Comparison of Presowing Wheat Treatments by Low-Temperature Plasma, Electric Field, Cold Hardening, and Action of Tebuconazole-Based Disinfectant
Abstract
:Featured Application
Abstract
1. Introduction
- To determine the effect of a combination of electrostatic field and plasma treatment;
- To determine the effect of the electrostatic field and plasma together with the effect of the disinfectant “Bunker”;
- To determine the effect of seed treatment with the electrostatic field and plasma on the relative frost resistance of hardened seedlings;
- To determine the effect of seed treatment with the electrostatic field and plasma on the relative frost resistance of hardened seedlings in combination with the effect of the disinfectant “Bunker”;
- To compare the effect of the presowing hardening of seeds by the action of low temperatures with the effects of the action of the plasma, electric field, and disinfectant.
2. Materials and Methods
2.1. Statistics and Experimental Design
2.2. Seed Material and Seedling Handling Methods
 (in Figure 1) with an electric field or/and plasma, the seeds were placed for germination in a thermostat
(in Figure 1) with an electric field or/and plasma, the seeds were placed for germination in a thermostat  immediately.
immediately. –
–  (in Figure 1). The first stage
(in Figure 1). The first stage  was 7 days at C. The second stage
was 7 days at C. The second stage  was 3 days at C.
was 3 days at C. after germination
after germination  in a thermostat were divided into samples of 50 pieces in gauze bags
in a thermostat were divided into samples of 50 pieces in gauze bags  . After the hardening
. After the hardening  –
–  , the seedlings were subjected to freezing
, the seedlings were subjected to freezing  for one day at temperatures of , , , and C consistently. One quarter of the seedlings were taken out on one day at C
for one day at temperatures of , , , and C consistently. One quarter of the seedlings were taken out on one day at C  ; after the second day at C, the second quarter of the seedlings was taken out
; after the second day at C, the second quarter of the seedlings was taken out  , the third one a day later at C
, the third one a day later at C  , and, finally, the last quarter after another day at C
, and, finally, the last quarter after another day at C  . After this, they were exposed to defrosting
. After this, they were exposed to defrosting  , thawing
, thawing  , and completion of growing
, and completion of growing  . The relative frost resistance of seedlings
. The relative frost resistance of seedlings  was determined as the proportion of seedlings that survived freezing. To determine the proportion
was determined as the proportion of seedlings that survived freezing. To determine the proportion  of surviving seedlings after the thawing stage
of surviving seedlings after the thawing stage  , the seedlings were laid out in groups of samples on one layer of filter paper and germinated
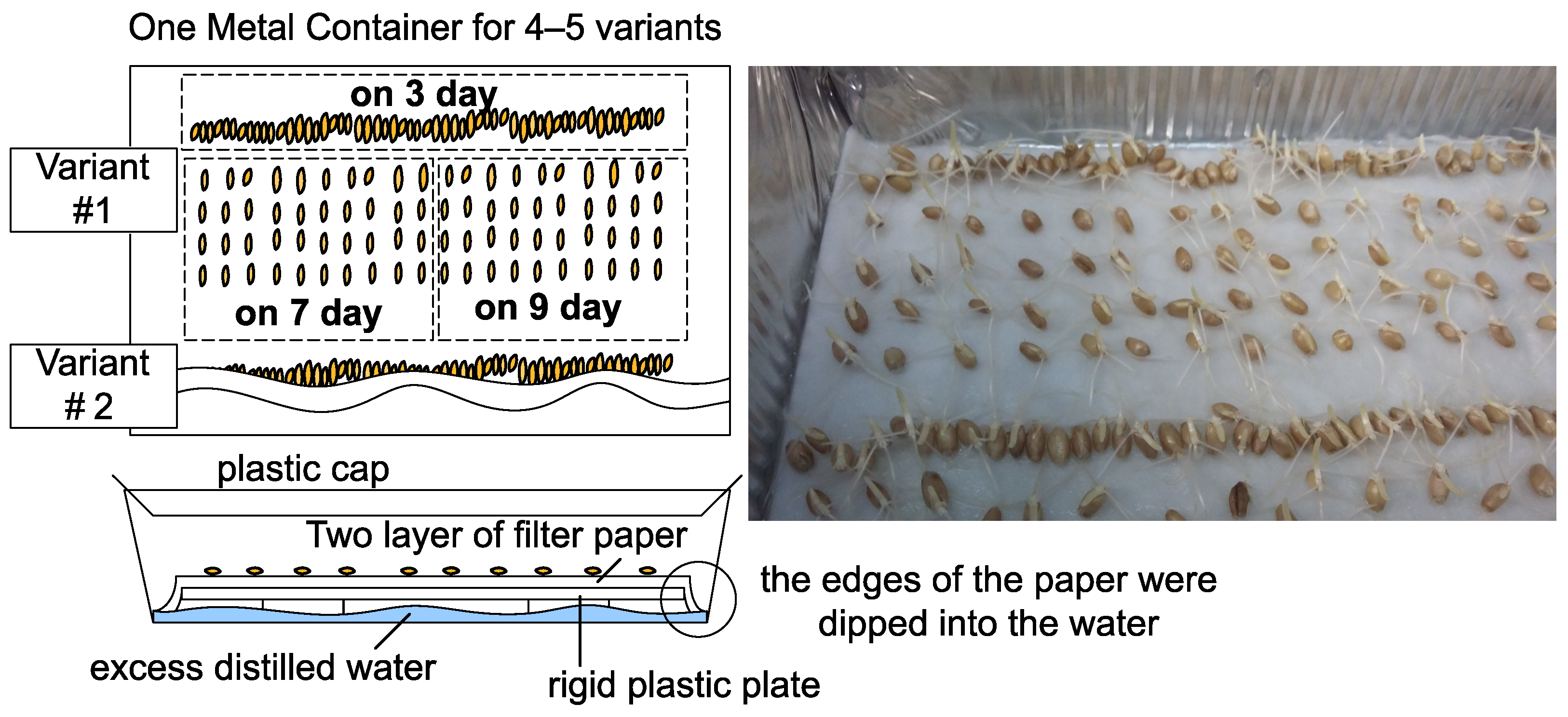
, the seedlings were laid out in groups of samples on one layer of filter paper and germinated  in the dark in distilled water for up to 7 days. We placed up to 20 samples in one container (Figure 3). Seedlings that started growing within 7 days were classified as survivors. A sample count example is shown in Figure 4.
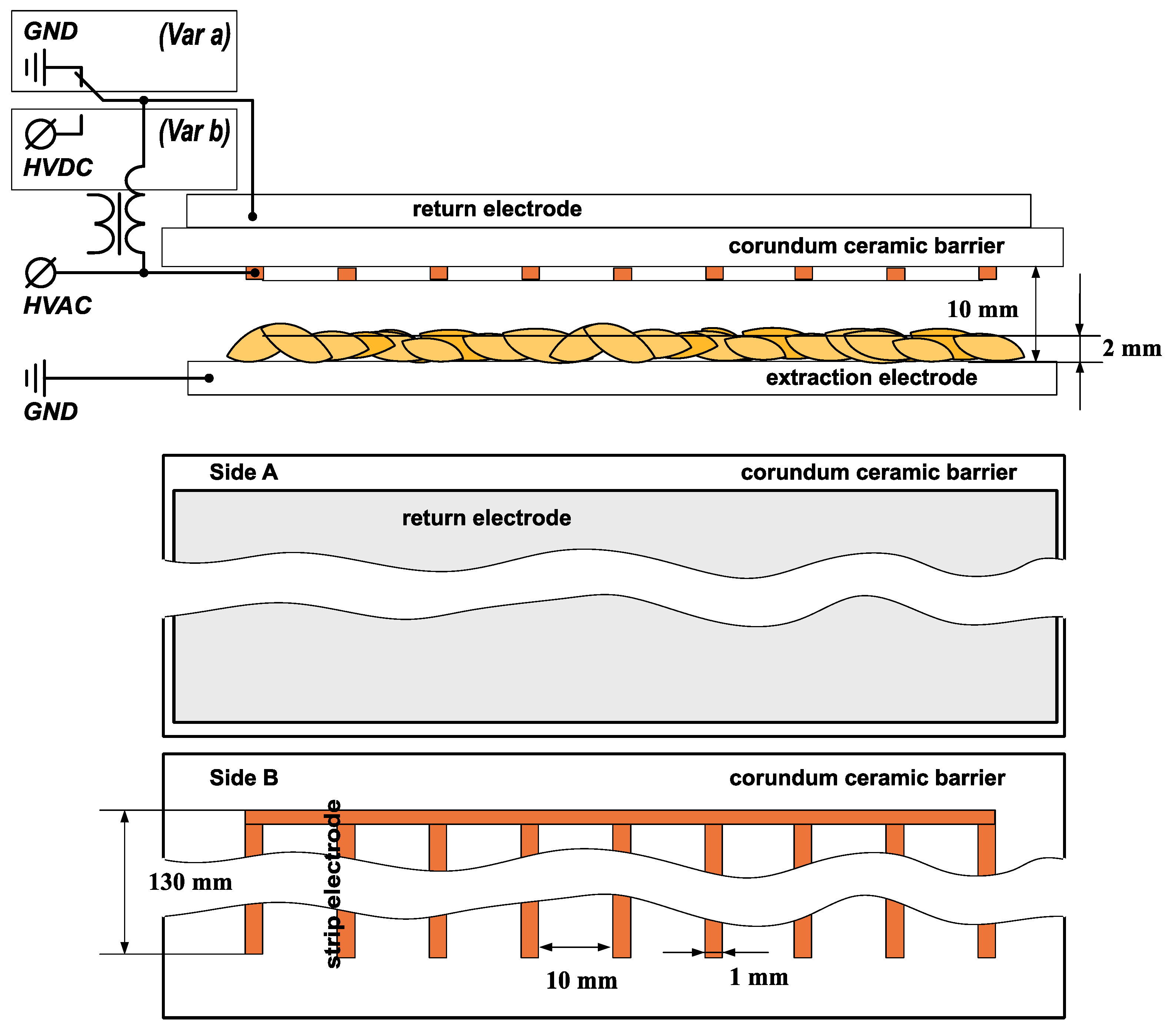
in the dark in distilled water for up to 7 days. We placed up to 20 samples in one container (Figure 3). Seedlings that started growing within 7 days were classified as survivors. A sample count example is shown in Figure 4.2.3. Experimental Setup and Seed Treatment
3. Results and Discussion
3.1. Experimental Data
3.2. Chemical Treatment
3.3. Cold Hardening
3.4. Constant Electric Field
3.5. Plasma Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McKevith, B. Nutritional aspects of cereals. Nutr. Bull. 2004, 29, 111–142. [Google Scholar] [CrossRef]
- Enghiad, A.; Ufer, D.; Countryman, A.M.; Thilmany, D.D. An Overview of Global Wheat Market Fundamentals in an Era of Climate Concerns. Int. J. Agron. 2017, 2017, 3931897. [Google Scholar] [CrossRef]
- FAO. Staple Foods: What Do People Eat? Available online: http://www.fao.org/docrep/u8480e/u8480e07.htm (accessed on 20 April 2022).
- FAO. Sustainable Food and Agriculture. Available online: https://www.fao.org/sustainability/en/ (accessed on 20 April 2022).
- FAO. The 2030 Agenda for Sustainable Development. Sustainable Development Goals. Available online: https://www.fao.org/sustainable-development-goals/overview/fao-and-the-2030-agenda-for-sustainable-development/sustainable-agriculture/en/ (accessed on 20 April 2022).
- Kaur, B.; Sandhu, K.S.; Kamal, R.; Kaur, K.; Singh, J.; Röder, M.S.; Muqaddasi, Q.H. Omics for the Improvement of Abiotic, Biotic, and Agronomic Traits in Major Cereal Crops: Applications, Challenges, and Prospects. Plants 2021, 10, 1989. [Google Scholar] [CrossRef]
- EnergyXPRT, Industrial Companies. Available online: https://www.energy-xprt.com/companies/?keyword=high-voltage (accessed on 20 April 2022).
- Attri, P.; Ishikawa, K.; Okumura, T.; Koga, K.; Shiratani, M. Plasma Agriculture from Laboratory to Farm: A Review. Processes 2020, 8, 1002. [Google Scholar] [CrossRef]
- Wannicke, N.; Wagner, R.; Stachowiak, J.; Nishime, T.M.C.; Ehlbeck, J.; Weltmann, K.D.; Brust, H. Efficiency of plasma-processed air for biological decontamination of crop seeds on the premise of unimpaired seed germination. Plasma Process. Polym. 2021, 18, 2000207. [Google Scholar] [CrossRef]
- Šimek, M.; Homola, T. Plasma-assisted agriculture: History, presence, and prospects—A review. Eur. Phys. J. 2021, 75, 1–31. [Google Scholar] [CrossRef]
- Adhikari, B.; Adhikari, M.; Park, G. The Effects of Plasma on Plant Growth, Development, and Sustainability. Appl. Sci. 2020, 10, 6045. [Google Scholar] [CrossRef]
- Mravlje, J.; Regvar, M.; Starič, P.; Mozetič, M.; Vogel-Mikuš, K. Cold Plasma Affects Germination and Fungal Community Structure of Buckwheat Seeds. Plants 2021, 10, 851. [Google Scholar] [CrossRef]
- Zahoranová, A.; Henselová, M.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Medvecká, V.; Černák, M. Effect of Cold Atmospheric Pressure Plasma on the Wheat Seedlings Vigor and on the Inactivation of Microorganisms on the Seeds Surface. Plasma Chem. Plasma Process. 2015, 36, 397–414. [Google Scholar] [CrossRef]
- Hoppanová, L.; Medvecká, V.; Dylíková, J.; Hudecová, D.; Kaliňáková, B.; Kryštofová, S.; Zahoranová, A. Low-temperature plasma applications in chemical fungicide treatment reduction. Acta Chim. Slovaca 2020, 13, 26–33. [Google Scholar] [CrossRef]
- Huang, P.; Xu, L.; Xie, Y. Biomedical Applications of Electromagnetic Detection: A Brief Review. Biosensors 2021, 11, 225. [Google Scholar] [CrossRef]
- Yudaev, I.; Mashkov, S.; Nugmanov, S.; Gridneva, T.; Syrkin, V.; Fatkhutdinov, M.; Kryuchin, P.; Daus, Y.; Vasiliev, S. Electrophysical Presowing Treatment of Seeds as a Way To Intensify Processes in the Crop Sector of Agriculture; RIO SamGAU: Kinel, Russia, 2020. (In Russian) [Google Scholar]
- Sokolova, M.; Hulka, L.; Pietsch, G.J. Influence of a Bias Voltage on the Characteristics of Surface Discharges in Dry Air. Plasma Process. Polym. 2005, 2, 162–169. [Google Scholar] [CrossRef]
- Avgust Company Products, JSC “August” Inc., Russia. Available online: https://avgust.com/docs/Avgust_portfolio_en.pdf (accessed on 30 January 2022).
- Korsukova, A.; Grabelnych, O.; Borovik, O.; Dorofeev, N.; Pobezhimova, T.; Voinikov, V. The Influence of the Treatment of Seeds by Tebuconazole on the Carbohydrates Content and Frost Resistance of Winter Wheat and Winter Rye. Agric. Chem. 2016, 7, 52–58. (In Russian) [Google Scholar]
- Demidenko, G.; Romanov, V. Impact of chemical protection on germination and growing of Tulunskaya 12 wheat germs. Bull. Novosibirsk State Agrar. Univ. 2017, 42, 42–48. (In Russian) [Google Scholar]
- Babaytseva, T. Influence of presowing treatment of seeds on yield and sowing quality of the winter grain crops. Bull. Izhevsk State Agric. Acad. 2018, 55, 12–21. (In Russian) [Google Scholar]
- Grabelnych, O.; Polykova, E.; Korsukova, A.; Zabanova, N.; Berezhnaya, E.; Lyubushkina, I.; Fedotova, O.; Stepanov, A.; Pobezhimova, T.; Dorofeev, N. Differently Directional Effects of Tebuconazole-Based Disinfectant of Seeds “Bunker” on the Growth of Winter Wheat Shoots and Roots. Ecology 2020, 34, 3–19. [Google Scholar] [CrossRef]
- Pobezhimova, T.; Korsukova, A.; Borovik, O.; Zabanova, N.; Dorofeev, N.; Grabelnych, O.; Voinikov, V. The Influence of Tebuconazole and Tebuconazole-Based Disinfectant “Bunker” on the Functioning of Winter Wheat Mitochondria. Biol. Membr. 2020, 37, 215–223. [Google Scholar] [CrossRef]
- Li, G.; Li, D.; Rao, H.; Liu, X. Potential neurotoxicity, immunotoxicity, and carcinogenicity induced by metribuzin and tebuconazole exposure in earthworms (Eisenia fetida) revealed by transcriptome analysis. Sci. Total. Environ. 2022, 807, 150760. [Google Scholar] [CrossRef]
- Murcia-Morales, M.; Heinzen, H.; Parrilla-Vázquez, P.; del Mar Gómez-Ramos, M.; Fernández-Alba, A.R. Presence and distribution of pesticides in apicultural products: A critical appraisal. TrAC Trends Anal. Chem. 2022, 146, 116506. [Google Scholar] [CrossRef]
- Wang, Y.; Ning, X.; Li, G.; Sang, N. New insights into potential estrogen agonistic activity of triazole fungicides and coupled metabolic disturbance. J. Hazard. Mater. 2022, 424, 127479. [Google Scholar] [CrossRef]
- Jisha, K.; Vijayakumari, K.; Puthur, J.T. Seed priming for abiotic stress tolerance: An overview. Acta Physiol. Plant. 2012, 35, 1381–1396. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Liu, S.; Zhu, X.; Song, F.; Liu, F. Chapter 12—Induction of cross tolerance by cold priming and acclimation in plants: Physiological, biochemical and molecular mechanisms. In Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants; Hossain, M.A., Liu, F., Burritt, D.J., Fujita, M., Huang, B., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 183–201. [Google Scholar] [CrossRef]
- Song, J.S.; Kim, S.B.; Ryu, S.; Oh, J.; Kim, D.S. Emerging Plasma Technology That Alleviates Crop Stress During the Early Growth Stages of Plants: A Review. Front. Plant Sci. 2020, 11, 988. [Google Scholar] [CrossRef]
- Waskow, A.; Avino, F.; Howling, A.; Furno, I. Entering the plasma agriculture field: An attempt to standardize protocols for plasma treatment of seeds. Plasma Process. Polym. 2022, 19, 2100152. [Google Scholar] [CrossRef]
- Plant Collection. The Core Facilities Center “Bioresource Center ”, The Siberian Institute of Plant Physiology and Biochemistry SB RAS, Russia. Available online: http://www.sifibr.irk.ru/en/collection.html (accessed on 20 April 2022).
- Tumanov, I. Methods for Determining the Frost Resistance of Plants; Nauka: Moscow, Russia, 1967. (In Russian) [Google Scholar]
- Gundareva, S.V.; Lazukin, A.V.; Nikitin, A.; Romanov, G. Pre-Sowing Treatment of Winter Wheat Seeds with a Surface Discharge: Freezing Tolerance. Technical Phys. Lett. 2021, 47, 849–852. [Google Scholar] [CrossRef]
- Laboratory of Mycology and Phytopathology, All-Russian Institute of Plant Protection (FSBSI VIZR), Russia. Available online: http://vizrspb.ru/en/research-departments/laboratory-2-mycology-and-phytopathology/ (accessed on 20 April 2022).
- Lazukin, A.V.; Nikitin, A.M.; Romanov, G.A. Surface discharge energy in an electrode system of parallel strips. Tech. Phys. Lett. 2021, 47, 12–14. (In Russian) [Google Scholar] [CrossRef]
- Lazukin, A.; Selivonin, I.; Pinchuk, M.; Moralev, I.; Krivov, S. Influence of the supply voltage period duration and the electrode configuration on the length of microdischarges in surface dielectric barrier discharge. Izv. Vuzov. Fiz. 2018, 61, 152–156. (In Russian) [Google Scholar]
- Krivov, S.A.; Moralev, I.A.; Lazukin, A.V.; Selivonin, I.V. Ion Wind in a Three-Electrode Surface Barrier Discharge Arrangement. IEEE Trans. Plasma Sci. 2020, 48, 2442–2447. [Google Scholar] [CrossRef]
- Lazukin, A.; Serdukov, Y.; Pinchuk, M.; Stepanova, O.; Krivov, S.; Lyubushkina, I. Treatment of spring wheat seeds by ozone generated from humid air and dry oxygen. Res. Agric. Eng. 2018, 64, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Lazukin, A.V.; Grabel’nykh, O.I.; Serdyukov, Y.A.; Pobezhimova, T.P.; Nurminskii, V.N.; Korsukova, A.V.; Krivov, S.A. The Effect of Surface Barrier Discharge Plasma Products on the Germination of Cereals. Technol. Phys. Lett. 2019, 45, 16–19. [Google Scholar] [CrossRef]
- Krivov, S.A.; Lazukin, A.V.; Serdyukov, Y.A.; Gundareva, S.V.; Romanov, G.A. Effect of constant high-voltage electric field on wheat seed germination. IOP SciNotes 2020, 1, 024002. [Google Scholar] [CrossRef]
 seed treatment,
seed treatment,  germination in thermostat,
germination in thermostat,  selection of seedlings after 40 h,
selection of seedlings after 40 h,  formation of samples of 50 pieces,
formation of samples of 50 pieces,  preparation of seed samples for hardening,
preparation of seed samples for hardening,  the first stage of cold hardening (7 days),
the first stage of cold hardening (7 days),  the second stage of cold hardening (3 days),
the second stage of cold hardening (3 days),  freezing,
freezing,  defrosting (24 h),
defrosting (24 h),  thawing (48 h),
thawing (48 h),  germination (7 days), and
germination (7 days), and  counting the proportion of surviving seedlings and
counting the proportion of surviving seedlings and  morphophysiological analysis of seedlings after 3, 7, and 9 days of germination. The arrows are marked with a circle for the seeds, and the arrows are solid for seedlings.
morphophysiological analysis of seedlings after 3, 7, and 9 days of germination. The arrows are marked with a circle for the seeds, and the arrows are solid for seedlings.
 seed treatment,
seed treatment,  germination in thermostat,
germination in thermostat,  selection of seedlings after 40 h,
selection of seedlings after 40 h,  formation of samples of 50 pieces,
formation of samples of 50 pieces,  preparation of seed samples for hardening,
preparation of seed samples for hardening,  the first stage of cold hardening (7 days),
the first stage of cold hardening (7 days),  the second stage of cold hardening (3 days),
the second stage of cold hardening (3 days),  freezing,
freezing,  defrosting (24 h),
defrosting (24 h),  thawing (48 h),
thawing (48 h),  germination (7 days), and
germination (7 days), and  counting the proportion of surviving seedlings and
counting the proportion of surviving seedlings and  morphophysiological analysis of seedlings after 3, 7, and 9 days of germination. The arrows are marked with a circle for the seeds, and the arrows are solid for seedlings.
morphophysiological analysis of seedlings after 3, 7, and 9 days of germination. The arrows are marked with a circle for the seeds, and the arrows are solid for seedlings.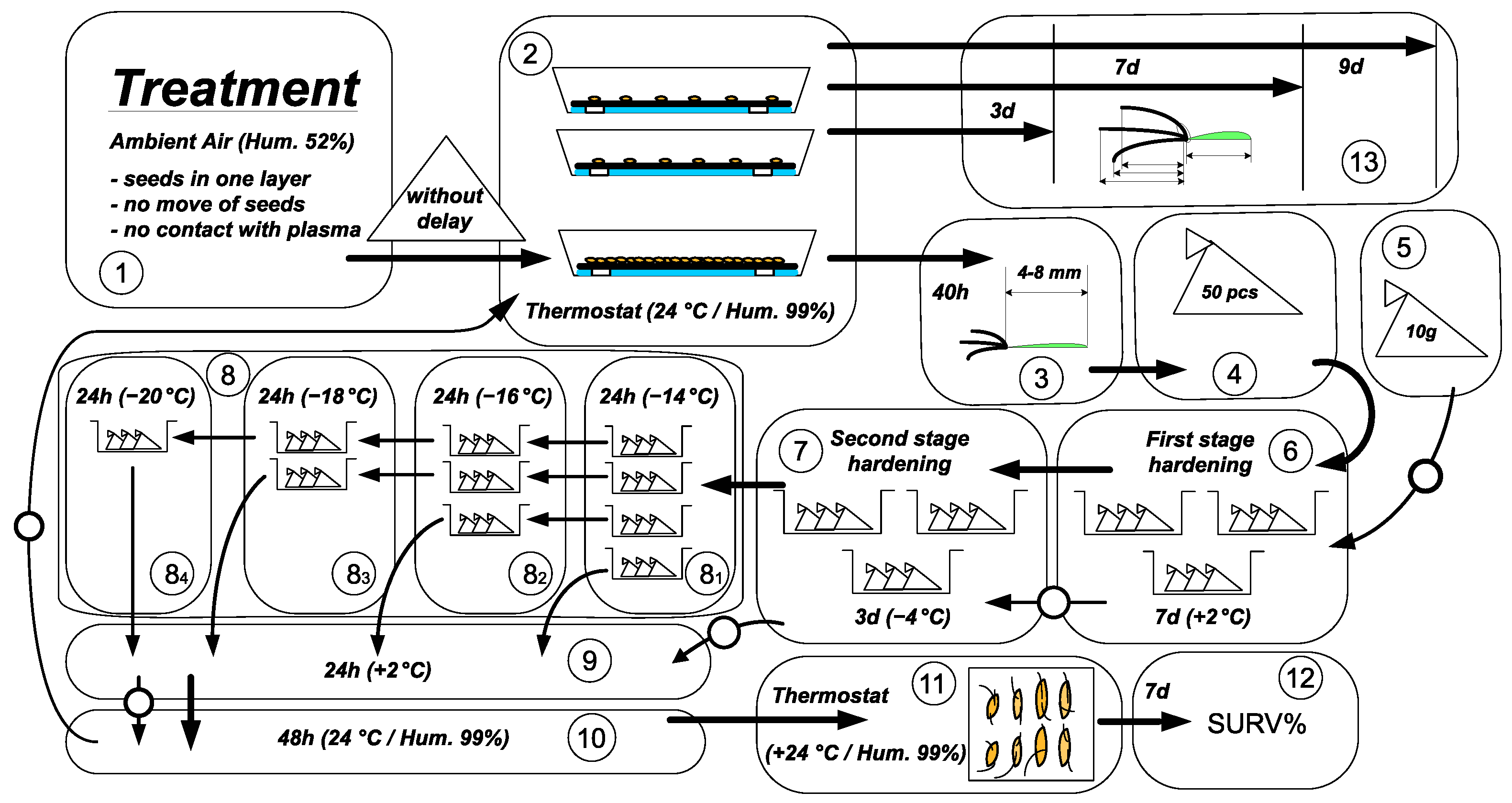

| Control | Cold Hardening | Bunker 0.5 | Bunker 1.5 | Bunker 0.5 16 kHz/1.5 kV | Bunker 1.5 5 kV/cm 1 min | Bunker 1.5 16 kHz/1.5 + 5 kV | |
|---|---|---|---|---|---|---|---|
| 3rd day of Germination | |||||||
| Shoot length % (mm) | |||||||
| Root length % (mm) | |||||||
| Total root length % (mm) | |||||||
| Raw mass of shoots | |||||||
| per plant % (mg/pcs) | |||||||
| Dry mass of shoots | |||||||
| per plant % (mg/pcs) | |||||||
| Raw mass of roots | |||||||
| per plant % (mg/pcs) | |||||||
| Dry mass of roots | |||||||
| per plant % (mg/pcs) | |||||||
| Germination % | |||||||
| Number of sets, pcs | 16 | 6 | 5 | 4 | 4 | 2 | 4 |
| Total number of plants, pcs | 767 | 233 | 347 | 366 | 135 | 80 | 130 |
| 7th day of Germination | |||||||
| Shoot length % (mm) | |||||||
| Root length % (mm) | |||||||
| Total root length % (mm) | |||||||
| Raw mass of shoots | |||||||
| per plant % (mg/pcs) | |||||||
| Dry mass of shoots | |||||||
| per plant % (mg/pcs) | |||||||
| Raw mass of roots | |||||||
| per plant % (mg/pcs) | |||||||
| Dry mass of roots | |||||||
| per plant % (mg/pcs) | |||||||
| Germination % | |||||||
| Number of sets, pcs | 11 | 3 | 5 | 4 | 4 | 2 | 2 |
| Total number of plants, pcs | 446 | 98 | 287 | 246 | 126 | 73 | 138 |
| 9th day of Germination | |||||||
| Shoot length % (mm) | |||||||
| Root length % (mm) | |||||||
| Total root length % (mm) | |||||||
| Raw mass of shoots | |||||||
| per plant % (mg/pcs) | |||||||
| Dry mass of shoots | |||||||
| per plant % (mg/pcs) | |||||||
| Raw mass of roots | |||||||
| per plant % (mg/pcs) | |||||||
| Dry mass of roots | |||||||
| per plant % (mg/pcs) | |||||||
| Germination % | |||||||
| Number of sets, pcs | 11 | 1 | 5 | 4 | 3 | 1 | 3 |
| Total number of plants, pcs | 543 | 46 | 266 | 253 | 91 | 31 | 104 |
| Control | 4.4 kHz/1.5 kV | 4.4 kHz/2.4 kV | 16 kHz/1.5 kV | 16 kHz/2.4 kV | 5 kV/cm 1 min | 5 kV/cm 10 min | 16 kHz /1.5 + 5 kV | |
|---|---|---|---|---|---|---|---|---|
| 3rd day of Germination | ||||||||
| Shoot length % (mm) | ||||||||
| Root length % (mm) | ||||||||
| Total root length % (mm) | ||||||||
| Raw mass of shoots | ||||||||
| per plant % (mg/pcs) | ||||||||
| Dry mass of shoots | ||||||||
| per plant % (mg/pcs) | ||||||||
| Raw mass of roots | ||||||||
| per plant % (mg/pcs) | ||||||||
| Dry mass of roots | ||||||||
| per plant % (mg/pcs) | ||||||||
| Germination % | ||||||||
| Number of sets, pcs | 16 | 4 | 4 | 12 | 3 | 3 | 8 | 8 |
| Total number of plants, pcs | 743 | 207 | 207 | 650 | 166 | 164 | 363 | 364 |
| 7th day of Germination | ||||||||
| Shoot length % (mm) | ||||||||
| Root length % (mm) | ||||||||
| Total root length % (mm) | ||||||||
| Raw mass of shoots | ||||||||
| per plant % (mg/pcs) | ||||||||
| Dry mass of shoots | ||||||||
| per plant % (mg/pcs) | ||||||||
| Raw mass of roots | ||||||||
| per plant % (mg/pcs) | ||||||||
| Dry mass of roots | ||||||||
| per plant % (mg/pcs) | ||||||||
| Germination % | ||||||||
| Number of sets, pcs | 13 | 4 | 5 | 8 | 4 | 5 | 6 | 9 |
| Total number of plants, pcs | 535 | 198 | 233 | 347 | 182 | 237 | 244 | 395 |
| 9th day of Germination | ||||||||
| Shoot length % (mm) | ||||||||
| Root length % (mm) | ||||||||
| Total root length % (mm) | ||||||||
| Raw mass of shoots | ||||||||
| per plant % (mg/pcs) | ||||||||
| Dry mass of shoots | ||||||||
| per plant % (mg/pcs) | ||||||||
| Raw mass of roots | ||||||||
| per plant % (mg/pcs) | ||||||||
| Dry mass of roots | ||||||||
| per plant % (mg/pcs) | ||||||||
| Germination % | ||||||||
| Number of sets, pcs | 12 | 4 | 5 | 11 | 4 | 5 | 6 | 5 |
| Total number of plants, pcs | 555 | 177 | 246 | 501 | 170 | 211 | 231 | 230 |
| C | C | C | C | |||||
|---|---|---|---|---|---|---|---|---|
| mean% | NR | mean% | NR | mean% | NR | mean% | NR | |
| Control | 64 | 52 | 55 | 62 | ||||
| Bunker 0.5 | 13 | 20 | 16 | 14 | ||||
| Bunker 1.5 | 10 | 5 | 3 | 4 | ||||
| Bunker 0.5; 16 kHz/1.5 kV | 5 | 5 | 9 | 10 | ||||
| Bunker 0.5; 5 kV/cm 1 min | 3 | 4 | 4 | 6 | ||||
| Bunker 0.5; 16 kHz/1.5 + 5 kV | 2 | 3 | 3 | 4 | ||||
| 4.4 kHz/1.5 kV | 7 | 7 | 7 | 6 | ||||
| 4.4 kHz/2.4 kV | 23 | 28 | 32 | 36 | ||||
| 16 kHz/1.5 kV | 27 | 15 | 25 | 27 | ||||
| 16 kHz/2.4 kV | 7 | 9 | 7 | 8 | ||||
| 5 kV/cm 1 min | 9 | 13 | 13 | 15 | ||||
| 5 kV/cm 10 min | 11 | 11 | 13 | 14 | ||||
| 16 kHz/1.5 kV + 5 kV | 11 | 16 | 17 | 17 |
| , kV | 1 | 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Frequency | Power, W | |||||||
| 4.4 kHz | ||||||||
| 16 kHz | 3 | |||||||
| Reference [22] | Current Values | |||||
|---|---|---|---|---|---|---|
| 7th day of Germination | ||||||
| Control | Bunker 0.5 | Bunker 1.5 | Control | Bunker 0.5 | Bunker 1.5 | |
| Shoot length % (mm) | ||||||
| Total root length % (mm) | ||||||
| Raw mass of shoots | ||||||
| per plant % (mg/pcs) | ||||||
| Dry mass of shoots | ||||||
| per plant % (mg/pcs) | ||||||
| Raw mass of roots | ||||||
| per plant % (mg/pcs) | ||||||
| Dry mass of roots | ||||||
| per plant % (mg/pcs) | ||||||
| 9th day of Germination | ||||||
| Shoot length % (mm) | ||||||
| Total root length % (mm) | ||||||
| Raw mass of shoots | ||||||
| per plant % (mg/pcs) | ||||||
| Dry mass of shoots | ||||||
| per plant % (mg/pcs) | ||||||
| Raw mass of roots | ||||||
| per plant % (mg/pcs) | ||||||
| Dry mass of roots | ||||||
| per plant % (mg/pcs) | ||||||
| Seedborne Fungi | Seed Contamination, % | |
|---|---|---|
| Control | Bunker 0.5 | |
| Alternaria | 92 | 85 |
| Bipolaris | 2 | 0 |
| Cladosporium | 1 | 0 |
| Epicoccum | 8 | 5 |
| Fusarium | 6 | 0 |
| Mucor | 1 | 0 |
| Penicillium | 5 | 4 |
| Trichothecium | 2 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazukin, A.; Pinchuk, M.; Korsukova, A.; Nikiforov, A.; Romanov, G.; Stepanova, O.; Grabelnych, O. Comparison of Presowing Wheat Treatments by Low-Temperature Plasma, Electric Field, Cold Hardening, and Action of Tebuconazole-Based Disinfectant. Appl. Sci. 2022, 12, 6447. https://doi.org/10.3390/app12136447
Lazukin A, Pinchuk M, Korsukova A, Nikiforov A, Romanov G, Stepanova O, Grabelnych O. Comparison of Presowing Wheat Treatments by Low-Temperature Plasma, Electric Field, Cold Hardening, and Action of Tebuconazole-Based Disinfectant. Applied Sciences. 2022; 12(13):6447. https://doi.org/10.3390/app12136447
Chicago/Turabian StyleLazukin, Alexander, Mikhail Pinchuk, Anna Korsukova, Anton Nikiforov, Gennadij Romanov, Olga Stepanova, and Olga Grabelnych. 2022. "Comparison of Presowing Wheat Treatments by Low-Temperature Plasma, Electric Field, Cold Hardening, and Action of Tebuconazole-Based Disinfectant" Applied Sciences 12, no. 13: 6447. https://doi.org/10.3390/app12136447
APA StyleLazukin, A., Pinchuk, M., Korsukova, A., Nikiforov, A., Romanov, G., Stepanova, O., & Grabelnych, O. (2022). Comparison of Presowing Wheat Treatments by Low-Temperature Plasma, Electric Field, Cold Hardening, and Action of Tebuconazole-Based Disinfectant. Applied Sciences, 12(13), 6447. https://doi.org/10.3390/app12136447







