Image Human Thorax Using Ultrasound Traveltime Tomography with Supervised Descent Method
Abstract
:1. Introduction
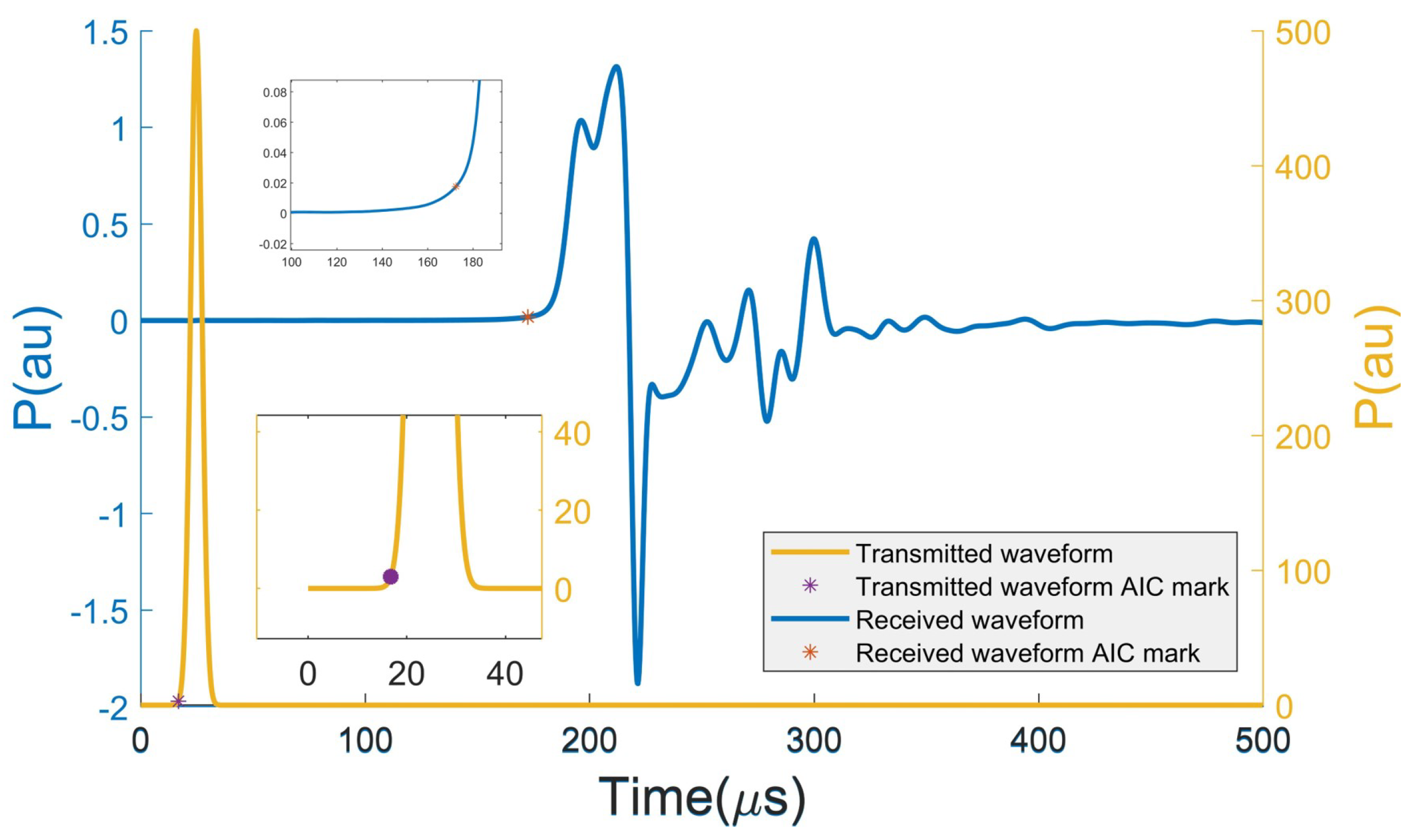
2. Forward Modeling for Computing Traveltime
3. Traveltime Tomography with SDM
3.1. Formulations of Inverse Problem with SDM
3.2. Offline Training Process
3.3. Online Predicting Process
4. Numerical Experiments
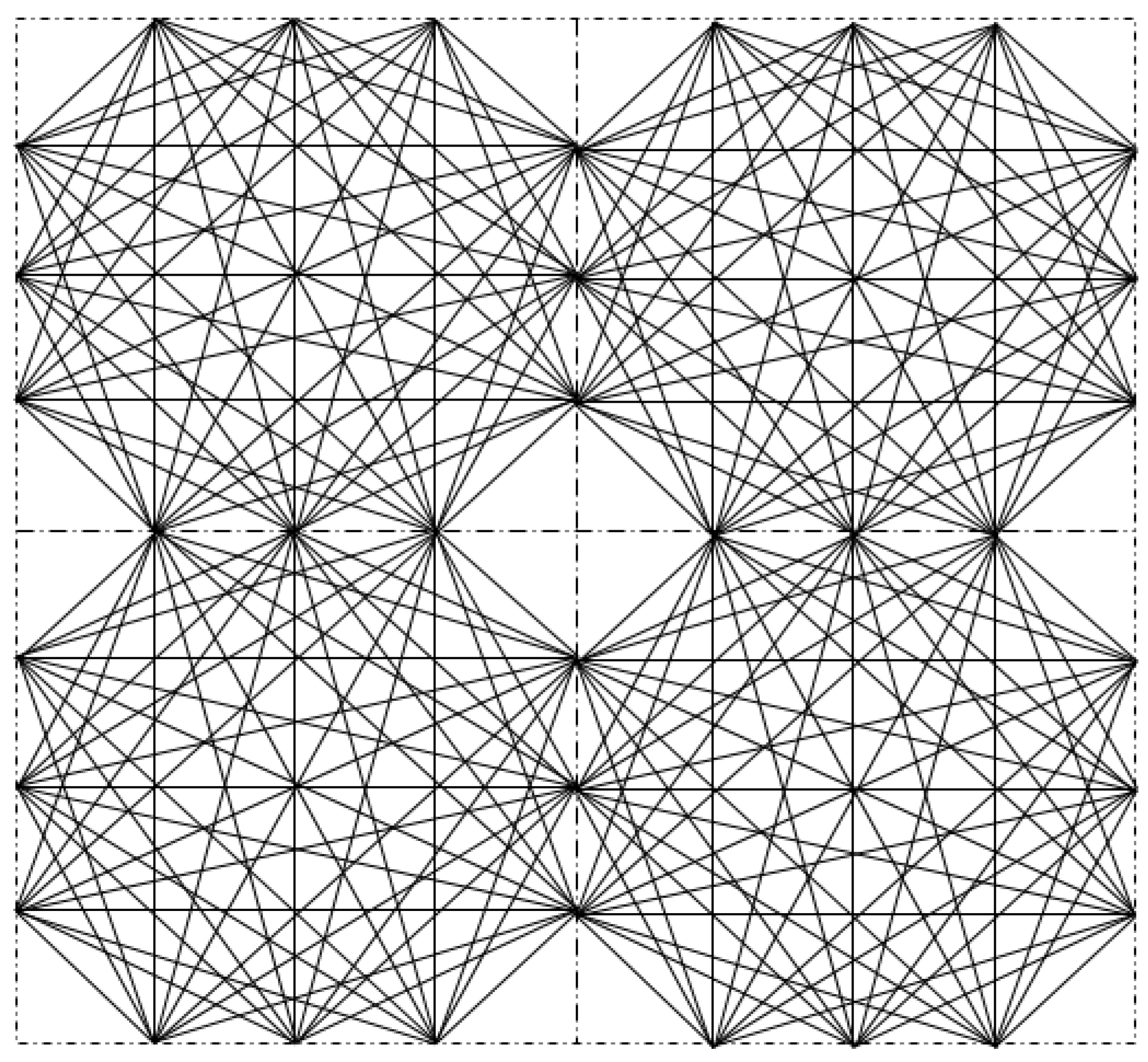
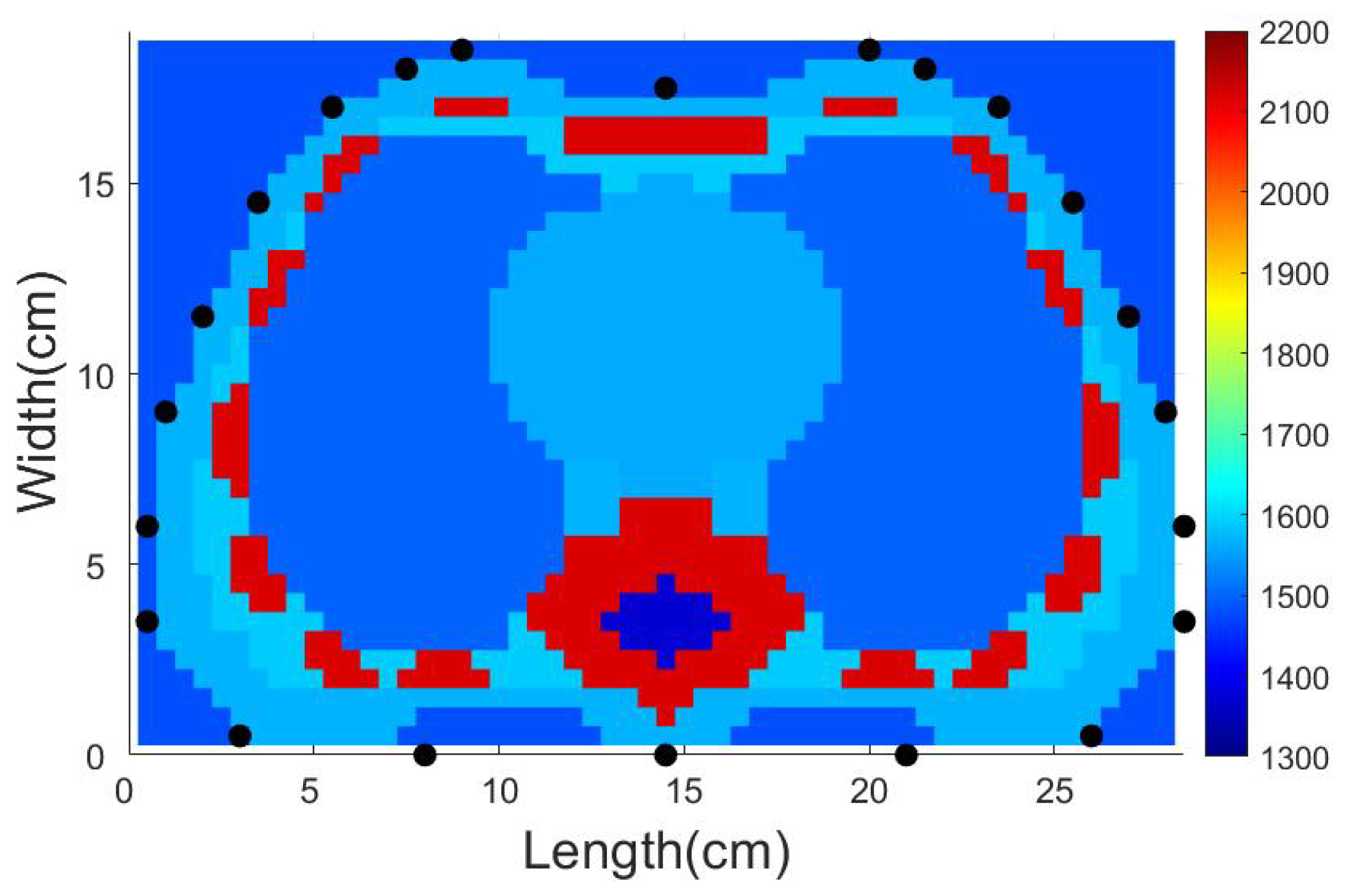
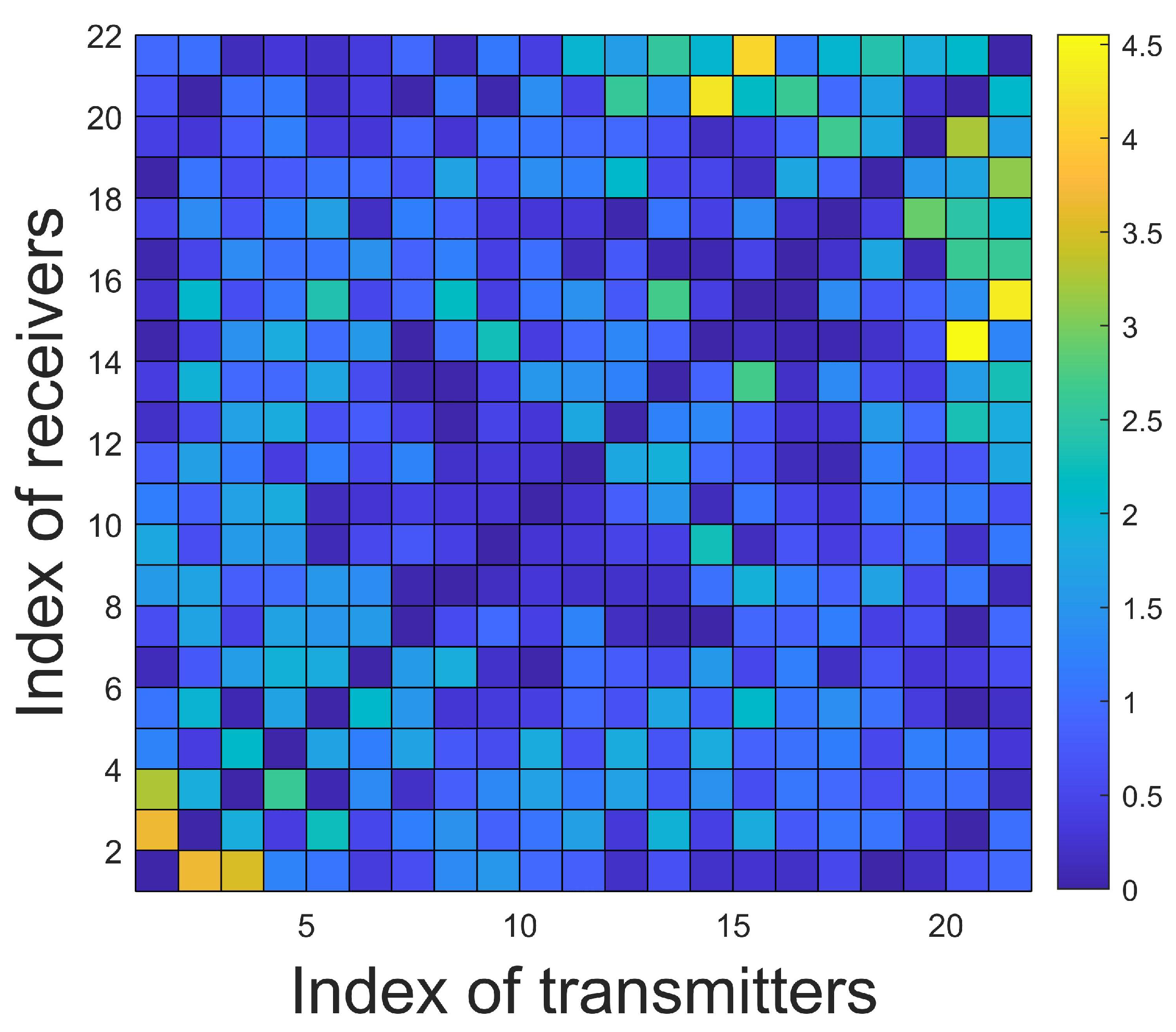
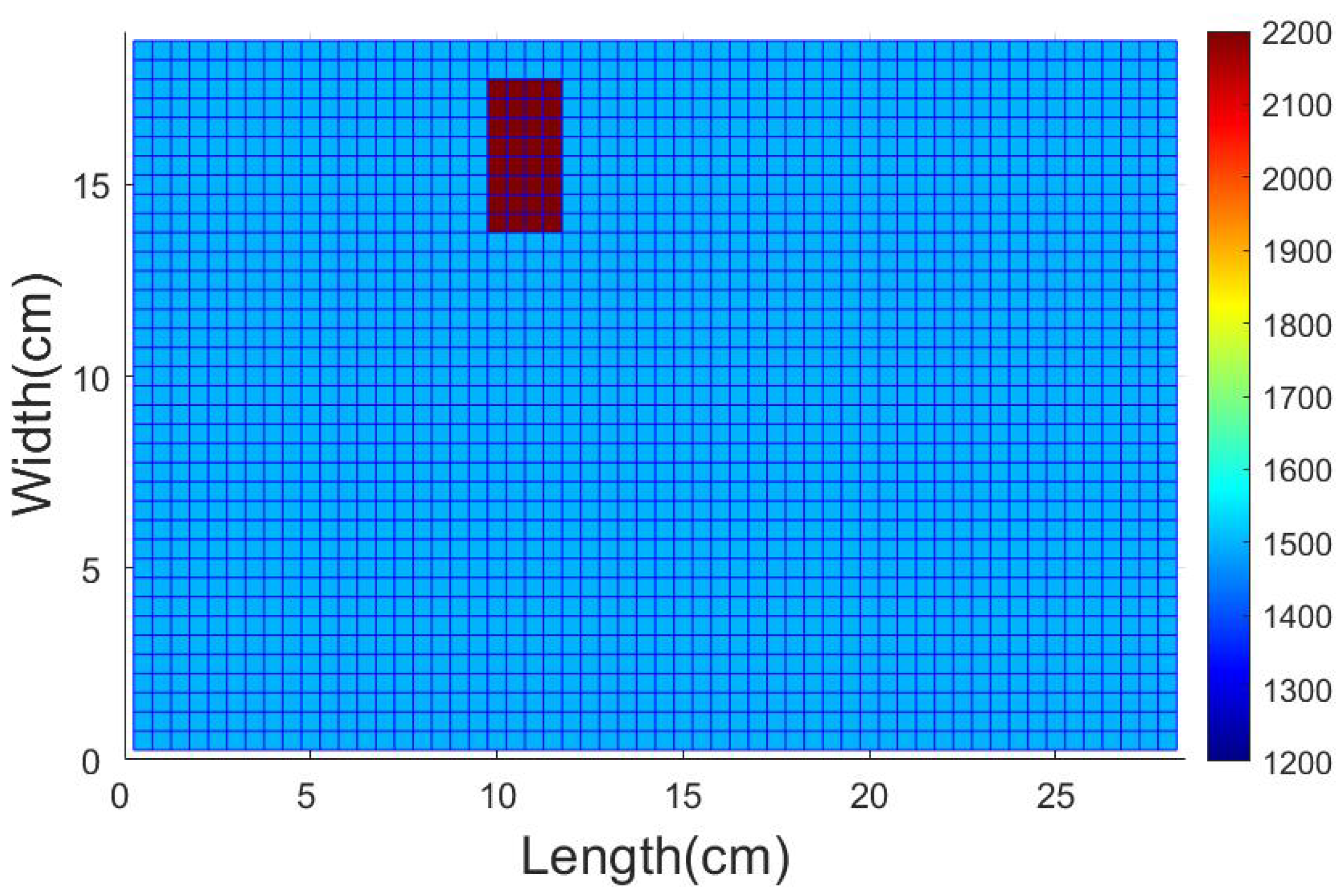
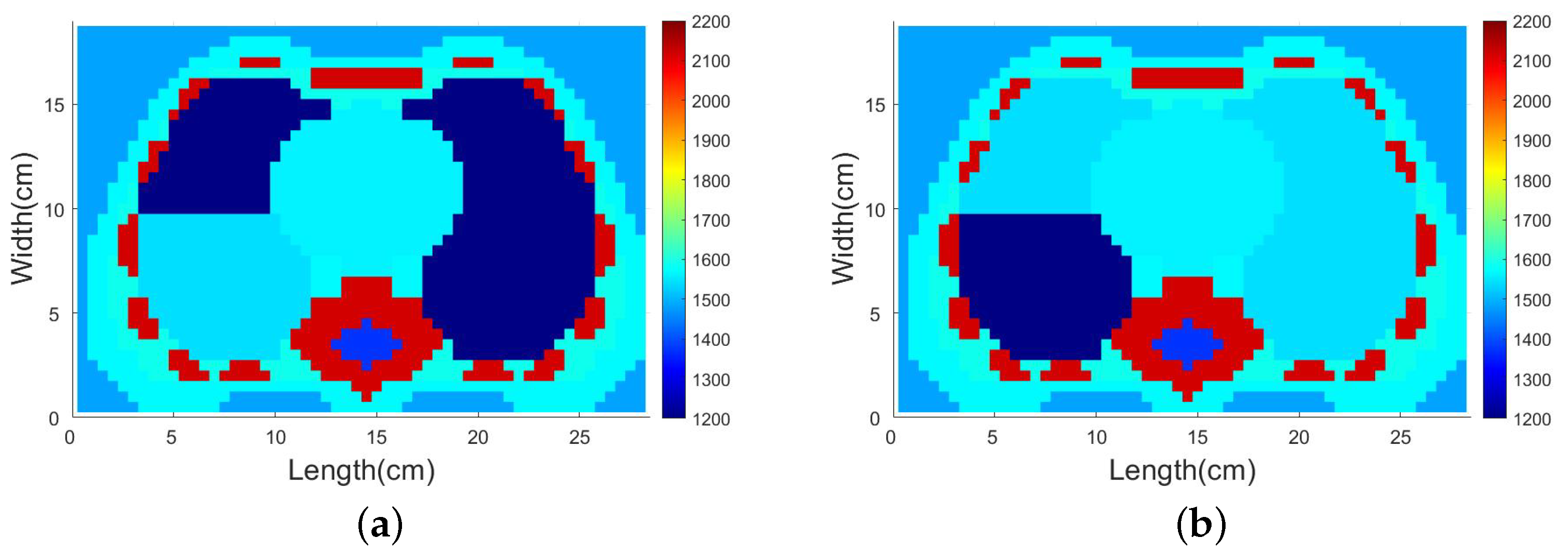
4.1. Description of the Test Domain and Forward Modeling
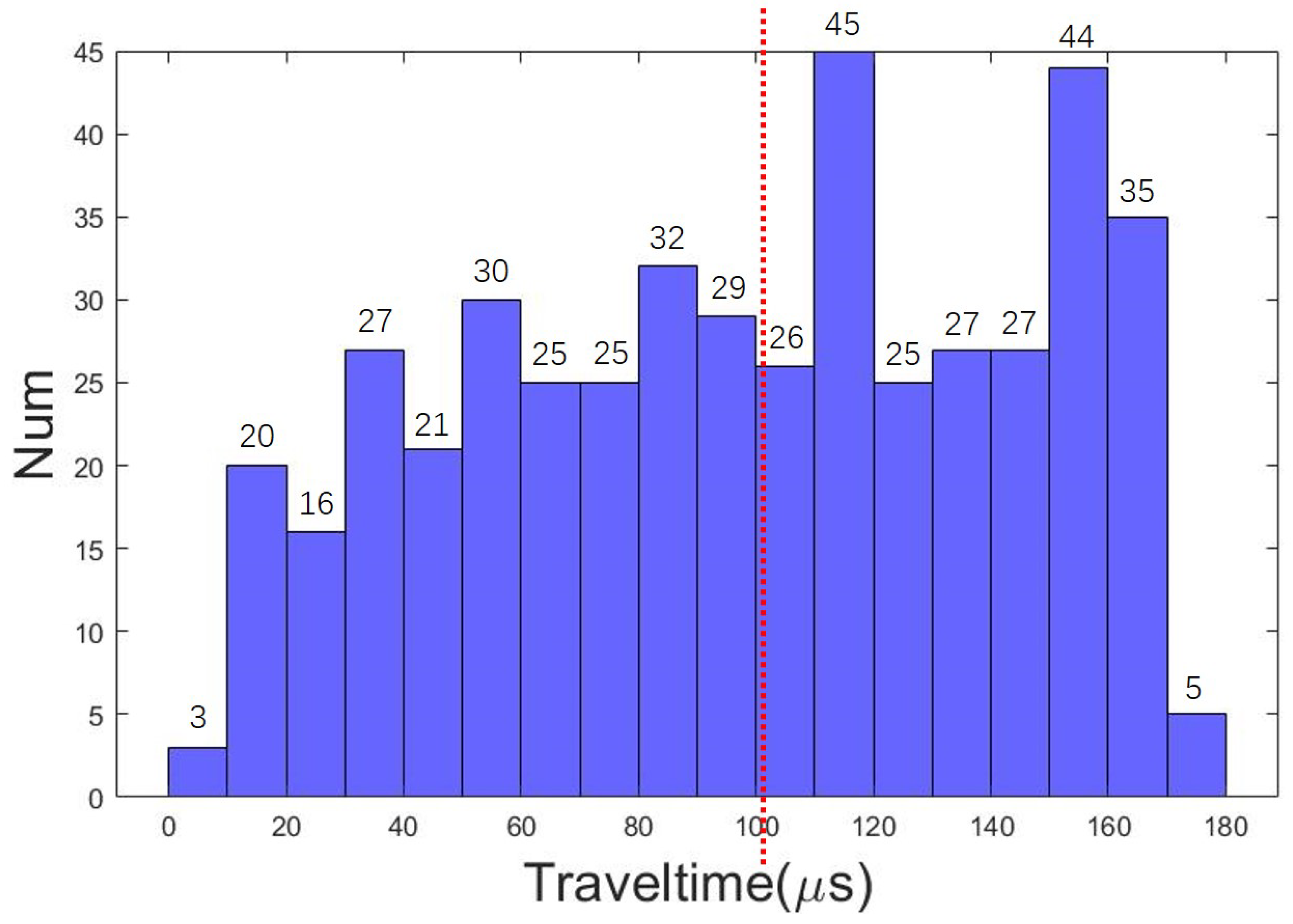
4.2. Details of the Training Set and the Training Process
4.3. Numerical Experiments
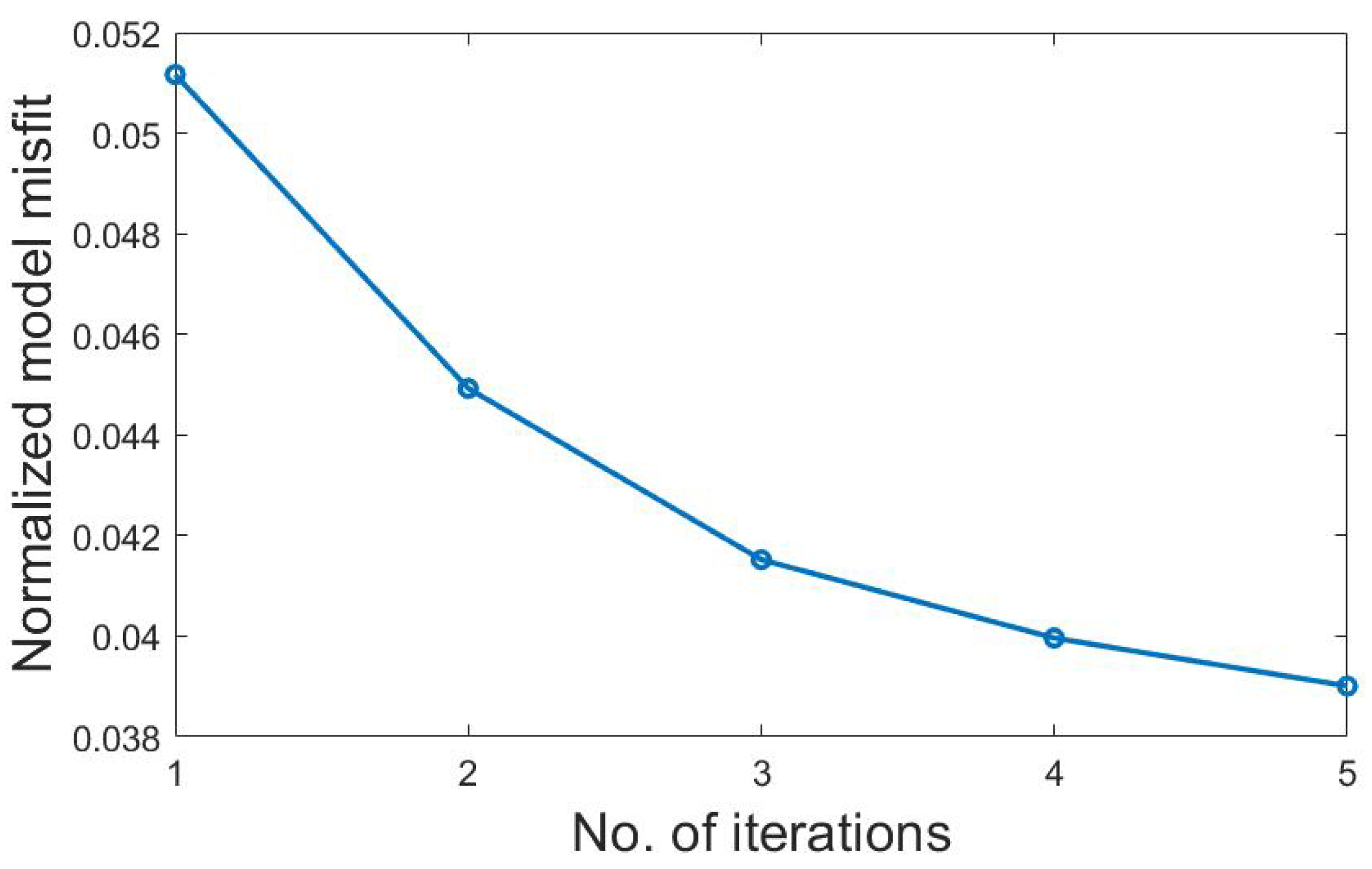
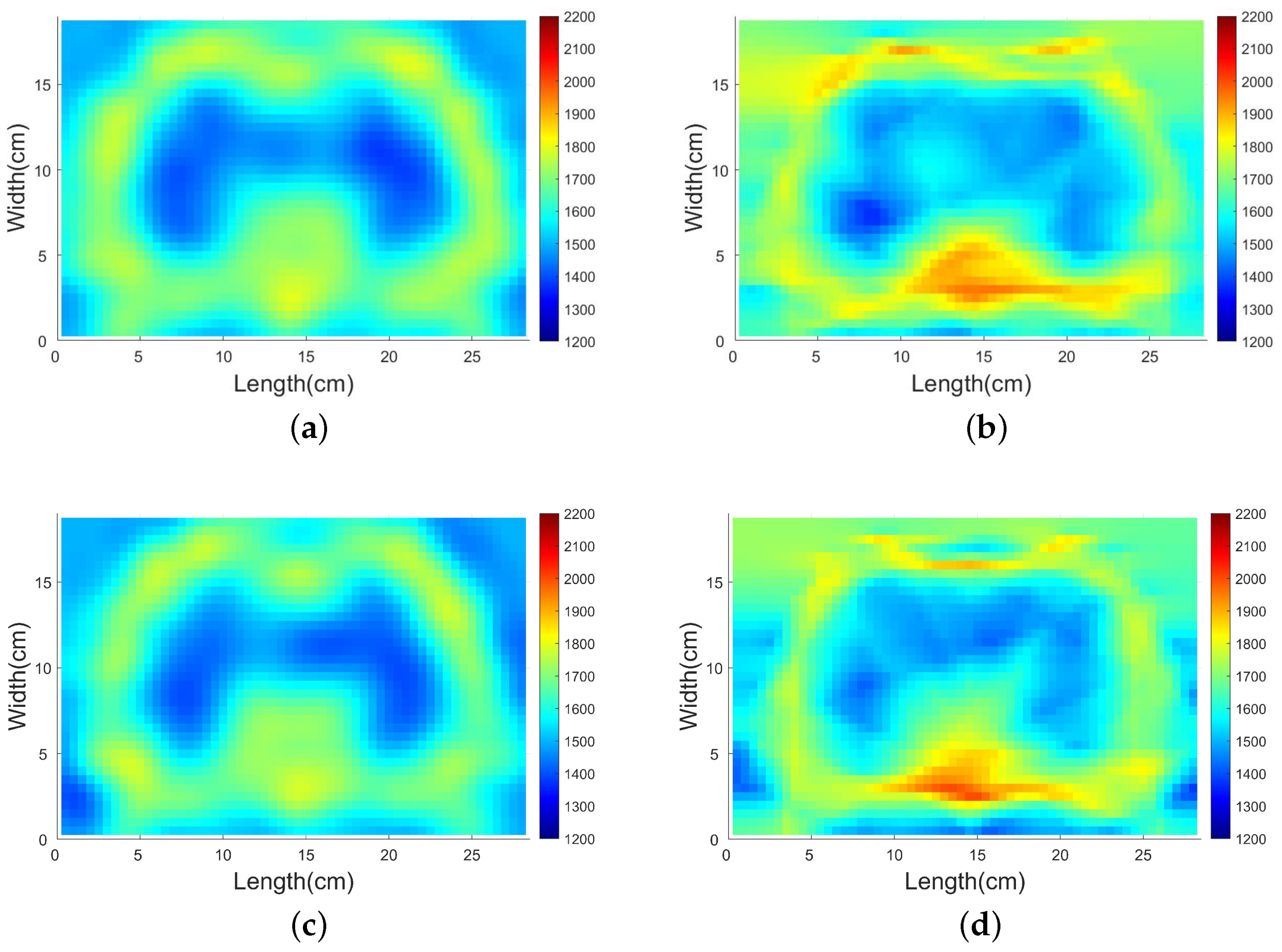
4.3.1. Comparison with Traditional Gradient-Based Method
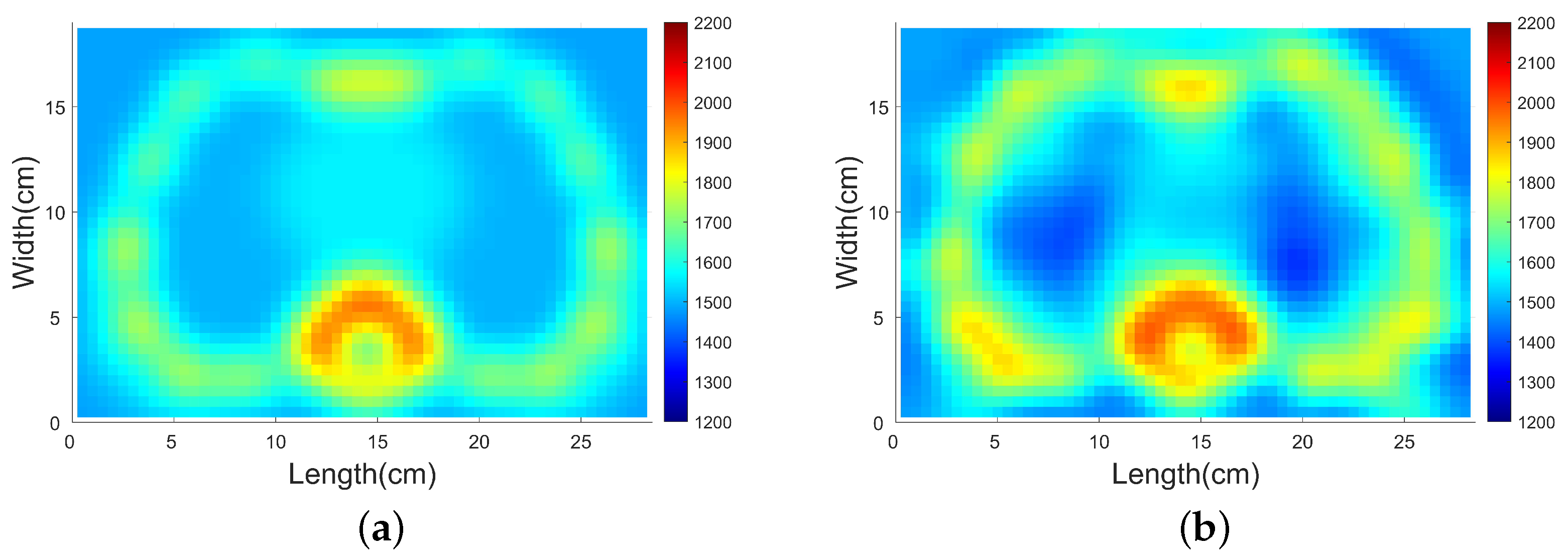
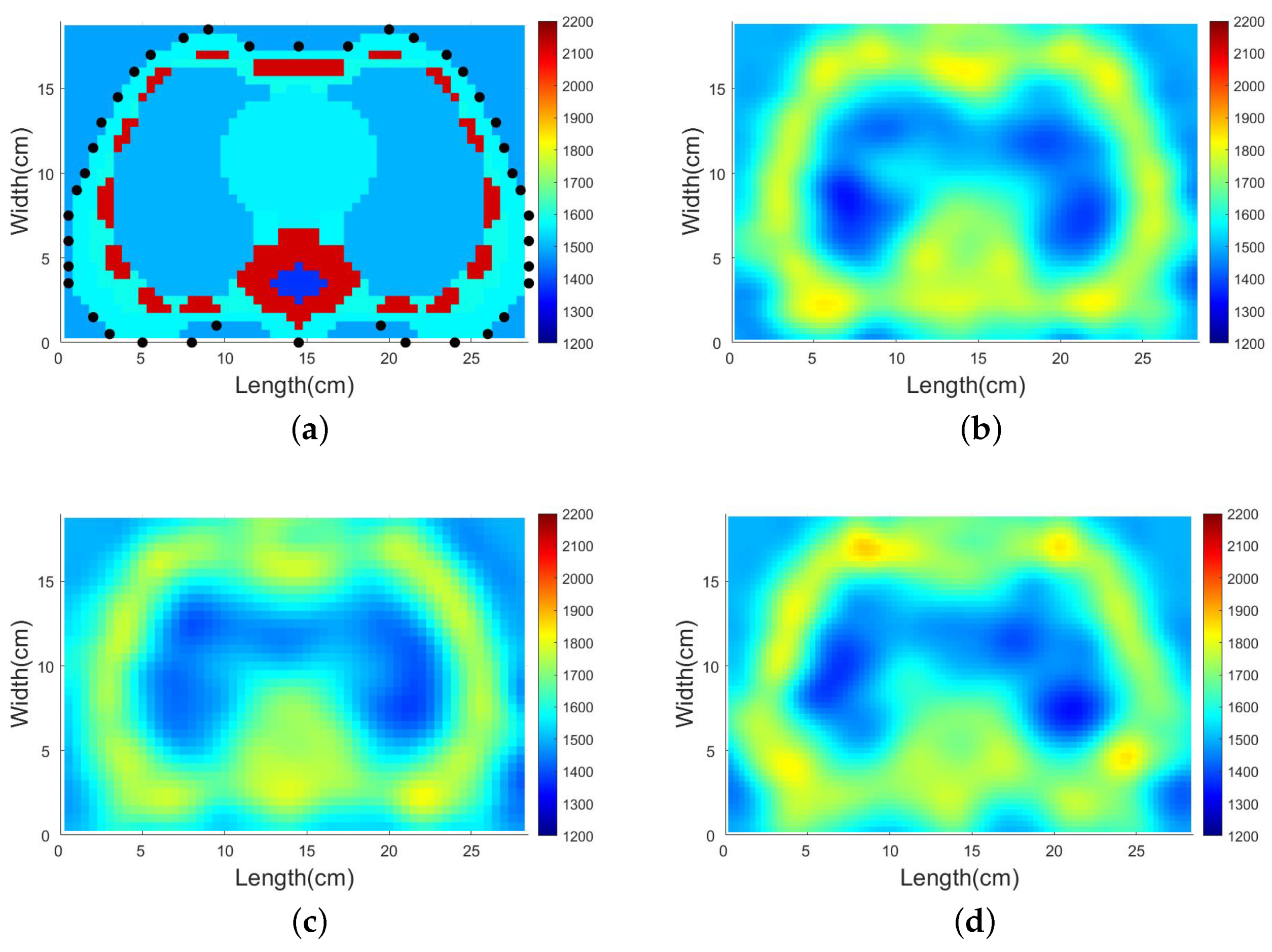
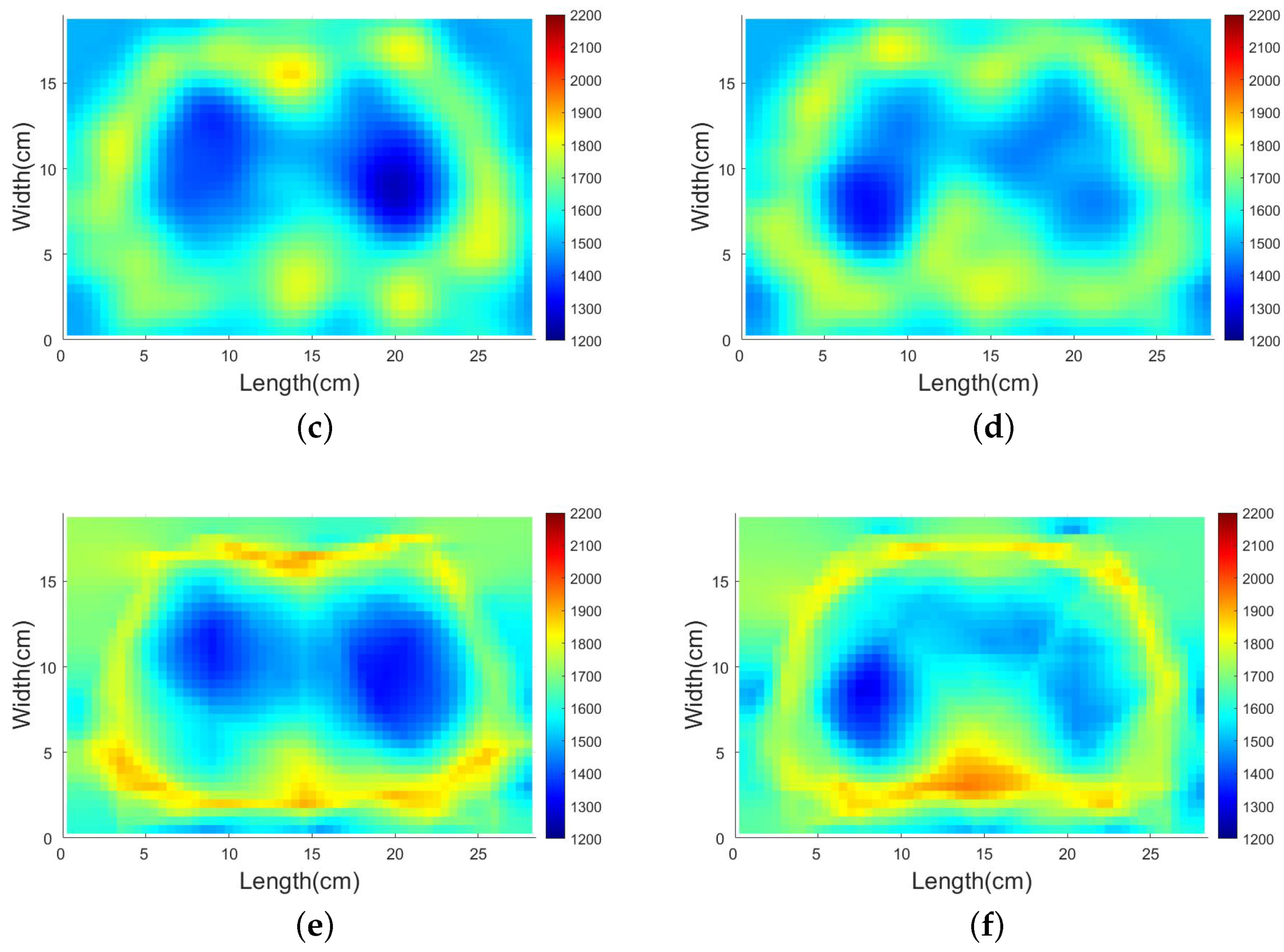
4.3.2. SDM Traveltime Inversion of Thorax on Different States
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SDM | Supervised Descent Method |
| SPR | Shortest Path Ray |
| DoI | Domain of Interest |
| NDE | Nondestructive Evaluation |
| CT | Computed Tomography |
| MRI | Magnetic Resonance Imaging |
| SNR | Signal to Noise Ratio |
| EIT | Electrical Impedance Tomography |
References
- Chiao, R.Y.; Thomas, L.J. Analytic evaluation of sampled aperture ultrasonic imaging techniques for NDE. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1994, 41, 484–493. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, J. Nondestructive evaluation of steel-concrete composite structure using high-frequency ultrasonic guided wave. Ultrasonics 2020, 103, 106096. [Google Scholar] [CrossRef]
- Liu, H.; Xia, H.; Zhuang, M.; Long, Z.; Liu, C.; Cui, J.; Xu, B.; Hu, Q.; Liu, Q.H. Reverse time migration of acoustic waves for imaging based defects detection for concrete and CFST structures. Mech. Syst. Signal Process. 2019, 117, 210–220. [Google Scholar] [CrossRef]
- Gardner, P.; Fuentes, R.; Dervilis, N.; Mineo, C.; Pierce, S.; Cross, E.; Worden, K. Machine learning at the interface of structural health monitoring and non-destructive evaluation. Philos. Trans. R. Soc. A 2020, 378, 20190581. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, L.; Perissinotto, A.; Dabala, M. Mechanical monitoring of fracture healing using ultrasound imaging. Clin. Orthop. Relat. Res. 1993, 293, 71–76. [Google Scholar] [CrossRef]
- Dong, F.; Jiang, Z.; Qiao, X.; Xu, L. Application of electrical resistance tomography to two-phase pipe flow parameters measurement. Flow Meas. Instrum. 2003, 14, 183–192. [Google Scholar] [CrossRef]
- Tan, C.; Li, X.; Liu, H.; Dong, F. An ultrasonic transmission/reflection tomography system for industrial multiphase flow imaging. IEEE Trans. Ind. Electron. 2019, 66, 9539–9548. [Google Scholar] [CrossRef]
- Yang, M.; Schlaberg, H.I.; Hoyle, B.S.; Beck, M.S.; Lenn, C. Real-time ultrasound process tomography for two-phase flow imaging using a reduced number of transducers. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1999, 46, 492–501. [Google Scholar] [CrossRef]
- Gonçalves, L.F.; Espinoza, J.; Romero, R.; Kusanovic, J.P.; Swope, B.; Nien, J.K.; Erez, O.; Soto, E.; Treadwell, M.C. Four-dimensional ultrasonography of the fetal heart using a novel Tomographic Ultrasound Imaging display. J. Perinat. Med. 2006, 34, 39–55. [Google Scholar] [CrossRef] [Green Version]
- Brekke, S.; Tegnander, E.; Torp, H.; Eik-Nes, S. Tissue Doppler gated (TDOG) dynamic three-dimensional ultrasound imaging of the fetal heart. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2004, 24, 192–198. [Google Scholar] [CrossRef]
- Papadacci, C.; Finel, V.; Villemain, O.; Tanter, M.; Pernot, M. 4D ultrafast ultrasound imaging of naturally occurring shear waves in the human heart. IEEE Trans. Med. Imaging 2020, 39, 4436–4444. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.Y.; Jiang, Z.Y.; Fu, T.T.; Wang, Q.M.; Zhu, Y.L.; Dai, M.; Wang, W.P.; Yu, J.H.; Ding, H. Transfer learning radiomics based on multimodal ultrasound imaging for staging liver fibrosis. Eur. Radiol. 2020, 30, 2973–2983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berzigotti, A.; Castera, L. Update on ultrasound imaging of liver fibrosis. J. Hepatol. 2013, 59, 180–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imbault, M.; Chauvet, D.; Gennisson, J.L.; Capelle, L.; Tanter, M. Intraoperative functional ultrasound imaging of human brain activity. Sci. Rep. 2017, 7, 7304. [Google Scholar] [CrossRef] [Green Version]
- Demene, C.; Baranger, J.; Bernal, M.; Delanoe, C.; Auvin, S.; Biran, V.; Alison, M.; Mairesse, J.; Harribaud, E.; Pernot, M.; et al. Functional ultrasound imaging of brain activity in human newborns. Sci. Transl. Med. 2017, 9, eaah6756. [Google Scholar] [CrossRef]
- Macé, E.; Montaldo, G.; Cohen, I.; Baulac, M.; Fink, M.; Tanter, M. Functional ultrasound imaging of the brain. Nat. Methods 2011, 8, 662–664. [Google Scholar] [CrossRef]
- Szabo, T.L. Diagnostic Ultrasound Imaging: Inside Out; Academic Press: Cambridge, MA, USA, 2004. [Google Scholar]
- Zhou, B.; Yang, X.; Zhang, X.; Curran, W.J.; Liu, T. Ultrasound elastography for lung disease assessment. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2020, 67, 2249–2257. [Google Scholar] [CrossRef]
- Wang, X.; Peng, Y.; Lu, L.; Lu, Z.; Bagheri, M.; Summers, R.M. Chestx-ray8: Hospital-scale chest X-ray database and benchmarks on weakly-supervised classification and localization of common thorax diseases. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Honolulu, HI, USA, 21–26 July 2017; pp. 2097–2106. [Google Scholar]
- Nehmeh, S.; Erdi, Y.; Pan, T.; Pevsner, A.; Rosenzweig, K.; Yorke, E.; Mageras, G.; Schoder, H.; Vernon, P.; Squire, O.; et al. Four-dimensional (4D) PET/CT imaging of the thorax: 4D PET/CT. Med. Phys. 2004, 31, 3179–3186. [Google Scholar] [CrossRef]
- Ates, O.F.; Taydas, O.; Dheir, H. Thorax magnetic resonance imaging findings in patients with coronavirus disease (COVID-19). Acad. Radiol. 2020, 27, 1373–1378. [Google Scholar] [CrossRef]
- Zhang, K.; Li, M.; Yang, F.; Xu, S.; Abubakar, A. Three-dimensional electrical impedance tomography with multiplicative regularization. IEEE Trans. Biomed. Eng. 2019, 66, 2470–2480. [Google Scholar] [CrossRef]
- Zhang, H.; Li, M.; Yang, F.; Xu, S.; Zhou, H.; Yang, Y.; Chen, L. A low-profile compact dual-band l-shape monopole antenna for microwave thorax monitoring. IEEE Antennas Wirel. Propag. Lett. 2020, 19, 448–452. [Google Scholar] [CrossRef]
- Martelius, L.; Heldt, H.; Lauerma, K. B-lines on pediatric lung sonography: Comparison with computed tomography. J. Ultrasound Med. 2016, 35, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Mento, F.; Soldati, G.; Prediletto, R.; Demi, M.; Demi, L. Quantitative lung ultrasound spectroscopy applied to the diagnosis of pulmonary fibrosis: The first clinical study. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 2265–2273. [Google Scholar] [CrossRef] [PubMed]
- Dargent, A.; Chatelain, E.; Kreitmann, L.; Quenot, J.P.; Cour, M.; Argaud, L.; COVID-LUS Study Group. Lung ultrasound score to monitor COVID-19 pneumonia progression in patients with ARDS. PLoS ONE 2020, 15, e0236312. [Google Scholar] [CrossRef]
- Wang, G.; Ji, X.; Xu, Y.; Xiang, X. Lung ultrasound: A promising tool to monitor ventilator-associated pneumonia in critically ill patients. Crit. Care 2016, 20, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Buonsenso, D.; Brancato, F.; Valentini, P.; Curatola, A.; Supino, M.; Musolino, A.M. The use of lung ultrasound to monitor the antibiotic response of community-acquired pneumonia in children: A preliminary hypothesis. J. Ultrasound Med. 2020, 39, 817–826. [Google Scholar] [CrossRef]
- Rueter, D.; Hauber, H.P.; Droeman, D.; Zabel, P.; Uhlig, S. Low-frequency ultrasound permeates the human thorax and lung: A novel approach to non-invasive monitoring. Ultraschall Der Med.-Eur. J. Ultrasound 2010, 31, 53–62. [Google Scholar] [CrossRef]
- Dai, Z.; Peng, Y.; Mansy, H.A.; Sandler, R.H.; Royston, T.J. Comparison of poroviscoelastic models for sound and vibration in the lungs. J. Vib. Acoust. 2014, 136, 050905. [Google Scholar] [CrossRef]
- Peng, Y.; Dai, Z.; Mansy, H.A.; Henry, B.M.; Sandler, R.H.; Balk, R.A.; Royston, T.J. Sound transmission in porcine thorax through airway insonification. Med. Biol. Eng. Comput. 2016, 54, 675–689. [Google Scholar] [CrossRef] [Green Version]
- Mansy, H.A.; Balk, R.A.; Warren, W.H.; Royston, T.J.; Dai, Z.; Peng, Y.; Sandler, R.H. Pneumothorax effects on pulmonary acoustic transmission. J. Appl. Physiol. 2015, 119, 250–257. [Google Scholar] [CrossRef] [Green Version]
- Palnitkar, H.; Henry, B.M.; Dai, Z.; Peng, Y.; Mansy, H.A.; Sandler, R.H.; Balk, R.A.; Royston, T.J. Sound transmission in human thorax through airway insonification: An experimental and computational study with diagnostic applications. Med. Biol. Eng. Comput. 2020, 58, 2239–2258. [Google Scholar] [CrossRef] [PubMed]
- Morenz, K.; Biller, H.; Wolfram, F.; Leonhadt, S.; Rüter, D.; Glaab, T.; Uhlig, S.; Hohlfeld, J.M. Detection of air trapping in chronic obstructive pulmonary disease by low frequency ultrasound. BMC Pulm. Med. 2012, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Pohlmann, A.; Sehati, S.; Young, D. Effect of changes in lung volume on acoustic transmission through the human respiratory system. Physiol. Meas. 2001, 22, 233. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Duric, N.; Littrup, P.; Huang, L. In vivo breast sound-speed imaging with ultrasound tomography. Ultrasound Med. Biol. 2009, 35, 1615–1628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duric, N.; Littrup, P.; Poulo, L.; Babkin, A.; Pevzner, R.; Holsapple, E.; Rama, O.; Glide, C. Detection of breast cancer with ultrasound tomography: First results with the Computed Ultrasound Risk Evaluation (CURE) prototype. Med. Phys. 2007, 34, 773–785. [Google Scholar] [CrossRef]
- Zhang, H.; Thurber, C.; Rowe, C. Automatic P-wave arrival detection and picking with multiscale wavelet analysis for single-component recordings. Bull. Seismol. Soc. Am. 2003, 93, 1904–1912. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Huang, L.; Duric, N.; Zhang, H.; Rowe, C. An improved automatic time-of-flight picker for medical ultrasound tomography. Ultrasonics 2009, 49, 61–72. [Google Scholar] [CrossRef] [Green Version]
- Fatemi, A.; Måsøy, S.E.; Rodriguez-Molares, A. Row–Column-Based Coherence Imaging Using a 2-D Array Transducer: A Row-Based Implementation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 2303–2311. [Google Scholar] [CrossRef]
- Wodicka, G.R.; Stevens, K.N.; Golub, H.L.; Cravalho, E.G.; Shannon, D.C. A model of acoustic transmission in the respiratory system. IEEE Trans. Biomed. Eng. 1989, 36, 925–934. [Google Scholar] [CrossRef]
- Picano, E.; Pellikka, P.A. Ultrasound of extravascular lung water: A new standard for pulmonary congestion. Eur. Heart J. 2016, 37, 2097–2104. [Google Scholar] [CrossRef] [Green Version]
- Porcel, J.M.; Light, R.W. Pleural effusions due to pulmonary embolism. Curr. Opin. Pulm. Med. 2008, 14, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; De la Torre, F. Supervised descent method and its applications to face alignment. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Portland, OR, USA, 23–28 June 2013; pp. 532–539. [Google Scholar]
- Guo, R.; Li, M.; Yang, F.; Xu, S.; Abubakar, A. Application of supervised descent method for 2D magnetotelluric data inversion. Geophysics 2020, 85, WA53–WA65. [Google Scholar] [CrossRef]
- Zhang, H.; Li, M.; Yang, F.; Xu, S.; Yin, Y.; Zhou, H.; Yang, Y.; Zeng, S.; Shao, J. A feasibility study of 2-d microwave thorax imaging based on the supervised descent method. Electronics 2021, 10, 352. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, R.; Li, M.; Yang, F.; Xu, S.; Abubakar, A. Supervised descent learning for thoracic electrical impedance tomography. IEEE Trans. Biomed. Eng. 2020, 68, 1360–1369. [Google Scholar] [CrossRef]
- Lin, M.; Liu, Y. Guided Wave Tomography Based on Supervised Descent Method for Quantitative Corrosion Imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 3624–3636. [Google Scholar] [CrossRef]
- Rawlinson, N.; Sambridge, M. Seismic traveltime tomography of the crust and lithosphere. Adv. Geophys. 2003, 46, 81–199. [Google Scholar]
- Moser, T. Shortest path calculation of seismic rays. Geophysics 1991, 56, 59–67. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, T.; Zhou, H.; Li, M.; Yang, F.; Xu, S.; Cao, Y. A Preliminary Experiment Based on One-step Measurement-trained Supervised Descent Method for Microwave Thorax Imaging. In Proceedings of the 2021 Photonics & Electromagnetics Research Symposium (PIERS), Hangzhou, China, 21–25 November 2021; pp. 1921–1930. [Google Scholar]
- Guo, R.; Li, M.; Yang, F.; Xu, S.; Abubakar, A. First arrival traveltime tomography using supervised descent learning technique. Inverse Probl. 2019, 35, 105008. [Google Scholar] [CrossRef]
- Tikhonov, A.N.; Arsenin, V.Y. Solutions of Ill-Posed Problems; VH Winston & Sons: New York, NY, USA, 1977. [Google Scholar]
- Song, X.; Li, M.; Yang, F.; Xu, S.; Abubakar, A. Feasibility study of acoustic imaging for human thorax using an acoustic contrast source inversion algorithm. J. Acoust. Soc. Am. 2018, 144, 2782–2792. [Google Scholar] [CrossRef]
- Song, X.; Li, M.; Yang, F.; Xu, S.; Abubakar, A. Study on joint inversion algorithm of acoustic and electromagnetic data in biomedical imaging. IEEE J. Multiscale Multiphysics Comput. Tech. 2019, 4, 2–11. [Google Scholar] [CrossRef]
- Hasgall, P.; Di Gennaro, F.; Baumgartner, C.; Neufeld, E.; Lloyd, B.; Gosselin, M.; Payne, D.; Klingenböck, A.; Kuster, N. IT’IS Database for Thermal and Electromagnetic Parameters of Biological Tissues, version 4.1; Elsevier: Boston, FL, USA, 2022. [Google Scholar]
- Treeby, B.E.; Budisky, J.; Wise, E.S.; Jaros, J.; Cox, B. Rapid calculation of acoustic fields from arbitrary continuous-wave sources. J. Acoust. Soc. Am. 2018, 143, 529–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, N. A method for reading and checking phase times in autoprocessing system of seismic wave data. Zisin 1985, 38, 365–379. [Google Scholar] [CrossRef] [Green Version]
- Kuhlman, J.E.; Singha, N.K. Complex disease of the pleural space: Radiographic and CT evaluation. Radiographics 1997, 17, 63–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Currie, G.P.; Alluri, R.; Christie, G.L.; Legge, J.S. Pneumothorax: An update. Postgrad. Med. J. 2007, 83, 461–465. [Google Scholar] [CrossRef] [PubMed]

| Tissue | Acoustic Velocity (m/s) |
|---|---|
| Heart | 1561 |
| Lung (deflated) | 1500 |
| Bone (cancellous) | 2117.5 |
| Bone Marrow (yellow) | 1371.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Guo, R.; Zhang, H.; Zhou, H.; Cao, Y.; Li, M.; Yang, F.; Xu, S. Image Human Thorax Using Ultrasound Traveltime Tomography with Supervised Descent Method. Appl. Sci. 2022, 12, 6763. https://doi.org/10.3390/app12136763
Zhang T, Guo R, Zhang H, Zhou H, Cao Y, Li M, Yang F, Xu S. Image Human Thorax Using Ultrasound Traveltime Tomography with Supervised Descent Method. Applied Sciences. 2022; 12(13):6763. https://doi.org/10.3390/app12136763
Chicago/Turabian StyleZhang, Tong, Rui Guo, Haolin Zhang, Hongyu Zhou, Yeyu Cao, Maokun Li, Fan Yang, and Shenheng Xu. 2022. "Image Human Thorax Using Ultrasound Traveltime Tomography with Supervised Descent Method" Applied Sciences 12, no. 13: 6763. https://doi.org/10.3390/app12136763
APA StyleZhang, T., Guo, R., Zhang, H., Zhou, H., Cao, Y., Li, M., Yang, F., & Xu, S. (2022). Image Human Thorax Using Ultrasound Traveltime Tomography with Supervised Descent Method. Applied Sciences, 12(13), 6763. https://doi.org/10.3390/app12136763







