Surface Modification of Materials by Atmospheric-Pressure Plasma to Improve Impregnation with Essential Oils for the Control of Tropilaelaps Mites in Honeybees (Apis mellifera)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Essential Oil Extraction
2.2. Materials
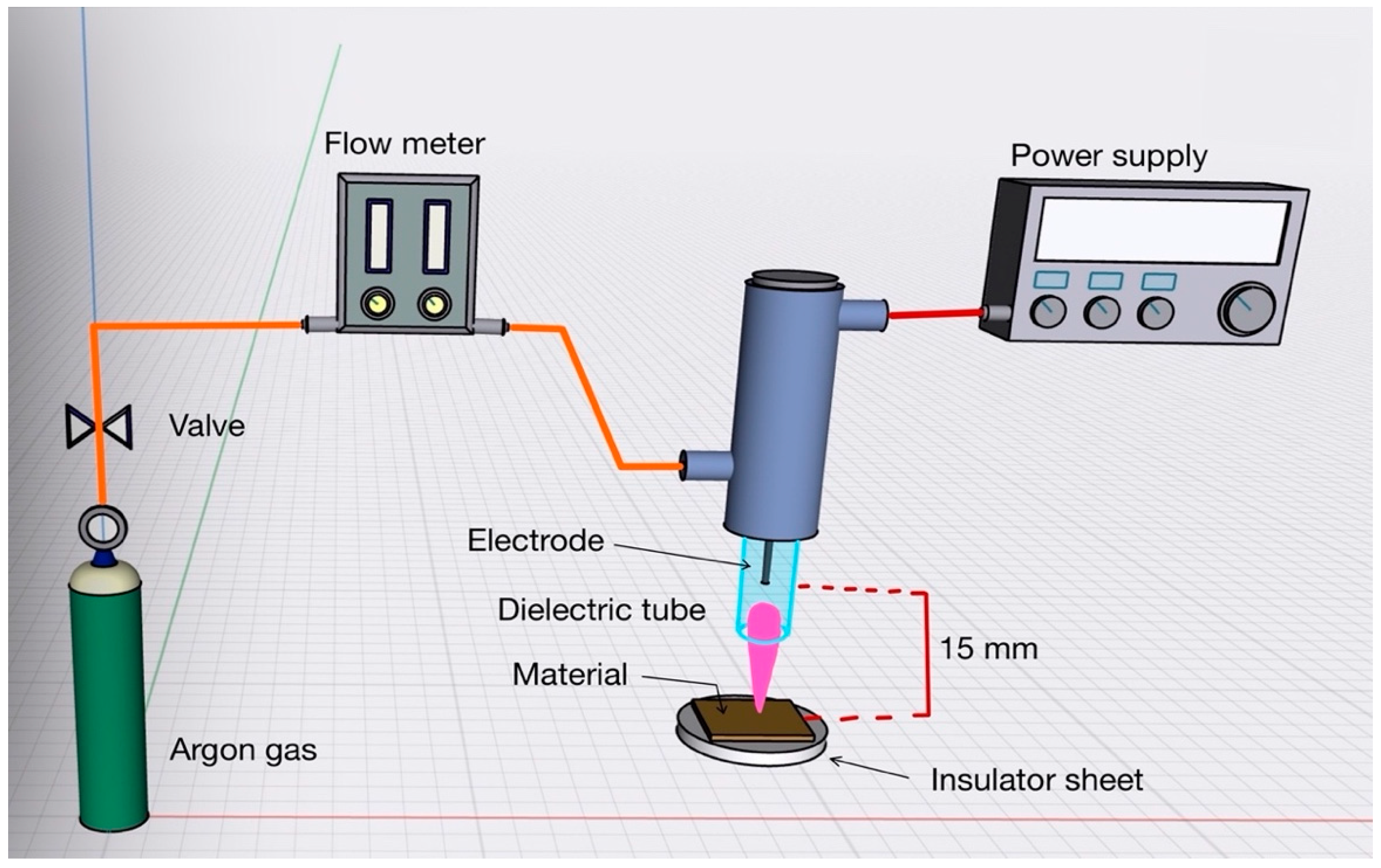
2.3. Atmospheric-Pressure Plasma Treatments
2.4. Impregnation Process
2.5. Analysis of Material Properties
2.5.1. Essential Oil Absorption Capacity
2.5.2. Essential Oil Holding Capacity
2.5.3. Essential Oil Release Rate
2.5.4. Surface Characterization
2.6. Essential Oil Impregnated Materials for Tropilaelaps spp. Mites Control under Field Conditions
2.7. Statistical Analysis
3. Results
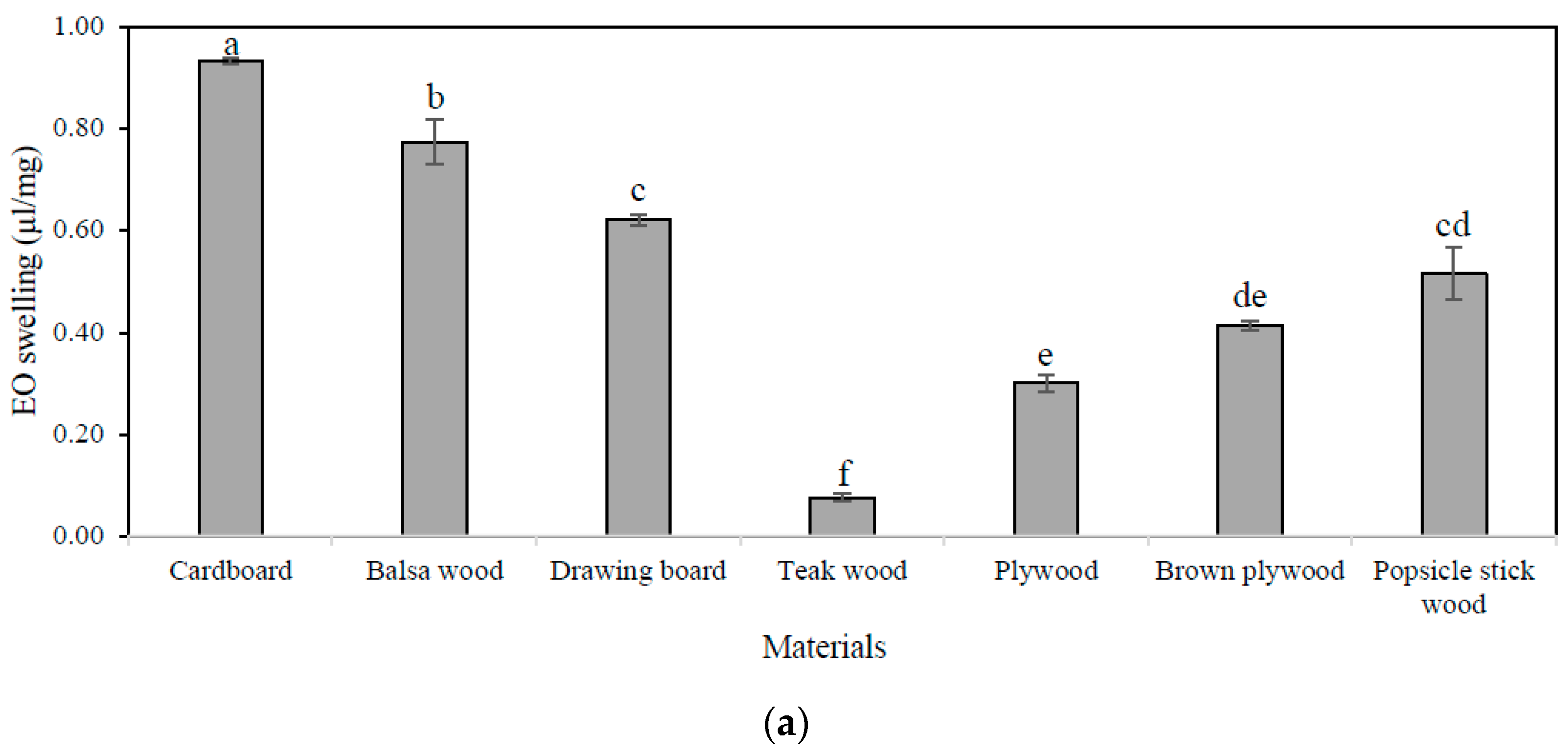
3.1. Essential Oil Absorption Capacity, Holding Capacity, and Release Rate of Materials
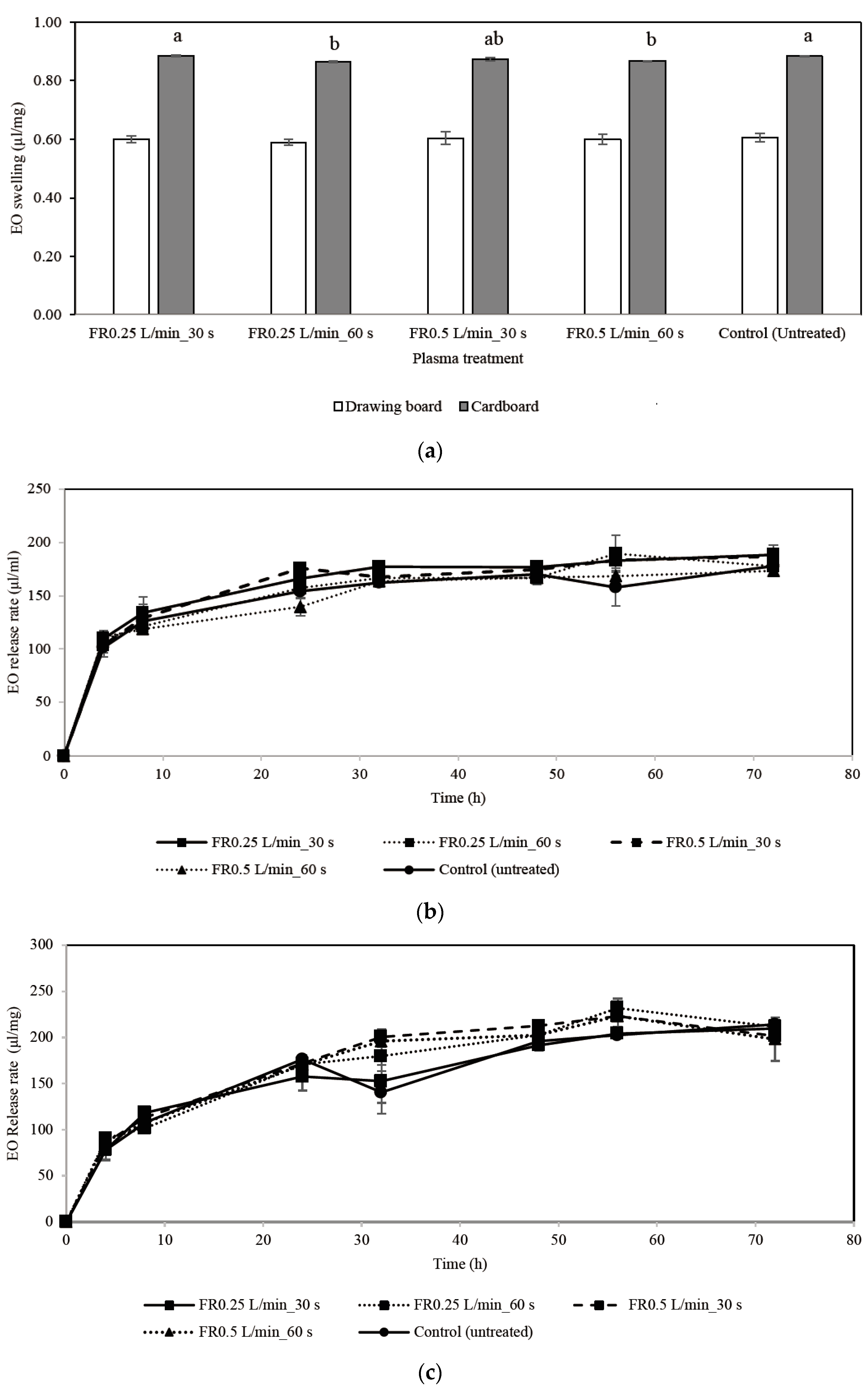
3.2. Effect of Atmospheric-Pressure Plasma on the Property of Materials
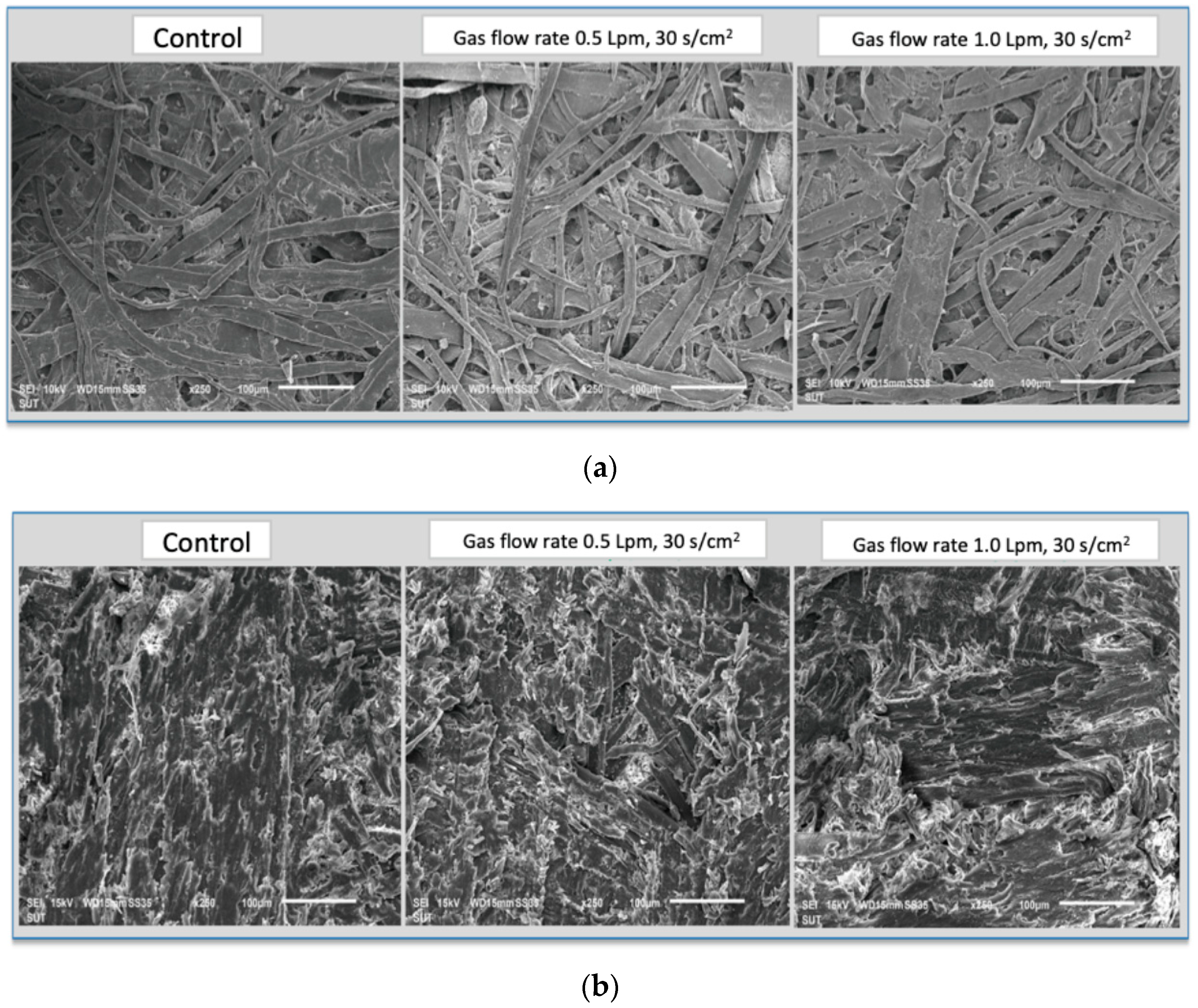
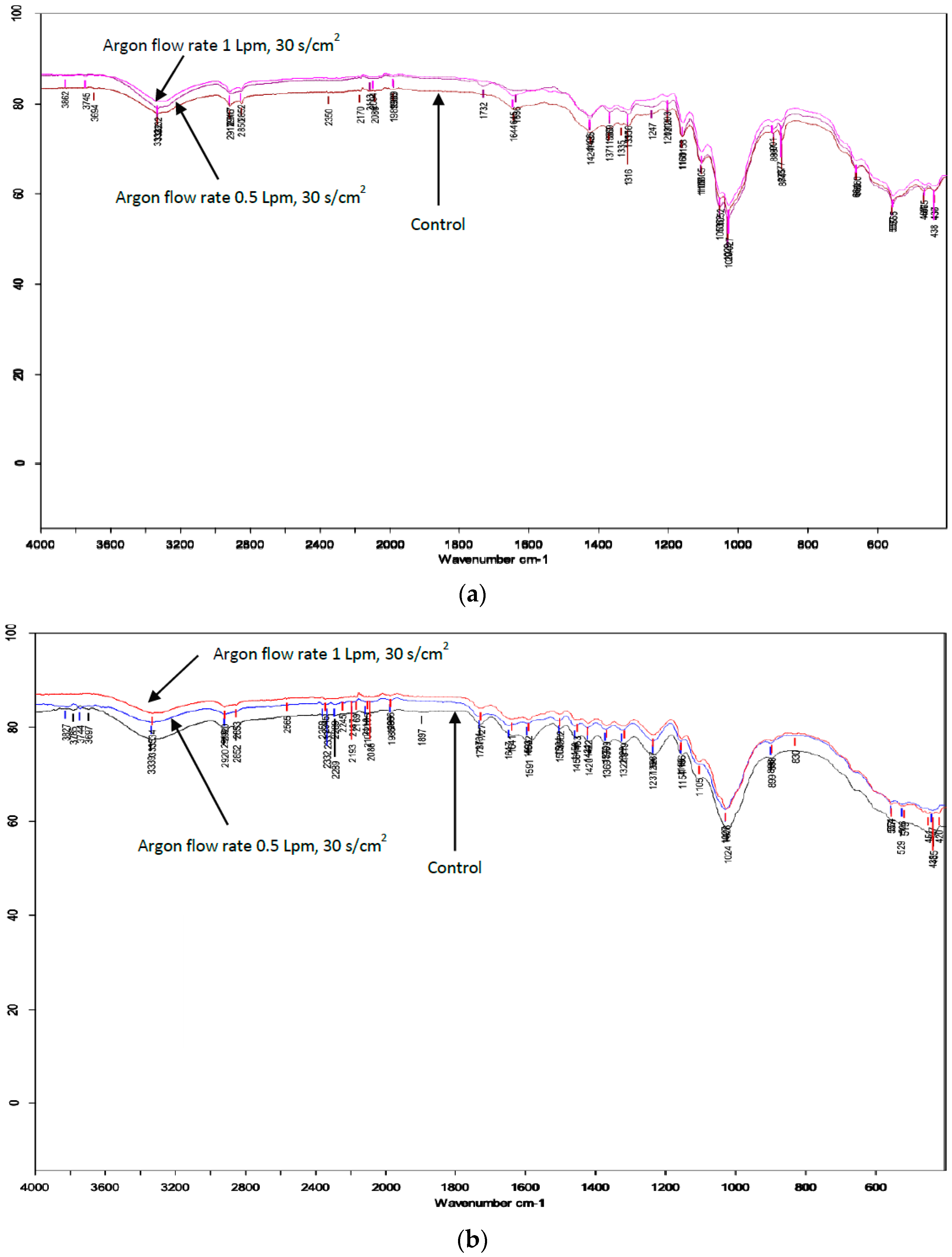
3.3. Surface Modification of Material after Atmospheric-Pressure Plasma Treatment
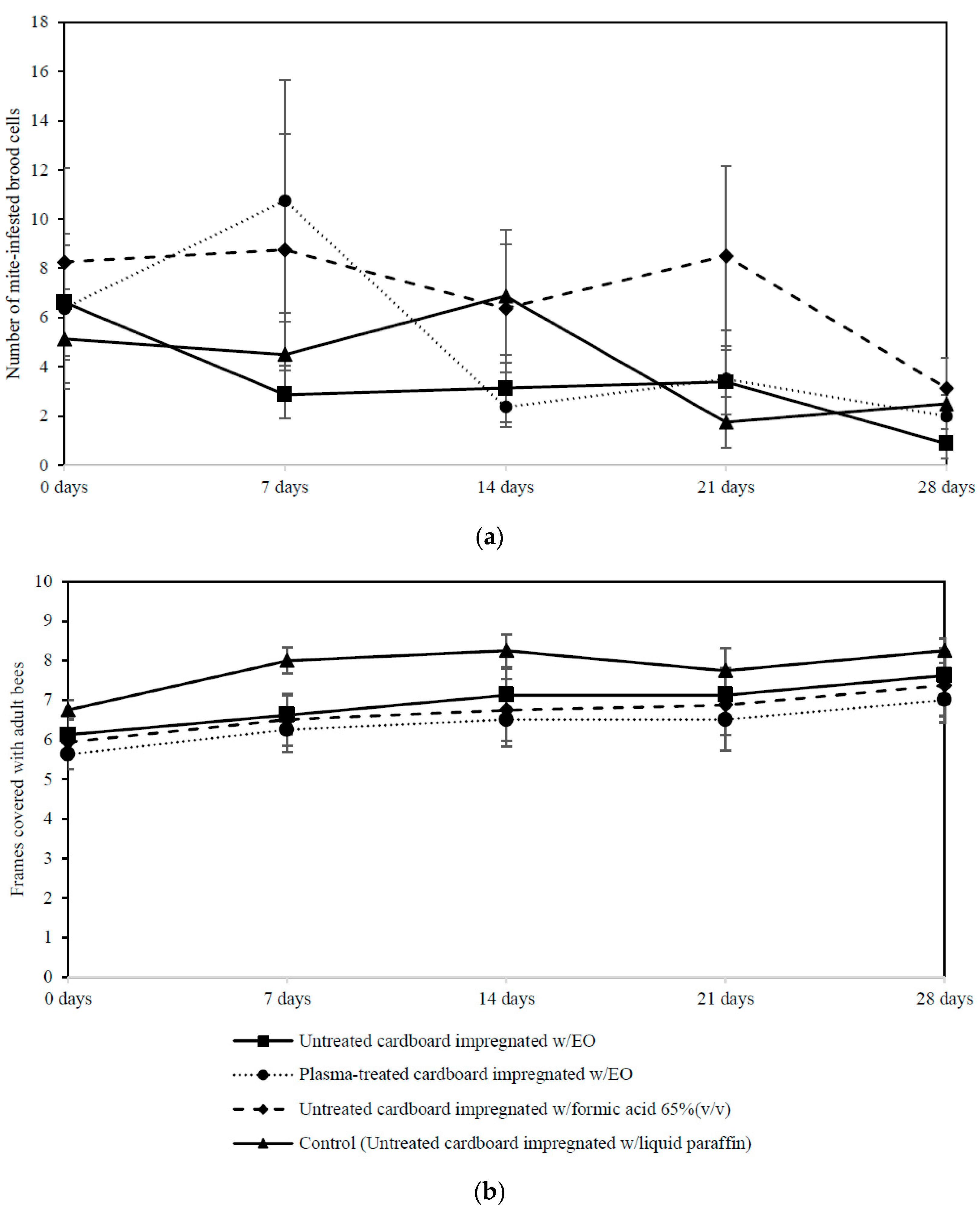
3.4. Efficacy of Plasma Treated Cardboard Impregnated with Essential Oil on Tropilaelaps spp. Mite Control under Field Conditions
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burgett, M.; Akratanakul, P.; Morse, R.A. Tropilaelaps clareae: A Parasite of Honeybees in South-East Asia. Bee World 1983, 64, 25–28. [Google Scholar] [CrossRef]
- Burgett, D.M.; Kitprasert, C. Evaluation of Apistan as a Control for Tropilaelaps clareae (Acari: Laelapidae), an Asian Honey Bee Brood Mite Parasite. Am. Bee J. 1990, 130, 51–53. [Google Scholar]
- Khongphinitbunjong, K.; de Guzman, L.I.; Burgett, M.D.; Rinderer, T.E.; Chantawannakul, P. Behavioral responses underpinning resistance and susceptibility of honeybees to Tropilaelaps mercedesae. Apidologie 2012, 43, 590–599. [Google Scholar] [CrossRef] [Green Version]
- De Guzman, L.I.; Williams, G.R.; Khongphinitbunjong, K.; Chantawannakul, P. Ecology, Life History, and Management of Tropilaelaps Mites. J. EconEntomol. 2017, 110, 319–332. [Google Scholar] [CrossRef] [Green Version]
- Thongsawang, T.; Rueangsom, P.; Boonyo, K.; Wongphruksasoong, V.; Suphanchaimat, R. Situation Analysis of Varroosis and Tropilaelaps Infestation of Honeybees in Thailand, 2017–2018. Vet. Med. 2021, 12, 169–176. [Google Scholar] [CrossRef]
- Luo, Q.H.; Zhou, T.; Dai, P.L.; Song, H.L.; Wu, Y.Y.; Wang, Q. Prevalence, Intensity and Associated Factor Analysis of Tropilaelaps mercedesae Infesting Apis mellifera in China. Exp. Appl. Acarol. 2011, 55, 135–146. [Google Scholar] [CrossRef]
- Camphor, E.S.W.; Hashmi, A.A.; Ritter, W.; Bowen, I.D. Seasonal Changes in Mite (Tropilaelaps clareae) and Honeybee (Apis mellifera) Populations in Apistan Treated and Untreated Colonies. Apiacta 2005, 40, 36–44. [Google Scholar]
- Kongpitak, P.; Polgar, G.; Heine, J. The Efficacy of Bayvarol® and Checkmite+® in The Control of Tropilaelaps mercedesae in The European Honey Bee (Apis mellifera) in Thailand. Apiacta 2008, 43, 12–16. [Google Scholar]
- Pettis, J.S.; Rose, R.; Chaimanee, V. Chemical and Cultural Control of Tropilaelaps mercedesae Mites in Honeybee (Apis mellifera) Colonies in Northern Thailand. PLoS ONE 2017, 12, e0188063. [Google Scholar] [CrossRef] [Green Version]
- Atwal, A.S.; Goyal, N.P. Infestation of Honeybee Colonies with Tropilaelaps, and Its Control. J. Apic. Res. 1971, 10, 137–142. [Google Scholar] [CrossRef]
- Haarmann, T.; Spivak, M.; Weaver, D.; Weaver, B.; Glenn, T. Effects of Fluvalinate and Coumaphos on Queen Honey Bees (Hymenoptera: Apidae) in Two Commercial Queen Rearing Operations. J. Econ. Entomol. 2002, 95, 28–35. [Google Scholar] [CrossRef]
- Burley, L.M.; Fell, R.D.; Saacke, R.G. Survival of Honey Bee (Hymenoptera: Apidae) Spermatozoa Incubated at Room Temperature from Drones Exposed to Miticides. J. Econ. Entomol. 2008, 101, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Mullin, C.A.; Frazier, M.; Frazier, J.L.; Ashcraft, S.; Simonds, R.; vanEngelsdorp, D.; Pettis, J.S. High Levels of Miticides and Agrochemicals in North American Apiaries: Implications for Honey Bee Health. PLoS ONE 2010, 5, e9754. [Google Scholar] [CrossRef] [Green Version]
- Damiani, N.; Gende, L.B.; Bailac, P.; Marcangeli, J.A.; Eguaras, M.J. Acaricidal and Insecticidal Activity of Essential Oils on Varroa destructor (Acari: Varroidae) and Apis mellifera (Hymenoptera: Apidae). Parasitol. Res. 2009, 106, 145–152. [Google Scholar] [CrossRef]
- Bendifallah, L.; Belguendouz, R.; Hamoudi, L.; Arab, K. Biological Activity of the Salvia officinalis L. (Lamiaceae) Essential Oil on Varroa destructor Infested Honeybees. Plants 2018, 7, 44. [Google Scholar] [CrossRef] [Green Version]
- Chaimanee, V.; Warrit, N.; Boonmee, T.; Pettis, J.S. Acaricidal Activity of Essential Oils for the Control of Honeybee (Apis mellifera) Mites Tropilaelaps mercedesae under Laboratory and Colony Conditions. Apidologie 2021, 52, 561–575. [Google Scholar] [CrossRef]
- Yanling, C.; Yingkuan, W.; Chen, P.; Deng, S.; Ruan, R. Non-Thermal Plasma Assisted Polymer Surface Modification and Synthesis: A Review. Int. J. Agric. Biol. Eng. 2014, 7, 1–9. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Linh, N.N.; Ghimire, B.; Pengkit, A.; Sornsakdanuphap, J.; Lee, S.-J.; Choi, E.H. Plasma and Nanomaterials: Fabrication and Biomedical Applications. Nanomaterials 2019, 9, 98. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Zhao, M.; Wang, L.; Qu, L.; Men, Y. Surface Modification of Polyester Fabrics by Atmospheric-Pressure Air/He Plasma for Color Strength and Adhesion Enhancement. Appl. Surf. Sci. 2017, 400, 304–311. [Google Scholar] [CrossRef]
- López-García, J.; Cupessala, F.; Humpolíček, P.; Lehocký, M. Physical and Morphological Changes of Poly(Tetrafluoroethylene) after Using Non-Thermal Plasma-Treatments. Materials 2018, 11, 2013. [Google Scholar] [CrossRef] [Green Version]
- Czylkowski, D.; Hrycak, B.; Sikora, A.; Moczała-Dusanowska, M.; Dors, M.; Jasiński, M. Surface Modification of Polycarbonate by an Atmospheric Pressure Argon Microwave Plasma Sheet. Materials 2019, 12, 2418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junka, A.; Żywicka, A.; Chodaczek, G.; Dziadas, M.; Czajkowska, J.; Duda-Madej, A.; Bartoszewicz, M.; Mikołajewicz, K.; Krasowski, G.; Szymczyk, P.; et al. Potential of Biocellulose Carrier Impregnated with Essential Oils to Fight Against Biofilms Formed on Hydroxyapatite. Sci. Rep. 2019, 9, 1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, T.P.; Haddi, K.; Corrêa, R.F.T.; Zapata, V.L.B.; Piau, T.B.; Souza, L.F.N.; Santos, S.-M.G.; Oliveira, E.E.; Jumbo, L.O.V.; Ribeiro, B.M.; et al. Prolonged Mosquitocidal Activity of Siparuna guianensis Essential Oil Encapsulated in Chitosan Nanoparticles. PLoS Negl. Trop. Dis. 2019, 13, e0007624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, P.; Preet, S.; Ananya; Singh, A. Preparation of Thymus vulgaris (L.) Essential Oil Nanoemulsion and Its Chitosan Encapsulation for Controlling Mosquito Vectors. Sci. Rep. 2022, 12, 4335. [Google Scholar] [CrossRef]
- Macgregor, M.; Vasilev, K. Perspective on Plasma Polymers for Applied Biomaterials Nanoengineering and the Recent Rise of Oxazolines. Materials 2019, 12, 191. [Google Scholar] [CrossRef] [Green Version]
- Denes, F.S.; Manolache, S. Macromolecular Plasma-Chemistry: An Emerging Field of Polymer Science. Prog. Polym. Sci. 2004, 29, 815–885. [Google Scholar] [CrossRef]
- Li, X.; Lin, J.; Qiu, Y. Influence of He/O2 Atmospheric-Pressure Plasma Jet Treatment on Subsequent Wet Desizing of Polyacrylate on PET Fabrics. Appl. Surf. Sci. 2012, 258, 2332–2338. [Google Scholar] [CrossRef]
- Dalei, G.; Das, S.; Prava Das, S. Non-Thermal Plasma Assisted Surface Nano-Textured Carboxymethyl Guar Gum/Chitosan Hydrogels for Biomedical Applications. RSC Adv. 2019, 9, 1705–1716. [Google Scholar] [CrossRef] [Green Version]
- Castro Vidaurre, E.F.; Achete, C.A.; Gallo, F.; Garcia, D.; Simão, R.; Habert, A.C. Surface Modification of Polymeric Materials by Plasma Treatment. Mat. Res. 2002, 5, 37–41. [Google Scholar] [CrossRef] [Green Version]
- Puliyalil, H.; Cvelbar, U. Selective Plasma Etching of Polymeric Substrates for Advanced Applications. Nanomaterials 2016, 6, 108. [Google Scholar] [CrossRef] [Green Version]
- Palumbo, F.; Lo Porto, C.; Favia, P. Plasma Nano-Texturing of Polymers for Wettability Control: Why, What and How. Coatings 2019, 9, 640. [Google Scholar] [CrossRef] [Green Version]
- Golda-Cepa, M.; Engvall, K.; Hakkarainen, M.; Kotarba, A. Recent Progress on Parylene C Polymer for Biomedical Applications: A Review. Prog. Org. Coat. 2020, 140, 105493. [Google Scholar] [CrossRef]
- Holländer, A.; Cosemans, P. Surface Technology for Additive Manufacturing. Plasma Processes Polym. 2020, 17, 1900155. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boonmee, T.; Wongthaveethong, L.; Sinpoo, C.; Disayathanoowat, T.; Pettis, J.S.; Chaimanee, V. Surface Modification of Materials by Atmospheric-Pressure Plasma to Improve Impregnation with Essential Oils for the Control of Tropilaelaps Mites in Honeybees (Apis mellifera). Appl. Sci. 2022, 12, 5800. https://doi.org/10.3390/app12125800
Boonmee T, Wongthaveethong L, Sinpoo C, Disayathanoowat T, Pettis JS, Chaimanee V. Surface Modification of Materials by Atmospheric-Pressure Plasma to Improve Impregnation with Essential Oils for the Control of Tropilaelaps Mites in Honeybees (Apis mellifera). Applied Sciences. 2022; 12(12):5800. https://doi.org/10.3390/app12125800
Chicago/Turabian StyleBoonmee, Thummanoon, Laedlugkana Wongthaveethong, Chainarong Sinpoo, Terd Disayathanoowat, Jeffery S. Pettis, and Veeranan Chaimanee. 2022. "Surface Modification of Materials by Atmospheric-Pressure Plasma to Improve Impregnation with Essential Oils for the Control of Tropilaelaps Mites in Honeybees (Apis mellifera)" Applied Sciences 12, no. 12: 5800. https://doi.org/10.3390/app12125800
APA StyleBoonmee, T., Wongthaveethong, L., Sinpoo, C., Disayathanoowat, T., Pettis, J. S., & Chaimanee, V. (2022). Surface Modification of Materials by Atmospheric-Pressure Plasma to Improve Impregnation with Essential Oils for the Control of Tropilaelaps Mites in Honeybees (Apis mellifera). Applied Sciences, 12(12), 5800. https://doi.org/10.3390/app12125800









