Peptidomic Characteristic of Peptides Generated in Dry-Cured Loins with Probiotic Strains of LAB during 360-Days Aging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Dry-Cured Meat Products
2.2. Extraction and Digestion Meat Protein
2.3. Peptide Identification
2.4. Data Analysis
3. Results and Discussion
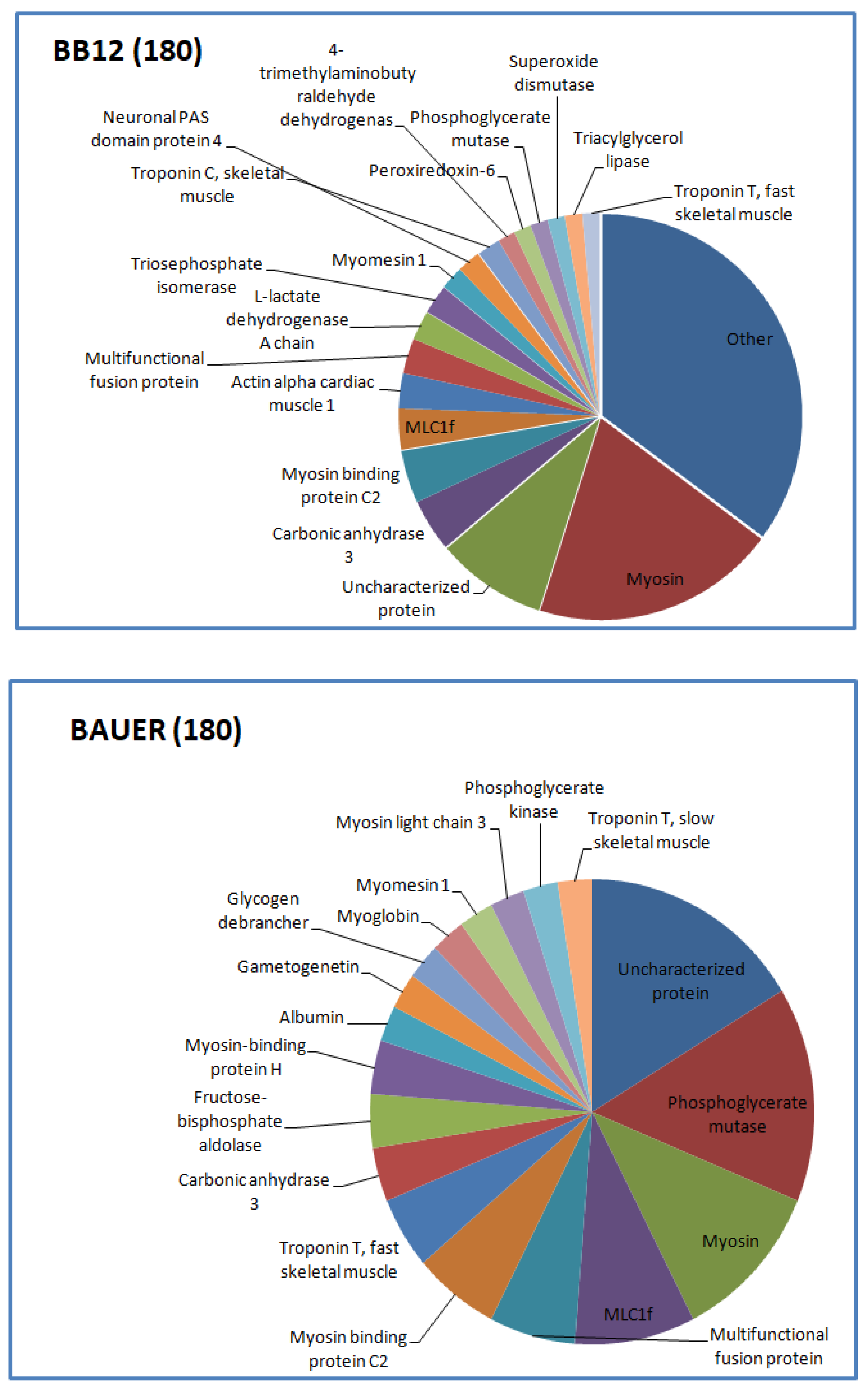
3.1. Peptide Profile of Digesta Depending on LAB Strains and Aging Time
3.2. Potentially Bioactive Peptides
| Biological Activity | Aging Time [Days] | References | |||||
|---|---|---|---|---|---|---|---|
| 28 | 90 | 180 | 270 | 360 | |||
| Antiradical | WSF | LOCK, BB12 * | BB12 | BAUER | BB12 | BB12 | [37] |
| SSF | BAUER | C, BAUER | C, BB12 | LOCK | BAUER | ||
| ACE-inhibitory activity | WSF | N.A. | LOCK | BB12, C | C, LOCK | BAUER | [38] |
| SSF | N.A. | C, LOCK, BAUER | BAUER | BB12 | BAUER | ||
| DPP-IV-inhibitory activity | WSF | BAUER | LOCK, BB12 | BB12 | LOCK, BAUER | C | [27] |
| SSF | C, BAUER | BB12 | BB12, LOCK | LOCK | BB12 | ||
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arihara, K.; Yokoyama, I.; Ohata, M. Bioactivities generated from meat proteins by enzymatic hydrolysis and the Maillard reaction. Meat Sci. 2021, 180, 108561. [Google Scholar] [CrossRef] [PubMed]
- Choe, J.; Seol, K.H.; Son, D.I.; Lee, H.J.; Lee, M.; Jo, C. Identification of angiotensin I-converting enzyme inhibitory peptides from enzymatic hydrolysates of pork loin. Int. J. Food Prop. 2019, 22, 1112–1121. [Google Scholar] [CrossRef] [Green Version]
- Wen, S.; Zhou, G.; Song, S.; Xu, X.; Voglmeir, J.; Liu, L.; Li, C. Discrimination of in vitro and in vivo digestion products of meat proteins from pork, beef, chicken, and fish. Proteomics 2015, 15, 3688–3698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escudero, E.; Aristoy, M.C.; Nishimura, H.; Arihara, K.; Toldrá, F. Antihypertensive effect and antioxidant activity of peptide fractions extracted from Spanish dry-cured ham. Meat Sci. 2012, 91, 306–311. [Google Scholar] [CrossRef]
- Escudero, E.; Sentandreu, M.A.; Arihara, K.; Toldra, F. Angiotensin I-converting enzyme inhibitory peptides generated from in vitro gastrointestinal digestion of pork meat. J. Agric. Food Chem. 2010, 58, 2895–2901. [Google Scholar] [CrossRef]
- Kaur, L.; Hui, S.X.; Morton, J.D.; Kaur, R.; Chian, F.M.; Boland, M. Endogenous proteolytic systems and meat tenderness: Influence of post-mortem storage and processing. Food Sci. Anim. Resour. 2021, 41, 589. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Bekhit, A.E.D.A. Role of calpain system in meat tenderness: A review. Food Sci. Hum. Wellness 2018, 7, 196–204. [Google Scholar] [CrossRef]
- Hu, S.; Zhou, G.; Xu, X.; Zhang, W.; Li, C. Contribution of cathepsin B and L to endogenous proteolysis in the course of modern Jinhua ham processing. Food Control 2022, 135, 108584. [Google Scholar] [CrossRef]
- Di Luccia, A.; Picariello, G.; Cacace, G.; Scaloni, A.; Faccia, M.; Liuzzi, V.; Musso, S.S. Proteomic analysis of water soluble and myofibrillar protein changes occurring in dry-cured hams. Meat Sci. 2005, 69, 479–491. [Google Scholar] [CrossRef]
- López, C.M.; Bru, E.; Vignolo, G.M.; Fadda, S.G. Identification of small peptides arising from hydrolysis of meat proteins in dry fermented sausages. Meat Sci. 2015, 104, 20–29. [Google Scholar] [CrossRef]
- Mora, L.; Escudero, E.; Toldrá, F. Characterization of the peptide profile in Spanish Teruel, Italian Parma and Belgian dry-cured hams and its potential bioactivity. Food Res. Int. 2016, 89, 638–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Guo, M.; Wang, Q.; Dong, J.; Lu, S.; Lyu, B.; Ma, X. Antioxidant activities of peptides derived from mutton ham, Xuanwei ham and Jinhua ham. Food Res. Int. 2021, 142, 110195. [Google Scholar] [CrossRef] [PubMed]
- Paolella, S.; Falavigna, C.; Faccini, A.; Virgili, R.; Sforza, S.; Dall’Asta, C.; Galaverna, G. Effect of dry-cured ham maturation time on simulated gastrointestinal digestion: Characterization of the released peptide fraction. Food Res. Int. 2015, 67, 136–144. [Google Scholar] [CrossRef]
- Martini, S.; Conte, A.; Tagliazucchi, D. Comparative peptidomic profile and bioactivities of cooked beef, pork, chicken and turkey meat after in vitro gastro-intestinal digestion. J. Proteom. 2019, 208, 103500. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, R.; Munekata, P.E.; Agregan, R.; Lorenzo, J.M. Effect of commercial starter cultures on free amino acid, biogenic amine and free fatty acid contents in dry-cured foal sausage. LWT-Food Sci. Technol. 2016, 71, 47–53. [Google Scholar] [CrossRef]
- Pogorzelska-Nowicka, E.; Atanasov, A.G.; Horbańczuk, J.; Wierzbicka, A. Bioactive compounds in functional meat products. Molecules 2018, 23, 307. [Google Scholar] [CrossRef] [Green Version]
- Kęska, P.; Stadnik, J.; Wójciak, K.M.; Neffe-Skocińska, K. Physico-chemical and proteolytic changes during cold storage of dry-cured pork loins with probiotic strains of LAB. Int. J. Food Sci. Technol. 2020, 55, 1069–1079. [Google Scholar] [CrossRef]
- Toldrá, F.; Gallego, M.; Reig, M.; Aristoy, M.C.; Mora, L. Bioactive peptides generated in the processing of dry-cured ham. Food Chem. 2020, 321, 126689. [Google Scholar] [CrossRef]
- Xing, L.; Li, G.; Toldrá, F.; Zhang, W. The physiological activity of bioactive peptides obtained from meat and meat by-products. Adv. Food Nutr. Res. 2021, 97, 147–185. [Google Scholar]
- Fernández, M.; Benito, M.J.; Martín, A.; Casquete, R.; Córdoba, J.J.; Córdoba, M.G. Influence of starter culture and a protease on the generation of ACE-inhibitory and antioxidant bioactive nitrogen compounds in Iberian dry-fermented sausage “salchichón”. Heliyon 2016, 2, e00093. [Google Scholar] [CrossRef] [Green Version]
- Champagne, C.P.; Ross, R.P.; Saarela, M.; Hansen, K.F.; Charalampopoulos, D. Recommendations for the viability assessment of probiotics as concentrated cultures and in food matrices. Int. J. Food Microbiol. 2011, 149, 185–193. [Google Scholar] [CrossRef]
- Libera, J.; Karwowska, M.; Stasiak, D.M.; Dolatowski, Z.J. Microbiological and physicochemical properties of dry-cured neck inoculated with probiotic of Bifidobacterium animalis ssp. lactis BB-12. Int. J. Food Sci. Technol. 2015, 50, 1560–1566. [Google Scholar] [CrossRef]
- Sionek, B.; Kołożyn-Krajewska, D.; Pasternok, I. Przeżywalność bakterii o właściwościach probiotycznych w kiełbasach surowo dojrzewających w czasie chłodniczego przechowywania. Żywność Nauka Technol. Jakość 2014, 21, 103–113. [Google Scholar]
- Stadnik, J.; Dolatowski, Z.J. Biogenic amines content during extended ageing of dry-cured pork loins inoculated with probiotics. Meat Sci. 2012, 91, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Neffe-Skocińska, K.; Okoń, A.; Kołożyn-Krajewska, D.; Dolatowski, Z. Amino acid profile and sensory characteristics of dry fermented pork loins produced with a mixture of probiotic starter cultures. J. Sci. Food Agric. 2017, 97, 2953–2960. [Google Scholar] [CrossRef]
- Wójciak, K.M.; Libera, J.; Stasiak, D.M.; Kołożyn-Krajewska, D. Technological aspect of Lactobacillus acidophilus Bauer, Bifidobacterium animalis BB-12 and Lactobacillus rhamnosus LOCK900 use in dry-fermented pork neck and sausage. J. Food Process. Preserv. 2017, 41, e12965. [Google Scholar] [CrossRef]
- Kęska, P.; Stadnik, J. Dipeptidyl Peptidase IV Inhibitory Peptides Generated in Dry-Cured Pork Loin during Aging and Gastrointestinal Digestion. Nutrients 2022, 14, 770. [Google Scholar] [CrossRef]
- Uniprot. Available online: https://www.uniprot.org/help/uniprotkb (accessed on 30 October 2021).
- De Almeida, M.A.; Saldaña, E.; da Silva Pinto, J.S.; Palacios, J.; Contreras-Castillo, C.J.; Sentandreu, M.A.; Fadda, S.G. A peptidomic approach of meat protein degradation in a low-sodium fermented sausage model using autochthonous starter cultures. Food Res. Int. 2018, 109, 368–379. [Google Scholar] [CrossRef]
- Sayd, T.; Dufour, C.; Chambon, C.; Buffière, C.; Remond, D.; Sante-Lhoutellier, V. Combined in vivo and in silico approaches for predicting the release of bioactive peptides from meat digestion. Food Chem. 2018, 249, 111–118. [Google Scholar] [CrossRef]
- Okoń, A.; Dolatowski, Z.J. Proteoliza białek w wędlinach surowo dojrzewających z udziałem szczepu probiotycznego Lactobacillus casei ŁOCK 0900. Żywność Nauka Technol. Jakość 2012, 19, 138–151. [Google Scholar]
- Okoń, A.; Dolatowski, Z.J. Effect of probiotic bacteria on free amino acid profile and sensory traits of raw-ripening pork sirloin during storage. Żywność Nauka Technol. Jakość 2014, 3, 92–107. [Google Scholar] [CrossRef]
- Okoń, A.; Stadnik, J.; Dolatowski, Z.J. Effect of Lactobacillus acidophilus Bauer and Bifidobacterium animalis ssp. Lactis BB12 on proteolytic changes in dry-cured loins. Food Sci. Biotechnol. 2017, 26, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Stadnik, J.; Dolatowski, Z.J. Changes in selected parameters related to proteolysis during ageing of dry-cured pork loins inoculated with probiotics. Food Chem. 2013, 139, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Stadnik, J.; Stasiak, D.M.; Dolatowski, Z.J. Proteolysis in dry-aged loins manufactured with sonicated pork and inoculated with Lactobacillus casei ŁOCK 0900 probiotic strain. Int. J. Food Sci. Technol. 2014, 49, 2578–2584. [Google Scholar] [CrossRef]
- Kęska, P.; Stadnik, J. Stability of antiradical activity of protein extracts and hydrolysates from dry-cured pork loins with probiotic strains of LAB. Nutrients 2018, 10, 521. [Google Scholar] [CrossRef] [Green Version]
- Kęska, P.; Stadnik, J. Ageing-time dependent changes of angiotensin I-converting enzyme-inhibiting activity of protein hydrolysates obtained from dry-cured pork loins inoculated with probiotic lactic acid bacteria. Int. J. Pept. Res. Ther. 2019, 25, 1173–1185. [Google Scholar] [CrossRef] [Green Version]
- Fontoura, R.; Daroit, D.J.; Corrêa, A.P.F.; Moresco, K.S.; Santi, L.; Beys-da-Silva, W.O.; Brandelli, A. Characterization of a novel antioxidant peptide from feather keratin hydrolysates. New Biotechnol. 2019, 49, 71–76. [Google Scholar] [CrossRef]
- Sinkiewicz, I.; Staroszczyk, H.; Śliwińska, A. Solubilization of keratins and functional properties of their isolates and hydrolysates. J. Food Biochem. 2018, 42, e12494. [Google Scholar] [CrossRef]
- Taraszkiewicz, A.; Sinkiewicz, I.; Sommer, A.; Dąbrowska, M.; Staroszczyk, H. Prediction of Bioactive Peptides from Chicken Feather and Pig Hair Keratins Using in Silico Analysis Based on Fragmentomic Approach. Curr. Pharm. Des. 2022, 28, 841–851. [Google Scholar] [CrossRef]
- Alahyaribeik, S.; Nazarpour, M. Effects of Bioactive Peptides Derived from Feather Keratin on Small Intestinal Function, Meat Quality, And Performance of Broiler Chicks; Research Square: Durham, UK, 2022. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Borrajo, P.; Amarowicz, R.; Lorenzo, J.M.; Franco, D. Peptidomic analysis of antioxidant peptides from porcine liver hydrolysates using SWATH-MS. J. Proteom. 2021, 232, 104037. [Google Scholar] [CrossRef]

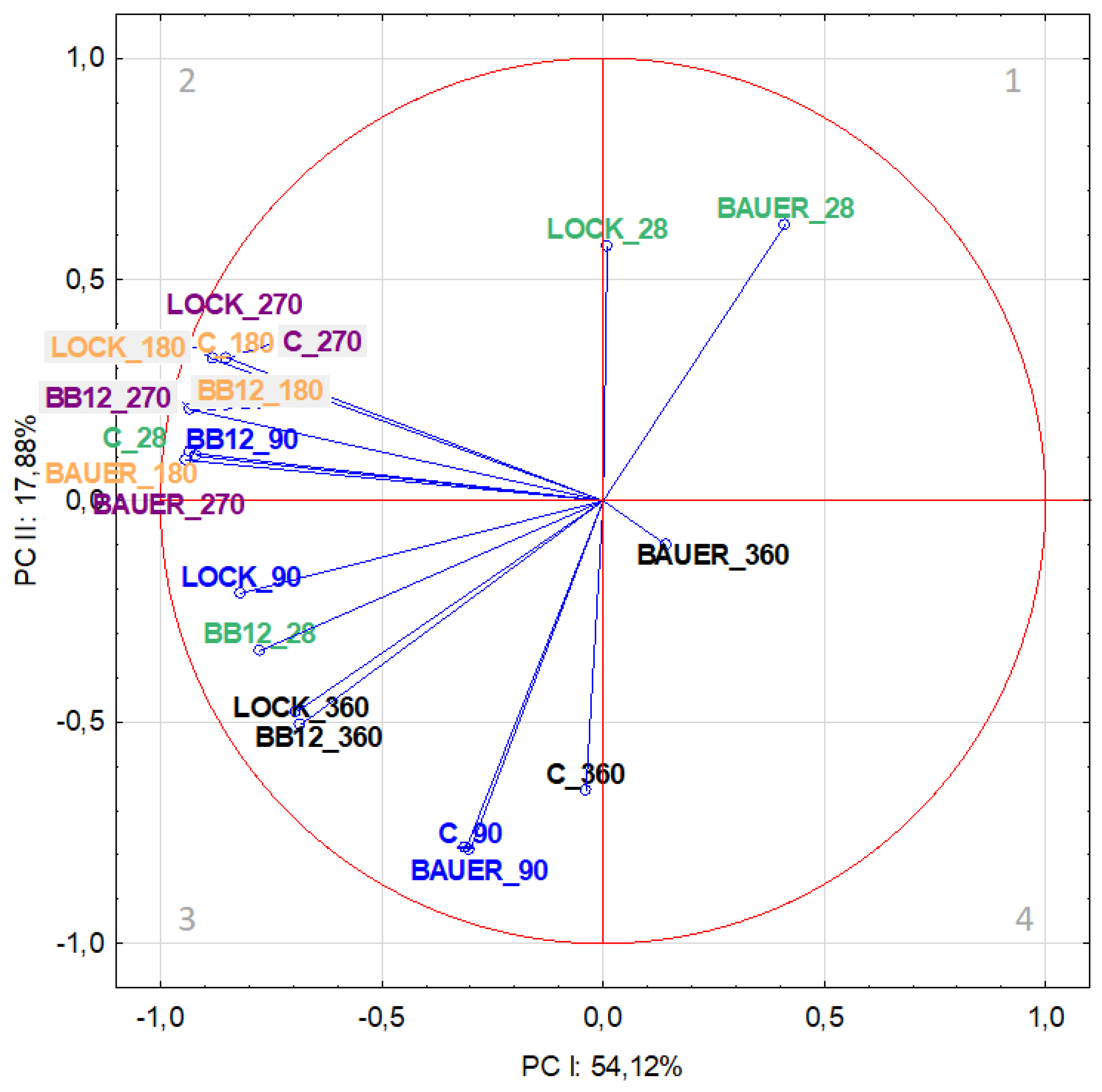

| WSF | SSF | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| VARIABLE | PC-I(37.49%) | PC-II(26.70%) | PC-III(9.95%) | PC-IV(7.24%) | VARIABLE | PC-I(54.12%) | PC-II(17.88) | PC-III(6.89%) | PC-IV(5.89%) |
| C (28) | –0.724435 | 0.262115 | −0.352099 | −0.331593 | C (28) | –0.93424 | 0.108496 | 0.047113 | −0.052446 |
| LOCK (28) | 0.269170 | –0.795678 | −0.216578 | −0.345634 | LOCK (28) | 0.00931 | 0.574768 | −0.331658 | 0.317299 |
| BB12 (28) | 0.246643 | –0.797257 | −0.160653 | −0.341932 | BB12 (28) | –0.77591 | –0.338803 | −0.368830 | −0.174170 |
| BAUER (28) | 0.375738 | –0.343222 | −0.400425 | 0.525156 | BAUER (28) | 0.41106 | 0.624094 | −0.428884 | 0.160513 |
| C (90) | 0.392323 | –0.507622 | −0.472681 | 0.380877 | C (90) | –0.30080 | –0.789736 | 0.095578 | 0.372307 |
| LOCK (90) | 0.362414 | –0.639137 | −0.426923 | 0.073803 | LOCK (90) | –0.81862 | –0.209054 | −0.321064 | −0.186710 |
| BB12 (90) | –0.864396 | –0.212517 | −0.033672 | 0.208543 | BB12 (90) | –0.91744 | 0.100880 | −0.027314 | −0.098820 |
| BAUER (90) | –0.484937 | –0.546784 | 0.449369 | 0.168381 | BAUER (90) | –0.30980 | –0.785863 | 0.086060 | 0.363812 |
| C (180) | –0.727642 | 0.251874 | −0.311022 | −0.322069 | C (180) | –0.84904 | 0.322890 | 0.198590 | 0.146240 |
| LOCK (180) | –0.834232 | 0.084192 | −0.326834 | −0.216638 | LOCK (180) | –0.88069 | 0.319656 | 0.190586 | 0.110626 |
| BB12 (180) | 0.000906 | 0.824066 | 0.172432 | −0.201925 | BB12 (180) | –0.93241 | 0.205737 | 0.106908 | 0.016286 |
| BAUER (180) | –0.110778 | –0.720010 | 0.409412 | −0.296903 | BAUER (180) | –0.94440 | 0.091946 | 0.013676 | −0.069686 |
| C (270) | 0.286562 | –0.763319 | −0.264908 | −0.301805 | C (270) | –0.84904 | 0.322890 | 0.198590 | 0.146240 |
| LOCK (270) | –0.125060 | –0.719110 | 0.431949 | −0.254824 | LOCK (270) | –0.88069 | 0.319656 | 0.190586 | 0.110626 |
| BB12 (270) | –0.906581 | –0.096689 | −0.223576 | 0.028201 | BB12 (270) | –0.93241 | 0.205737 | 0.106908 | 0.016286 |
| BAUER (270) | –0.904469 | –0.103652 | −0.193721 | 0.075228 | BAUER (270) | –0.94440 | 0.091946 | 0.013676 | −0.069686 |
| C (360) | –0.558538 | –0.529942 | 0.427206 | 0.173273 | C (360) | –0.03862 | –0.654224 | 0.375348 | 0.224751 |
| LOCK (360) | –0.817993 | –0.328129 | 0.111945 | 0.254292 | LOCK (360) | –0.68275 | –0.505517 | −0.397019 | −0.125809 |
| BB12 (360) | –0.893838 | –0.011469 | −0.270146 | −0.045758 | BB12 (360) | –0.69149 | –0.476146 | −0.414102 | −0.139977 |
| BAUER (360) | –0.822440 | –0.312895 | 0.110344 | 0.255456 | BAUER (360) | 0.14147 | –0.098988 | 0.423680 | −0.734828 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kęska, P.; Stadnik, J. Peptidomic Characteristic of Peptides Generated in Dry-Cured Loins with Probiotic Strains of LAB during 360-Days Aging. Appl. Sci. 2022, 12, 6036. https://doi.org/10.3390/app12126036
Kęska P, Stadnik J. Peptidomic Characteristic of Peptides Generated in Dry-Cured Loins with Probiotic Strains of LAB during 360-Days Aging. Applied Sciences. 2022; 12(12):6036. https://doi.org/10.3390/app12126036
Chicago/Turabian StyleKęska, Paulina, and Joanna Stadnik. 2022. "Peptidomic Characteristic of Peptides Generated in Dry-Cured Loins with Probiotic Strains of LAB during 360-Days Aging" Applied Sciences 12, no. 12: 6036. https://doi.org/10.3390/app12126036
APA StyleKęska, P., & Stadnik, J. (2022). Peptidomic Characteristic of Peptides Generated in Dry-Cured Loins with Probiotic Strains of LAB during 360-Days Aging. Applied Sciences, 12(12), 6036. https://doi.org/10.3390/app12126036







