Modeling of a Double Gas Hydrate Particle Ignition
Abstract
:1. Introduction
2. Mathematical Model
2.1. Physical Problem Statement
2.2. Mathematical Model and Numerical Methods
3. Results and Discussion
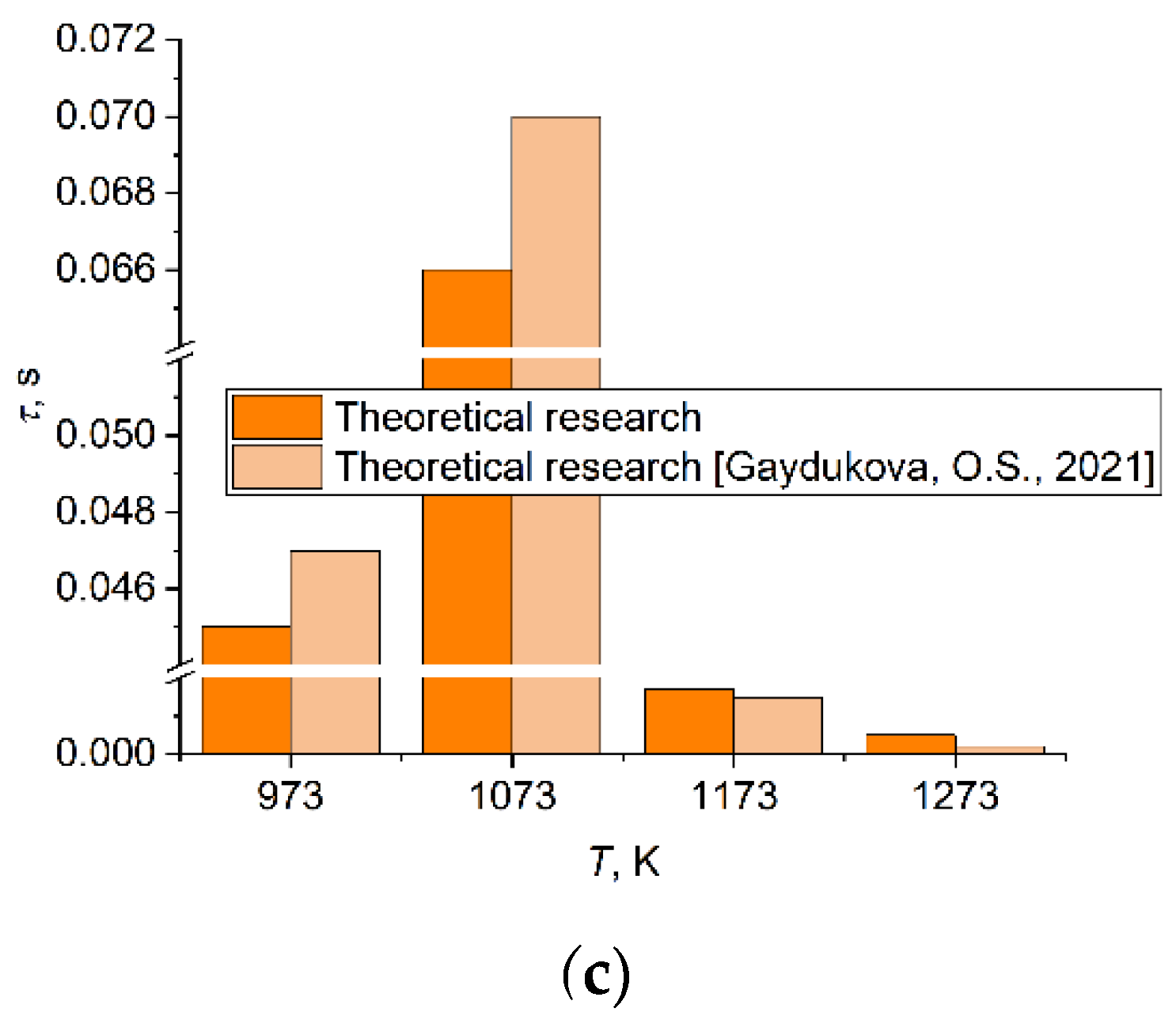
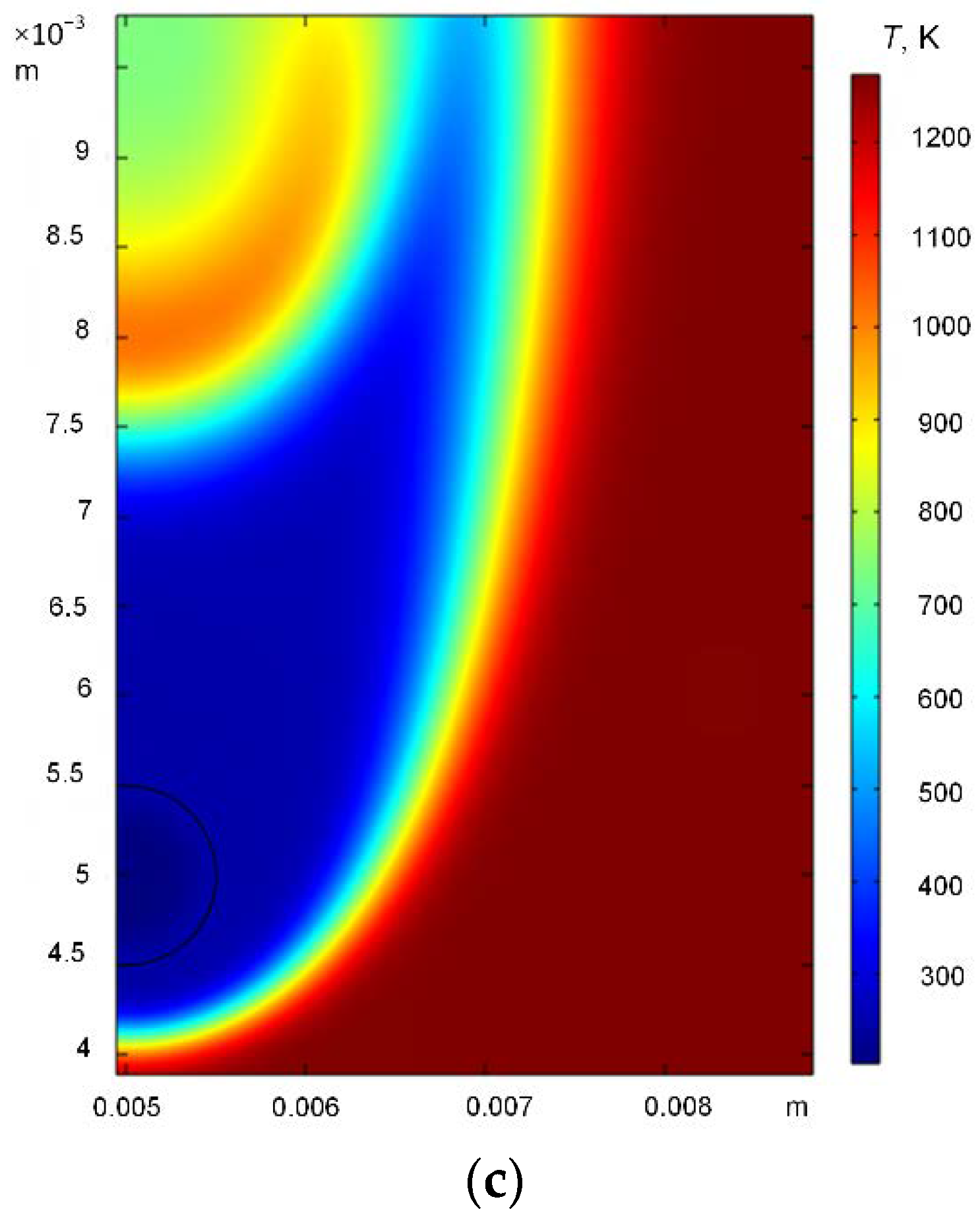
3.1. Effect of Heating Source Temperature
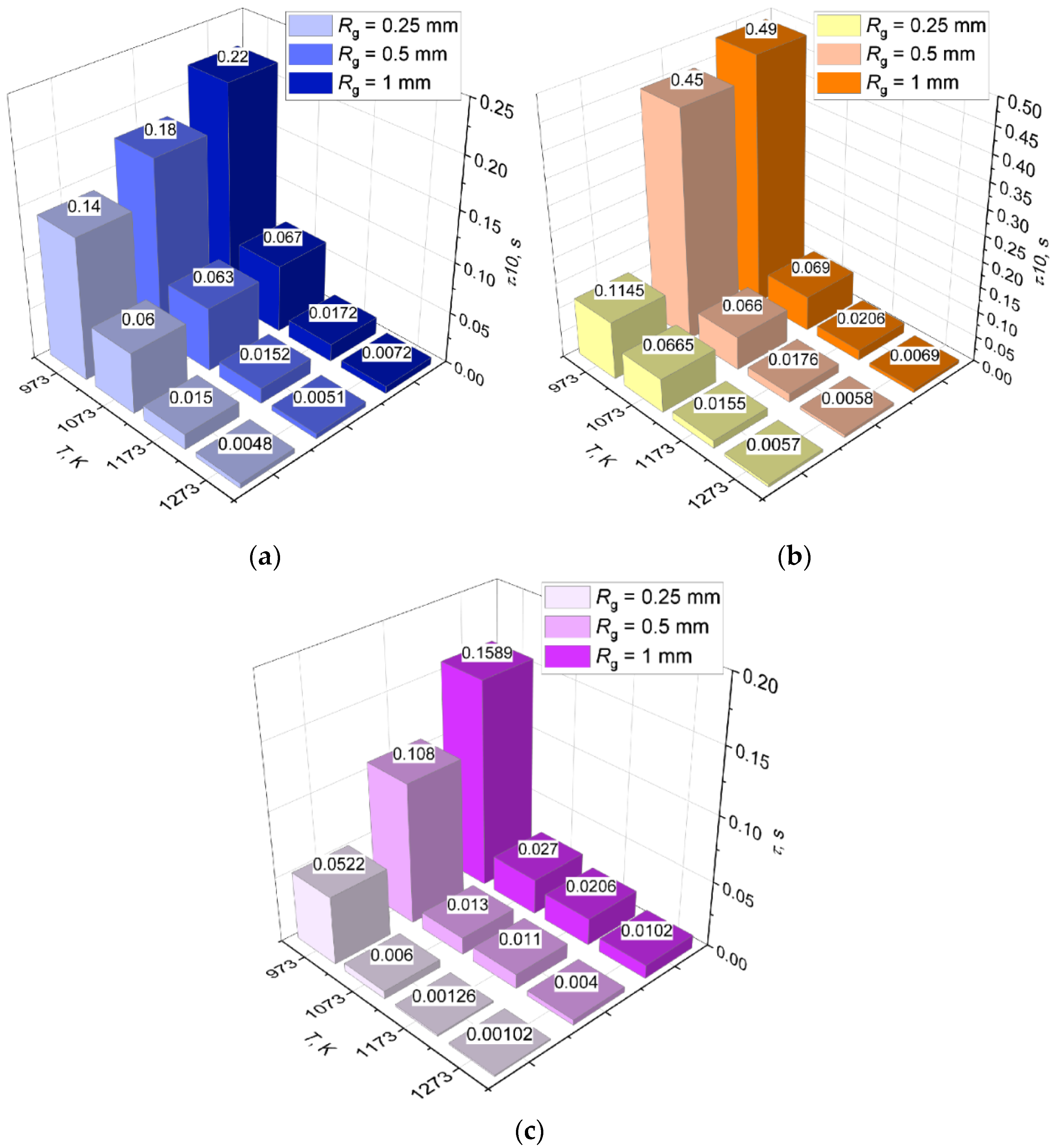
3.2. Effect of Hydrate Particle Size
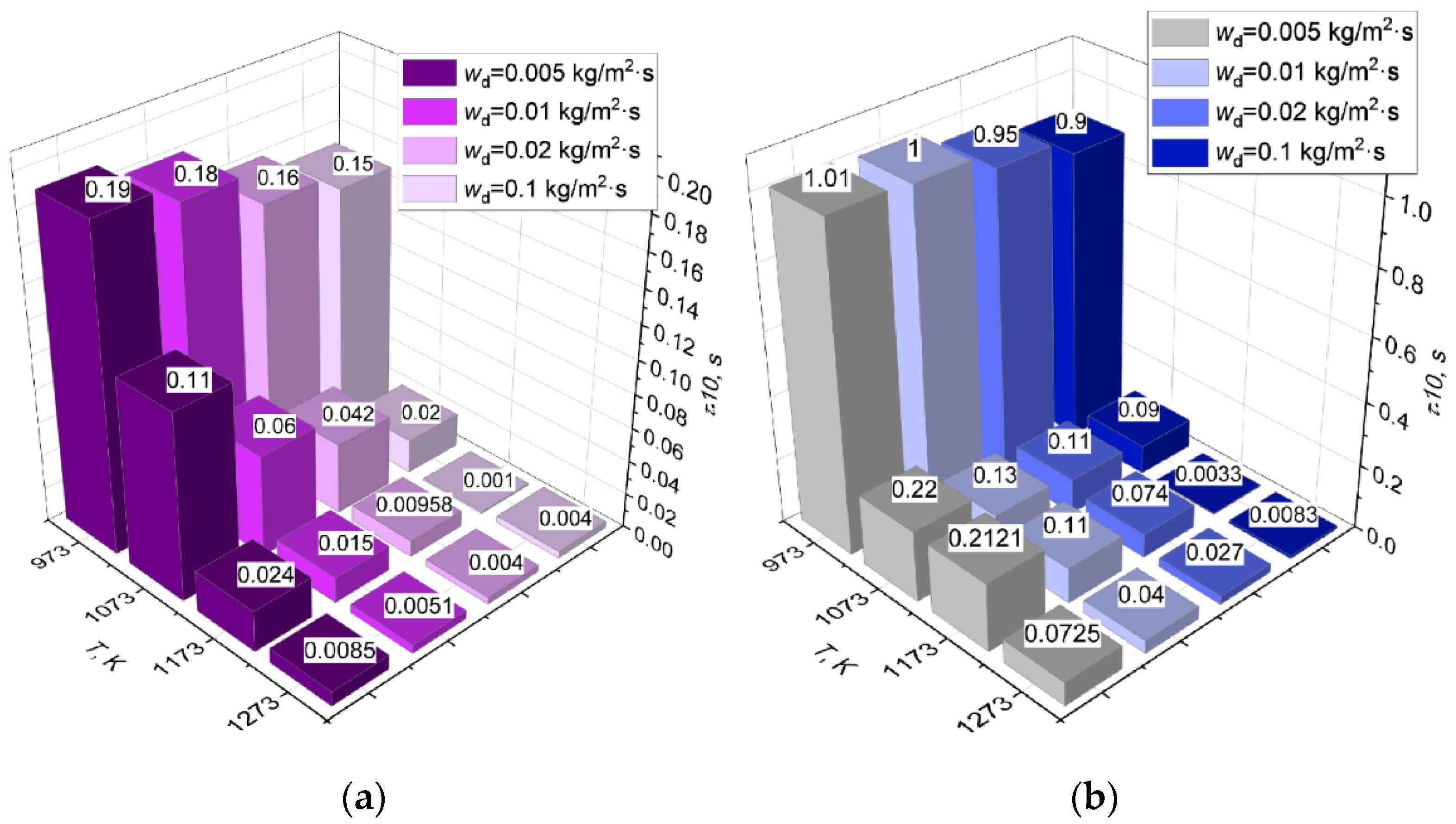
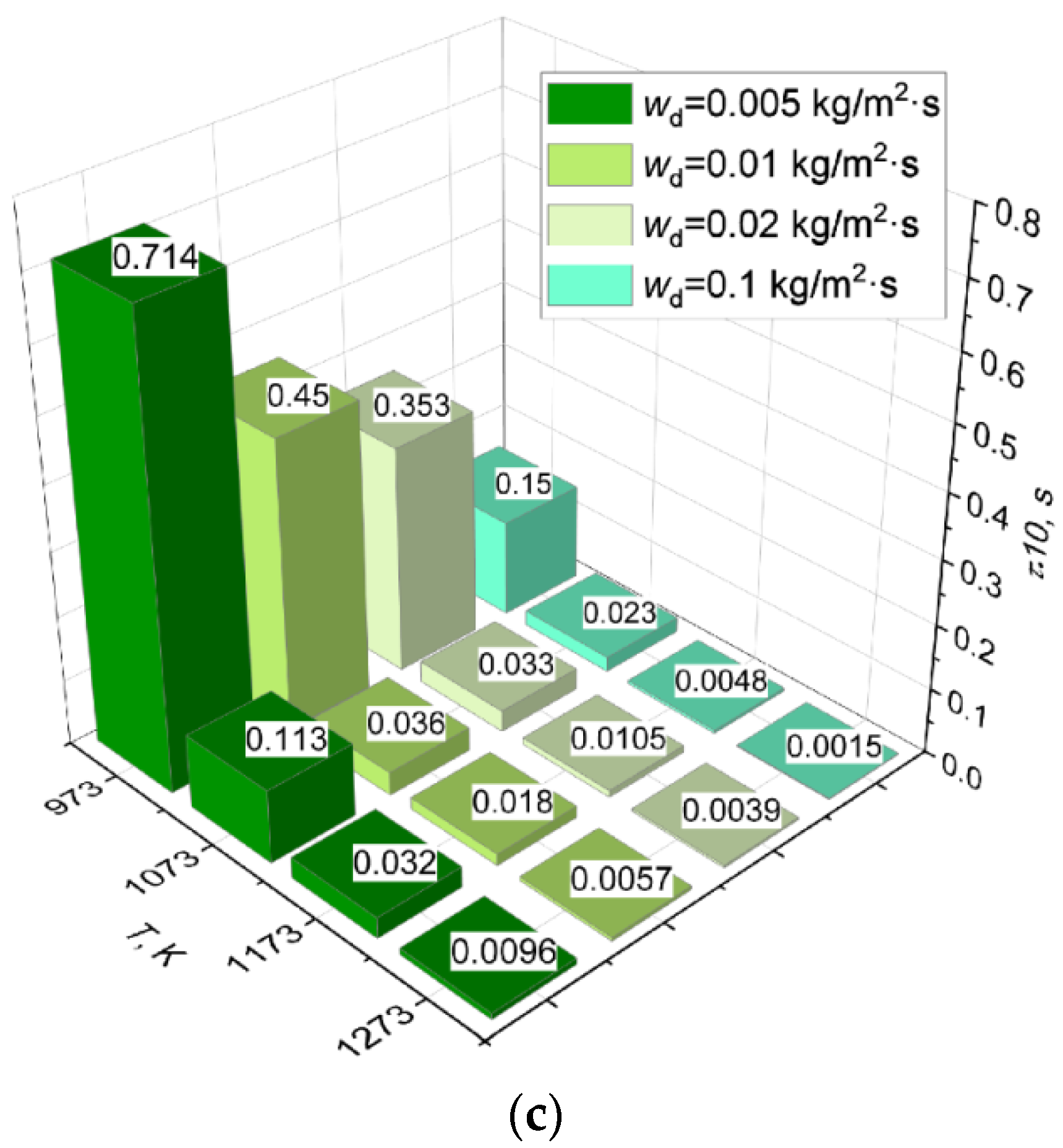
3.3. Effect of Dissociation and Evaporation Rates
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cui, G.; Dong, Z.; Wang, S.; Xing, X.; Shan, T.; Li, Z. Effect of the water on the flame characteristics of methane hydrate combustion. Appl. Energy 2020, 259, 114205. [Google Scholar] [CrossRef]
- Hečko, D.; Malcho, M.; Mičko, P.; Kantová, N.Č.; Kolková, Z.; Hrabovský, P.; Belány, P. Controlled production of natural gas hydrates in an experimental device with an internal circulation circuit. Appl. Sci. 2022, 12, 312. [Google Scholar] [CrossRef]
- Lee, T.; Lee, J.Y.; Ahn, T.; Son, H.A. Numerical Simulation of Gas Hydrate Production Using the Cyclic Depressurization Method in the Ulleung Basin of the Korea East Sea. Appl. Sci. 2021, 11, 9748. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, J.; Chen, Z.; Bai, Y.; Su, H.; Zhao, E.; Li, G. Enhancing hot water flooding in hydrate bearing layers through a novel staged production method. Energy 2021, 217, 119319. [Google Scholar] [CrossRef]
- Chen, H.; Han, B.; Lang, C.; Wen, M.; Fan, B.; Liu, Z. Hydrates for cold storage: Formation characteristics, stability, and promoters. Appl. Sci. 2021, 11, 10470. [Google Scholar] [CrossRef]
- Luketa, A.; Blanchat, T. The phoenix series large-scale methane gas burner experiments and liquid methane pool fires experiments on water. Combust. Flame 2015, 162, 4497–4513. [Google Scholar] [CrossRef]
- Misyura, S.Y.; Manakov, A.Y.; Nyashina, G.S.; Gaidukova, O.S.; Morozov, V.S.; Skiba, S.S. Gas hydrate combustion in five method of combustion organization. Entropy 2020, 22, 710. [Google Scholar] [CrossRef]
- Chien, Y.C.; Dunn-Rankin, D. Combustion characteristics of methane hydrate flames. Energies 2019, 12, 1939. [Google Scholar] [CrossRef]
- Wu, F.-H.; Chao, Y.-C. A Study of Methane Hydrate Combustion Phenomenon Using a Cylindrical Porous Burner. Combust. Sci. Technol. 2016, 188, 1983–2002. [Google Scholar] [CrossRef]
- Maruyama, Y.; Fuse, M.J.; Yokomori, T.; Ohmura, R.; Watanabe, S.; Iwasaki, T.; Iwabuchi, W.; Ueda, T. Experimental investigation of flame spreading over pure methane hydrate in a laminar boundary layer. Proc. Combust. Inst. 2013, 34, 2131–2138. [Google Scholar] [CrossRef]
- Yoshioka, T.; Yamamoto, Y.; Yokomori, T.; Ohmura, R.; Ueda, T. Experimental study on combustion of a methane hydrate sphere. Exp. Fluids 2015, 56, 192. [Google Scholar] [CrossRef]
- Rossi, F.; Gambelli, A.M.; Sharma, D.K.; Castellani, B.; Nicolini, A.; Castaldi, M.J. Experiments on methane hydrates formation in seabed deposits and gas recovery adopting carbon dioxide replacement strategies. Appl. Therm. Eng. 2019, 148, 371–381. [Google Scholar] [CrossRef]
- Misyura, S.Y. Comparing the dissociation kinetics of various gas hydrates during combustion: Assessment of key factors to improve combustion efficiency. Appl. Energy 2020, 270, 115042. [Google Scholar] [CrossRef]
- Chen, B.; Sun, H.; Zhao, G.; Wang, B.; Zhao, Y.; Yang, M. Experimental observation of methane hydrate dissociation via different depressurization modes under water phase flow. Fuel 2021, 283, 118908. [Google Scholar] [CrossRef]
- Ji, J.; Lin, S.; Zhao, C.; Li, K.; Gao, Z. Experimental study on initial temperature influence on flame spread characteristics of diesel and gasoline-diesel blends. Fuel 2016, 178, 283–289. [Google Scholar] [CrossRef]
- Lv, X.; Shi, B.; Zhou, S.; Wang, S.; Huang, W.; Sun, X. Study on the decomposition mechanism of natural gas hydrate particles and its microscopic agglomeration characteristics. Appl. Sci. 2018, 8, 2464. [Google Scholar] [CrossRef]
- Sahith, S.J.K.; Pedapati, S.R.; Lal, B. Investigation on gas hydrates formation and dissociation in multiphase gas dominant transmission pipelines. Appl. Sci. 2020, 10, 5052. [Google Scholar] [CrossRef]
- Lee, S.; Padilla, R.; Dunn-Rankin, D.; Pham, T.; Kwon, O.C. Extinction limits and structure of counterflow nonpremixed H2O-laden CH4/air flames. Energy 2015, 93, 442–450. [Google Scholar] [CrossRef]
- Chen, X.R.; Li, X.S.; Chen, Z.Y.; Zhang, Y.; Yan, K.F.; Lv, Q.N. Experimental investigation into the combustion characteristics of propane hydrates in porous media. Energies 2015, 8, 1242–1255. [Google Scholar] [CrossRef]
- Padilla, R.E.; Escofet-Martin, D.; Pham, T.; Pitz, W.J.; Dunn-Rankin, D. Structure and behavior of water-laden CH4/air counterflow diffusion flames. Combust. Flame 2018, 196, 439–451. [Google Scholar] [CrossRef]
- Jordà Juanós, A.; Sirignano, W.A. Pressure effects on real-gas laminar counterflow. Combust. Flame 2017, 181, 54–70. [Google Scholar] [CrossRef]
- Bar-Kohany, T.; Sirignano, W.A. Transient combustion of a methane-hydrate sphere. Combust. Flame 2016, 163, 284–300. [Google Scholar] [CrossRef]
- Cui, G.; Wang, S.; Dong, Z.; Xing, X.; Shan, T.; Li, Z. Effects of the diameter and the initial center temperature on the combustion characteristics of methane hydrate spheres. Appl. Energy 2020, 257, 114058. [Google Scholar] [CrossRef]
- Wang, S.; Cui, G.; Bi, H.; Liu, C.; Dong, Z.; Xing, X.; Li, Z.; Liu, J. Effect analysis on flame characteristics in the combustion of methane hydrate spheres under natural convective flow conditions. J. Nat. Gas Sci. Eng. 2020, 83, 103578. [Google Scholar] [CrossRef]
- Cui, G.; Dong, Z.; Xie, K.; Wang, S.; Guo, T.; Liu, J.; Xing, X.; Li, Z. Experimental study on the effect of airflow conditions on the combustion characteristics of methane hydrate. Fuel 2021, 300, 120926. [Google Scholar] [CrossRef]
- Gao, J.; Hossain, A.; Nakamura, Y. Flame base structures of micro-jet hydrogen/methane diffusion flames. Proc. Combust. Inst. 2017, 36, 4209–4216. [Google Scholar] [CrossRef]
- Wu, F.H.; Padilla, R.E.; Dunn-Rankin, D.; Chen, G.B.; Chao, Y.C. Thermal structure of methane hydrate fueled flames. Proc. Combust. Inst. 2017, 36, 4391–4398. [Google Scholar] [CrossRef]
- Sizikov, A.A.; Vlasov, V.A.; Stoporev, A.S.; Manakov, A.Y. Decomposition Kinetics and Self-Preservation of Methane Hydrate Particles in Crude Oil Dispersions: Experiments and Theory. Energy Fuels 2019, 33, 12353–12365. [Google Scholar] [CrossRef]
- Dagan, Y.; Bar-Kohany, T. Flame propagation through three-phase methane-hydrate particles. Combust. Flame 2018, 193, 25–35. [Google Scholar] [CrossRef]
- Chiglintseva, A.S.; Gimaltdinov, I.K.; Bayanov, I.M.; Stolpovsky, M.V. Modeling of the combustion process of methane hydrate taking into account the kinetics of the decomposition process. J. Phys. Conf. Ser. 2021, 2094, 022053. [Google Scholar] [CrossRef]
- Gaydukova, O.S.; Misyura, S.Y.; Strizhak, P.A. Investigating regularities of gas hydrate ignition on a heated surface: Experiments and modelling. Combust. Flame 2021, 228, 78–88. [Google Scholar] [CrossRef]
- Cui, G.; Dong, Z.; Xie, K.; Wang, S.; Guo, T.; Liu, J.; Xing, X.; Li, Z. Effects of gas content and ambient temperature on combustion characteristics of methane hydrate spheres. J. Nat. Gas Sci. Eng. 2021, 88, 103842. [Google Scholar] [CrossRef]
- Veluswamy, H.P.; Kumar, A.; Kumar, R.; Linga, P. An innovative approach to enhance methane hydrate formation kinetics with leucine for energy storage application. Appl. Energy 2017, 188, 190–199. [Google Scholar] [CrossRef]
- Sloan, E.D., Jr.; Koh, C.A.; Koh, C.A. Clathrate Hydrates of Natural Gases; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Holder, G.D.; Hand, J.H. Multiple-phase equilibria in hydrates from methane, ethane, propane and water mixtures. AIChE J. 1982, 28, 440–447. [Google Scholar] [CrossRef]
- Gaidukova, O.S.; Misyura, S.Y.; Strizhak, P.A. Study of the gas hydrates ignition under heating different schemes. J. Eng. Phys. Thermophys. 2022, in press. [Google Scholar]
- Kuznetsov, G.V.; Strizhak, P.A. Heat and mass transfer at ignition of liquid fuel droplets spreading over the surface of massive hot bodies. J. Eng. Thermophys. 2010, 19, 75–84. [Google Scholar] [CrossRef]
- Kutateladze, S.S.; Leont’ev, A.I. Heat Transfer, Mass Transfer, and Friction in Turbulent Boundary Layers; Hemisphere: New York, NY, USA, 1989. [Google Scholar]
- Misyura, S.Y.; Donskoy, I.G.; Manakov, A.Y.; Morozov, V.S.; Strizhak, P.A.; Skiba, S.S.; Sagidullin, A.K. Studying the influence of key parameters on the methane hydrate dissociation in order to improve the storage efficiency. J. Energy Storage 2021, 44, 103288. [Google Scholar] [CrossRef]
- Misyura, S.Y.; Donskoy, I.G. Dissociation of a powder layer of methane gas hydrate in a wide range of temperatures and heat fluxes. Powder Technol. 2022, 397, 117017. [Google Scholar] [CrossRef]
- Frenklach, M.; Bornside, D.E. Shock-initiated ignition in methane-propane mixtures. Combust. Flame 1984, 56, 1–27. [Google Scholar] [CrossRef]
- Pomerantsev, V.V.; Arefyev, M.K.; Akhmedov, D.B. Fundamentals of Practical Combustion; Energoatom: Leningrad, Russia, 1986. [Google Scholar]
- Schädel, B.T.; Duisberg, M.; Deutschmann, O. Steam reforming of methane, ethane, propane, butane, and natural gas over a rhodium-based catalyst. Catal. Today 2009, 142, 42–51. [Google Scholar] [CrossRef]
- Grabowski, R.; Słoczyński, J. Kinetics of oxidative dehydrogenation of propane and ethane on VO x/SiO2 pure and with potassium additive. Chem. Eng. Process. Process Intensif. 2005, 44, 1082–1093. [Google Scholar] [CrossRef]
- Misyura, S.Y.; Donskoy, I.G. Dissociation of gas hydrate for a single particle and for a thick layer of particles: The effect of self-preservation on the dissociation kinetics of the gas hydrate layer. Fuel 2022, 314, 122759. [Google Scholar] [CrossRef]
- Chen, Z.; Li, D.; Zhang, S.; Liao, X.; Zhou, B.; Chen, D. A well-test model for gas hydrate dissociation considering a dynamic interface. Fuel 2022, 314, 123053. [Google Scholar] [CrossRef]
- Yang, M.; Sun, H.; Chen, B.; Song, Y. Effects of water-gas two-phase flow on methane hydrate dissociation in porous media. Fuel 2019, 255, 115637. [Google Scholar] [CrossRef]
- Misyura, S.Y.; Morozov, V.S. Influence of Air Velocity on Non-Isothermal Decay and Combustion of Gas Hydrate. J. Eng. Thermophys. 2021, 30, 374–382. [Google Scholar] [CrossRef]
- Misyura, S.Y.; Donskoy, I.G. Co-modeling of methane hydrate dissociation and combustion in a boundary layer. Combust. Flame 2022, 238, 111912. [Google Scholar] [CrossRef]
- Misyura, S.Y. Dissociation of various gas hydrates (methane hydrate, double gas hydrates of methane-propane and methane-isopropanol) during combustion: Assessing the combustion efficiency. Energy 2020, 206, 118120. [Google Scholar] [CrossRef]







| Constant | Designation | Value | Measuring Unit |
|---|---|---|---|
| Activation energy of oxidation reaction of fuel vapors | Ea | 190 × 103 | J/mol |
| Pre-exponential factor of oxidation reaction of fuel vapors | k0 | 7.4 × 108 | s−1 |
| Thermal effect of oxidation reaction | Qr | 14.644 × 106 | J/kg |
| Heat of water evaporation | Qevap | 2.2 × 106 | J/kg |
| Heat of ice melting | Qmelt | 3.4 × 105 | J/kg |
| Mass rate of gas hydrate dissociation | wd | 0.01 | kg/(m2∙s) |
| Heat of gas hydrate dissociation | Qd | 108 × 103 | J/mol |
| Mass rate of ice melting | wmelt | 0.01 | kg/(m2∙s) |
| Mass rate of water evaporation | wevap | 0.01 | kg/(m2∙s) |
| External medium (heating source) temperature | T | 973–1273 | K |
| Initial temperature of gas hydrate | Tg | 203 | K |
| Air flow rate | Ua | 1 | m/s |
| Hydrate structure porosity | φ | 0.05 | – |
| Thermal conductivity coefficient of gas hydrate | λ | 1.33 | W/(m·K) |
| Gas hydrate density | ρ | 909 | kg/m2 |
| Specific heat capacity of gas hydrate | C | 2200 | J/(kg·K) |
| T, K | Methane-Propane Hydrate τ, s at Ea = 190 × 103 J/mol, k0 = 7.4 × 108 s−1 | Methane τ, s at Ea = 103.8 × 103 J/mol, k0 = 5.6 × 1012 s−1 | Propane τ, s at Ea = 61.5 × 103 J/mol, k0 = 4.2 × 1011 s−1 | Ethane τ, s at Ea = 76 × 103 J/mol, k0 = 1.04 × 105 s−1 |
|---|---|---|---|---|
| 973 | 1.8 × 10−2 | 5.07 × 10−7 | 1.70 × 10−7 | 6.1 × 10−4 |
| 1073 | 6.3 × 10−3 | 4.80 × 10−7 | 1.05 × 10−7 | 3.46 × 10−4 |
| 1173 | 1.52 × 10−3 | 1.94 × 10−7 | 6.74 × 10−8 | 2.36 × 10−4 |
| 1273 | 5.1 × 10−4 | 1.13 × 10−7 | 4.43 × 10−8 | 2.14 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaidukova, O.; Misyura, S.; Razumov, D.; Strizhak, P. Modeling of a Double Gas Hydrate Particle Ignition. Appl. Sci. 2022, 12, 5953. https://doi.org/10.3390/app12125953
Gaidukova O, Misyura S, Razumov D, Strizhak P. Modeling of a Double Gas Hydrate Particle Ignition. Applied Sciences. 2022; 12(12):5953. https://doi.org/10.3390/app12125953
Chicago/Turabian StyleGaidukova, Olga, Sergey Misyura, Dmitrii Razumov, and Pavel Strizhak. 2022. "Modeling of a Double Gas Hydrate Particle Ignition" Applied Sciences 12, no. 12: 5953. https://doi.org/10.3390/app12125953
APA StyleGaidukova, O., Misyura, S., Razumov, D., & Strizhak, P. (2022). Modeling of a Double Gas Hydrate Particle Ignition. Applied Sciences, 12(12), 5953. https://doi.org/10.3390/app12125953








