Abstract
(1) Background: Endoscopic ultrasound-guided radiofrequency ablation (EUS-RFA) is an alternative treatment for pancreatic tumors. Currently, EUS-RFA has been trialled to treat hepatic tumors. However, little has been reported about optimal settings for EUS-RFA in patients with hepatic tumors. We evaluated the ablation effect after in vivo RFA using a new EUS-RFA electrode in a pig model. (2) Methods Four pigs were used for the in vivo test. The in vivo testing was divided into two tests based on the length of the RFA electrode (0.5, 0.7, 1 or 1.5 cm), the ablation power (30 W or 50 W), and the ablation time (10 or 15 s). In test one, ablation effect was evaluated based on the electrode length and power. In test two, ablation effect was assessed based on power and time. (3) Results: In test one, the ablation width and depth correlated with the length of the electrode and power (0.5 cm, 10 W, 10 s: width 0.46 cm, depth 0.65 cm vs. 1.5 cm, 75 W, 10 s: width 0.77 cm, depth 1.80 cm). In test two, ablation width and depth were similar when RFA was set at 1.5cm, 50 W, and 10 s or 1 cm, 30 W, and 15 s (0.65 cm, 1.14 cm vs. 0.65cm, 1.26 cm). (4) Conclusions: The relationship between electrode length, ablation power, and ablation time, and the resulting ablation effect in pig livers suggest that EUS-RFA produces effective ablation while minimizing thermal injury.
1. Introduction
Radiofrequency ablation (RFA) has been considered as safe and effective for the treatment of tumors of the liver, lung, and kidney [1]. Traditionally, RFA is performed percutaneously under US or CT guidance [2]. However, this procedure is unsuitable for patients with massive ascites. Percutaneous RFA is also technically challenging in ablation of tumors located in the caudate lobe of the liver. Recently, endoscopic ultrasound (EUS) has emerged as an essential diagnostic examination for the management of various gastrointestinal, hepatobiliary, and pancreatic diseases. Therefore, EUS-guided RFA (EUS-RFA) has been increasingly utilized as an alternative therapeutic option for the treatment of various pancreatobiliary tumors [1,3,4]. As the experience of EUS-RFA in pancreatic tumors has been accumulated, EUS-RFA has also been tried for hepatic tumors [5,6].
However, there has been concern regarding RFA-related adverse events such as thermal injury to structures around the RFA probe as well as its technical limitations [7]. There is also a paucity of preclinical feasibility studies of EUS-RFA involving normal tissue in vivo. Therefore, we aimed to evaluate the ablation effect after in vivo RFA using a new EUS-RFA electrode at various probe-electrode lengths, power settings, and ablation times in a live pig model.
2. Materials and Methods
2.1. RFA Device
A newly designed 19-gauge RFA electrode and a VIVA radiofrequency (RF) generator (STARmed, Goyang, Korea) were used for RFA (Figure 1). The total length of the electrode, including delivery system, was 150 cm. The exposed distal end of the electrode was needle-shaped and echogenic. The various lengths of the exposed tip were as follows: 0.5 cm, 0.7 cm, 1 cm, and 1.5 cm. RF energy was delivered by the VIVA RF generator. During ablation, the RF electrode was cooled and internally perfused with circulating chilled saline solution (0 °C) delivered via a pump to prevent overheating.
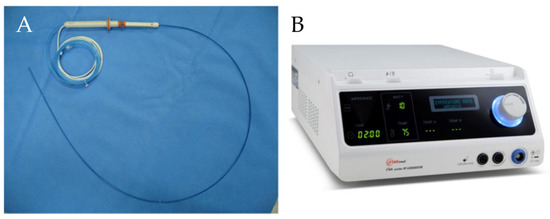
Figure 1.
Endoscopic radiofrequency electrode and power generator (STARmed, Seoul, Korea). (A) 19-gauge endoscopic radiofrequency ablation electrode. (B) A VIVA radiofrequency power generator.
2.2. In Vivo Testing
Four mini pigs (Sus scrofa; mean body weight, 30 kg) were used for the in vivo experimental study. All mini pigs were given only water for 24 h and fasted overnight before the procedures. The pigs were intubated and administered 1.5% isoflurane (Forane; JW Pharmaceutical, Seoul, Korea). Cardiopulmonary parameters were monitored throughout the procedure. An upper midline incision was made from the xiphoid process to the umbilicus, and RFA was performed under general anesthesia.
In vivo testing was divided into two tests based on the length of the exposed distal end of the RFA electrode, the electrical energy, and the ablation time. In test one, two pigs were divided into four groups, which were assigned based on the length of the exposed distal end of the RFA electrode and RFA power (Group A: 0.5 cm, 10 W, 10 s; Group B: 0.7 cm, 25 W 10 s; Group C: 1 cm, 50 W, 10 s; Group D: 1.5 cm, 75 W, 10 s). In test two, three pigs were divided into three groups which were assigned based on RFA power and ablation time (Group A: 1 cm, 30 W, 10 s; Group B: 1 cm, 30 W, 15 s; Group C: 1 cm, 50 W, 10 s). All procedures were repeated five times, after which the pigs were euthanized. Livers were immediately surgically excised for gross examination of damage and tissue response. For gross analysis, the area of visible coagulation and necrosis was measured using calipers in fresh tissue. The animal care and use committee of our institution approved this animal study protocol (IACUC No. 2016-14-063).
2.3. Statistical Analysis
Results are expressed as the mean ± standard deviation (SD). Statistical analysis was performed using SPSS® Statistics 22.0 software (SPSS Inc., Chicago, IL, USA).
3. Results
Outcomes of RFA
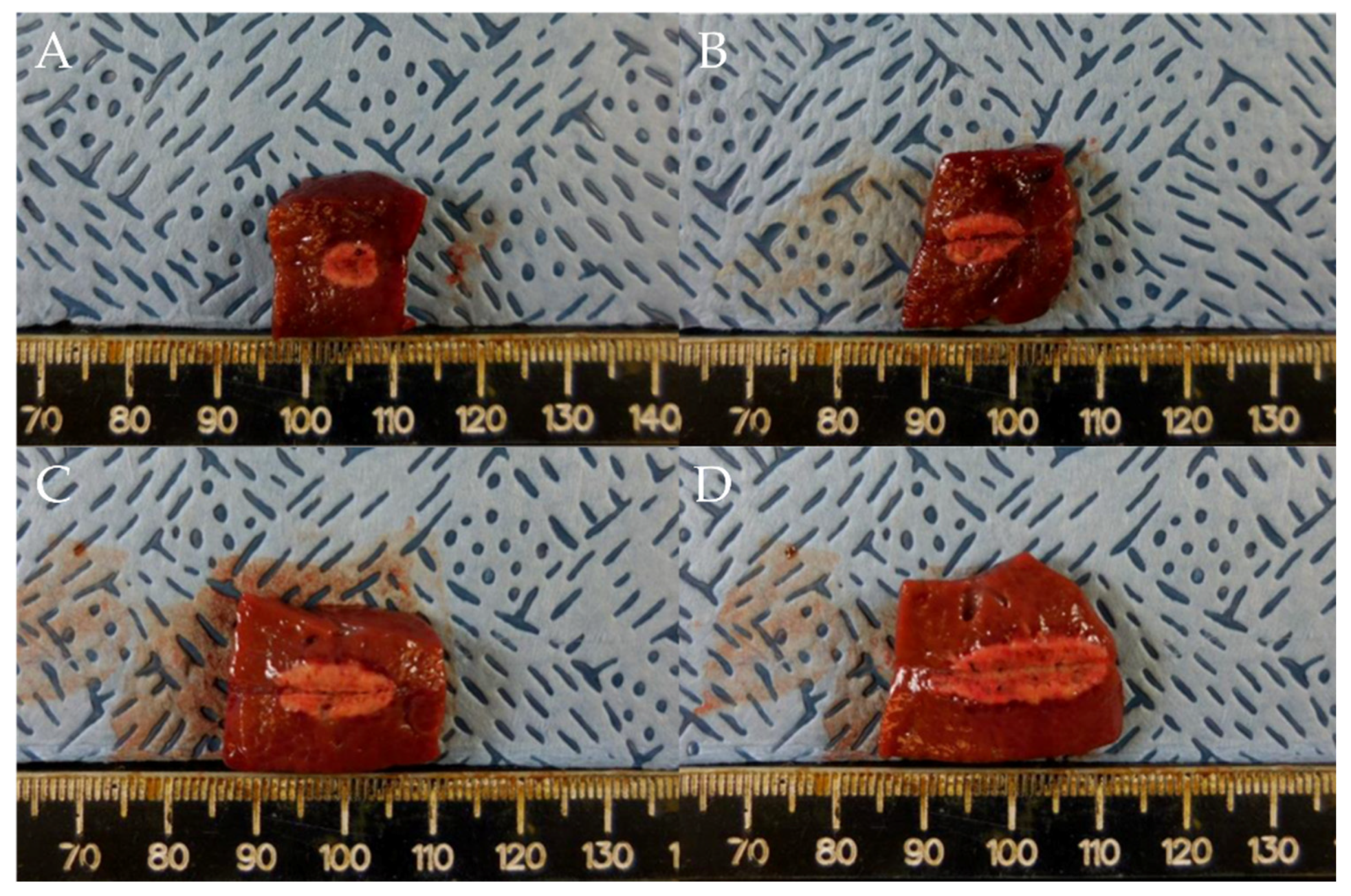
Gross necropsy findings showed a well-demarcated ablated lesion that could be distinguished from surrounding liver parenchyma by a whitish peripheral rim (Figure 2).
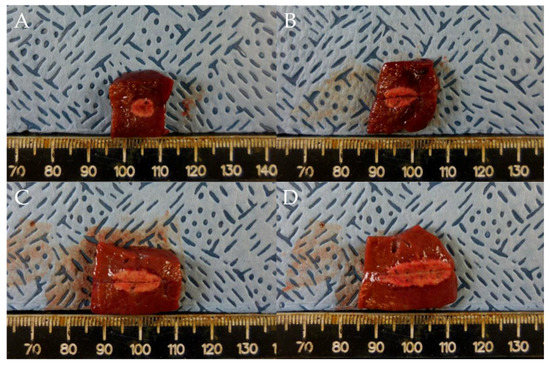
Figure 2.
Comparison of ablation zones based on electrode robe length and ablation power. (A) 0.5 cm, 10 W, 10 s. (B) 0.7 cm, 25 W, 10 s (C) 1 cm, 50 W, 10 s. (D) 1.5 cm, 75 W, 10 s. Specimens exhibit a well-demarcated ablated lesion that is distinguishable from the surrounding liver parenchyma.
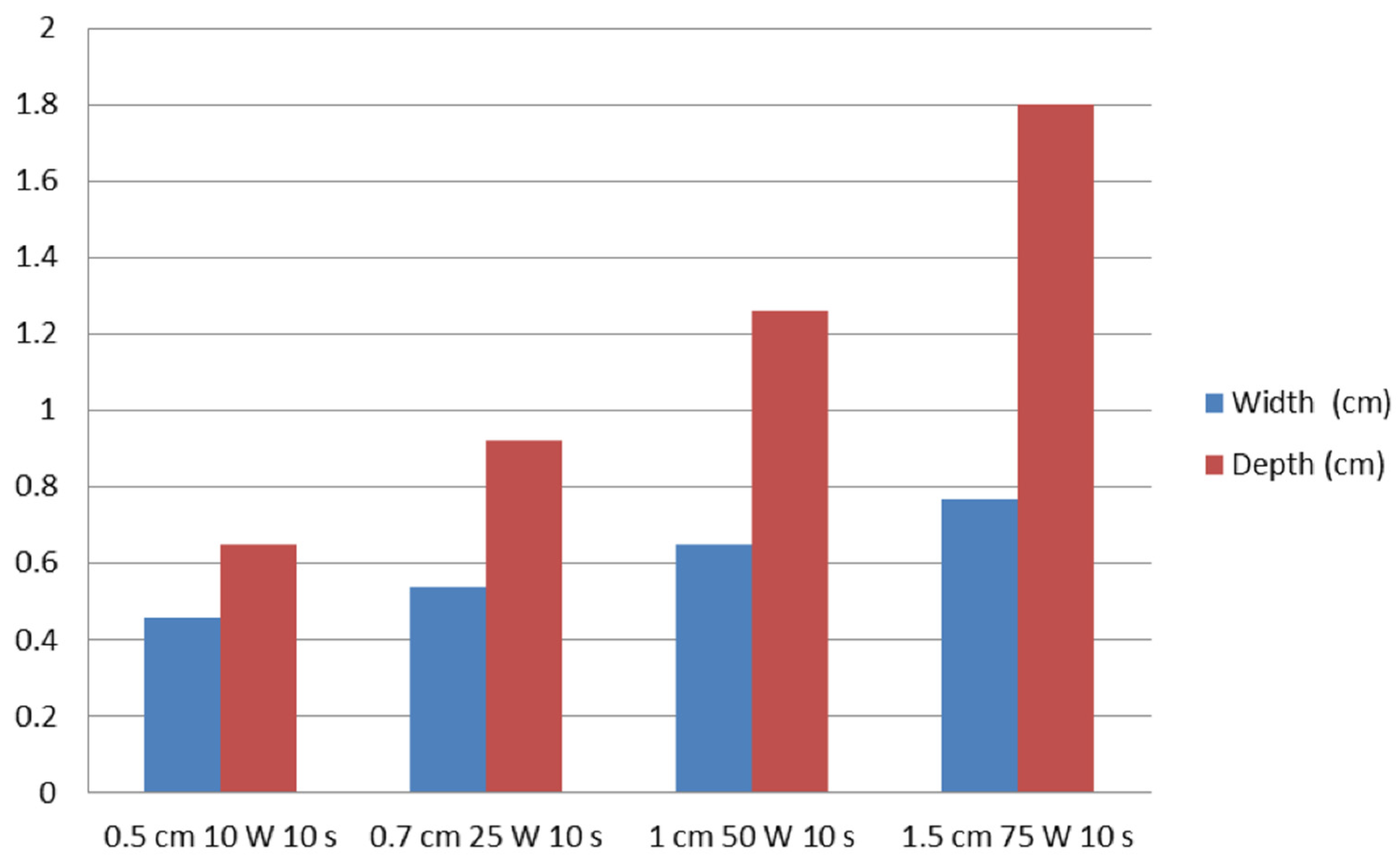
In test one, at 0.5, 0.7, 1, and 1.5 cm probe tip lengths, the mean maximal injury widths were 0.46 ± 0.07, 0.54 ± 0.05, 0.65 ± 0.05, and 0.77 ± 0.02 cm, respectively, while the mean maximal injury depths were 0.65 ± 0.07, 0.92 ± 0.08, 1.26 ± 0.14, and 1.80 ± 0.11 cm, respectively (Table 1, Figure 2 and Figure 3). Therefore, injury width and depth increased with electrode length and RFA power. The roll-off phenomenon occurred between 7 s and 8 s in each procedure.

Table 1.
Ablation effects based on the length of the exposed distal end of the RFA electrode and RFA power.
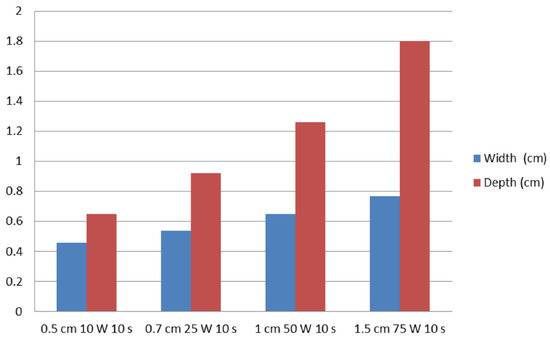
Figure 3.
Relationships of ablation effect on probe tip length and RFA power. The vertical axis represents the width (cm) and depth (cm) of ablation zones.
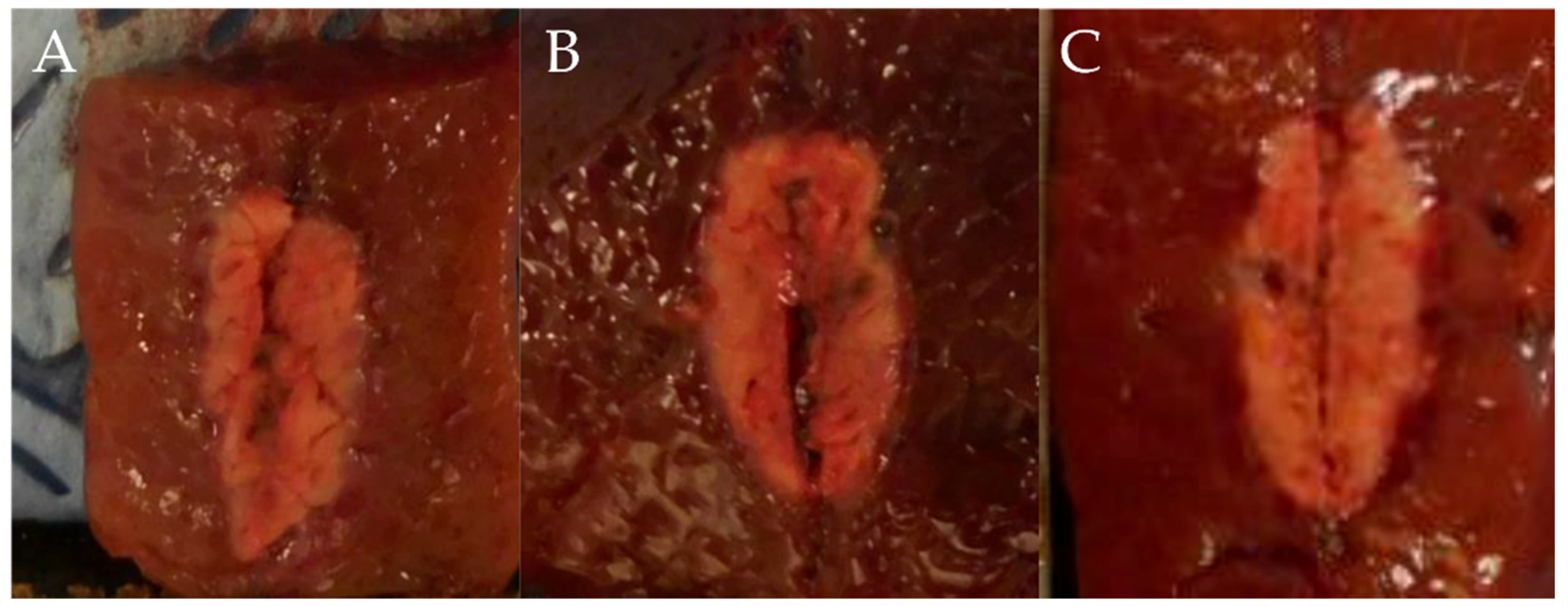
In test two, in groups A (1 cm, 30 W, 10 s), B (1 cm, 30 W, 15 s), and C (1 cm, 50 W, 10 s), the mean maximal injury widths were 0.56 ± 0.05, 0.65 ± 0.06, and 0.65 ± 0.05 cm, respectively, while the mean maximal injury depths were 1.06 ± 0.29, 1.14 ± 0.05, and 1.26 ± 0.09 cm, respectively (Table 2, Figure 4 and Figure 5). Therefore, the maximal injury width and depth were similar between groups B and C (Table 2). The roll-off phenomenon occurred between 12 and 15 s in group B.

Table 2.
Ablation effects based on the RFA power and ablation time.
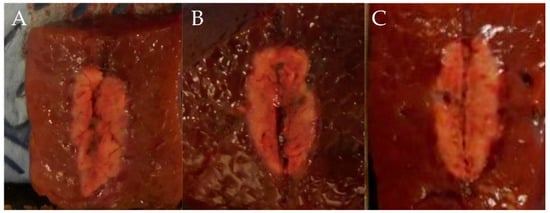
Figure 4.
Comparison of ablation zones based on the ablation power and ablation time. (A) 1 cm, 30 W, 10 s (B) 1 cm, 30 W, 15 s (C) 1 cm, 50 W, 10 s.
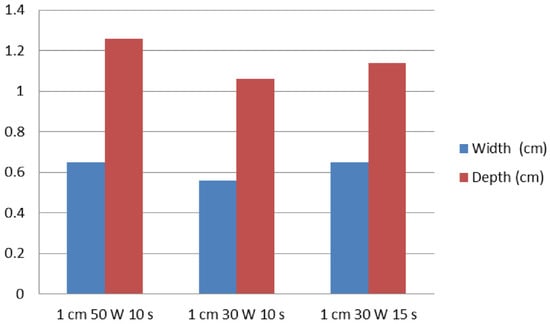
Figure 5.
A graph of ablation effect according to the RFA power and ablation time. The vertical axis represents the width (cm) and depth (cm) of ablation zones.
4. Discussion
Recently, EUS-RFA has been investigated as a treatment for patients with various pancreatic tumors [1,3,7,8,9,10]. Previous studies showed promising clinical outcomes for treating various pancreatic tumors with acceptable adverse events [1,3,4,11]. Recently, EUS-RFA has been applied in patients with hepatocellular carcinoma [5,6]. When hepatic lesions are located in the left lobe and in the caudate lobe, however, percutaneous RFA may be difficult. Furthermore, the subcapsular/subdiaphragmatic location may have an intrinsic risk of thermal injury, resulting in pleural effusion or hemothorax and a higher risk of tumor seeding/tumor recurrence [12]. EUS-RFA could be beneficial when percutaneous RFA is not applicable in patients with hepatic tumors.
However, overall clinical experience with EUS-RFA is limited, therefore, there is uncertainties of clinical efficacy and concerns about EUS-RFA related adverse events. Therefore, preclinical validation is mandatory for the application of EUS-RFA. Ex vivo experiments are easy to perform, however, these may produce different ablation results than in vivo studies since blood flow is absent. Proximity to large blood vessels also plays a significant role in heat transmission. Blood flow acts as a “heat sink” and cools nearby tissue, thereby limiting the ablation effect [13,14]. Although it is more difficult to perform in vivo tests, they do produce more realistic data. In the present study, therefore, we evaluated the effects of a novel RFA electrode using pig livers in vivo as a virtual mass to correctly measure the effects probe and procedure variables have on the width and depth of ablation.
Our results showed that the ablation effects correlate with the length of electrode. There is a possibility that the shortest probe length (0.5 cm) at the weakest power (10 W) may lead to insufficient ablation. In contrast, when treating with the longest probe length (1.5 cm) at the strongest power (75 W), the ablation effect can be increased for ablation of hepatic lesions. Furthermore, we also compared ablation effects based on ablation power and time, including the settings currently used for EUA-RFA for pancreatic tumors (1 cm, 50 W, 10 s) [1,3,6]. Ablation effects increased with ablation power (50 W) and time (15 s), unpredictable roll-off phenomena occurred at extended ablation time. This might decrease the ablation effect in target lesions. Unlike pancreatic lesions, hepatic lesions are surrounded by a large parenchyma, safety margins in the liver are larger than in the pancreas. In terms of optimal settings for EUS-RFA, its efficacy for ablation of hepatic tumors, therefore, longer probe with stronger power will be effective for treating hepatic tumors.
A major limitation of this study is that RFA was conducted only in a small number of pigs. Secondly, we could not evaluate possible late adverse events, such as bleeding, or perforation. In addition, the ablation effect on liver may not be representative of the effect in human hepatic tumors, particularly when considering differences in blood supply and the diverse cytologic milieu. However, we tested various RFA settings in the pig liver and tried to find the trend of ablation effects according to different parameters and electrode lengths. Considering that RFA ablation effect is more dependent on the electrical characteristics of tissue such as impedance and conductivity than histological characteristics, we think these baseline data can give some insight on the ablation of liver tissue. Further studies in the animal and human liver will be required in order to obtain more representative data.
Despite the limitations described previously, the present study describes the relationship between electrode length, ablation power, and ablation time and ablation effect in live pig livers. EUS-RFA are suitable for effective ablation while minimizing thermal injury. Further large number of preclinical studies of RFA in the porcine liver are warranted to verify our results before human trials can be performed.
Author Contributions
Conceptualization, D.-W.S.; methodology, T.-J.S.; software, S.-H.C. and D.O.; validation, G.H. and T.-J.S.; formal analysis, S.-H.C. and D.O.; investigation, S.-H.C. and D.O.; resources, D.-W.S.; data curation, S.-H.C., D.O. and G.H.; writing—original draft preparation, S.-H.C. and D.O.; writing—review and editing, D.-W.S.; visualization, S.-H.C. and D.O.; supervision, D.-W.S.; project administration, D.-W.S.; funding acquisition, D.-W.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Korean Health Technology R and D project, Ministry of Health and Welfare, Republic of Korea (HI16C1163).
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board (or Ethics Committee) of Asan Medical Center (IACUC No. 2016-14-063).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Song, T.J.; Seo, D.W.; Lakhtakia, S.; Reddy, N.; Oh, D.W.; Park, D.H.; Lee, S.S.; Lee, S.K.; Kim, M.H. Initial experience of EUS-guided radiofrequency ablation of unresectable pancreatic cancer. Gastrointest. Endosc. 2016, 83, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Yang, W.; Yan, K.; Gao, W.; Dai, Y.; Wang, Y.B.; Zhang, X.P.; Yin, S.S. Treatment efficacy of radiofrequency ablation of 338 patients with hepatic malignant tumor and the relevant complications. World J. Gastroenterol. 2005, 11, 6395–6401. [Google Scholar] [CrossRef]
- Choi, J.H.; Seo, D.W.; Song, T.J.; Park, D.H.; Lee, S.S.; Lee, S.K.; Kim, M.H. Endoscopic ultrasound-guided radiofrequency ablation for management of benign solid pancreatic tumors. Endoscopy 2018, 50, 1099–1104. [Google Scholar] [CrossRef]
- Oh, D.; Ko, S.W.; Seo, D.W.; Hong, S.M.; Kim, J.H.; Song, T.J.; Park, D.H.; Lee, S.K.; Kim, M.H. Endoscopic ultrasound-guided radiofrequency ablation of pancreatic microcystic serous cystic neoplasms: A retrospective study. Endoscopy 2021, 53, 739–743. [Google Scholar] [CrossRef]
- De Nucci, G.; Della Corte, C.; Reati, R.; Imperatore, N.; Arena, I.; Larghi, A.; Manes, G. Endoscopic ultrasound-guided radiofrequency ablation for hepatocellular carcinoma in cirrhosis: A case report test for efficacy and future perspectives. Endosc. Int. Open 2020, 8, E1713–E1716. [Google Scholar] [CrossRef] [PubMed]
- Attili, F.; Boškoski, I.; Bove, V.; Familiari, P.; Costamagna, G. EUS-guided radiofrequency ablation of a hepatocellular carcinoma of the liver. VideoGIE 2018, 3, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Viera, F.T.; Ghittoni, G.; Cobianchi, L.; Rosa, L.L.; Siciliani, L.; Bortolotto, C.; Veronese, L.; Vercelli, A.; Gallotti, A.; et al. Radiofrequency ablation of pancreatic neuroendocrine tumors: A pilot study of feasibility, efficacy, and safety. Pancreas 2014, 43, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Crino, S.F.; D’Onofrio, M.; Bernardoni, L.; Frulloni, L.; Iannelli, M.; Malleo, G.; Paiella, S.; Larghi, A.; Gabbrielli, A. EUS-guided radiofrequency ablation (EUS-RFA) of solid pancreatic neoplasm using an 18-gauge needle electrode: Feasibility, safety, and technical success. J. Gastrointestin. Liver Dis. 2018, 27, 67–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakhtakia, S.; Ramchandani, M.; Galasso, D.; Gupta, R.; Venugopal, S.; Kalpala, R.; Reddy, D.N. EUS-guided radiofrequency ablation for management of pancreatic insulinoma by using a novel needle electrode (with videos). Gastrointest. Endosc. 2016, 83, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Scopelliti, F.; Pea, A.; Conigliaro, R.; Butturini, G.; Frigerio, I.; Regi, P.; Giardino, A.; Bertani, H.; Paini, M.; Pederzoli, P.; et al. Technique, safety, and feasibility of EUS-guided radiofrequency ablation in unresectable pancreatic cancer. Surg. Endosc. 2018, 32, 4022–4028. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.; Seo, D.W.; Song, T.J.; Park, D.H.; Lee, S.K.; Kim, M.H. Clinical outcomes of EUS-guided radiofrequency ablation for unresectable pancreatic cancer: A prospective observational study. Endosc. Ultrasound 2022, 11, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Berzigotti, A.; Seijo, S.; Reverter, E.; Bosch, J. Assessing portal hypertension in liver diseases. Expert Rev. Gastroenterol. Hepatol. 2013, 7, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Chiou, Y.Y.; Chou, Y.H. Radiofrequency ablation of hepatocellular carcinoma. J. Med. Ultrasound 2008, 16, 272–284. [Google Scholar] [CrossRef] [Green Version]
- Lencioni, R.; Crocetti, L.; Cioni, D.; Della Pina, C.; Bartolozzi, C. Percutaneous radiofrequency ablation of hepatic colorectal metastases: Technique, indications, results, and new promises. Investig. Radiol. 2004, 39, 689–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).