Nitrate and Dissolved Organic Carbon Release in Sandy Soils at Different Liquid/Solid Ratios Amended with Graphene and Classical Soil Improvers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soils and Improvers Characteristics
2.2. Batch Experiments
2.3. Graphene’s NO3− Sorption Isotherm
3. Results and Discussions
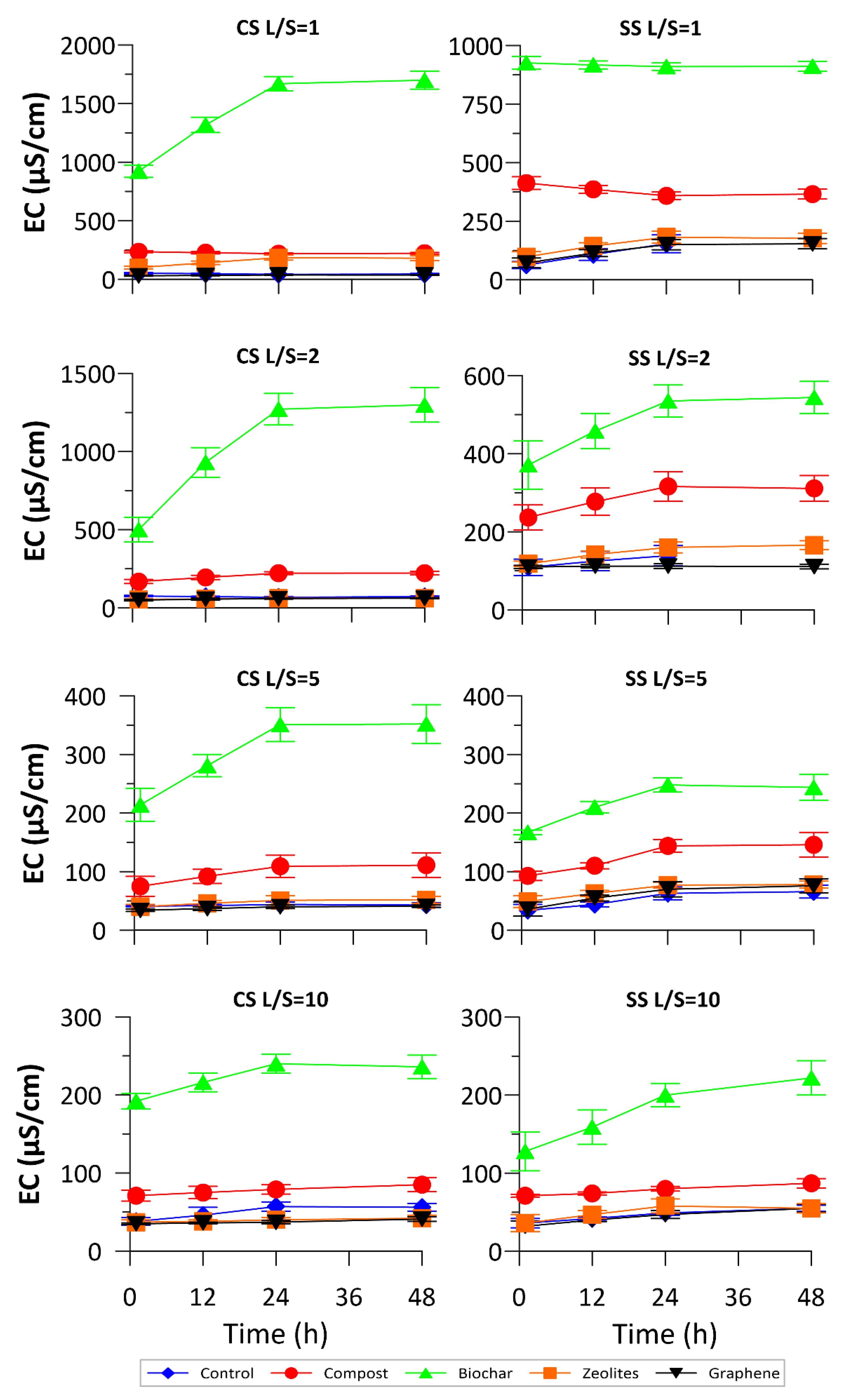
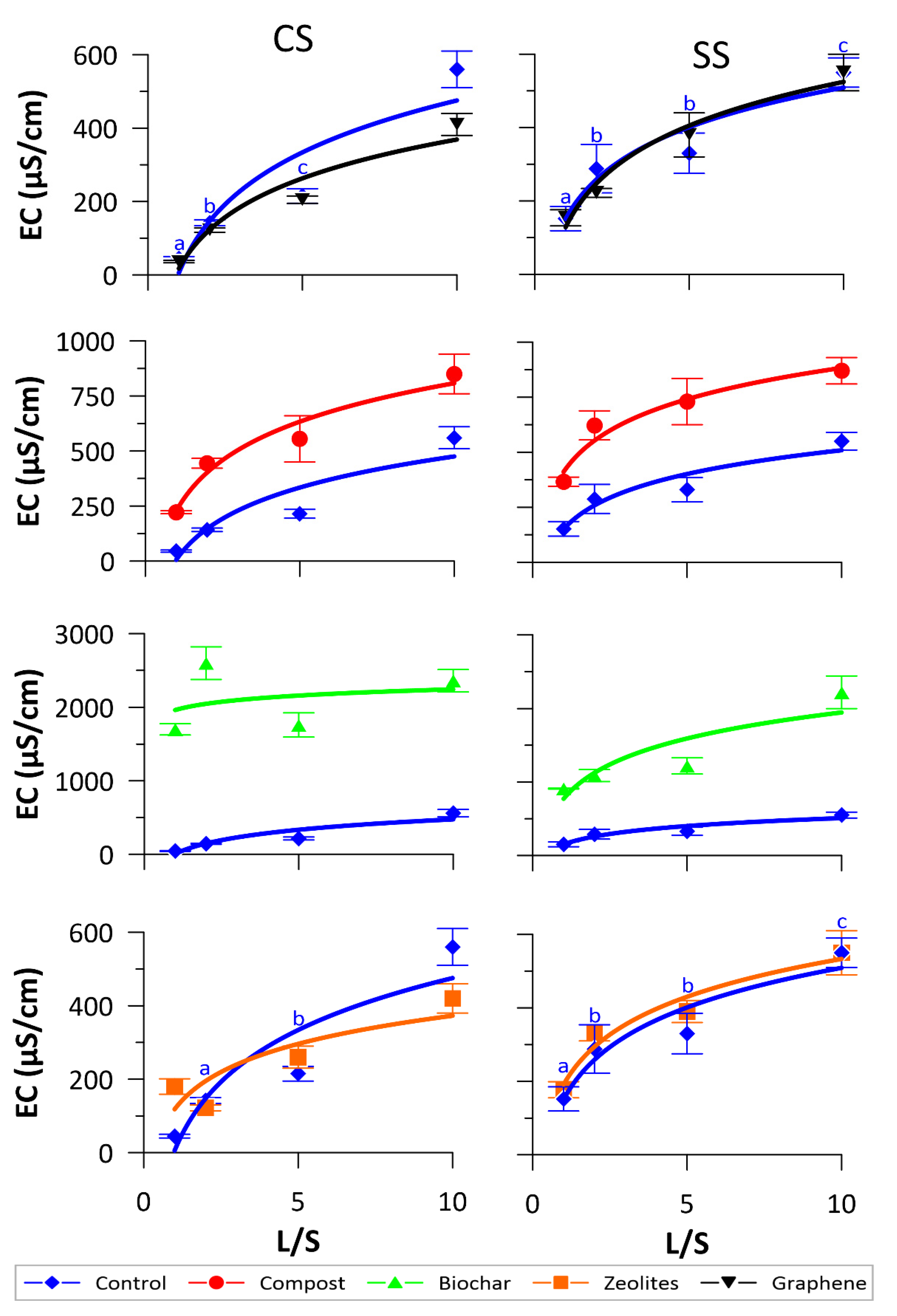
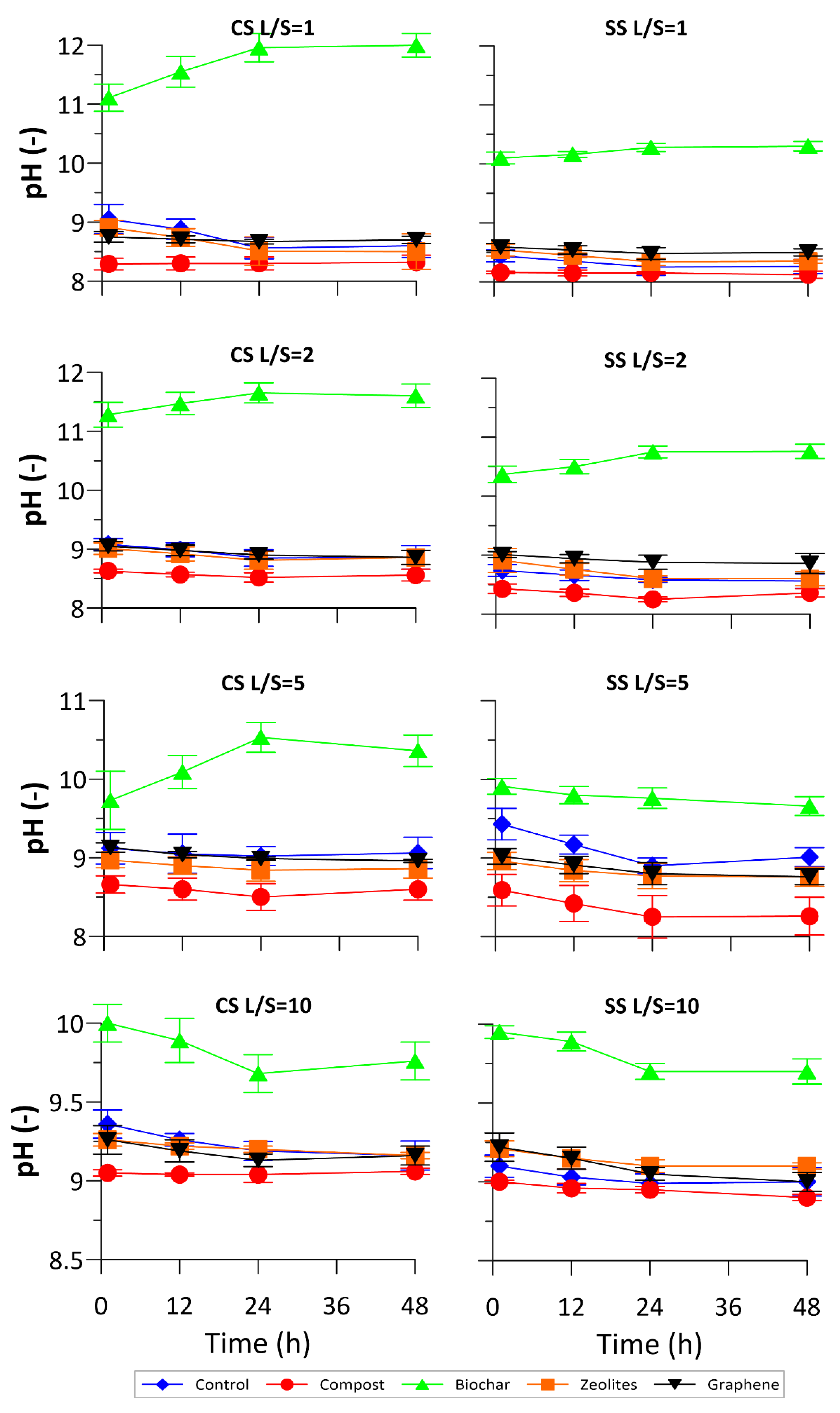
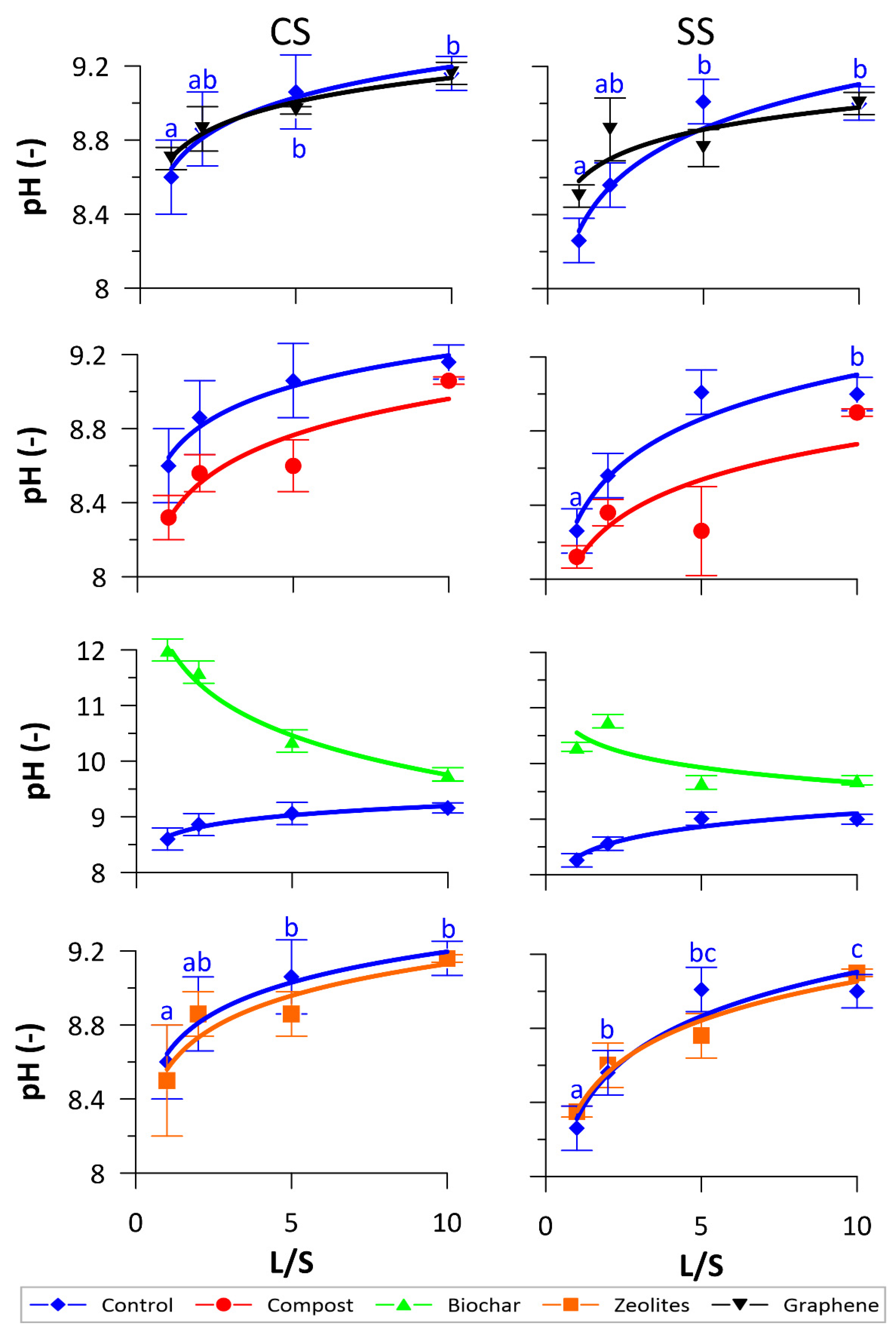
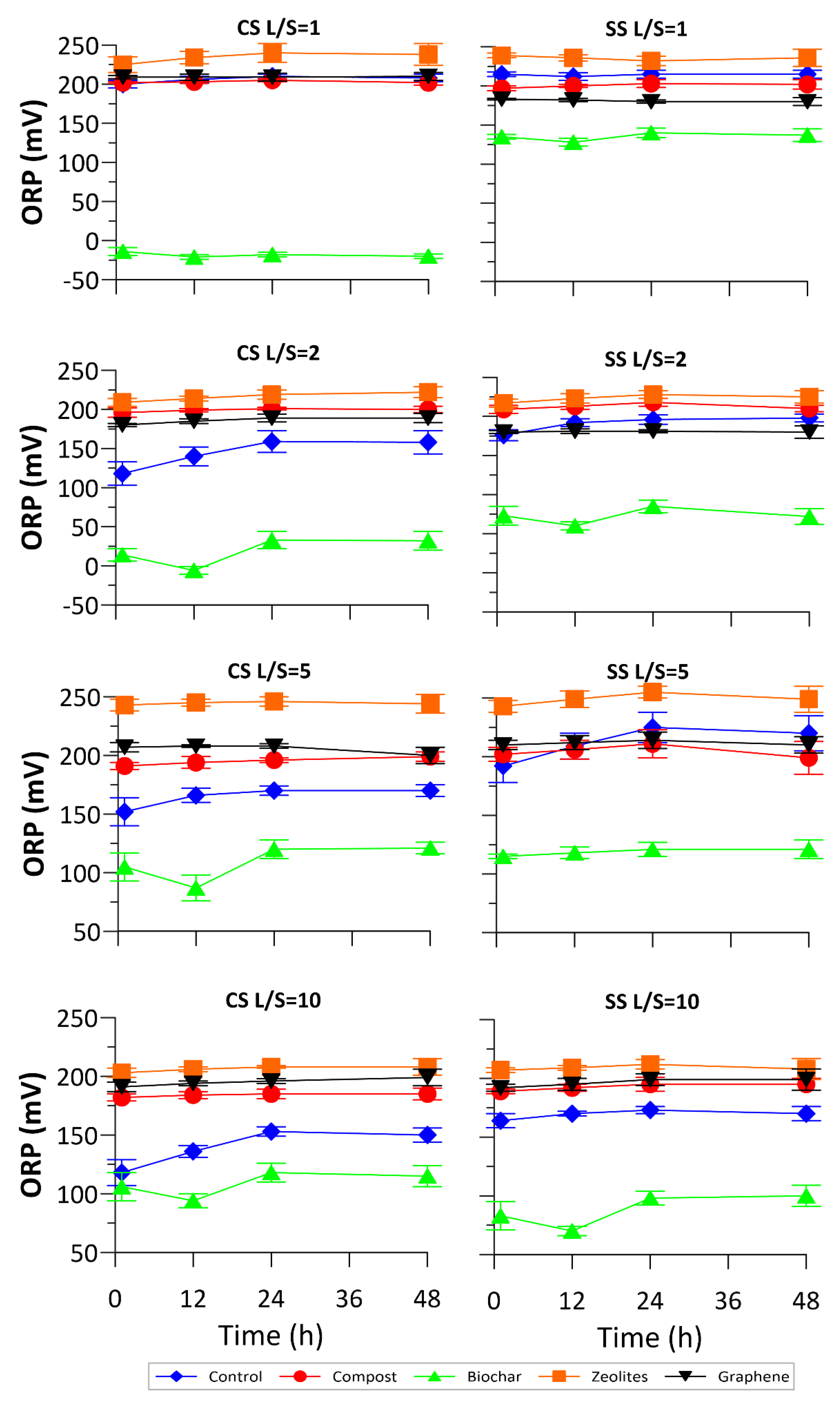
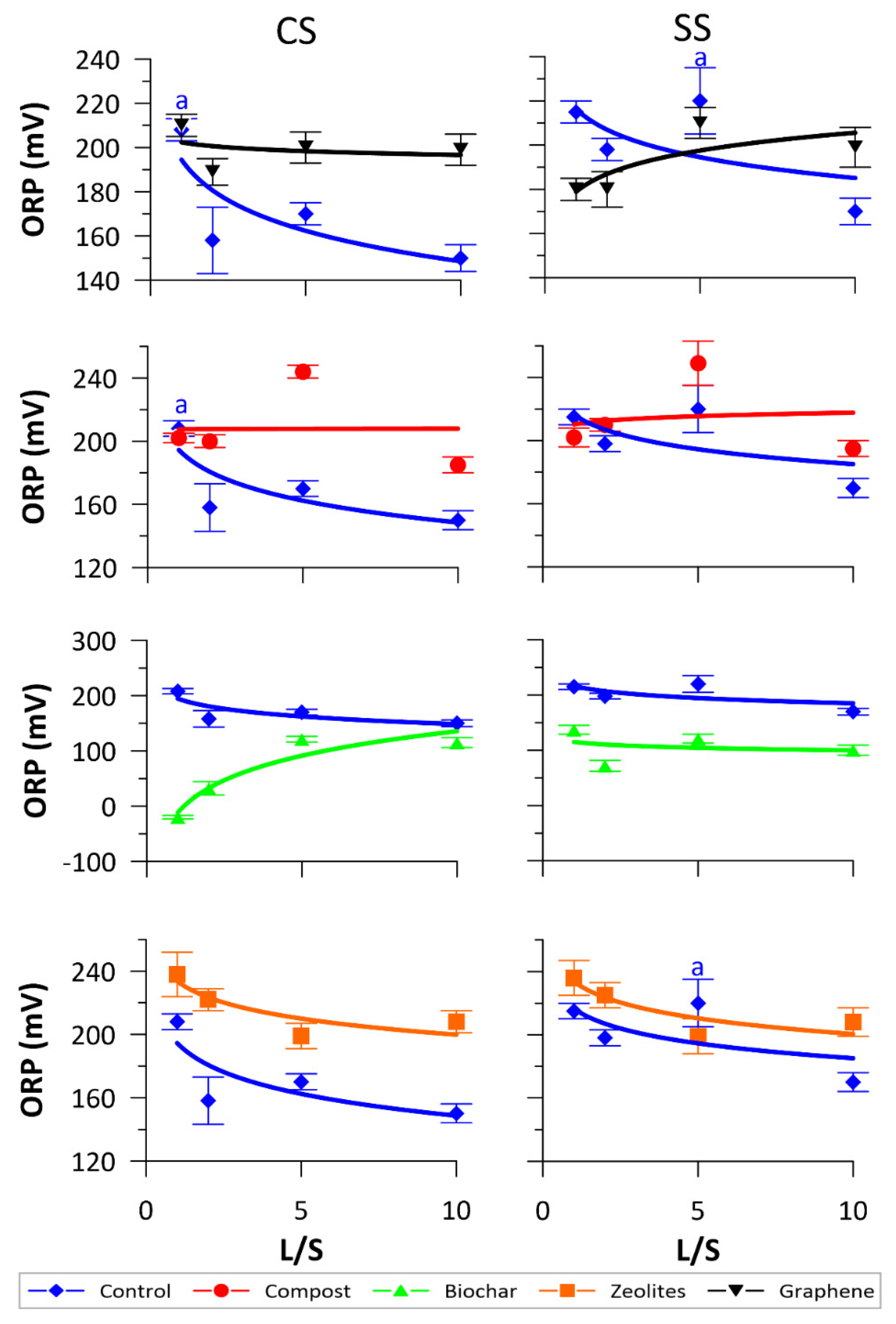
3.1. Physical-Chemical Monitoring
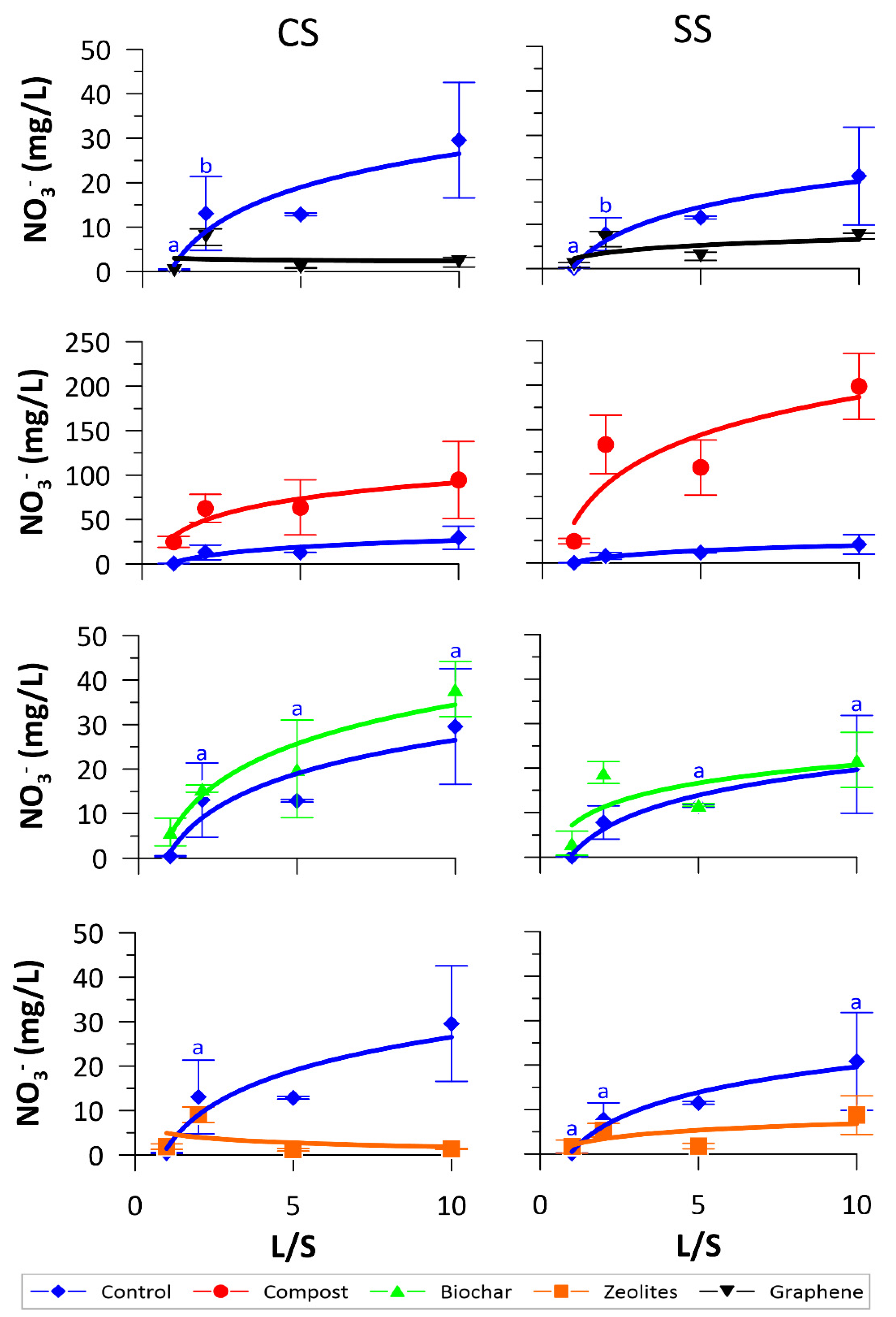
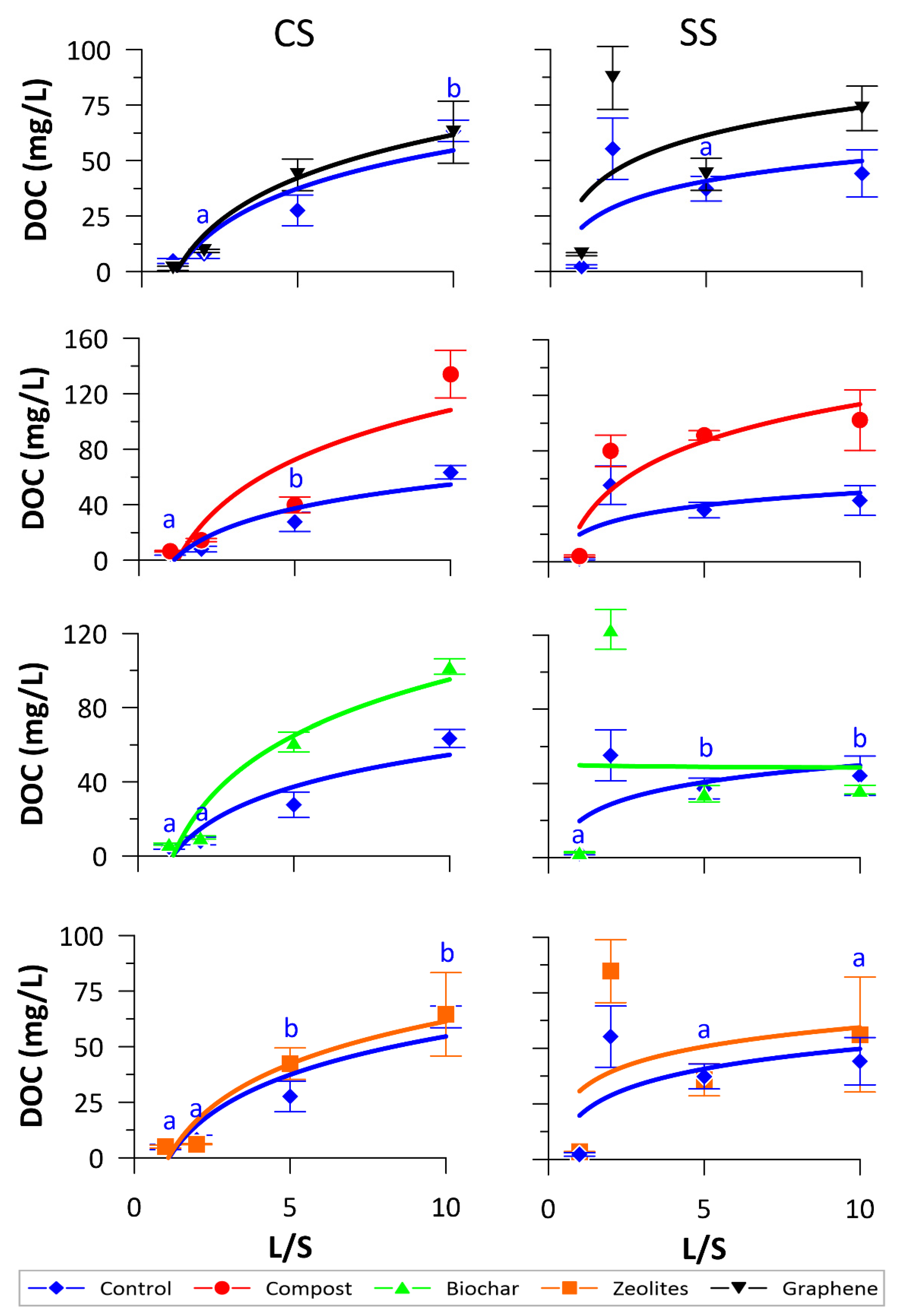
3.2. NO3− and DOC Release
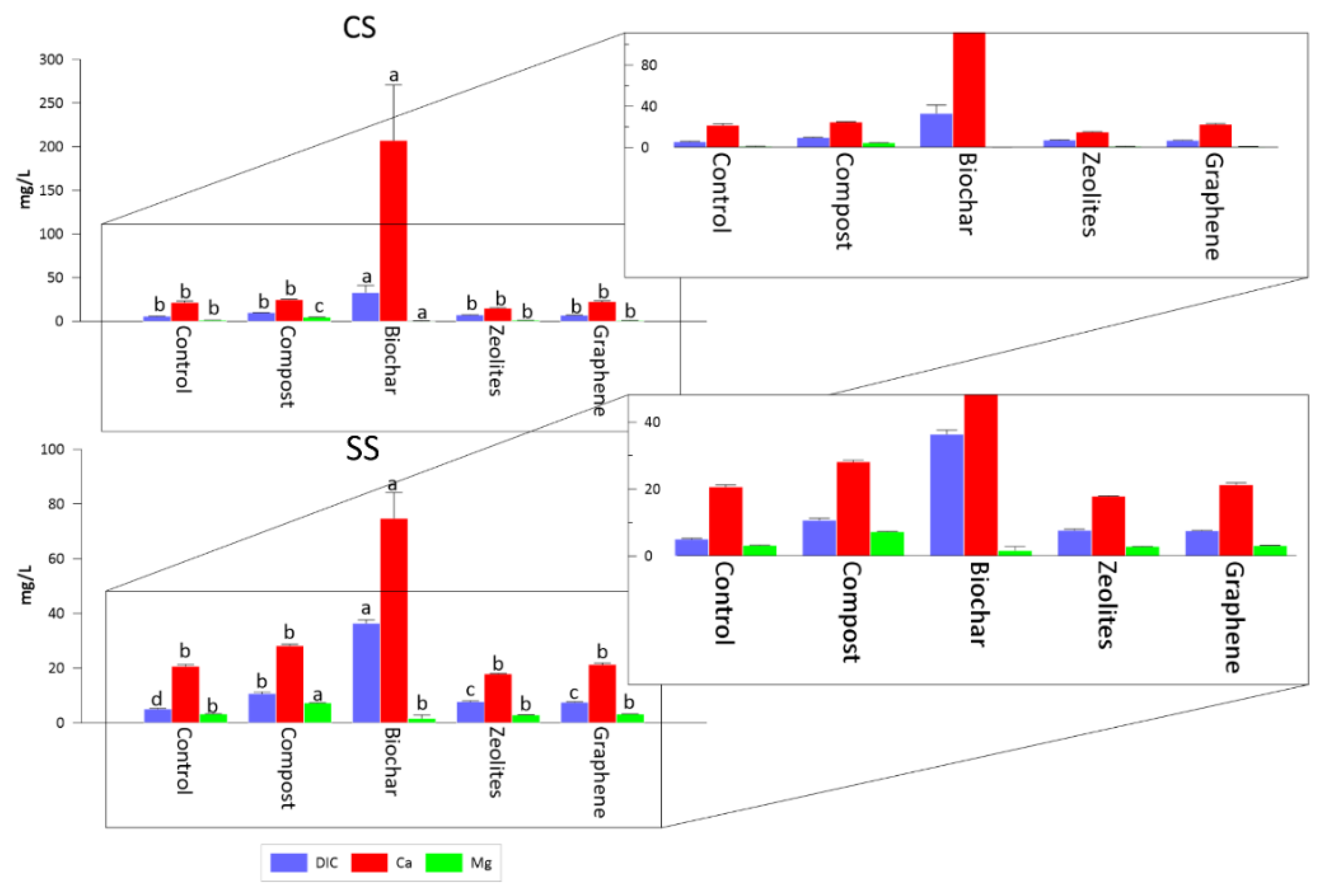
3.3. DIC, Ca and Mg in L/S = 5 Batches
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, A.; Chua, C.K.; Latiff, N.M.; Loo, A.H.; Wong, C.H.A.; Eng, A.Y.S.; Bonanni, A.; Pumera, M. Graphene and Its Electrochemistry—An Update. Chem. Soc. Rev. 2016, 45, 2458–2493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghadiri, S.K.; Nasseri, S.; Nabizadeh, R.; Khoobi, M.; Nazmara, S.; Mahvi, A.H. Adsorption of Nitrate onto Anionic Bio-Graphene Nanosheet from Aqueous Solutions: Isotherm and Kinetic Study. J. Mol. Liq. 2017, 242, 1111–1117. [Google Scholar] [CrossRef]
- Feng, L.; Liu, Z. Graphene in Biomedicine: Opportunities and Challenges. Nanomedicine 2011, 6, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Shen, H.; Wang, Y.; Chu, X.; Xie, J.; Zhou, N.; Shen, J. Biomedical Application of Graphene: From Drug Delivery, Tumor Therapy, to Theranostics. Colloids Surf. B Biointerfaces 2020, 185, 110596. [Google Scholar] [CrossRef]
- Kusmartsev, F.V.; Wu, W.M.; Pierpoint, M.P.; Yung, K.C. Application of Graphene Within Optoelectronic Devices and Transistors. In Applied Spectroscopy and the Science of Nanomaterials; Misra, P., Ed.; Springer Singapore: Singapore, 2015; pp. 191–221. ISBN 978-981-287-242-5. [Google Scholar]
- Mortazavi, V.; Moosavi, A.; Nouri-Borujerdi, A. Enhancing Water Desalination in Graphene-Based Membranes via an Oscillating Electric Field. Desalination 2020, 495, 114672. [Google Scholar] [CrossRef]
- Gareeb, R.Y.; Elnouby, M.; Hasan, M.A.; Ticu, S.; Popa, A.R.; Bungău, S.G.; Hafez, E.E. New Trend for Using the Reduced Graphene Oxide as Effective and Eco-Friendly Nematicide. Mater. Plast. 2019, 56, 59–64. [Google Scholar] [CrossRef]
- Randviir, E.P.; Brownson, D.A.C.; Banks, C.E. A Decade of Graphene Research: Production, Applications and Outlook. Mater. Today 2014, 17, 426–432. [Google Scholar] [CrossRef]
- Costa, G.M.; da Hussain, C.M. Ethical, Legal, Social and Economics Issues of Graphene. Compr. Anal. Chem. 2020, 91, 263–279. [Google Scholar] [CrossRef]
- Korhonen, J.; Honkasalo, A.; Seppälä, J. Circular Economy: The Concept and Its Limitations. Ecol. Econ. 2018, 143, 37–46. [Google Scholar] [CrossRef]
- Alessandrino, L.; Colombani, N.; Eusebi, A.L.; Aschonitis, V.; Mastrocicco, M. Testing Graphene versus Classical Soil Improvers in a Sandy Calcisol. Catena 2022, 208, 105754. [Google Scholar] [CrossRef]
- Lord, E.I.; Anthony, S.G.; Goodlass, G. Agricultural Nitrogen Balance and Water Quality in the UK. Soil Use Manag. 2002, 18, 363–369. [Google Scholar] [CrossRef]
- Schröder, J.J.; Scholefield, D.; Cabral, F.; Hofman, G. The Effects of Nutrient Losses from Agriculture on Ground and Surface Water Quality: The Position of Science in Developing Indicators for Regulation. Environ. Sci. Pol. 2004, 7, 15–23. [Google Scholar] [CrossRef]
- Delay, M.; Lager, T.; Schulz, H.D.; Frimmel, F.H. Comparison of Leaching Tests to Determine and Quantify the Release of Inorganic Contaminants in Demolition Waste. Waste Manag. 2007, 27, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Schuwirth, N.; Hofmann, T. Comparability of and Alternatives to Leaching Tests for the Assessment of the Emission of Inorganic Soil Contamination (11 Pp). J. Soils Sediments 2006, 6, 102–112. [Google Scholar] [CrossRef]
- Celis, R.; Real, M.; Hermosín, M.C.; Cornejo, J. Sorption and Leaching Behaviour of Polar Aromatic Acids in Agricultural Soils by Batch and Column Leaching Tests. Eur. J. Soil Sci. 2005, 56, 287–297. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Veerawattananun, S.; Ito, M.; Hiroyoshi, N.; Igarashi, T. Pyrite Oxidation in the Presence of Hematite and Alumina: I. Batch Leaching Experiments and Kinetic Modeling Calculations. Sci. Total Environ. 2017, 580, 687–698. [Google Scholar] [CrossRef]
- Di Giuseppe, D.; Faccini, B.; Mastrocicco, M.; Colombani, N.; Coltorti, M. Reclamation influence and background geochemistry of neutral saline soils in the Po River Delta Plain (Northern Italy). Environ. Earth Sci. 2014, 72, 2457–2473. [Google Scholar] [CrossRef]
- Sakanakura, H.; Ito, K.; Tang, J.; Nakagawa, M.; Ishimori, H. Determining Adsorption Parameters of Potentially Contaminant-Releasing Materials Using Batch Tests with Differing Liquid-Solid Ratios. Materials 2021, 14, 2534. [Google Scholar] [CrossRef]
- Sahuquillo, A.; Rigol, A.; Rauret, G. Overview of the Use of Leaching/Extraction Tests for Risk Assessment of Trace Metals in Contaminated Soils and Sediments. TrAC Trends Anal. Chem. 2003, 22, 152–159. [Google Scholar] [CrossRef]
- Iden, S.C.; Durner, W. Multiple Batch Extraction Test to Estimate Contaminant Release Parameters Using a Bayesian Approach. J. Contam. Hydrol. 2008, 95, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Grathwohl, P.; Susset, B. Comparison of Percolation to Batch and Sequential Leaching Tests: Theory and Data. Waste Manag. 2009, 29, 2681–2688. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.; Peacock, M.; Evans, C.D.; Page, S.E.; Whelan, M.J.; Gauci, V.; Kho, L.K. Quantifying Tropical Peatland Dissolved Organic Carbon (DOC) Using UV-Visible Spectroscopy. Water Res. 2017, 115, 229–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Zhang, L.; Xu, C.; Vaidyanathan, S. Dissolved Inorganic Carbon Speciation in Aquatic Environments and Its Application to Monitor Algal Carbon Uptake. Sci. Total Environ. 2016, 541, 1282–1295. [Google Scholar] [CrossRef] [PubMed]
- Bendz, D.; Tüchsen, P.L.; Christensen, T.H. The Dissolution Kinetics of Major Elements in Municipal Solid Waste Incineration Bottom Ash Particles. J. Contam. Hydrol. 2007, 94, 178–194. [Google Scholar] [CrossRef]
- Mastrocicco, M.; Colombani, N.; Palpacelli, S. Fertilizers Mobilization in Alluvial Aquifer: Laboratory Experiments. Environ. Earth Sci. 2009, 56, 1371–1381. [Google Scholar] [CrossRef]
- Boretti, A.; Al-Zubaidy, S.; Vaclavikova, M.; Al-Abri, M.; Castelletto, S.; Mikhalovsky, S. Outlook for Graphene-Based Desalination Membranes. NPJ Clean Water 2018, 1, 5. [Google Scholar] [CrossRef]
- Kookana, R.S.; Sarmah, A.K.; Van Zwieten, L.; Krull, E.; Singh, B. Biochar Application to Soil. Agronomic and Environmental Benefits and Unintended Consequences. Adv. Agron. 2011, 112, 103–143. [Google Scholar] [CrossRef]
- Ramesh, K.; Reddy, D.D. Chapter Four—Zeolites and Their Potential Uses in Agriculture. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2011; Volume 113, pp. 219–241. [Google Scholar]
- Chefetz, B.; Hatcher, P.G.; Hadar, Y.; Chen, Y. Chemical and Biological Characterization of Organic Matter during Composting of Municipal Solid Waste. J. Environ. Qual. 1996, 25, 776–785. [Google Scholar] [CrossRef]
- Booth, M.S.; Stark, J.M.; Rastetter, E. Controls on Nitrogen Cycling in Terrestrial Ecosystems: A Synthetic Analysis of Literature Data. Ecol. Monogr. 2005, 75, 139–157. [Google Scholar] [CrossRef] [Green Version]
- Novak, S.M.; Fiorelli, J.L. Greenhouse Gases and Ammonia Emissions from Organic Mixed Crop-Dairy Systems: A Critical Review of Mitigation Options. Agron. Sustain. Dev. 2010, 30, 215–236. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.R.; Minhas, P.S. Strategies for Managing Saline/Alkali Waters for Sustainable Agricultural Production in South Asia. Agric. Water Manag. 2005, 78, 136–151. [Google Scholar] [CrossRef]
- Hu, Y.-B.; Wolf-Gladrow, D.A.; Dieckmann, G.S.; Völker, C.; Nehrke, G. A Laboratory Study of Ikaite (CaCO3·6H2O) Precipitation as a Function of PH, Salinity, Temperature and Phosphate Concentration. Mar. Chem. 2014, 162, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Morse, J.W.; Gledhill, D.K.; Millero, F.J. CaCo3 Precipitation Kinetics in Waters from the Great Bahama Bank:: Implications for the Relationship between Bank Hydrochemistry and Whitings. Geochim. Cosmochim. Acta 2003, 67, 2819–2826. [Google Scholar] [CrossRef]
- Joseph, S.D.; Camps-Arbestain, M.; Lin, Y.; Munroe, P.; Chia, C.H.; Hook, J.; Van Zwieten, L.; Kimber, S.; Cowie, A.; Singh, B.P.; et al. An Investigation into the Reactions of Biochar in Soil. Soil Res. 2010, 48, 501–515. [Google Scholar] [CrossRef]
- Qiu, Y.; Wang, Z.; Owens, A.C.E.; Kulaots, I.; Chen, Y.; Kane, A.B.; Hurt, R.H. Antioxidant Chemistry of Graphene-Based Materials and Its Role in Oxidation Protection Technology. Nanoscale 2014, 6, 11744–11755. [Google Scholar] [CrossRef] [Green Version]
- Sharpley, A.N. Effect of Soil PH on Cation and Anion Solubility. Commun. Soil Sci. Plant Anal. 1991, 22, 827–841. [Google Scholar] [CrossRef]
- Jiang, M.; Feng, L.; Zheng, X.; Chen, Y. Bio-Denitrification Performance Enhanced by Graphene-Facilitated Iron Acquisition. Water Res. 2020, 180, 115916. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality [Electronic Resource]: Incorporating 1st and 2nd Addenda, Vol.1, Recommendations. 2008. Available online: https://apps.who.int/iris/handle/10665/204411 (accessed on 1 March 2022).
- Hernandez-Soriano, M.C.; Kerré, B.; Kopittke, P.M.; Horemans, B.; Smolders, E. Biochar Affects Carbon Composition and Stability in Soil: A Combined Spectroscopy-Microscopy Study. Sci. Rep. 2016, 6, 25127. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) Framework Integrates Plant Litter Decomposition with Soil Organic Matter Stabilization: Do Labile Plant Inputs Form Stable Soil Organic Matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef] [Green Version]
- Bungau, S.; Behl, T.; Aleya, L.; Bourgeade, P.; Aloui-Sossé, B.; Purza, A.L.; Abid, A.; Samuel, A.D. Expatiating the Impact of Anthropogenic Aspects and Climatic Factors on Long-Term Soil Monitoring and Management. Environ. Sci. Pollut. Res. 2021, 28, 30528–30550. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kaur, I.; Sehgal, A.; Singh, S.; Sharma, N.; Bhatia, S.; Al-Harrasi, A.; Bungau, S. The Dichotomy of Nanotechnology as the Cutting Edge of Agriculture: Nano-Farming as an Asset versus Nanotoxicity. Chemosphere 2022, 288, 132533. [Google Scholar] [CrossRef] [PubMed]
- Rowley, M.C.; Grand, S.; Verrecchia, É.P. Calcium-Mediated Stabilisation of Soil Organic Carbon. Biogeochemistry 2018, 137, 27–49. [Google Scholar] [CrossRef] [Green Version]
| Sand | Silt | Clay | Carbonates | pH | |
|---|---|---|---|---|---|
| CS | 96.8 ± 2.4 | 2.5 ± 1.6 | 0.7 ± 1.4 | 87.9 ± 4.2 | 7.8 ± 0.2 |
| SS | 96.9 ± 0.2 | 2.7 ± 0.1 | 0.4 ± 0.2 | 5.3 ± 0.6 | 8.1 ± 0.2 |
| Compost | 90.6 ± 2.0 | 8.8 ± 1.0 | 0.5 ± 0.1 | 0.2 ± 0.03 | 6.7 ± 0.1 |
| Biochar | 80.6 ± 5.5 | 4.9 ± 1.7 | 14.3 ± 4.6 | 2.1 ± 0.6 | 9.9 ± 0.1 |
| Zeolites | 3.2 ± 0.3 | 7.7 ± 3.4 | 89.2 ± 3.2 | 0.1 ± 0.02 | 8.1 ± 0.1 |
| Graphene | 0.0 ± 0.0 | 0.0 ± 0.0 | 100.0 ± 0.0 | 0.0 ± 0.0 | 7.1 ± 0.1 |
| Compost | Zeolites | Biochar | Graphene | |
|---|---|---|---|---|
| Type | Cow manure vermicompost | Clinoptilolite 67.5% Mordenite 32.5% | Wood gasification biochar | GR006050 |
| Manufacturer | LTDG® | SBM LIFE SCIENCE® | BioDea® | Directa Plus® |
| Mix (% d.w.) | 1.8 | 0.9 | 4.5 | 0.015 |
| NO3− | |||||
|---|---|---|---|---|---|
| L/S ratios (CS) | Control | Compost | Biochar | Zeolites | Graphene |
| 1 | 0.45 ± 0.12a | 24.83 ± 6.2a | 5.87 ± 1.1a | 1.86 ± 0.62b | 0.1 ± 0.01a |
| 2 | 6.52 ± 4.15b | 31.24 ± 7.9b | 7.79 ± 0.39b | 4.52 ± 0.87c | 3.86 ± 0.93 |
| 5 | 2.57 ± 0.06ab | 12.73 ± 6.2c | 4.00 ± 2.2a | 0.23 ± 0.06a | 0.15 ± 0.01a |
| 10 | 2.95 ± 1.3ab | 9.44 ± 4.34c | 3.79 ± 0.62a | 0.13 ± 0.01a | 0.21 ± 0.11a |
| F ratio | 4.159 | 7.927 | 2.796 | 43.31 | 46.917 |
| p value | 0.047 | 0.009 | 0.109 | <0.001 | <0.001 |
| L/S ratios (SS) | Control | Compost | Biochar | Zeolites | Graphene |
| 1 | 0.17 ± 0.06a | 24.5 ± 3.1a | 3.14 ± 2.72a | 0.66 ± 1.48ab | 0.82 ± 0.62a |
| 2 | 3.88 ± 1.86b | 66.56 ± 16.5b | 9.49 ± 1.24b | 2.66 ± 0.76c | 3.33 ± 0.86b |
| 5 | 2.29 ± 0.06ab | 21.46 ± 6.2a | 2.33 ± 0.06a | 0.35 ± 0.12b | 0.55 ± 0.18a |
| 10 | 2.08 ± 1.1ab | 19.86 ± 3.72a | 2.18 ± 0.62a | 0.87 ± 0.43ab | 0.73 ± 0.06a |
| F ratio | 5.898 | 18.031 | 15.686 | 4.079 | 17.479 |
| p value | 0.02 | <0.001 | 0.001 | 0.05 | <0.001 |
| DOC | |||||
| L/S ratios (CS) | Control | Compost | Biochar | Zeolites | Graphene |
| 1 | 4.85 ± 1.15a | 6.5 ± 0.55a | 6.46 ± 0.58a | 5.06 ± 0.63a | 1.5 ± 0.92b |
| 2 | 4.04 ± 1.04a | 7.29 ± 0.56a | 4.99 ± 0.42b | 3.06 ± 0.1a | 4.67 ± 0.36a |
| 5 | 5.52 ± 1.37a | 7.99 ± 1.13a | 12.3 ± 1.07c | 8.47 ± 1.41b | 8.71 ± 1.41c |
| 10 | 6.34 ± 0.48a | 13.41 ± 1.7b | 10.21 ± 0.41d | 6.45 ± 1.87ab | 6.28 ± 1.39a |
| F ratio | 2.514 | 24.344 | 72.024 | 11.228 | 22.209 |
| p value | 0.132 | <0.001 | <0.001 | 0.003 | <0.001 |
| L/S ratios (SS) | Control | Compost | Biochar | Zeolites | Graphene |
| 1 | 2.23 ± 0.76a | 4.16 ± 0.74b | 2.86 ± 0.32a | 3.48 ± 0.09a | 7.76 ± 0.7a |
| 2 | 3.48 ± 1.29a | 8.07 ± 0.62a | 2.13 ± 0.31b | 6.54 ± 1.35a | 5.18 ± 0.35a |
| 5 | 7.46 ± 1.11b | 18.23 ± 0.69c | 6.89 ± 0.88c | 7.16 ± 1.44a | 8.75 ± 1.44a |
| 10 | 4.41 ± 1.06a | 10.21 ± 2.17a | 3.66 ± 0.23ab | 5.61 ± 2.58a | 7.34 ± 1.01a |
| F ratio | 12.825 | 68.512 | 50.99 | 2.917 | 7.335 |
| p value | 0.002 | <0.001 | <0.001 | 0.1 | 0.011 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alessandrino, L.; Colombani, N.; Aschonitis, V.G.; Mastrocicco, M. Nitrate and Dissolved Organic Carbon Release in Sandy Soils at Different Liquid/Solid Ratios Amended with Graphene and Classical Soil Improvers. Appl. Sci. 2022, 12, 6220. https://doi.org/10.3390/app12126220
Alessandrino L, Colombani N, Aschonitis VG, Mastrocicco M. Nitrate and Dissolved Organic Carbon Release in Sandy Soils at Different Liquid/Solid Ratios Amended with Graphene and Classical Soil Improvers. Applied Sciences. 2022; 12(12):6220. https://doi.org/10.3390/app12126220
Chicago/Turabian StyleAlessandrino, Luigi, Nicolò Colombani, Vassilis George Aschonitis, and Micòl Mastrocicco. 2022. "Nitrate and Dissolved Organic Carbon Release in Sandy Soils at Different Liquid/Solid Ratios Amended with Graphene and Classical Soil Improvers" Applied Sciences 12, no. 12: 6220. https://doi.org/10.3390/app12126220
APA StyleAlessandrino, L., Colombani, N., Aschonitis, V. G., & Mastrocicco, M. (2022). Nitrate and Dissolved Organic Carbon Release in Sandy Soils at Different Liquid/Solid Ratios Amended with Graphene and Classical Soil Improvers. Applied Sciences, 12(12), 6220. https://doi.org/10.3390/app12126220








