.9Sb0.1O3 coated titanium electrodes. Port. Electrochim. Acta 1999, 17, 149–156. [Google Scholar] [CrossRef]
- Yao, B.; Chandrasekaran, S.; Zhang, J.; Xiao, W.; Qian, F.; Zhu, C.; Duoss, E.B.; Spadaccini, C.M.; Worsley, M.A.; Li, Y. Efficient 3D printed pseudocapacitive electrodes with ultrahigh MnO2 loading. Joule 2019, 3, 459–470. [Google Scholar] [CrossRef] [Green Version]
- Raza, S.A.; Naqvi, S.Q.; Usman, A.; Jennings, J.R.; Soon, Y.W. Spectroscopic study of the interaction between rhodamine B and graphene. J. Photochem. Photobiol. A Chem. 2021, 418, 113417. [Google Scholar] [CrossRef]
- Ahmed, R.M.; Saif, M. Optical properties of Rhodamine B dye doped in transparent polymers for sensor application. Chin. J. Phys. 2013, 51, 511–521. [Google Scholar] [CrossRef]
- Mchedlov-Petrosyan, N.O.; Kholin, Y.V. Aggregation of Rhodamine B in water. Russ. J. Appl. Chem. 2004, 77, 414–422. [Google Scholar] [CrossRef]
- Chandra, S.; Das, P.; Bag, S.; Laha, D.; Pramanik, P. Synthesis, functionalization and bioimaging applications of highly fluorescent carbon nanoparticles. Nanoscale 2011, 3, 1533–1540. [Google Scholar] [CrossRef]
- Chen, F.; Zhao, J.; Hidaka, H. Highly selective deethylation of rhodamine B: Adsorption and photooxidation pathways of the dye on the TiO2TiO2/SiO2SiO composite photocatalyst. Int. J. Photoenergy 2003, 5, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Liang, L.; Cheng, L.; Zhang, Y.; Wang, Q.; Wu, Q.; Xue, Y.; Meng, X. Efficiency and mechanisms of Rhodamine B degradation in Fenton-like systems based on zero-valent iron. RSC Adv. 2020, 10, 28509–28515. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Liu, S.; Zhang, H.; Wang, X.; Wang, J. New insight into the selective photocatalytic oxidation of RhB through a strategy of modulating radical generation. RSC Adv. 2018, 8, 13625–13634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, K.; Yang, S.; He, H.; Sun, C.; Gu, C.; Ju, Y. Visible light-driven photocatalytic degradation of Rhodamine B over NaBiO3: Pathways and mechanism. J. Phys. Chem. A 2009, 113, 10024–10032. [Google Scholar] [CrossRef] [PubMed]


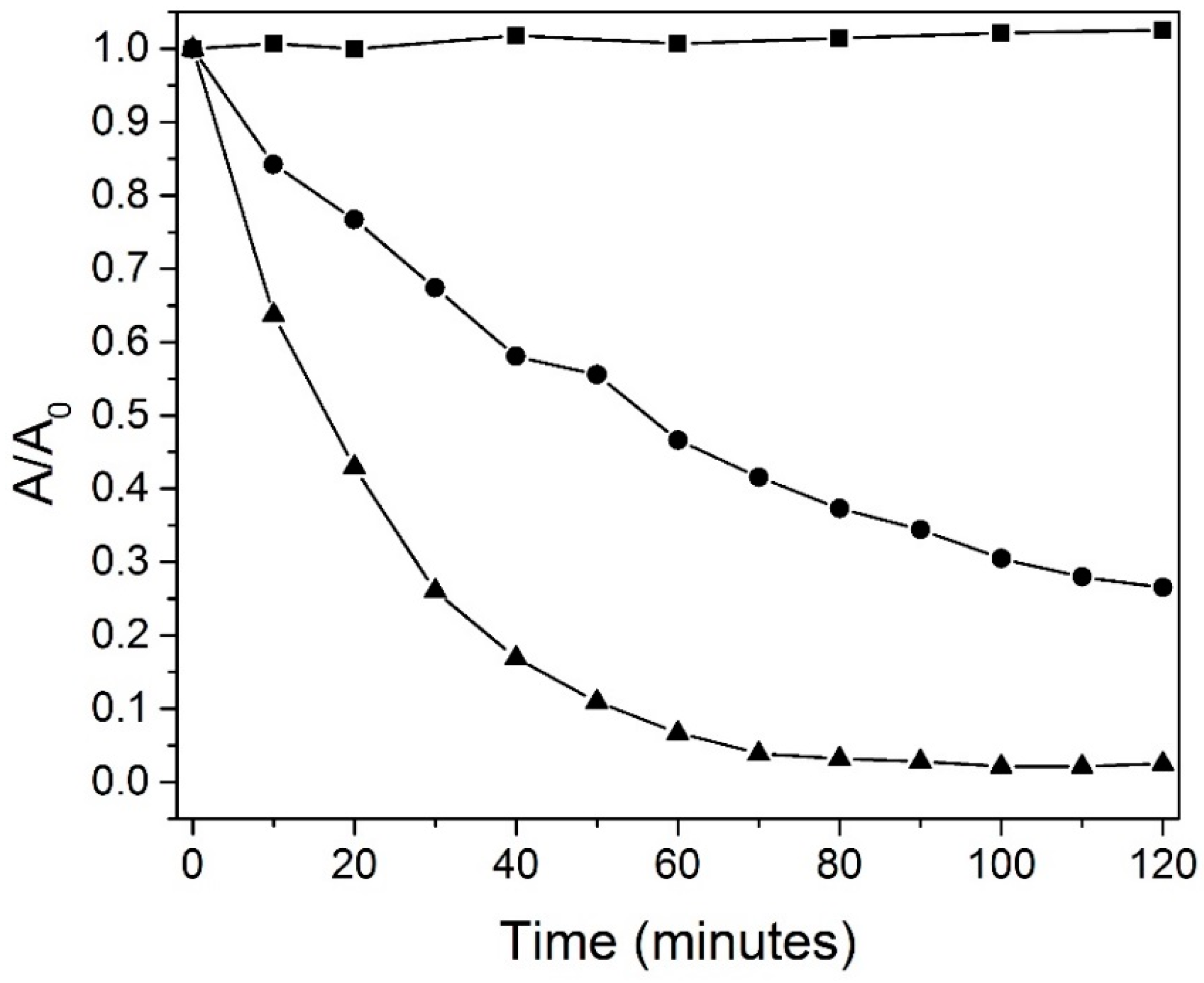

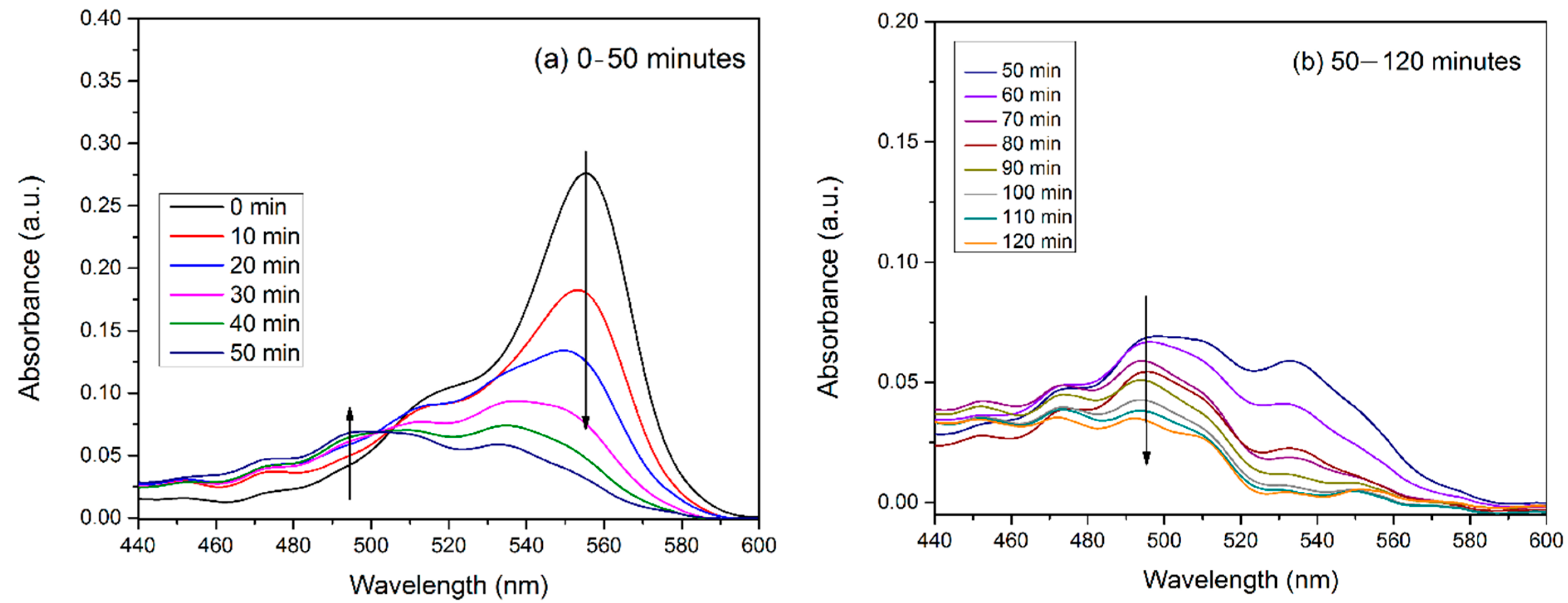

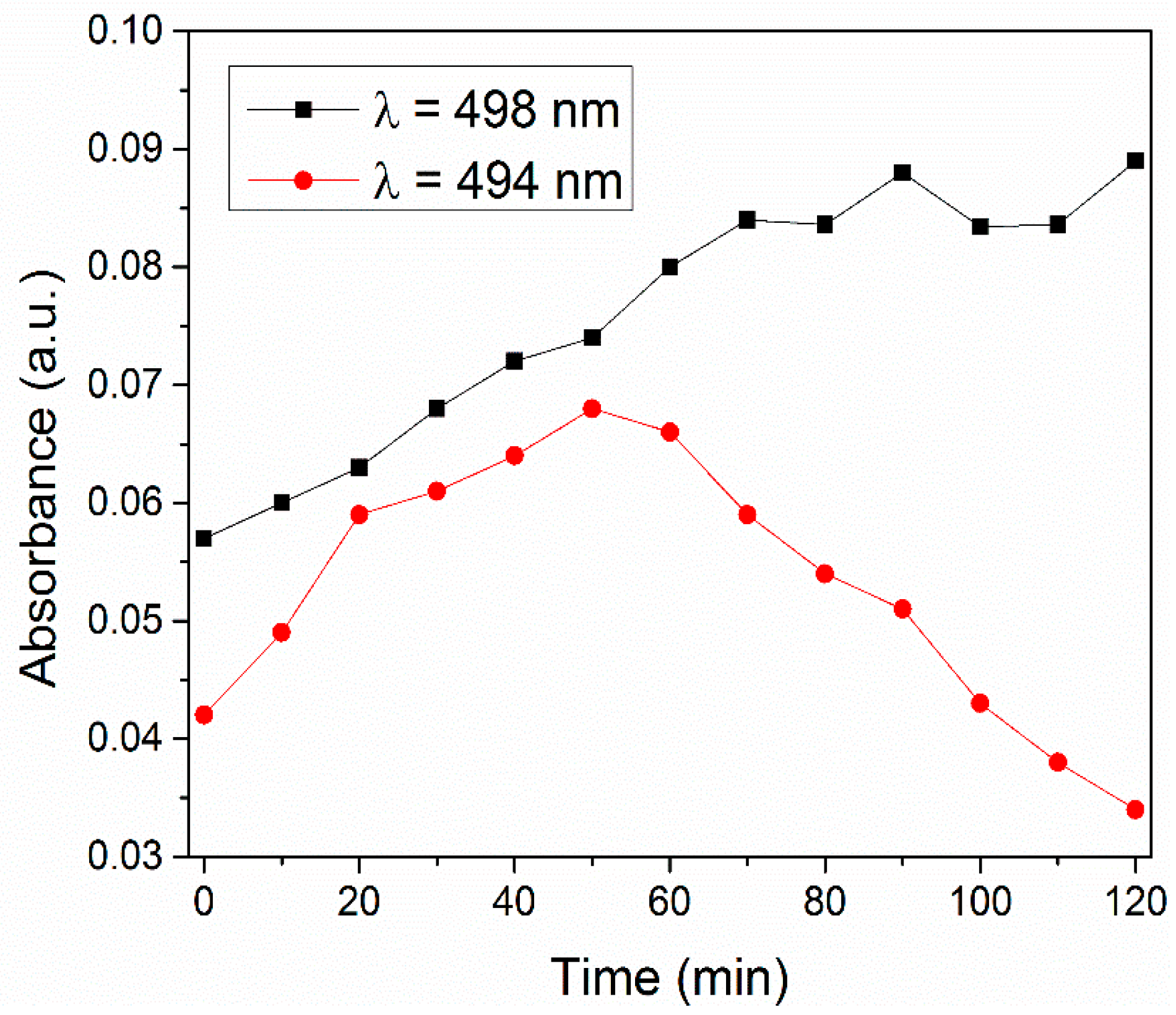
| MnO2-Type | Catalyst Concentration (g/L) | Volume and Concentration of Rhodamine B | Degradation Percent of RhB | Source |
|---|---|---|---|---|
| α-MnO2 | 0.2 (α-MnO2/PMS system; PMS-0.20 g/L) | 100 mL; 20 mg/L | 99% in 90 min | [26] |
| MnO2 aerogel | 0.5 | 10 mL; 5 mg/L (pH = 2.5) | 100% in 40 min | [27] |
| α-MnO2 nanorods | 0.2 | 50 mL; 40 mg/L (pH = 6.6) | 94.4% in 80 min | [28] |
| α-MnO2 | 1 | 30 mL; 10 mg/L (pH = 7) | 90% in 60 min | [29] |
| α-MnO2 | 0.0045 | 100 mL, 0.25 mg/L | 80% in 70 min (UV light) | [30] |
| Electrode Code | α-MnO2 (mg) | Vulcan (mg) | Nafion Solution (µL) | Ethanol (µL) | DMF (µL) |
|---|---|---|---|---|---|
| G | - | - | - | - | - |
| GV-EtOH | - | 2 | - | 500 | - |
| GN-EtOH | - | - | 10 | 500 | - |
| GV-N-EtOH | - | 2 | 10 | 500 | - |
| GMnO2-EtOH | 3 | - | - | 500 | - |
| GMnO2-V-EtOH | 3 | 2 | - | 500 | - |
| GMnO2-N-EtOH | 3 | - | 10 | 500 | - |
| GMnO2-V-N-EtOH | 3 | 2 | 10 | 500 | - |
| GV-DMF | - | 2 | - | - | 500 |
| GN-DMF | - | - | 10 | - | 500 |
| GV-N-DMF | - | 2 | 10 | - | 500 |
| GMnO2-DMF | 3 | - | - | - | 500 |
| GMnO2-V-DMF | 3 | 2 | - | - | 500 |
| GMnO2-N-DMF | 3 | - | 10 | - | 500 |
| GMnO2-V-N-DMF | 3 | 2 | 10 | - | 500 |
| Electrode Code | Cdl (mF cm−2) | R2 | Rf | EASA (cm2) |
|---|---|---|---|---|
| G | 1.323 | 0.9965 | 22.05 | 6.174 |
| GV-EtOH | 2.49 | 0.9976 | 41.5 | 11.62 |
| GN-EtOH | 1.281 | 0.9936 | 21.35 | 5.978 |
| GV-N-EtOH | 1.63 | 0.9922 | 27.17 | 7.6076 |
| GMnO2-EtOH | 2.382 | 0.9967 | 39.7 | 11.116 |
| GMnO2-V-EtOH | 3.444 | 0.9977 | 57.4 | 16.072 |
| GMnO2-N-EtOH | 1.676 | 0.9938 | 27.93 | 7.8204 |
| GMnO2-V-N-EtOH | 2 | 0.9973 | 33.33 | 9.3324 |
| GV-DMF | 2.69 | 0.9593 | 44.83 | 12.554 |
| GN-DMF | 1.309 | 0.9925 | 21.82 | 6.1096 |
| GV-N-DMF | 1.76 | 0.9516 | 29.33 | 8.2124 |
| GMnO2-DMF | 2.66 | 0.9807 | 44.33 | 12.4124 |
| GMnO2-V-DMF | 3.41 | 0.9584 | 56.83 | 15.9124 |
| GMnO2-N-DMF | 1.512 | 0.9647 | 25.2 | 7.056 |
| GMnO2-V-N-DMF | 2.12 | 0.9966 | 35.33 | 9.8924 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taranu, B.-O.; Novaconi, S.D.; Ivanovici, M.; Gonçalves, J.N.; Rus, F.S. α-MnO2 Nanowire Structure Obtained at Low Temperature with Aspects in Environmental Remediation and Sustainable Energy Applications. Appl. Sci. 2022, 12, 6821. https://doi.org/10.3390/app12136821
Taranu B-O, Novaconi SD, Ivanovici M, Gonçalves JN, Rus FS. α-MnO2 Nanowire Structure Obtained at Low Temperature with Aspects in Environmental Remediation and Sustainable Energy Applications. Applied Sciences. 2022; 12(13):6821. https://doi.org/10.3390/app12136821
Chicago/Turabian StyleTaranu, Bogdan-Ovidiu, Stefan Danica Novaconi, Madalina Ivanovici, João Nuno Gonçalves, and Florina Stefania Rus. 2022. "α-MnO2 Nanowire Structure Obtained at Low Temperature with Aspects in Environmental Remediation and Sustainable Energy Applications" Applied Sciences 12, no. 13: 6821. https://doi.org/10.3390/app12136821
APA StyleTaranu, B.-O., Novaconi, S. D., Ivanovici, M., Gonçalves, J. N., & Rus, F. S. (2022). α-MnO2 Nanowire Structure Obtained at Low Temperature with Aspects in Environmental Remediation and Sustainable Energy Applications. Applied Sciences, 12(13), 6821. https://doi.org/10.3390/app12136821






