Featured Application
Inoculation strategies for microalgae and bacteria consortium for the treatment of paper pulp industry effluents with an increase in biomass sedimentation and algae biomass recovery.
Abstract
The microalgae–bacteria consortium is a promising and sustainable alternative for industrial wastewater treatment, since it may allow good removal of organic matter and nutrients, as well as the possibility of producing products with added value from the algae biomass. This research investigated the best bacterial and microalgae inoculation ratio for system start-up and evaluation of removing organic matter (as chemical oxygen demand (COD)), ammoniacal nitrogen (NH4+–N), nitrite nitrogen (NO2−–N), nitrate nitrogen (NO3−–N), phosphate phosphorus (PO43−–P) and biomass formation parameters in six photobioreactors with a total volume of 1000 mL. Reactors were operated for 14 days with the following ratios of pulp mill biomass aerobic (BA) and Scenedesmus sp. microalgae (MA): 0:1 (PBR1), 1:0 (PBR2), 1:1 (PBR3), 3:1 (PBR4), 5:1 (PBR5), and 1:3 (PBR6). Results show that COD removal was observed in just two days of operation in PBR4, PBR5, and PBR6, whereas for the other reactors (with a lower rate of initial inoculation) it took five days. The PBR5 and PBR6 performed better in terms of NH4+–N removal, with 86.81% and 77.11%, respectively, which can be attributed to assimilation by microalgae and nitrification by bacteria. PBR6, with the highest concentration of microalgae, had the higher PO43−–P removal (86%), showing the advantage of algae in consortium with bacteria for phosphorus uptake. PBR4 and PBR5, with the highest BA, led to a better biomass production and sedimentability on the second day of operation, with flocculation efficiencies values over 90%. Regarding the formation of extracellular polymeric substances (EPS), protein production was substantially higher in PBR4 and PBR5, with more BA, with average concentrations of 49.90 mg/L and 49.05 mg/L, respectively. The presence of cyanobacteria and Chlorophyceae was identified in all reactors except PBR1 (only MA), which may indicate a good formation and structuring of the microalgae–bacteria consortium. Scanning electron microscopy (SEM) analysis revealed that filamentous microalgae were employed as a foundation for the fixation of bacteria and other algae colonies.
1. Introduction
The contamination of aquatic systems is increased due to the increase in wastewater produced by population growth and the expansion of industrial processes [1,2]. To avoid negative environmental impacts, biological wastewater processes are widely used for domestic and industrial wastewater treatment [3,4]. Conventional wastewater treatment processes, such as activated sludge, stabilization ponds, or percolating filters, present technical restrictions and high operating costs when it comes to nutrient removal and biomass harvesting [5]. According to Ummalyma et al. [6], biomass harvesting for the creation of goods with added value is identified as the stage with the greatest cost of the production process—20% to 30% of the total production.
Therefore, new technologies for industrial wastewater treatment must be investigated. New and innovative systems that use the microalgae–bacteria consortium seem to be a sustainable and environmentally friendly technological alternative, requiring less area to operate [7] and promoting more efficient and less expensive wastewater treatment techniques [8] than the conventional processes. The production of photosynthetic oxygen, which is utilized by aerobic bacteria to oxidize organic materials and ammonia, and carbon dioxide (CO2) sequestering by algae, are major benefits of this system, since it eliminates the need for artificial oxygen and avoids the release of carbon to the atmosphere. Biological sequestration of CO2 by algae is a future field of research since its photosynthetic capability can efficiently capture carbon [9]. Algae and bacteria also uptake nutrients for their synthesis and maintenance.
In addition to the economic aspects and nutrient removal, systems that use microalgae and bacterial bioflocculation, in the form of a microalgae–bacteria consortium (MABA), show the potential for resource recovery and valorization, promoting new added-values in the scope of circular economy [10]. Thus, wastewater treatment plants (WWTP) that use microalgae–bacteria aggregates are effective for biomass growth, allowing for more sedimentation and biomass extraction [11].
Despite MABA’s wide use to domestic wastewater, this synergistic consortium can also be used to treat industrial effluents. However, no research has been found on the use of MABA for the treatment of wastewater from the paper pulp industry. However, satisfactory outcomes with aerobic systems for pulp and paper wastewater have already been reported in the literature [12,13] as well as interesting results using microalgae species such as Scenedesmus sp. and Chlorella vulgaris to treat paper industry effluents [14,15,16,17]. The symbiotic process between microalgae and bacteria is also good for removing pharmaceutical substances, and has been already used to remove antibiotics, antihypertensives, and psychiatric pharmaceuticals under appropriate operating conditions [18,19].
The use of MABA for the treatment of pulp and paper wastewater may provide the following advantages: (i) biomass production with potential for byproducts commercialization such as animal feed, biopolymers, and biofuels; (ii) low operating costs because it is a clean and sustainable alternative; (iii) carbon capture by microalgae, which can generate additional revenue in the future around carbon credits; and (iv) generation of high quality algal biomass, which can be sold to power plants and converted into electricity [16,20,21,22,23].
Wastewater generated in the paper and pulp industries originates from activities such as debarking wood, manufacturing paper, recycling fibers, and generating cellulose, among others [24]. In general, the operational processes include chemical, mechanical, thermo-mechanical, and chemical–mechanical technologies, which need a large amount of water consumption. Furthermore, these processes generate a large volume of effluents containing a variety of pollutants, as well as high operating costs for their treatment with classical technologies [15,25].
In this context, noting the need to fill some gaps left by the classical wastewater processes, such as energy consumption, gas emissions, construction area, and the importance of improving the circular economy of systems, increasing sedimentability and biomass harvest, the use of MABA for treating paper and pulp wastewater arises, it seems, as an alternative that is less expensive and with greater efficiency in removing organic matter and nutrients. The novelty of this study consists of evaluating different ratios of microalgae and aerobic sludge inoculum for the suitable treatment of a paper pulp wastewater, with the goal of removing organic matter and nutrients, as well as evaluating consortium formation, sedimentability, and biomass composition and, therefore, aids future work in deciding on the initial concentration of microalgae and bacterial in inoculums.
2. Materials and Methods
2.1. Characterization of Aerobic Biomass, Species of Microalgae, and Wastewater
The microalgae strain used in this study was Scenedesmus sp., collected in Portugal and supplied by Aqualgae (Viana do Castelo, Portugal). The culture was adapted in 1 L Erlenmeyer, with a constant air supply (2 L/min) and photosynthetic radiation of 100 µmol/m2.s, an average temperature of 25 ± 2 °C, and a photoperiod of 12 h of light and 12 h of darkness. The culture medium was GoldMedium Fresh-Water Species (GM-FWS), also supplied by Aqualgae (Viana do Castelo, Portugal), with a concentration inoculum of volatile suspended solids (VSS) of 735.00 ± 70.36 mg/L. The activated sludge used for inoculation and the development of the microalgae–bacteria consortium came from the bottom disposal of an aerobic reactor from a paper pulp industry WWTP located in Portugal. The average sludge VSS concentration was 3045.00 ± 210.99 mg/L and the VSS concentration in the paper pulp industry wastewater was 365.00 ± 20.21 mg/L. The experimental setup is shown in Figure 1.
Figure 1.
Schematic representation of the experimental setup.
2.2. Configuration of Photobioreactors
The operational setup consisted of six photobioreactors (PBR), with different microalgae and aerobic sludge inoculum ratios. To serve as a control, two photobioreactors were inoculated with only aerobic sludge and only microalgae. The experiment lasted for 14 days. Leong et al. [26] suggest an initial concentration of VSS of 100 mg/L. The six photobioreactors were inoculated with the ratios of microalgae and activated sludge (MA:AS) presented in Table 1 and afterwards were filled with wastewater from the paper pulp industry with a total working volume of 1 L.

Table 1.
Characteristics of the initial inoculation concentration of photobioreactors.
A constant air injection of 2 L/min was used to aerate all photobioreactors. The PBRs were kept in continuous agitation with a magnetic stirrer at 100 rpm during the experiment to avoid biomass sedimentation. The average temperature was kept at 25 ± 2 °C, with a light and dark cycle of 12 h:12 h and a radiation of 200 µmol/m2.s, all while using an LED intensity-adjustable system based on the LED MAXLED Pro (Fajozes, Portugal). A similar strategy was used by Su et al. [27] to evaluate the synergistic effects between microalgae and bacteria. It is important to mention that these operational factors have a direct impact on the system’s balance and overall efficiency. During the operation of the PBRs, the pH was monitored daily and continuously adjusted to around 7.5 ± 0.5, using a 1M HCL solution to maintain the system equilibrium.
2.3. Analytical Methods
On days 0, 2, 5, 7, 9, 12, and 14, a water sample was collected to determine pH, dissolved oxygen (DO), temperature, chemical oxygen demand (COD), VSS, total nitrogen (TN), ammoniacal nitrogen (NH4+–N), nitrite nitrogen (NO2−–N), nitrate nitrogen (NO3−–N), and phosphate phosphorus (PO43−–P). The temperature, pH, and DO were analyzed using the probes Sentix41 and Oxical and a Multi 340i meter (WTW, Germany). To assess the concentration in the liquid phase, the water samples were filtered through 0.45 µm membranes to remove solids. The colorimetric method was used to analyze COD, NH4+–N, NO2−–N, NO3−–N, and PO43–P, using the RFID model DR3900 technology (Hach, Loveland, CO, USA) and the cuvette tests LCK 114, LCK 303, LCK 342, LCK 339, and LCK 350, respectively. TN and VSS were carried out through standard methods [28].
Chlorophyll (CHL) analyses were also performed in all water samples to monitor the development of microalgae; regardless of the species, this parameter indicates the productivity of algal biomass of the symbiotic process. The analysis of chlorophyll “a” and “b” was performed according to the methodology indicated by Jeffrey et al. [29] and Saseendran et al. [30], where an aliquot of 30 mL of mixed liquor from each photobioreactor was centrifuged for 10 min at 4000 rpm. The supernatant was then discarded, and 30 mL of acetone and methanol (2:1 v/v) were added. The solution was then introduced into a Sonics Materials, Vibracell (Newtown, CT, USA) sonicator for 100 s, resulting in cell breakage and pigment extraction. After sonication, the samples were conditioned in the dark, with aluminum foil around the falcon tubes, for 60 min in a refrigerator at 4 °C. Sequentially, the samples were centrifuged for 10 min at 4000 rpm at room temperature. Finally, the chlorophyll-a and the chlorophyll-b optical density (OD) was measured at wavelengths of 665 nm, 645 nm, and 630 nm in the supernatant, using a Spectronic Helios Gamma UV-Vis Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Equations (1) and (2) were used for the calculation of chlorophyll “a” and “b” (mg/L), respectively [29].
where chlorophyll-a is the concentration of chlorophyll a (mg/L), chlorophyll-b is the concentration of chlorophyll b (mg/L), OD665 is the wavelength at 665 nm, OD645 is the wavelength at 645 nm, OD630 is the wavelength at 630 nm, V1 is the volume of solvent (2:1) (L) and V is the volume of sample (L).
A sedimentation test was also performed for all water samples to assess the biomass’s sedimentation capability, following the methodology described by Leong et al. [26]. A 50 mL aliquot of the mixed liquor sample was withdrawn, and its initial optical density (OD) was measured at a wavelength of 650 nm, after the sample was allowed to rest for 20 min before being tested for OD at the same wavelength (the new aliquot was extracted up to a maximum of 2.5 cm from the surface). Equation (3) shows the biomass flocculation efficiency (%):
where FE is the flocculation efficiency (%), F is the OD650 after 20 min of sedimentation and I is the corresponding OD of the homogenized sample’s OD650 (T = 0).
Regarding the composition of the biomass, the extracellular polymeric substances (EPS) were extracted according to Arcila and Buitrón [31]. Initially, 30 mL of mixed liquor was centrifuged at 4000 rpm for 20 min. The liquor was vortexed after the supernatant was removed and 15 mL of saline solution (NaCl) was added. The samples were then placed at a temperature of 90 °C for 60 min. After that, each sample was centrifuged at 4000 rpm for an additional 20 min, and then a 1.2 mm membrane was used to filter it. According to Dubois et al. [32] and Lowry et al. [33], after completing the previous stage, the sample was sent for examination of polysaccharides (PS) and proteins (PN).
The lipid content was extracted using 0.5 g of dry biomass from photobioreactors, applied to Soxhlet for 8 h, and extracted using 2:1 methanol:chloroform with continuous extraction, as described in Bligh and Dyer [34]. After extraction, 10 mL of the extract was transferred to a screw-on test tube that had already been weighed on an analytical balance for lipid quantification of the extracted oil. The solvent was evaporated in a water bath at 100 °C until a mass of 5 to 10 mg was obtained. Finally, as defined by Leong et al. [26], the total dry lipid was determined by being calculated by dividing the yield of dry lipid weight after solvent extracted (g) by the yield of dry biomass (g) using gravimetry.
2.4. Analysis of the Microbial Community
Optical microscopy with a camera and software was used to observe the species and morphological structures present in the mixed liquor and during the granule formation process (Nikon Labophot 2 Microscope, Japan & Leica Application Suite “LAS” v4.13, Leica, Germany). Finally, the samples were separated for scanning electron microscopy (SEM) analysis and placed in a glutaraldehyde solution for 24 h at a temperature of roughly 4 °C in the refrigerator. Following that, the samples were washed and dehydrated in a 50%, 60%, 70%, 80%, 90%, and 100% alcohol solution (alcohol:distilled water) for 10 min each. The samples were fixed to a support material and covered with gold by physical spraying under a low vacuum to continue the procedure [35]. The analysis was carried out using a high-performance scanning electron microscope (S-3400N).
3. Results and Discussion
3.1. Monitoring of Control Parameters in Photobioreactors
The temperature in the PBRs ranged from 24 to 26 °C. This parameter is directly related to light incidence and is a key control parameter for the system’s balance, as well as DO concentrations, pH, species dynamics, and the total efficiency of photobioreactors using the microalgae–bacteria consortia. This factor stayed constant during the period of the 14-day experiment, for the fact that microalgae grow best at temperatures around 25 °C. According to Ras et al. [36], high temperatures in microalgae systems can directly damage all of the enzymes in the microalgae cells, as well as generate a thermal stress that primarily affects the proteins and membrane structures.
The initial pH of the systems, after of inoculation of microalgae and aerobic sludge, was in the range of 7.89 ± 0.16 in the six PBRs, as shown in Figure 2. After two days of monitoring, the species started to emerge in the system, and pH increased to 8.92, 8.85, 9.13, 9.23, 9.18, and 9.53 in PBR1, PBR2, PBR3, PBR4, PBR5, and PBR6, respectively. This circumstance indicates that the medium’s photosynthetic activity was increasing, allowing for more organic matter degradation [37]. To avoid nutrient removal mechanisms such as ammonia volatilization and phosphorus (P) precipitation, the pH was maintained with 1M HCl during the operative time. Because of the alkaline pH range, the process of spontaneous autoflocculation of microalgae could occur [38]. On the other hand, some microalgae species are unable to resist acidic conditions [39]. The pH of all photobioreactors was kept close to neutral for these reasons.
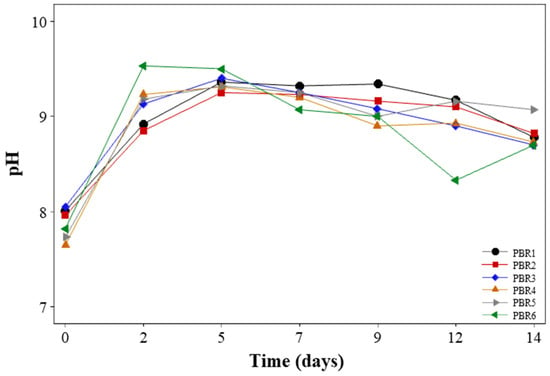
Figure 2.
Variation of pH in photobioreactors during operation.
Higher values of DO were observed in the photobioreactors, with values of 17.06 mg/L, 17.44 mg/L, and 17.73 mg/L for PBR4, PBR5, and PBR6, respectively. These values indicate that the medium has a higher photosynthetic activity, implying that microalgae in PBRs are growing at a faster rate. The high concentration of DO in the system can have a direct impact on biomass productivity as well as macroscopic compositions, including proteins, carbohydrates, and lipids [40]. When DO is above 4 mg/L, it may favor biomass synthesis, whereas when it is below, it may result in an incomplete breakdown of organic molecules [41]. PBR1, PBR2, and PBR3 had the lowest concentration of organic carbon in the medium, as well as the lowest microalgae growth rate and the lowest concentration of suspended particles in the medium; these latter two characteristics will be examined further in later sections.
3.2. Influence on Microalgae–Bacteria Inoculation Ratio on Organic Matter Removal
The performance of organic matter removal was measured in terms of COD, for which average concentration in the paper pulp wastewater was 1010.5 ± 63.30 mg/L. PBR1, PBR2, and PBR3 had the lowest initial inoculation ratio and showed a slower COD degradation, resulting in a progressive treatment performance during the monitoring days. After two days of operation, the COD removal efficiencies (RE) were 22.05%, 13.72%, and 35.74% for PBR1, PBR2, and PBR3, respectively. Given that bacteria and microalgae are involved in the decomposition of organic components, this could be due to a reduced concentration of microalgae and bacteria in the medium.
In two days, the synergistic process between microalgae and bacteria presented COD RE of 53.90%, 55.90%, and 64.29% for PBR4, PBR5, and PBR6, respectively, which had the greater inoculation ratios (Figure 3). Both organic matter oxidation by bacteria and its assimilation by microalgae had occurred simultaneously when these organisms were in symbiosis in a consortium, generating mutualistic transfer of CO2 and DO [42]. In general, until the fifth day of operation, all PBRs performed well in terms of organic matter removal. Afterwards, there was a stability period in terms of carbonaceous material removal, with final COD concentrations in the effluent of PBRs below 390 mg/L. The final concentrations of COD were 356 mg/L, 273 mg/L, 279 mg/L, 200 mg/L, 175 mg/L, and 210 mg/L for PBR1, PBR2, PBR3, PBR4, PBR5, and PBR6, respectively, corresponding to RE of 67.59%, 75.80%, 75.21%, 83.03%, 85.50%, and 82.04%, respectively.
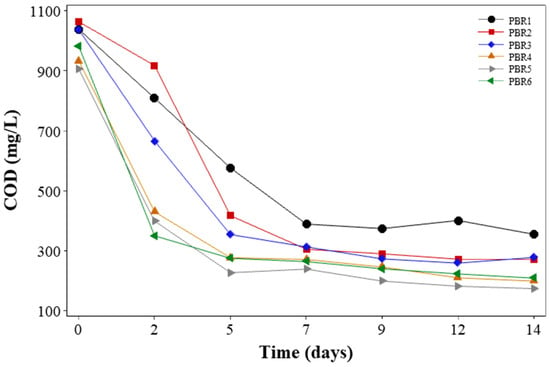
Figure 3.
Variation of soluble COD concentrations in PBR effluents.
In the final of the 14-day operation period, the lowest COD effluent concentration was detected for the PBR5 (initial ratio of 1:5 microalgae–bacteria). This can be explained due to the higher rate of organic carbon oxidation by the bacteria compared with algae as stated by Kampschreur et al. [43]. Usha et al. [17], working with Scenesdesmus sp. and a paper pulp wastewater, with an initial dilution of 60%, obtained a COD RE of 89% after 28 days of operation, but without controlling the ratio of microalgae–bacteria, since bacteria only came from the wastewater.
3.3. Influence of Microalgae–Bacteria Inoculation Ratio on Nitrogen and Phosphorus Removal
The photobioreactors with the highest inoculation concentration, whether of microalgae or bacteria, had the greatest removal of NH4+–N, with average RE around 56%, 86%, and 77%, respectively. This indicates the occurrence of simultaneous nitrification or assimilation of nitrogen (N). It is believed that the loss of N in the system due to volatilization is minimal in systems with the microalgae–bacteria consortium, because there is a natural control of the environment with the operating time, and the synergistic effect between the microalgae and bacteria also allows this form of nitrogenous material to be incorporated into the biomass, preventing the formation of greenhouse gases [44]. Cai et al. [45] also reported that the consumption of NH4+–N is slightly faster during the light cycle, due to the active production of ATP and NADPH when microalgae undergo photosynthesis.
The initial concentration of nitrate (NO3−–N) in the six photobioreactors was low, around 1.98 ± 0.61 mg/L. Accumulation of nitrate in the medium occurred in PBR1 and PBR2 (only with microalgae inoculation and only with bacterial inoculation) with final concentrations of 3.61 mg/L and 2.79 mg/L, respectively. Final nitrate concentrations in the soluble effluent for PBR3 (1:1), PBR4 (1:3), PBR5 (1:5), and PBR6 (3:1) were 1.81 mg/L, 1.17 mg/L, 1.06 mg/L, and 0.86 mg/L, respectively. It is possible that simultaneous nitrification and denitrification occurred in a single granular structure in these systems, during the granulation process with microalgae and bacteria, which coexist with distinct growth rates [46]. Furthermore, Leong et al. [26] verified that the nitrification process carried out by the bacteria in the symbiotic system favored the assimilation of oxidized forms of nitrogen (NO2- NO3-) by the microalgae Chlorella vulgaris.
TN removal in PBRs that used microalgae/bacteria inoculation yielded results of 41.69%, 40.00%, 56.31%, and 46.86% for PBR3, PBR4, PBR5, and PBR6, respectively. The photobioreactor that started with a 1:5 inoculation ratio (PBR5) performed better, which could be due to a better nitrification process in the medium, as previously mentioned, allowing for more TN removal. The low initial concentration of ammoniacal nitrogen in the paper pulp industry effluent may have influenced the lower TN removal values; even in systems with different amounts of microalgae and bacteria, nutrient removal mechanisms may differ [27]. The mechanisms of N removal using a microalgae–bacteria consortia for industrial wastewater treatment need more investigation to clarify all the pathways, since some non-conventional pathways can be installed in specific conditions of pH and DO. Anaerobic ammonia oxidation, algae nitrification, heterotrophic nitrification, autotrophic denitrifies, and chemolithoautotrophics able to nitrify and denitrify in the presence and absence of oxygen can have an important role in N removal [47,48].
Regarding the performance in the removal of P (PO43−–P) in the photobioreactors, a reduction of 43.32%, 64.99%, 77.38%, 82.15%, 85.83%, and 93.49% for PBR1, PBR2, PBR3, PBR4, PBR5, and PBR6, respectively, was observed at end of the experiments. The PBR6 performed better in the removal of PO43−–P, which could be due to the increased microalgae concentration in the medium. The final concentration value for PO43−–P observed for PBR5 and PBR6 was around 0.93 mg/L and 0.32 mg/L, respectively, indicating that a higher concentration of microalgae/bacteria inoculation allows for better P assimilation and incorporation in the biomass. The lower removal values were observed in PBR1 and PBR2, with inoculation ratio of 1:0 and 0:1 microalgae:bacteria, and, taking into account the observations of Leong et al. [26], these results can be related to the low symbiosis between microalgae and bacteria for P removal by the two pathways.
Thus, the P concentration was two to three times greater than that of total N, but PBRs with the highest inoculation ratio showed that P removal was above 80%. Su et al. [27], using domestic wastewater treatment and similar photobioreactor operation for 14 days, reported P removal efficiency of 54% for PBR with only microalgae inoculation and 10.6% for PBR with only activated sludge inoculation. The authors hypothesize that the inhibition of light between algal cells in the first condition resulted in limited autotrophic growth in the medium. The process of growth and photosynthesis of the microalgae was also affected without the additional supply of CO2 provided by the bacteria (sludge). The low aggregation process of bacteria with microalgae, which may also suggest the aerobic sludge’s low P removal capacity, may have influenced PBR with bacteria only. Additionally, the absence of anaerobic and anoxic phases in this system allows for P-accumulating organisms to remove the waste (PAOs) [49,50].
Table 2 shows RE for N and P for different studies in photobioreactors. Gentili [14] and Tao et al. [51], using the species Scenedesmus sp. and Chlorella vulgaris, obtained removals of NH4−–N and PO43−–P above 90%. In contrast, Ahmad et al. [52] and Liu et al. [53], treating domestic wastewater with microalgae inoculation, reported NH4−–N RE between 94% and 98%, but a low P removal. Lin et al. [54], using a 1:3 algae/bacteria ratio for treating industrial dyes wastewater, with an initial VSS concentration in the mixed liquor of 3000 mg/L, achieved an 83% RE for NH4−–N, but only around 30.2% for P removal. It is worth noting that the initial NH4−–N concentration was 20 mg/L to 30 mg/L and the P concentration was 5 mg/L to 6.5 mg/L.

Table 2.
Comparison of NH4−–N and PO43−–P removal efficiencies in photobioreactors with various types of wastewaters.
3.4. Influence of the Microalgae–Bacteria Inoculation Ratio on the Growth and Formation of Biomass
Figure 4 shows a high variation in VSS concentration for the different PBRs, which is due to different inoculation ratios that impacted on the system’s starting values. Biomass produced in photobioreactors may contain a mixture of microalgae, bacteria, and other debris. PBR1 and PBR2 started with the lowest biomass concentration (445.98 ± 15.57 mg/L) associated only with microalgae inoculation and aerobic sludge inoculation, respectively, but these values also include a VSS fraction that came from the paper pulp wastewater. PBR1 showed lower biomass growth due to the lack of bacteria inoculation, despite the fact that this system start-up strategy provides a greater synergistic process and biomass growth in the system.
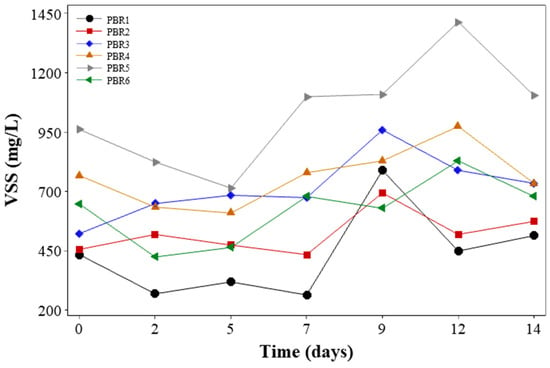
Figure 4.
Variation of VSS concentration throughout the experimental period.
During the 14 days of operation, the mean VSS concentration in PBR1, PBR2, PBR3, PBR4, PBR5, and PBR6 was 434.97 ± 104.99 mg/L, 520.00 ± 88.20 mg/L, 685.00 ± 135.01 mg/L, 768.11 ± 122.69 mg/L, 1100.00 ± 227.65 mg/L, and 648.37 ± 137.97 mg/L mg/L, respectively. The biomass seems to stabilize around the sixth day of operation, and afterwards, there was no significant variation in the PBRs medium. As discussed by Zhao et al. [55], this circumstance can be explained due to the good formation of the microalgae–bacteria consortium. As the biomass increases in size and becomes increasingly dense and compact after formation and structuring, biomass loss from variations in control parameters and substrate concentrations is low.
According to Liu et al. [56], in continuous feed systems, better maturation and granular density can only be detected after the 30th day of operation. In addition, biological aggregates are considered predominantly granular when at least 50% of the particles present in the systems are larger than 0.20 mm [57], enabling a better understanding of this process of biomass generation. Liu et al. [53] and Zhao et al. [55] applied variations in aeration and lighting to photobioreactors in sequential batches, and obtained dense granules with a high abundance of microalgae from the seventh day of operation. The photogranules maturation conditions aided in determining the critical factors for growing the granular consortium. As the photobioreactors were only used for 14 days and with a single batch, the results obtained are only indicative of how the symbiotic process of microalgae and bacteria occurs in the treatment of industrial paper pulp wastewater. Bacterial algal biomass is a consortium of photoautotrophs, chemoautotrophs, and heterotrophs that is stable and unified inside an EPS matrix [58] and can be classified as a spectrum of biogranules with applications in environmental engineering [59].
Another important factor for evaluating the formation of biomass is the chlorophyll content present in the biomass, helping in the evaluation of biomass productivity in photobioreactors. This factor is also important for the evaluation of satisfactory values of the microalgae:bacteria ratio used in the inoculation for starting the system. For a better understanding of the behavior of the systems, the total chlorophyll (chlorophyll-a and chlorophyll-b) was extracted from the samples, and their concentrations were calculated through Equations (1) and (2). Results are shown in Figure 5. This parameter allows for examining the degree of pigment production in microalgae [60]. For PBR1, PBR2, PBR3, PBR4, PBR5, and PBR6, the final mean values obtained from the sum of chlorophyll-a and chlorophyll-b were 0.67 ± 0.11 mg/L, 0.31 ± 0.03 mg/L, 0.59 ± 0.04 mg/L, 0.43 ± 0.01 mg/L, 0.54 ± 0.06 mg/L, and 0.69 ± 0.13 mg/L.
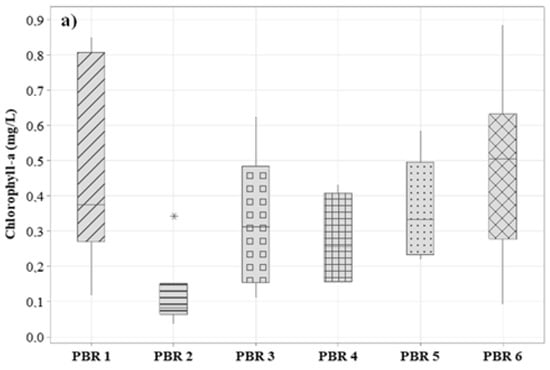
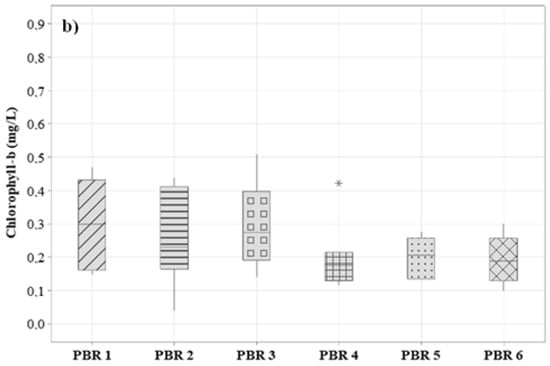
Figure 5.
Variation of the concentration of (a) chlorophyll-a and (b) chlorophyll-b during the operational period.
The highest concentration of total chlorophyll was found in PBR1 and PBR6, where a higher concentration of microalgae was inoculated, which may have contributed to the greater development of these microorganisms in the medium. PBR3, PBR4, and PBR5 with bacteria and microalgae inoculation had chlorophyll (a + b) values around 0.50 mg/L, indicating that even with a high concentration of suspended solids, there was good algal biomass productivity. The lowest total chlorophyll productivity was observed in PBR2 with an average of 0.30 mg/L, which is related to the high concentration of suspended solids in the water column, which hinders light penetration and thus limits the growth of photoautotrophic organisms. It is also worth noting that there was no initial inoculation of microalgae in PBR2.
Flocculation efficiencies (FE) were calculated trough Equation (3) for the biomass generated in the photobioreactors. The average values obtained during the 14 days were 55.65 ± 7.40%, 82.49 ± 9.70%, 82.06 ± 8.95%, 94.23 ± 2.92%, 94.89 ± 1.57%, and 91.15 ± 3.30% for PBR1, PBR2, PBR3, PBR4, PBR5, and PBR6, respectively. PBR4, PBR5, and PBR6 worked with a higher concentration and inoculation ratio of microalgae:bacteria, respectively, 1:3, 1:5, and 3:1, having presented a FE above 90% (i.e., a high biomass sedimentation capacity). This may indicate a more stable microalgae–bacteria consortium development with good bioflocculation, producing a biomass with greater potential as well as efficiency of the possibility of harvesting for resource recovery [27]. In similar work, Leong et al. [26] reported a FE of 42.37 ± 0.25%, which is lower than the values found in this study, but for domestic wastewater treatment and an inoculation ratio of 1:0.75.
The PBR1, with only microalgae inoculation (Scenedesmus sp.), produced a sinuous behavior with a lack of linearity in the biomass generated. As a result, the sedimentation process was impacted by cell wall properties as well as aspects related to the microscopic nature of microalgae. In this way, the satisfactory flocculation and sedimentation of biomass using the microalgae–bacteria consortium can be attributed to the start of the system with aerobic sludge inoculation. The good sedimentability of these reactors is typical of the aerobic processes of activated sludge, which, in turn, present more stable microbial aggregates allowing larger and denser sizes of granules.
3.5. Assessment of the Biomass Generated in Photogranules and Its Composition
It is known that the structure of the microalgae–bacteria consortium that is developed in photobioreactors is associated with extracellular polymeric substances (EPS) [61], relatively composed of carbohydrates (polysaccharides), proteins, and lipids. The evaluation of this EPS and its fractions is important because they can act as a “glue” for photogranule cell adhesion and their fractions can affect biomass viscosity [62]. Table 3 shows the values and behavior of the biomass found at the end of the photobioreactors operation.

Table 3.
Characterization of proteins (EPS-PN) and polysaccharides (EPS-PS) present in the biomass.
The protein content in PBR3, PBR4, and PBR5 was 44.50 ± 4.18 mg/gVSS, 49.90 ± 2.06 a mg/gVSS, and 49.05 ± 21.18 mg/gVSS, indicating that biomass has a higher stabilization capacity and that the microalgae–bacteria consortium is mature and structured, as also noted [55]. According to Barreiro-Vescovo et al. [63], the protein content extracted from the biomass with microalgae could be used to produce animal feed and food supplements.
Carbohydrate concentrations (EPS-PS) were higher in PBR1 and PBR2, which only used microalgae inoculation or bacterial inoculation, respectively. Concentrations in PBR4, PBR5, and PBR6 were 9.21 ± 1.66 mg/gVSS, 8.16 ± 0.52 mg/gVSS, and 12.65 ± 0.37 mg/gVSS, respectively. Carbohydrate concentrations vary with operating conditions and microalgae species present in the medium [64]. PBR4 and PBR5 were inoculated with 100 mg/L of VSS from the microalgae Scenedesmus sp., and the polysaccharide values found in these PBRs are similar to the values found by Valdez et al. [65], of an algal biomass composed mainly of Scenedesmus sp.
The oil content values for the PBR1, PBR2, PBR3, PBR4, PBR5, and PBR6 were 19.88 ± 0.96%, 1.76 ± 0.83%, 13.11 ± 0.74%, 7.40 ± 0.45%, 18.69 ± 0.15%, and 22.61 ± 1.01% at the end of system operation. Thus, the photobioreactors with the best lipid production performance were PBR1, PBR5, and PBR6, which may be related to a higher final concentration of microalgae in the mixed liquor. The rapid doubling time of microalgae aids in the production and storage of saturated neutral lipids, resulting in a higher biomass yield for biofuel production [66]. PBR2 had the lowest oil content, which can be attributed to the system’s initial inoculation with a 0:100 ratio (microalgae:bacteria). The oil content at the end of the batches may be related to the nutritional stress that the species went through during this feeding regime, since organic load did not reach the medium. This fact can be associated with the growth rate of organisms, which increased the cell density until nutrient depletion occurs, as noted in the experiments of Chen et al. [67]. Then, as the growth rate slows, increased storage of high-energy compounds, such as lipids, is possible due to changes in metabolic processes.
3.6. Microalgae and Bacteria Synergistic Process Behavior
Figure 6 shows microscopic photos of the algae and bacteria interaction at 40× and 10× magnification at the end of the 14-day system monitoring. PBR1, which exclusively used microalgae inoculation, was able to keep the initial Scenedesmus sp. culture alive. This may mean that the paper pulp wastewater is not toxic for this microalga. PBR2 appears to have grown by the photoautotrophic route of microalgae, primarily filamentous cyanobacteria such as Planktothrix sp., even though the system began with only bacteria present in the aerobic sludge.
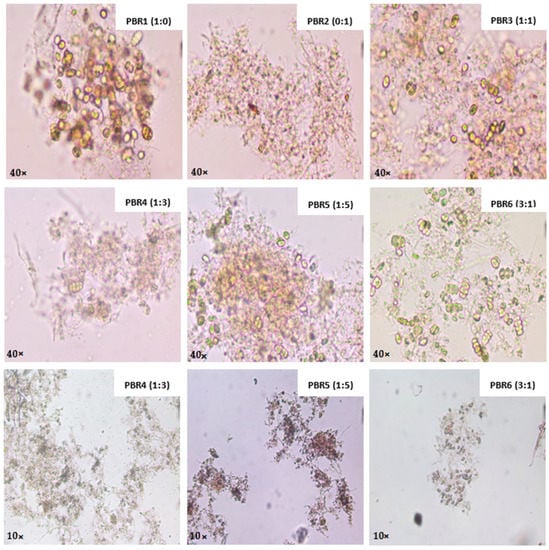
Figure 6.
Microalgae–bacteria consortia in photobioreactors after 14 days of operation.
As previously mentioned, a good process of bioaglutination was observed in the biomass of PBR3, PBR4, PBR5, and PBR6, which worked with inoculation of microalgae and bacteria [51], with the presence of morphological structures of cyanobacteria, such as Planktothrix sp., and filamentous microalgae, such as Stigeoclonium sp., having been observed. Finally, in PBR4 and PBR5, the biomass was found to be more stable, with more aggregated structures, as illustrated in the photographs with 10× magnification. There are still research gaps and challenges for better understanding the relation between algae and bacteria, such as identification of the best-performing algal strains and bacteria species, optimization of control parameters, overcoming seasonal variations of the effluent quality, and biomass harvesting, among others [68].
This development feature would mean the start of maturation in the photogranules, noted after scanning electron microscopy (SEM) study of floccular structures and filamentous bacteria in the biomass (Figure 7). The dominance of green microalgae in the nucleus of the granular structure was detected, as also found in the study of Arcila and Buitrón [31]. EPS excreted during in the symbiotic phase also aid in the production of granules [27]; thus, the pH control could also have influenced the good formation of photogranules, since high pH could have reduced microalgae and bacteria’s aggregation performance. Finally, fibers found in the effluent of wastewater from the paper pulp sector may have aided the photogranules structure and stability.
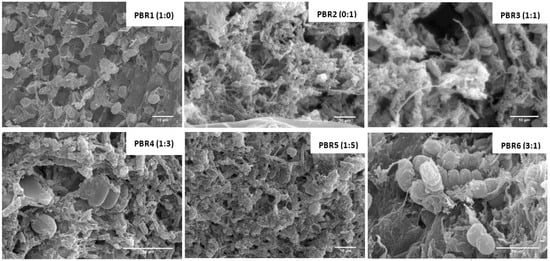
Figure 7.
The structuring process of the photogranules in photobioreactors.
4. Conclusions
The results of this research show that the symbiotic process between microalgae and bacteria was present in treatment of paper pulp wastewater, allowing good efficiency in the removal of organic matter and nutrients while increasing biomass harvesting and producing sedimentable photogranules. The PBR5 (1:5 microalgae:bacteria ratio) showed a better RE (85.5% COD, 86% NH4, and 56.31% TN), which may be related to the higher concentration of bacteria that contribute to the rapid degradation of these compounds. The PBR6 (3:1 microalgae:bacteria ratio) showed better P removal (85.83%), indicating good absorption and assimilation in the biomass by the highest concentration of microalgae inoculation. The PBR4, PBR5, and PBR6 showed flocculation efficiencies greater than 91%, indicating more structured and stabilized photogranules. The PBR4 and PBR5 (1:3 and 1:5 microalgae:bacteria ratio, respectively) showed a greater possibility of harvesting the biomass to generate bioproducts with added value, as evidenced by good biomass growth, good sedimentability, higher concentration of protein content, good level of lipid generation, and also allied to a good performance of wastewater treatment.
Author Contributions
Conceptualization, J.S.; methodology, J.S. and A.C.; formal analysis, J.S.; experiment, J.S. and A.C.; microscope analysis, J.S. and A.P.G.; investigation, J.S.; visualization, J.S., A.C., A.P.G., R.S. and A.A.; writing—original draft, J.S.; writing—review and editing, J.S., R.S. and A.A.; resources, R.S. and A.A.; project administration, R.S. and A.A.; supervision, R.S. and A.A. All authors have read and agreed to the published version of the manuscript.
Funding
Josivaldo Satiro received a PhD incentive grant from Santander Totta Bank 2021-UBI with a total value of 6.000€.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to the Foundation for Science and Technology (FCT, Portugal) for financial support by national funds through the projects UIDB/00195/2020 (FibEnTech) and UIDB/04035/2020 (GeoBioTec).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chan, Y.J.; Chong, M.F.; Law, C.L.; Hassell, D. A review on anaerobic-aerobic treatment of industrial and municipal wastewater. Chem. Eng. J. 2009, 155, 1–18. [Google Scholar] [CrossRef]
- Gallego-Schmid, A.; Tarpani, R.R.Z. Life cycle assessment of wastewater treatment in developing countries: A review. Water Res. 2019, 153, 63–79. [Google Scholar] [CrossRef] [Green Version]
- Goli, A.; Shamiri, A.; Khosroyar, S.; Talaiekhozani, A.; Sanaye, R.; Azizi, K. A review on different aerobic and anaerobic treatment methods in dairy industry wastewater. J. Environ. Treat. Tech. 2019, 6, 113–141. [Google Scholar]
- Eilertsen, H.C.; Eriksen, G.K.; Bergum, J.-S.; Strømholt, J.; Elvevoll, E.; Eilertsen, K.-E.; Heimstad, E.S.; Giæver, I.H.; Israelsen, L.; Svenning, J.B.; et al. Mass Cultivation of Microalgae: I. Experiences with Vertical Column Airlift Photobioreactors, Diatoms and CO2 Sequestration. Appl. Sci. 2022, 12, 3082. [Google Scholar] [CrossRef]
- De Godos, I.; Vargas, V.A.; Blanco, S.; González, M.C.G.; Soto, R.; Garcia-Encina, P.A.; Becares, E.; Muñoz, R. A comparative evaluation of microalgae for the degradation of piggery wastewater under photosynthetic oxygenation. Bioresour. Technol. 2010, 101, 5150–5158. [Google Scholar] [CrossRef] [PubMed]
- Ummalyma, S.B.; Gnansounou, E.; Sukumaran, R.K.; Sindhu, R.; Pandey, A.; Sahoo, D. Bioflocculation: An alternative strategy for harvesting of microalgae—An overview. Bioresour. Technol. 2017, 242, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Posadas, E.; Muñoz, R.; Guieysse, B. Integrating nutrient removal and solid management restricts the feasibility of algal biofuel generation via wastewater treatment. Algal Res. 2017, 22, 39–46. [Google Scholar] [CrossRef]
- Chandra, R.; Iqbal, H.M.N.; Vishal, G.; Lee, H.-S.; Nagra, S. Algal biorefinery: A sustainable approach to valorize algal-based biomass towards multiple product recovery. Bioresour. Technol. 2019, 278, 346–359. [Google Scholar] [CrossRef]
- Viswanaathan, S.; Perumal, P.K.; Sundaram, S. Integrated Approach for Carbon Sequestration and Wastewater Treatment Using Algal-Bacterial Consortia: Opportunities and Challenges. Sustainability 2022, 14, 1075. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Park, J.; Ralph, P.J.; Craggs, R.J. Improved microalgal productivity and nutrient removal through operating wastewater high rate algal ponds in series. Algal Res. 2019, 47, 101850. [Google Scholar] [CrossRef]
- Hende, S.V.D.; Carré, E.; Cocaud, E.; Beelen, V.; Boon, N.; Vervaeren, H. Bioresource Technology Treatment of industrial wastewaters by microalgal bacterial flocs in sequencing batch reactors. Bioresour. Technol. 2014, 161, 245–254. [Google Scholar] [CrossRef]
- Lang, X.; Li, Q.; Xu, Y.; Ji, M.; Yan, G.; Guo, S. Aerobic denitrifiers with petroleum metabolizing ability isolated from caprolactam sewage treatment pool. Bioresour. Technol. 2019, 290, 121719. [Google Scholar] [CrossRef]
- Show, K.-Y.; Lee, D.-J. Anaerobic Treatment. In Current Developments in Biotechnology and Bioengineering. Biological Treatment of Industrial Effluents; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 8; pp. 205–230. [Google Scholar] [CrossRef]
- Gentili, F.G. Microalgal biomass and lipid production in mixed municipal, dairy, pulp and paper wastewater together with added flue gases. Bioresour. Technol. 2014, 169, 27–32. [Google Scholar] [CrossRef]
- Porto, B.; Gonçalves, A.L.; Esteves, A.F.; De Souza, S.M.A.G.U.; De Souza, A.A.U.; Vilar, V.J.P.; Pires, J.C.M. Microalgal growth in paper industry effluent: Coupling biomass production with nutrients removal. Appl. Sci. 2020, 10, 3009. [Google Scholar] [CrossRef]
- Silva, M.; Gonçalves, A.; Vilar, V.; Pires, J. Experimental and techno-economic study on the use of microalgae for paper industry effluents remediation. Sustainability 2021, 13, 1314. [Google Scholar] [CrossRef]
- Usha, M.T.; Sarat Chandra, T.; Sarada, R.; Chauhan, V.S. Removal of nutrients and organic pollution load from pulp and paper mill effluent by microalgae in outdoor open pond. Bioresour. Technol. 2016, 214, 856–860. [Google Scholar] [CrossRef]
- Gallardo-Altamirano, M.; Maza-Márquez, P.; Montemurro, N.; Rodelas, B.; Osorio, F.; Pozo, C. Linking microbial diversity and population dynamics to the removal efficiency of pharmaceutically active compounds (PhACs) in an anaerobic/anoxic/aerobic (A2O) system. Chemosphere 2019, 233, 828–842. [Google Scholar] [CrossRef]
- Xiong, J.-Q.; Kurade, M.B.; Abou-Shanab, R.A.I.; Ji, M.-K.; Choi, J.; Kim, J.O.; Jeon, B.-H. Biodegradation of carbamazepine using freshwater microalgae Chlamydomonas mexicana and Scenedesmus obliquus and the determination of its metabolic fate. Bioresour. Technol. 2016, 205, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, S.; Richards, R.; McGinn, P. Screening of two freshwater green microalgae in pulp and paper mill wastewater effluents in Nova Scotia, Canada. Water Sci. Technol. 2021, 83, 1483–1498. [Google Scholar] [CrossRef]
- Chandra, R.; Rohit, M.; Swamy, Y.; Mohan, S.V. Regulatory function of organic carbon supplementation on biodiesel production during growth and nutrient stress phases of mixotrophic microalgae cultivation. Bioresour. Technol. 2014, 165, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, B.; Chen, W.-Y.; Raman, A.A.A.; Ibrahim, S. Microalgae lipid and biomass for biofuel production: A comprehensive review on lipid enhancement strategies and their effects on fatty acid composition. Renew. Sustain. Energy Rev. 2018, 97, 200–232. [Google Scholar] [CrossRef]
- Mohan, S.V.; Rohit, M.; Chiranjeevi, P.; Chandra, R.; Navaneeth, B. Heterotrophic microalgae cultivation to synergize biodiesel production with waste remediation: Progress and perspectives. Bioresour. Technol. 2015, 184, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, O.; Yerushalmi, L.; Haghighat, F. Wastewater treatment in the pulp-and-paper industry: A review of treatment processes and the associated greenhouse gas emission. J. Environ. Manag. 2015, 158, 146–157. [Google Scholar] [CrossRef]
- Boguniewicz-Zablocka, J.; Klosok-Bazan, I.; Naddeo, V.; Mozejko, C.A. Cost-effective removal of COD in the pre-treatment of wastewater from the paper industry. Water Sci. Technol. 2020, 81, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Leong, W.-H.; Lim, J.-W.; Lam, M.K.; Uemura, Y.; Ho, C.-D.; Ho, Y.-C. Co-cultivation of activated sludge and microalgae for the simultaneous enhancements of nitrogen-rich wastewater bioremediation and lipid production. J. Taiwan Inst. Chem. Eng. 2018, 87, 216–224. [Google Scholar] [CrossRef]
- Su, Y.; Mennerich, A.; Urban, B. Synergistic cooperation between wastewater-born algae and activated sludge for wastewater treatment: Influence of algae and sludge inoculation ratios. Bioresour. Technol. 2012, 105, 67–73. [Google Scholar] [CrossRef] [PubMed]
- APHA; AWWA; WPCF. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA; American Water Works Association: Denver, CO, USA; Water Environment Federation: Alexandria, VA, USA, 2017. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; Humphrey, G.F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Kumar, S.S.; Saramma, A.V. A revised method for pigment extraction from marine nannoplanktonic algal cultures. J. Biomass Util. 2013, 4, 47–52. [Google Scholar]
- Arcila, J.S.; Buitrón, G. Influence of solar irradiance levels on the formation of microalgae-bacteria aggregates for municipal wastewater treatment. Algal Res. 2017, 27, 190–197. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method Of Total Lipid Extraction And Purification. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar]
- Talbot, M.J.; White, R.G. Methanol fixation of plant tissue for Scanning Electron Microscopy improves preservation of tissue morphology and dimensions. Plant Methods 2013, 9, 36. [Google Scholar] [CrossRef] [Green Version]
- Ras, M.; Steyer, J.-P.; Bernard, O. Temperature effect on microalgae: A crucial factor for outdoor production. Rev. Environ. Sci. Biotechnol. 2015, 12, 153–164. [Google Scholar] [CrossRef] [Green Version]
- García, J.; Green, B.; Lundquist, T.; Mujeriego, R.; Hernández-Mariné, M.; Oswald, W. Long term diurnal variations in contaminant removal in high rate ponds treating urban wastewater. Bioresour. Technol. 2006, 97, 1709–1715. [Google Scholar] [CrossRef]
- Young, P.; Phasey, J.; Wallis, I.; Vandamme, D.; Fallowfield, H. Autoflocculation of microalgae, via magnesium hydroxide precipitation, in a high rate algal pond treating municipal wastewater in the South Australian Riverland. Algal Res. 2021, 59, 102418. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T. Renewable and sustainable bioenergies production from palm oil mill effluent (POME): Win-win strategies toward better environmental protection. Biotechnol. Adv. 2011, 29, 124–141. [Google Scholar] [CrossRef]
- Uggetti, E.; Sialve, B.; Hamelin, J.; Bonnafous, A.; Steyer, J.-P. CO2 addition to increase biomass production and control microalgae species in high rate algal ponds treating wastewater. J. CO2 Util. 2018, 28, 292–298. [Google Scholar] [CrossRef]
- Tang, C.-C.; Zuo, W.; Tian, Y.; Sun, N.; Wang, Z.-W.; Zhang, J. Effect of aeration rate on performance and stability of algal-bacterial symbiosis system to treat domestic wastewater in sequencing batch reactors. Bioresour. Technol. 2016, 222, 156–164. [Google Scholar] [CrossRef]
- Coggins, L.X.; Larma, I.; Hinchliffe, A.; Props, R.; Ghadouani, A. Flow cytometry for rapid characterisation of microbial community dynamics in waste stabilisation ponds. Water Res. 2020, 169, 115243. [Google Scholar] [CrossRef]
- Kampschreur, M.J.; Temmink, H.; Kleerebezem, R.; Jetten, M.S.; van Loosdrecht, M. Nitrous oxide emission during wastewater treatment. Water Res. 2009, 43, 4093–4103. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, S.; Modin, O.; Mijakovic, I. Technologies for biological removal and recovery of nitrogen from wastewater. Biotechnol. Adv. 2020, 43, 107570. [Google Scholar] [CrossRef]
- Cai, W.; Zhao, Z.; Li, D.; Lei, Z.; Zhang, Z.; Lee, D.-J. Algae granulation for nutrients uptake and algae harvesting during wastewater treatment. Chemosphere 2019, 214, 55–59. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Lei, Z. Microalgal-bacterial aggregates for wastewater treatment: A mini-review. Bioresour. Technol. Rep. 2019, 8, 100–199. [Google Scholar] [CrossRef]
- Albuquerque, A.; Oliveira, J.; Semitela, S.; Amaral, L. Influence of bed media characteristics on ammonia and nitrate removal in shallow horizontal subsurface flow constructed wetlands. Bioresour. Technol. 2009, 100, 6269–6277. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, X.; Zhou, X.; Chen, C. Recent Advances in Autotrophic Biological Nitrogen Removal. Water 2022, 14, 1101. [Google Scholar] [CrossRef]
- Wang, L.; Zhan, H.; Wang, Q.; Wu, G.; Cui, D. Enhanced aerobic granulation by inoculating dewatered activated sludge under short settling time in a sequencing batch reactor. Bioresour. Technol. 2019, 286, 121386. [Google Scholar] [CrossRef]
- Oehmen, A.; Lemos, P.C.; Carvalho, G.; Yuan, Z.; Keller, J.; Blackall, L.L.; Reis, M.A. Advances in enhanced biological phosphorus removal: From micro to macro scale. Water Res. 2007, 41, 2271–2300. [Google Scholar] [CrossRef]
- Ahmad, J.S.M.; Cai, W.; Zhao, Z.; Zhang, Z.; Shimizu, K.; Lei, Z.; Lee, D.-J. Stability of algal-bacterial granules in continuous-flow reactors to treat varying strength domestic wastewater. Bioresour. Technol. 2017, 244, 225–233. [Google Scholar] [CrossRef]
- Liu, L.; Zeng, Z.; Bee, M.; Gibson, V.; Wei, L.; Huang, X.; Liu, C. Characteristics and performance of aerobic algae-bacteria granular consortia in a photo-sequencing batch reactor. J. Hazard. Mater. 2018, 349, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Cao, P.; Xu, X.; Ye, B. Algal-bacterial symbiosis system treating high-load printing and dyeing wastewater in continuous-flow reactors under natural light. Water 2019, 11, 469. [Google Scholar] [CrossRef] [Green Version]
- Tao, R.; Kinnunen, V.; Praveenkumar, R.; Lakaniemi, A.M.; Rintala, J.A. Comparison of Scenedesmus acuminatus and Chlorella vulgaris cultivation in liquid digestates from anaerobic digestion of pulp and paper industry and municipal wastewater treatment sludge. J. Appl. Phycol. 2017, 29, 2845–2856. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, X.; Cai, W.; Lei, Z.; Shimizu, K.; Zhang, Z.; Utsumi, M.; Lee, D.-J. Response of algal-bacterial granular system to low carbon wastewater: Focus on granular stability, nutrients removal and accumulation. Bioresour. Technol. 2018, 268, 221–229. [Google Scholar] [CrossRef]
- Liu, L.; Fan, H.; Liu, Y.; Liu, C.; Huang, X. Development of algae-bacteria granular consortia in photo-sequencing batch reactor. Bioresour. Technol. 2017, 232, 64–71. [Google Scholar] [CrossRef]
- Daudt, G.C.; Xavier, J.A.; Meotti, B.; Guimarães, L.B.; da Costa, R.H.R. Researching new ways to reduce N2O emission from a granular sludge sequencing batch reactor treating domestic wastewater under subtropical climate conditions. Braz. J. Chem. Eng. 2019, 36, 209–220. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Wu, Y.; Esquivel-Elizondo, S.; Dolfing, J.; Rittmann, B.E. Using Microbial Aggregates to Entrap Aqueous Phosphorus. Trends Biotechnol. 2020, 38, 1292–1303. [Google Scholar] [CrossRef]
- Milferstedt, K.; Hamelin, J.; Park, C.; Jung, J.; Hwang, Y.; Cho, S.-K.; Jung, K.-W.; Kim, D.-H. Biogranules applied in environmental engineering. Int. J. Hydrogen Energy 2017, 42, 27801–27811. [Google Scholar] [CrossRef]
- Sanchini, A.; Grosjean, M. Quantification of chlorophyll a, chlorophyll b and pheopigments a in lake sediments through deconvolution of bulk UV–VIS absorption spectra. J. Paleolimnol. 2020, 64, 243–256. [Google Scholar] [CrossRef]
- Arcila, J.S.; Buitrón, G. Microalgae-bacteria aggregates: Effect of the hydraulic retention time on the municipal wastewater treatment, biomass settleability and methane potential. J. Chem. Technol. Biotechnol. 2016, 91, 2862–2870. [Google Scholar] [CrossRef]
- Quijano, G.; Arcila, J.S.; Buitrón, G. Microalgal-bacterial aggregates: Applications and perspectives for wastewater treatment. Biotechnol. Adv. 2017, 35, 772–781. [Google Scholar] [CrossRef]
- Barreiro-Vescovo, S.; González-Fernández, C.; Ballesteros, M.; de Godos, I. Activity determination of an algal-bacterial consortium developed during wastewater treatment based on oxygen evolution. J. Water Process Eng. 2020, 36, 101278. [Google Scholar] [CrossRef]
- Biller, P.; Ross, A. Potential yields and properties of oil from the hydrothermal liquefaction of microalgae with different biochemical content. Bioresour. Technol. 2011, 102, 215–225. [Google Scholar] [CrossRef]
- Valdez, P.J.; Tocco, V.J.; Savage, P.E. A general kinetic model for the hydrothermal liquefaction of microalgae. Bioresour. Technol. 2014, 163, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Salian, K.; Strezov, V. Biofuels From Microalgae. In Encyclopedia of Sustainable Technologies; Elsevier: Amsterdam, The Netherlands, 2017; Volume 3; pp. 107–120. [Google Scholar] [CrossRef]
- Chen, C.Y.; Yeh, K.L.; Aisyah, R.; Lee, D.J.; Chang, J.S. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: A critical review. Bioresour. Technol. 2011, 102, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Valchev, D.; Ribarova, I. A Review on the Reliability and the Readiness Level of Microalgae-Based Nutrient Recovery Technologies for Secondary Treated Effluent in Municipal Wastewater Treatment Plants. Processes 2022, 10, 399. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).