Effect of Rehabilitation on Brain Functional Connectivity in a Stroke Patient Affected by Conduction Aphasia
Abstract
:1. Introduction
2. Background
3. Materials and Methods
3.1. Case Description
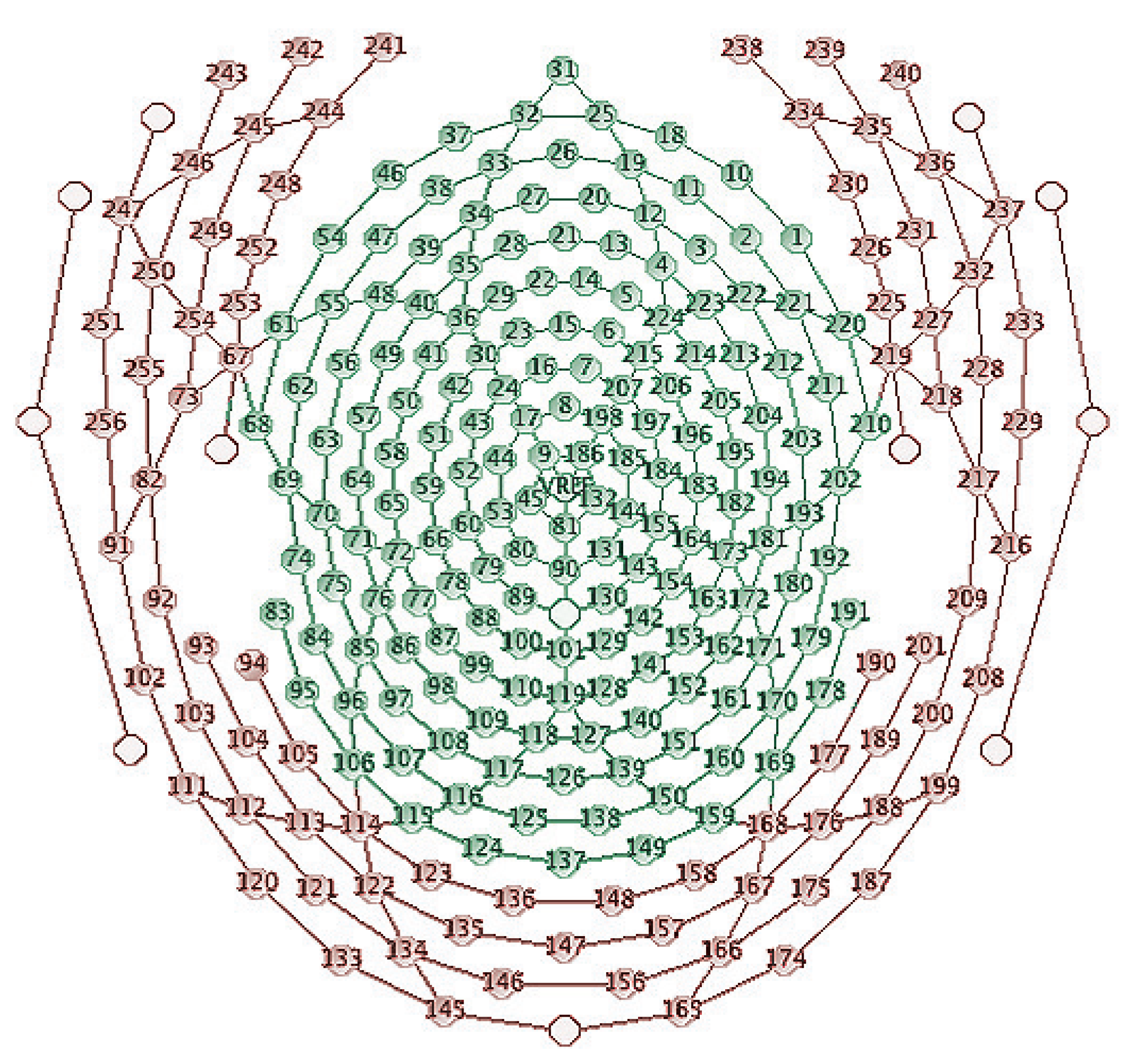
3.2. Brain Network Analysis
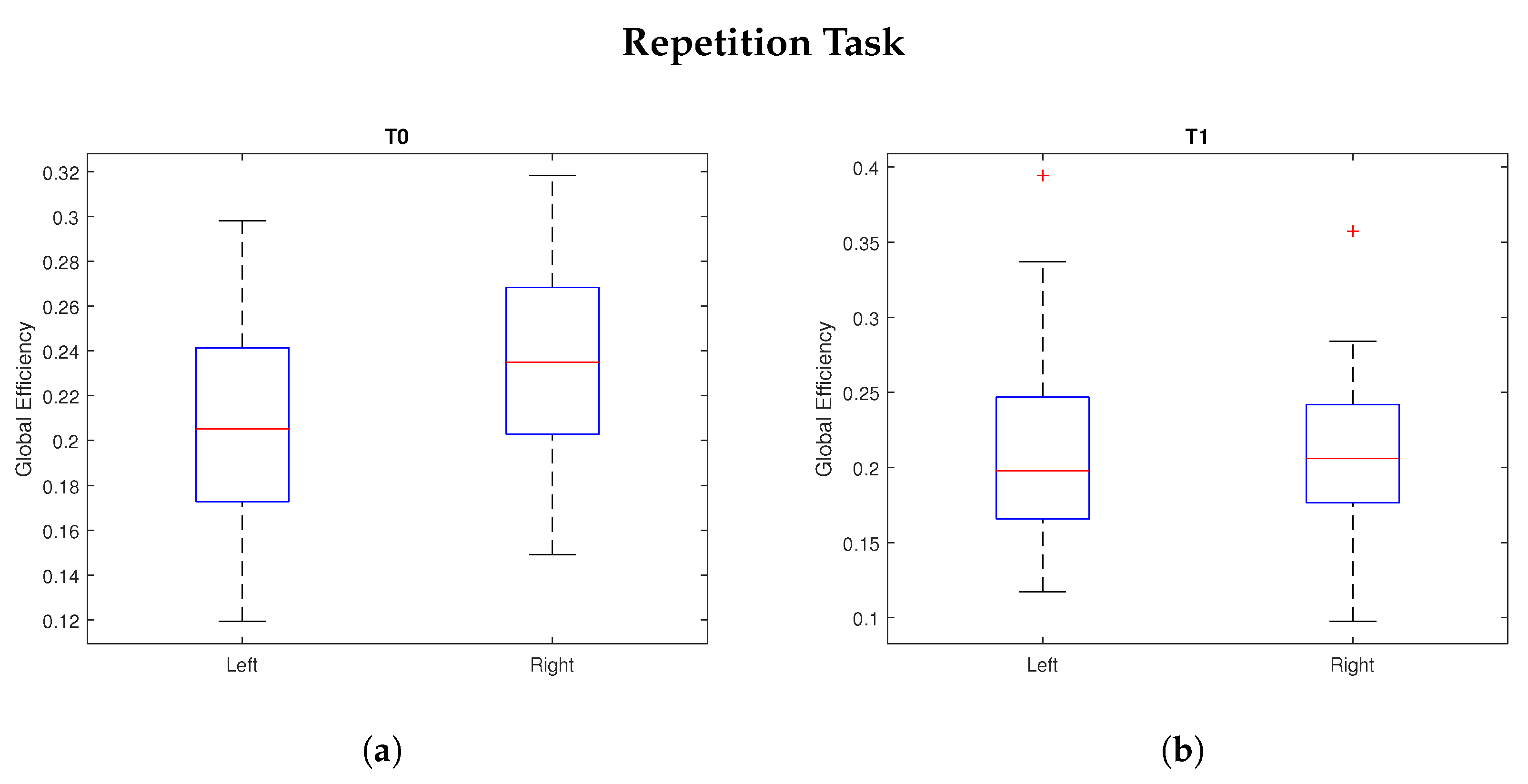
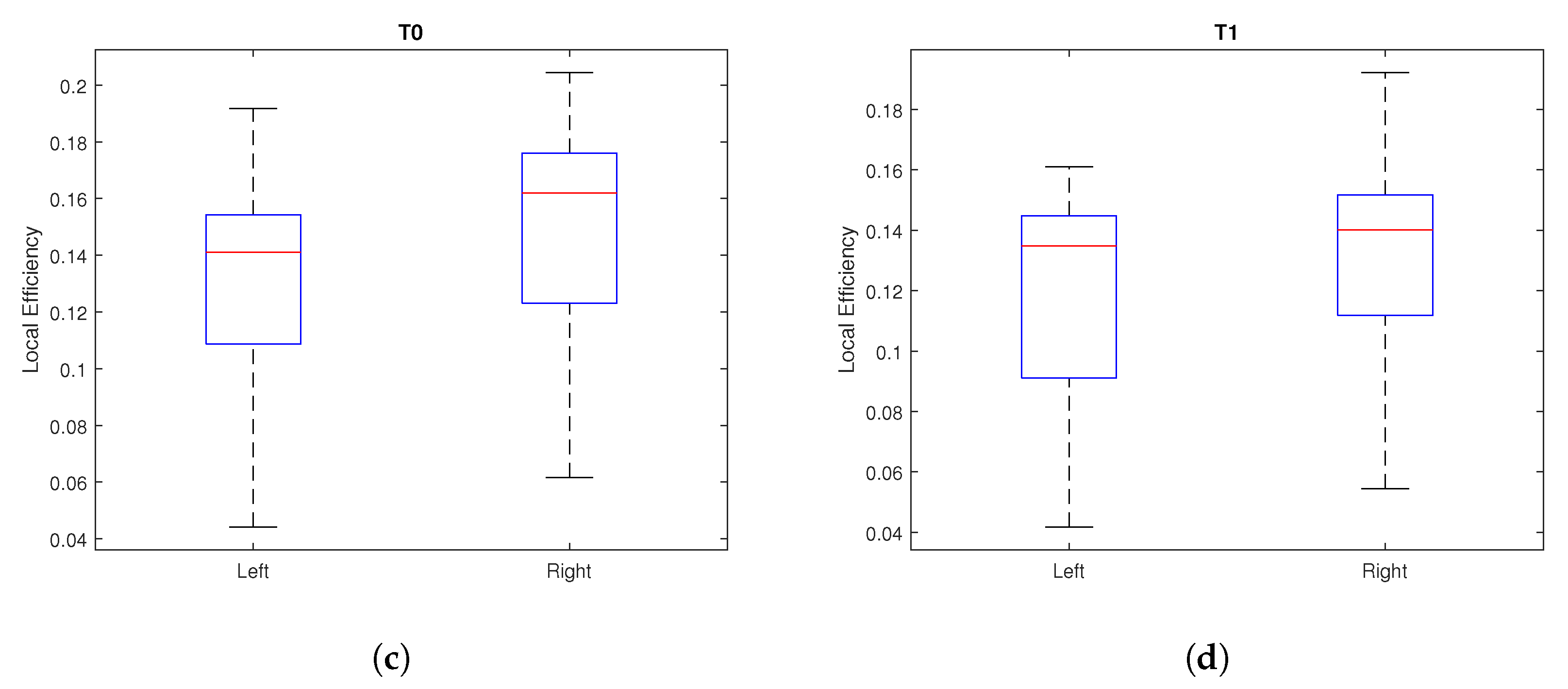
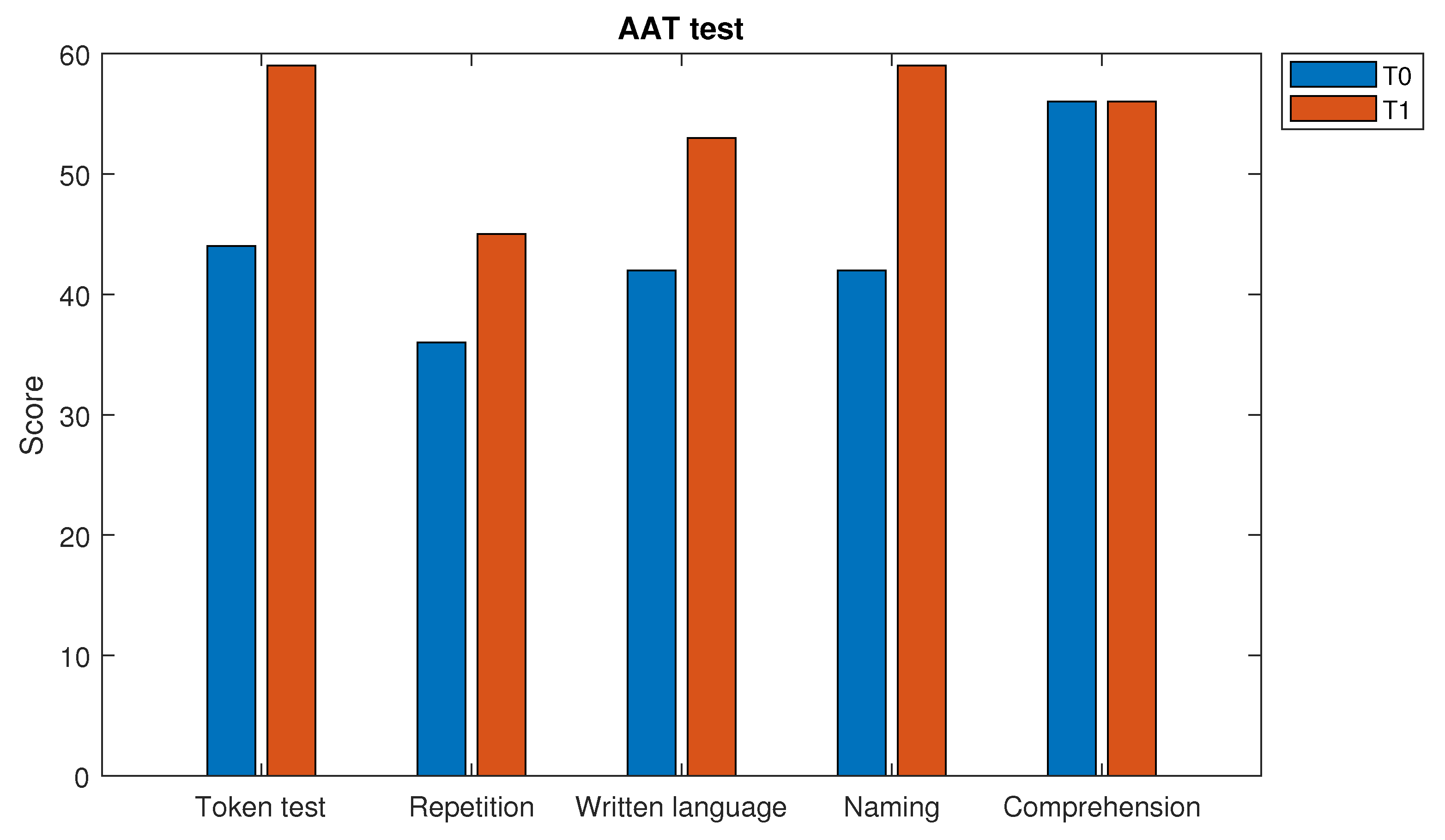
4. Results
5. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Stroke Organization. Annual Report. 2019. Available online: https://www.world-stroke.org (accessed on 11 April 2022).
- World Health Organization. Cerebrovascular Disorders: A Clinical and Research Classification; World Health Organization: Geneva, Switzerland, 1978. [Google Scholar]
- Gresham, G.E.; Stason, W.B.; Duncan, P.W. Post-Stroke Rehabilitation; Diane Publishing: Darby, PA, USA, 2004; Volume 95. [Google Scholar]
- Wernicke, C. Der Aphasische Symptomencomplex: Eine Psychologische Studie auf Anatomischer Basis; Cohn & Weigert: Breslau, Poland, 1874. [Google Scholar]
- Ardila, A. A review of conduction aphasia. Curr. Neurol. Neurosci. Rep. 2010, 10, 499–503. [Google Scholar] [CrossRef]
- Geschwind, N. Disconnexion syndromes in animals and man. Brain 1965, 88, 585. [Google Scholar] [CrossRef] [Green Version]
- Hickok, G.; Erhard, P.; Kassubek, J.; Helms-Tillery, A.K.; Naeve-Velguth, S.; Strupp, J.P.; Strick, P.L.; Ugurbil, K. A functional magnetic resonance imaging study of the role of left posterior superior temporal gyrus in speech production: Implications for the explanation of conduction aphasia. Neurosci. Lett. 2000, 287, 156–160. [Google Scholar] [CrossRef]
- Angrilli, A.; Elbert, T.; Cusumano, S.; Stegagno, L.; Rockstroh, B. Temporal dynamics of linguistic processes are reorganized in aphasics’ cortex: An EEG mapping study. Neuroimage 2003, 20, 657–666. [Google Scholar] [CrossRef] [Green Version]
- Breier, J.I.; Castillo, E.M.; Boake, C.; Billingsley, R.; Maher, L.; Francisco, G.; Papanicolaou, A.C. Spatiotemporal patterns of language-specific brain activity in patients with chronic aphasia after stroke using magnetoencephalography. Neuroimage 2004, 23, 1308–1316. [Google Scholar] [CrossRef]
- Sarasso, S.; Määttä, S.; Ferrarelli, F.; Poryazova, R.; Tononi, G.; Small, S.L. Plastic changes following imitation-based speech and language therapy for aphasia: A high-density sleep EEG study. Neurorehabilit. Neural Repair 2014, 28, 129–138. [Google Scholar] [CrossRef] [Green Version]
- Spironelli, C.; Angrilli, A. Brain plasticity in aphasic patients: Intra-and inter-hemispheric reorganisation of the whole linguistic network probed by N150 and N350 components. Sci. Rep. 2015, 5, 12541. [Google Scholar] [CrossRef] [Green Version]
- Thompson, C.K.; den Ouden, D.B. Neuroimaging and recovery of language in aphasia. Curr. Neurol. Neurosci. Rep. 2008, 8, 475. [Google Scholar] [CrossRef]
- Mattioli, F.; Ambrosi, C.; Mascaro, L.; Scarpazza, C.; Pasquali, P.; Frugoni, M.; Magoni, M.; Biagi, L.; Gasparotti, R. Early aphasia rehabilitation is associated with functional reactivation of the left inferior frontal gyrus: A pilot study. Stroke 2014, 45, 545–552. [Google Scholar] [CrossRef] [Green Version]
- Stefaniak, J.D.; Halai, A.D.; Ralph, M.A.L. The neural and neurocomputational bases of recovery from post-stroke aphasia. Nat. Rev. Neurol. 2020, 16, 43–55. [Google Scholar] [CrossRef]
- Formica, C.; De Salvo, S.; Micchìa, K.; La Foresta, F.; Dattola, S.; Mammone, N.; Corallo, F.; Ciavola, A.; Arcadi, F.A.; Marino, S.; et al. Cortical Reorganization after Rehabilitation in a Patient with Conduction Aphasia Using High-Density EEG. Appl. Sci. 2020, 10, 5281. [Google Scholar] [CrossRef]
- Rubinov, M.; Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 2010, 52, 1059–1069. [Google Scholar] [CrossRef]
- Achard, S.; Bullmore, E. Efficiency and cost of economical brain functional networks. PLoS Comput. Biol. 2007, 3, e17. [Google Scholar] [CrossRef]
- Bullmore, E.; Sporns, O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009, 10, 186–198. [Google Scholar] [CrossRef]
- Latora, V.; Marchiori, M. Efficient behavior of small-world networks. Phys. Rev. Lett. 2001, 87, 198701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caliandro, P.; Vecchio, F.; Miraglia, F.; Reale, G.; Della Marca, G.; La Torre, G.; Lacidogna, G.; Iacovelli, C.; Padua, L.; Bramanti, P.; et al. Small-world characteristics of cortical connectivity changes in acute stroke. Neurorehabilit. Neural Repair 2017, 31, 81–94. [Google Scholar] [CrossRef] [Green Version]
- Mammone, N.; De Salvo, S.; Marino, S.; Bonanno, L.; Ieracitano, C.; Dattola, S.; La Foresta, F.; Morabito, F.C. Estimating the Asymmetry of Brain Network Organization in Stroke Patients from High-Density EEG Signals. In Neural Approaches to Dynamics of Signal Exchanges; Springer: Singapore, 2020; pp. 475–483. [Google Scholar]
- Fallani, F.D.V.; Pichiorri, F.; Morone, G.; Molinari, M.; Babiloni, F.; Cincotti, F.; Mattia, D. Multiscale topological properties of functional brain networks during motor imagery after stroke. Neuroimage 2013, 83, 438–449. [Google Scholar] [CrossRef] [Green Version]
- Philips, G.R.; Daly, J.J.; Príncipe, J.C. Topographical measures of functional connectivity as biomarkers for post-stroke motor recovery. J. Neuroeng. Rehabil. 2017, 14, 1–16. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D. Instantaneous and lagged measurements of linear and nonlinear dependence between groups of multivariate time series: Frequency decomposition. arXiv 2007, arXiv:0711.1455. [Google Scholar]
- Shaphiro, S.; Wilk, M. An analysis of variance test for normality. Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Gibbons, J.; Chakraborti, S. Nonparametric Statistical Inference; Springer: Cham, Switzerland, 2011. [Google Scholar]
- Huber, W.; Poeck, K.; Weniger, D.; Willmes, K. AAT-Aachener Aphasie Test; Hogrefe: Göttingen, Germany, 1983. [Google Scholar]
- Van Meijeren-Pont, W.; Tamminga, S.J.; Fiocco, M.; Avila, A.G.; Volker, G.; Janssen, S.M.; Vlieland, T.P.V.; Oosterveer, D.M.; SCORE Study Group. Patient Activation During the First 6 Months After the Start of Stroke Rehabilitation. Arch. Phys. Med. Rehabil. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Vostrỳ, M.; Zilcher, L. Combined Therapy for Patients after Ischemic Stroke from The Point View of Comprehensive Rehabilitation. J. Educ. Cult. Soc. 2020, 11, 119–125. [Google Scholar] [CrossRef]
- Montalbán, M.A.; Arrogante, O. Rehabilitation through virtual reality therapy after a stroke: A literature review. Rev. Cient. Soc. Esp. Enferm. Neurol. 2020, 52, 19–27. [Google Scholar] [CrossRef]
- Knecht, S.; Dräger, B.; Deppe, M.; Bobe, L.; Lohmann, H.; Flöel, A.; Ringelstein, E.B.; Henningsen, H. Handedness and hemispheric language dominance in healthy humans. Brain 2000, 123, 2512–2518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesulam, M.M.; Weintraub, S.; Rogalski, E.J.; Wieneke, C.; Geula, C.; Bigio, E.H. Asymmetry and heterogeneity of Alzheimer’s and frontotemporal pathology in primary progressive aphasia. Brain 2014, 137, 1176–1192. [Google Scholar] [CrossRef] [Green Version]
- Fridriksson, J.; Yourganov, G.; Bonilha, L.; Basilakos, A.; Den Ouden, D.B.; Rorden, C. Revealing the dual streams of speech processing. Proc. Natl. Acad. Sci. USA 2016, 113, 15108–15113. [Google Scholar] [CrossRef] [Green Version]
- Mahowald, K.; Fedorenko, E. Reliable individual-level neural markers of high-level language processing: A necessary precursor for relating neural variability to behavioral and genetic variability. Neuroimage 2016, 139, 74–93. [Google Scholar] [CrossRef]
- Cocquyt, E.M.; De Ley, L.; Santens, P.; Van Borsel, J.; De Letter, M. The role of the right hemisphere in the recovery of stroke-related aphasia: A systematic review. J. Neurolinguist. 2017, 44, 68–90. [Google Scholar] [CrossRef] [Green Version]
- Vinehout, K.; Schmit, B.D.; Schindler-Ivens, S. Lower limb task-based functional connectivity is altered in stroke. Brain Connect. 2019, 9, 365–377. [Google Scholar] [CrossRef]
- Grefkes, C.; Nowak, D.A.; Eickhoff, S.B.; Dafotakis, M.; Küst, J.; Karbe, H.; Fink, G.R. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann. Neurol. 2008, 63, 236–246. [Google Scholar] [CrossRef]



| Network Parameters | Naming Task p-Value | Repetition Task p-Value | Reading Task p-Value | |||
|---|---|---|---|---|---|---|
| T0 | T1 | T0 | T1 | T0 | T1 | |
| Global Efficiency | 0.0456 * | 0.6728 | 0.0136 * | 0.5098 | 0.0193 * | 0.3922 |
| Local Efficiency | 0.0597 | 0.5341 | 0.0023 * | 0.1992 | 0.0033 * | 0.1340 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dattola, S.; La Foresta, F. Effect of Rehabilitation on Brain Functional Connectivity in a Stroke Patient Affected by Conduction Aphasia. Appl. Sci. 2022, 12, 5991. https://doi.org/10.3390/app12125991
Dattola S, La Foresta F. Effect of Rehabilitation on Brain Functional Connectivity in a Stroke Patient Affected by Conduction Aphasia. Applied Sciences. 2022; 12(12):5991. https://doi.org/10.3390/app12125991
Chicago/Turabian StyleDattola, Serena, and Fabio La Foresta. 2022. "Effect of Rehabilitation on Brain Functional Connectivity in a Stroke Patient Affected by Conduction Aphasia" Applied Sciences 12, no. 12: 5991. https://doi.org/10.3390/app12125991
APA StyleDattola, S., & La Foresta, F. (2022). Effect of Rehabilitation on Brain Functional Connectivity in a Stroke Patient Affected by Conduction Aphasia. Applied Sciences, 12(12), 5991. https://doi.org/10.3390/app12125991







