Photoreactor-Initiated Acetaldehyde Conversion Rate of a TiO2-Surface-Treated Alumina Photocatalyst Prepared Using the Sol–Gel Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Alumina Substrate Preparation
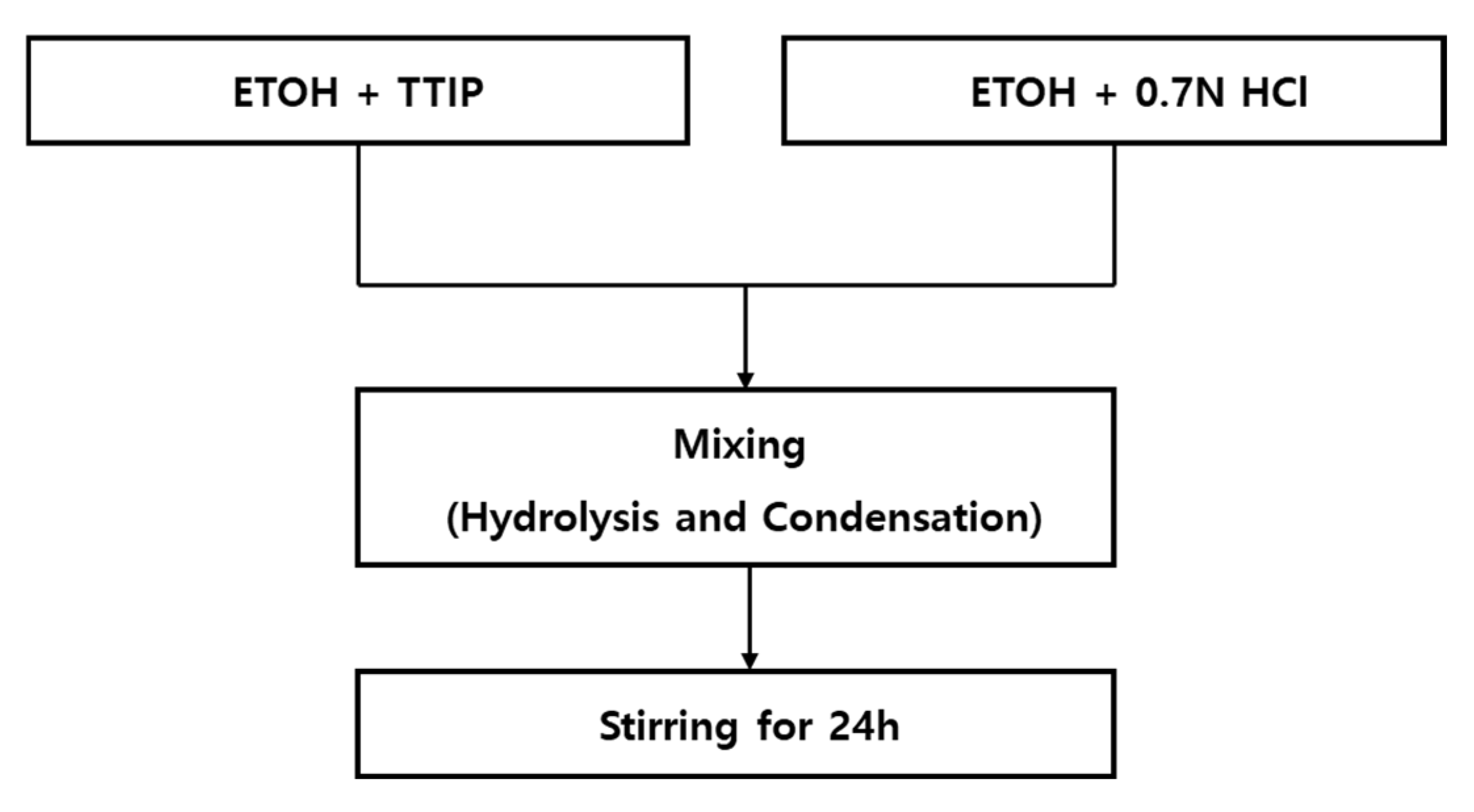
2.3. Preparation of the TiO2 Photocatalyst Using the Sol–Gel Method
2.4. Photoreactor Manufacturing and the TiO2–Alumina Catalyst Immobilization
2.5. Acetaldehyde Degradation Experiments
2.6. Conversion(X) of Acetaldehyde
2.7. Characterization
3. Results and Discussion
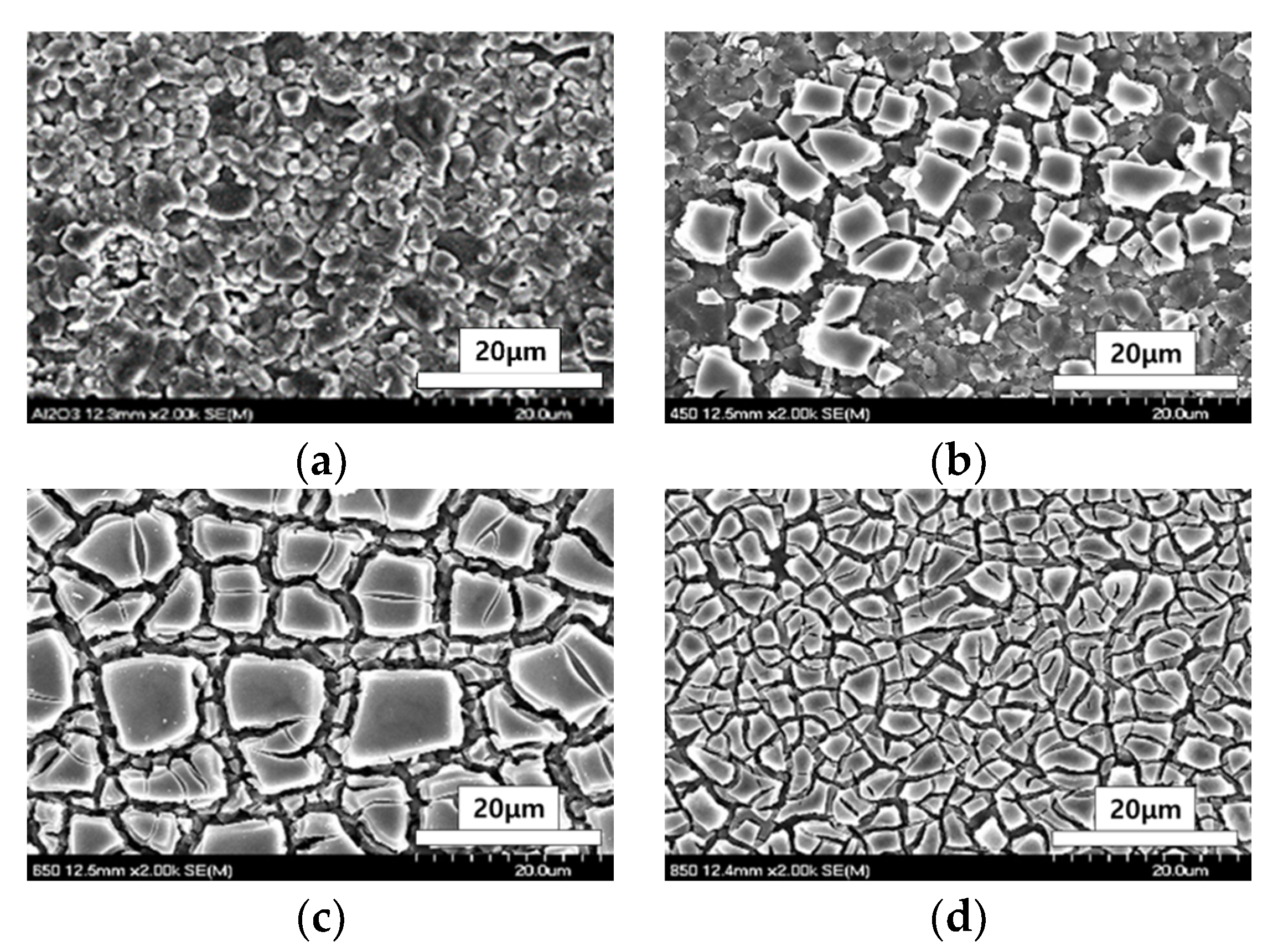
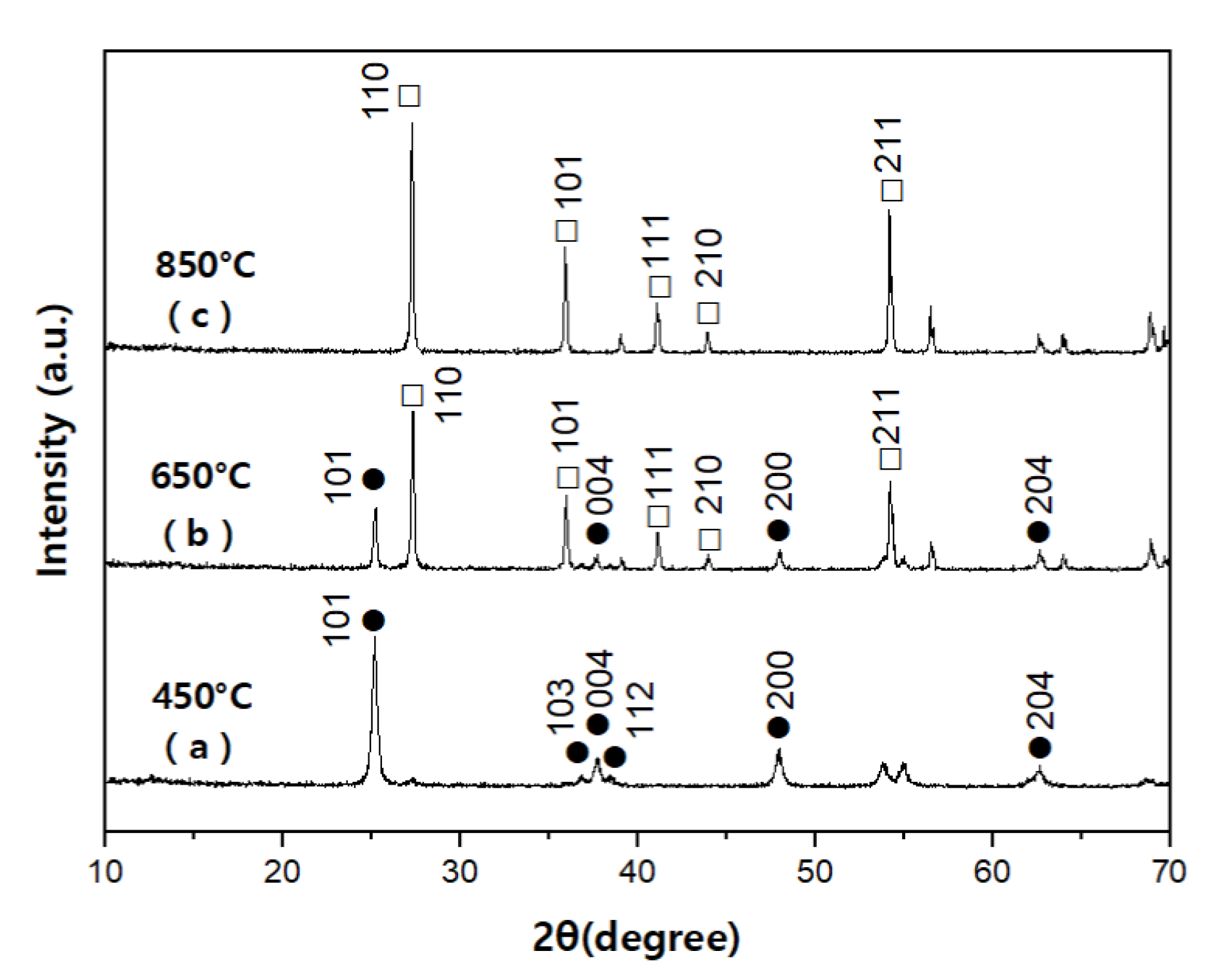
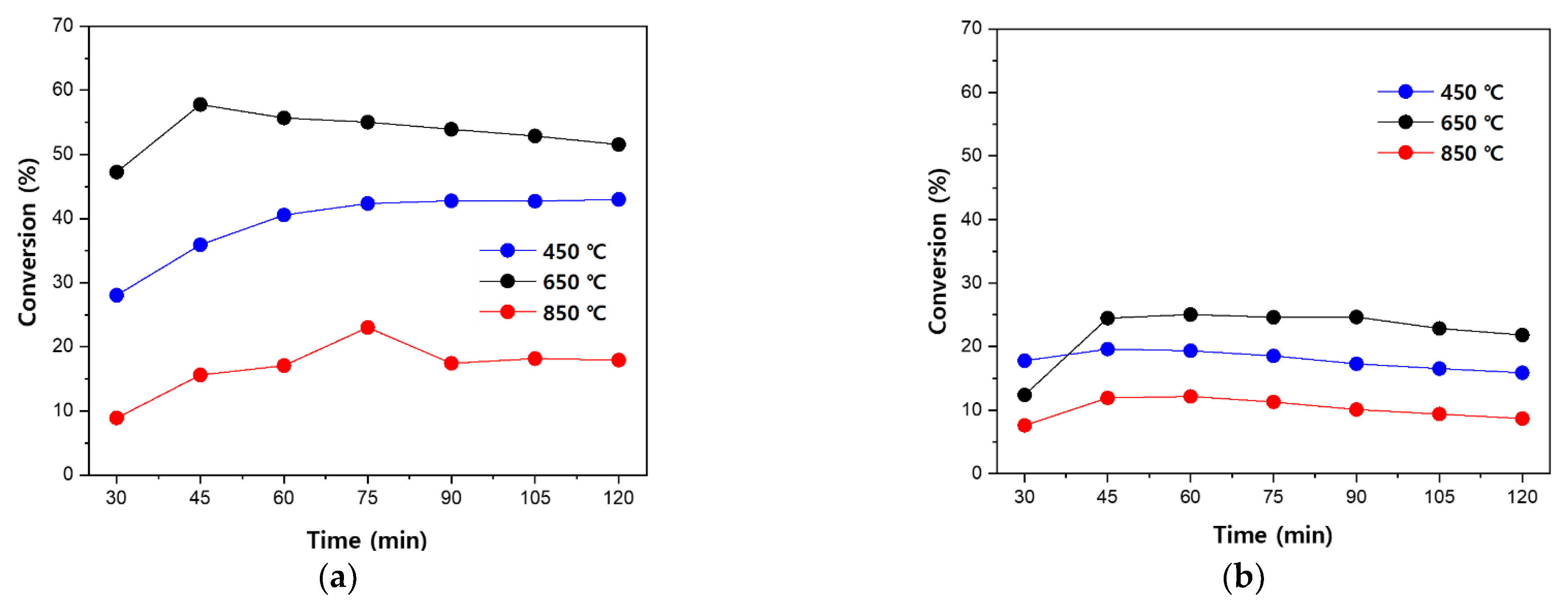
3.1. Photodegradation Characteristics of Acetaldehyde against Heat-Treatment Temperature during Catalyst Preparation
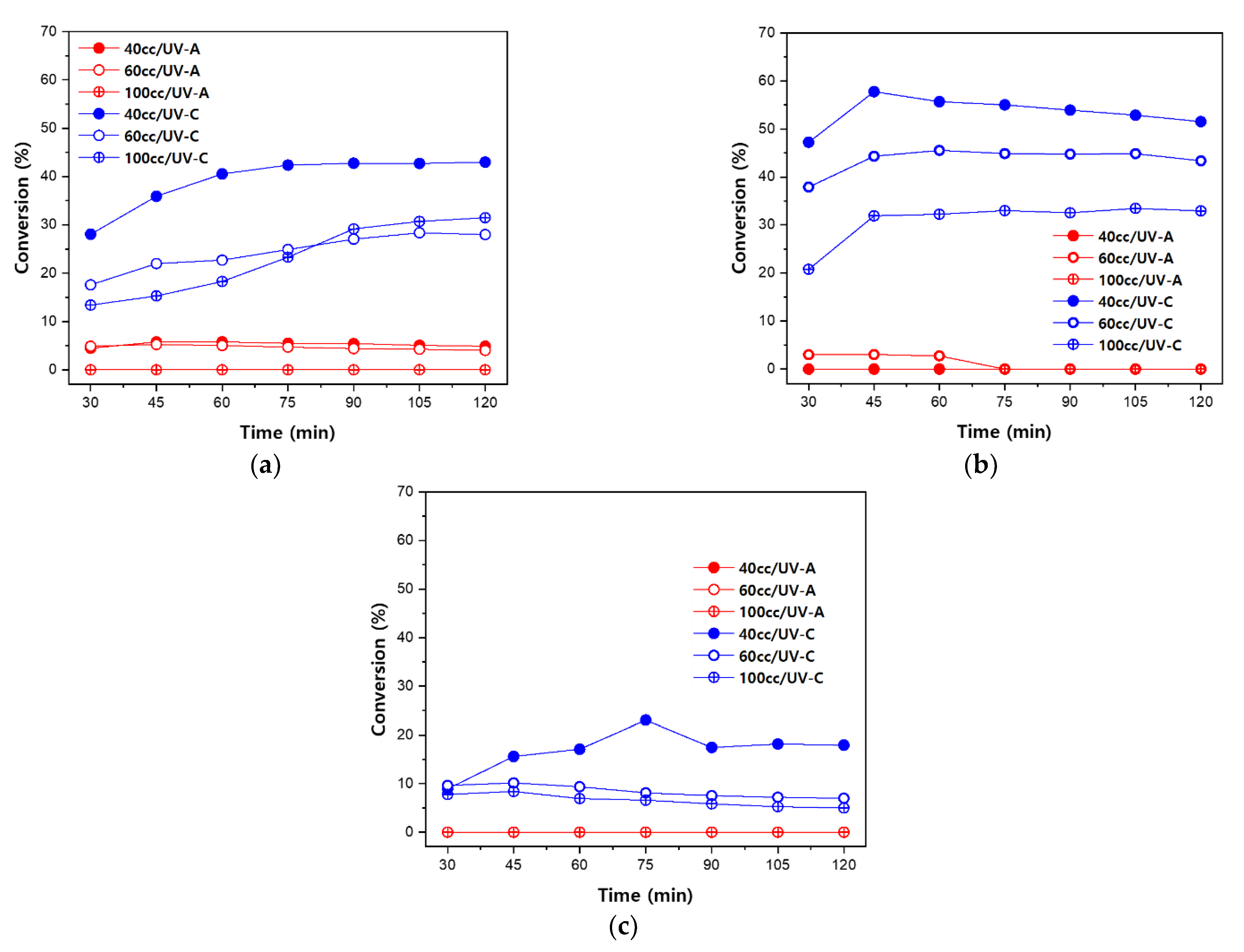
3.2. Acetaldehyde Degradation Characteristics according to Changes in Flow Rate
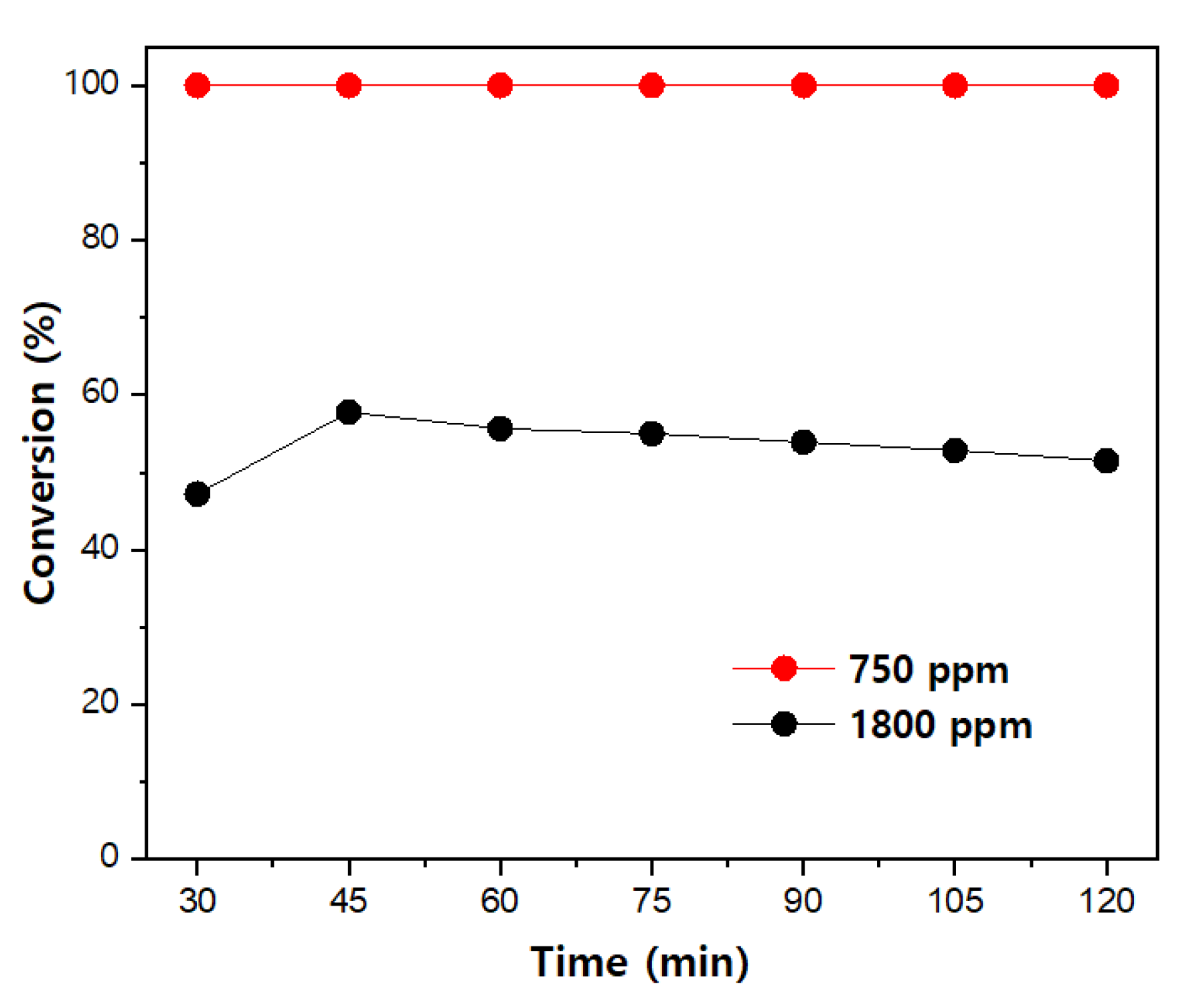
3.3. Acetaldehyde Degradation Characteristics according to the Initial Inflow Concentration
3.4. Acetaldehyde Degradation Characteristics according to Light Source Conditions
3.5. Effects of Moisture on Organic Matter Degradation Characteristics of the Catalysts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, Y.H.; Lin, C.C.; Hsu, S.C. Comparison of conventional and green building materials in respect of VOC emissions and ozone impact on secondary carvonyl emissions. Build Environ. 2015, 87, 274–282. [Google Scholar] [CrossRef]
- Yu, B.F.; Hu, Z.B.; Liu, M.; Yang, H.L.; Kong, Q.X.; Liu, Y.H. Review of research on air-conditioning systems and indoor air quality control for human health. Int. J. Refrig. 2009, 32, 3–20. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, D. Photocatalysis: Basic principles, diverse forms of implementations and emerging scientific opportunities. Adv. Energy Mater. 2017, 7, 1700841. [Google Scholar] [CrossRef] [Green Version]
- Weng, S.K.; Jing, W.L.; Woon, C.C.; Yean, L.P.; Lan, C.S. An overview of photocatalytic degradation: Photocatalyst, mechanisms, and development of photcatalytic membrane. Environ. Sci. Pollut. Res. Int. 2020, 27, 2522–2565. [Google Scholar]
- Fajar, B.L. Photocatalyst Principle and Application. Basic Concept. 2014. Available online: https://www.slideshare.net/fajarbudilaksono5/photocatalyst-principle-and-application (accessed on 5 March 2022).
- Marta, I.L.; Natalia, Q. Advanced Oxidation Processes for Water and Wastewater Treatment. Recent Pat. Eng. 2010, 4, 217–241. [Google Scholar]
- Rasoul, M.; Janan, P.; Ebrahim, K. Photocatalytic Removal of Nox Gas from Air by TiO2/Polymer Composite Nanofibers. Nanochem Res. 2018, 3, 212–218. [Google Scholar]
- Janusz, L.; Yi, H.Y.; Jeffrey, C.S.W. Removal of NOx by Photocatalytic Process. J. Photochem. Photobiol. C Photochem. Rev. 2013, 14, 29–52. [Google Scholar]
- Lee, W.I.; Choi, G.J.; Do, Y.R. Effect of Au and WO3 on the Surface Structure and Photocatalytic Activity of TiO2. Bull. Korean Chem. Soc. 1997, 18, 667–670. [Google Scholar]
- Saini, K.K.; Sharma, S.D.; Chanderkant; Kar, M.; Singh, D.; Sharma, C.P. Structural and optical properties of TiO2 thin films derived by sol–gel dip coating process. J. Non-Cryst. Solids 2007, 353, 2469–2473. [Google Scholar] [CrossRef]
- Son, H.S. Removal of gaseous benzene using TiO2 photocatalyst coated on ceramic and glass. J. Korean Soc. Atmos. Environ. 2003, 19, 57–66. [Google Scholar]
- Burns, G.P. Titanium dioxide dielectric films formed by rapid thermal oxidation. J. Appl. Phys. 1989, 65, 2095. [Google Scholar] [CrossRef]
- Schiller, S.; Beister, G.; Sieber, W.; Schirmer, G.; Hacker, E. Influence of deposition parameters on the optical and structural properties of TiO2 films produced by reactive d.c. plasmatron sputtering. Thin Solid Films 1981, 83, 239. [Google Scholar] [CrossRef]
- Suhail, M.H.; Mohan Rao, G.; Mohan, S.D. C reactive magnetron sputtering of titanium-structural and optical characterization of TiO2 films. J. Appl. Phys. 1992, 71, 1421. [Google Scholar] [CrossRef]
- Okimura, K.; Maeda, N.; Shibata, A. Characteristics of rutile TiO2 films prepared by r.f. magnetron sputtering at a low temperature. Thin Solid Films 1996, 281, 427–430. [Google Scholar] [CrossRef]
- Rice, G.W. Laser-Driven Pyrolysis: Synthesis of TiO2 from Titanium Isopropoxide. J. Am. Ceram. Soc. 1987, 70, C117–C120. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, Y.F.; Brodie, D.E. Photoconducting TiO2 prepared by spray pyrolysis using TiCl4. Thin Solid Films 1992, 213, 265–270. [Google Scholar] [CrossRef]
- Yeung, K.S.; Lam, Y.W. A simple chemical vapour deposition method for depositing thin TiO2 films. Thin Solid Films 1983, 109, 169–178. [Google Scholar] [CrossRef]
- Lu, J.P.; Wang, J.; Raj, R. Solution precursor chemical vapor deposition of titanium oxide thin films. Thin Solid Films 1991, 204, L13–L17. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, J.Y.; Choa, Y.H.; Higuchi, M.; Mizutani, N. Preparation of TiO2 thin film by liquid sprayed mist CVD method. Mater. Sci. Eng. B 2004, 107, 289–294. [Google Scholar] [CrossRef]
- Wang, W.B.; Yanguas-Gil, A.; Yang, Y.; Kim, D.Y.; Girolami, G.S.; Abelson, J.R. Chemical vapor deposition of TiO2 thin films from a new halogen-free precursor. J. Vac. Sci. Technol. A 2014, 32, 061502. [Google Scholar] [CrossRef]
- Hussin, R.; Choy, K.L.; Hou, X. Growth of TiO2 Thin Films by Atomic Layer Deposition (ALD). Adv. Mater. Res. 2016, 1133, 352–356. [Google Scholar] [CrossRef]
- Lai, X.; Weng, Y.; Chen, G.; Que, S.; Zhou, X.; Yan, Q.; Wu, C.; Guo, T.; Sun, J.; Zhang, Y. Morphology regulation of TiO2 thin film by ALD growth temperature and its applications to encapsulation and light extraction. J. Mater. Sci. Mater. Electron. 2020, 31, 21316–21324. [Google Scholar] [CrossRef]
- Yoko, T.; Yuasa, A.; Kamiya, K.; Sakka, S. Sol-Gel-Derived TiO2 Film Semiconductor Electrode for Photocleavage of Water: Preparation and Effects of Postheating Treatment on the Photoelectrochemical Behavior. J. Electrochem. Soc. 1991, 138, 2279. [Google Scholar] [CrossRef]
- Vorotilov, K.A.; Orlova, E.V.; Petrovsky, V.I. Sol-gel TiO2 films on silicon substrates. Thin Solid Films 1992, 207, 180–184. [Google Scholar] [CrossRef]
- Nagpal, V.J.; Davis, R.M.; Desu, S.B. Novel thin films of titanium dioxide particles synthesized by a sol-gel process. J. Mater. Res. 1995, 10, 3068–3078. [Google Scholar] [CrossRef]
- Su, C.; Hong, B.-Y.; Tseng, C.-M. Sol–gel preparation and photocatalysis of titanium dioxide. Catal. Today 2004, 96, 119–126. [Google Scholar] [CrossRef]
- Bach, H.; Krause, D. Thin Films on Glass; Springer: Heidelberg, Germany, 1997. [Google Scholar]
- Yoko, T.; Yuasa, A.; Kamiya, K.; Sakka, S. Sol-Gel Derived TiO2 Film Semiconductor Electrode for Photocleavage of Water: Electrode Reaction Mechanism (Commemoration Issue Dedicated to Professor Tetsuya HANAI on the Occasion of His Retirement). Bull. Inst. Chem. Res. Kyoto Univ. 1991, 69, 311. [Google Scholar]
- Xuzhuang, Y.; Yang, D.; Huaiyong, Z.; Jiangwen, L.; Martins, W.N.; Frost, R.; Daniel, L.; Yuenian, S. Mesoporous structure with size controllable anatase attached on silicate layers for efficient photocatalysis. J. Phys. Chem. C 2009, 113, 8243–8248. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Basu, S. Clay supported TiO2 nanoparticles for photocatalytic degradation of environmental pollutants: A review. J. Environ. Chem. Eng. 2018, 6, 6088–6107. [Google Scholar] [CrossRef]
- Szczepanik, B. Photocatalytic degradation of organic contaminants over clay-TiO2 nanocomposites: A review. Appl. Clay Sci. 2017, 141, 227–239. [Google Scholar] [CrossRef]
- Danfa, S.; Martins, R.C.; Quina, M.J.; Gomes, J. Supported TiO2 in ceramic materials for the photocatalytic degradation of contaminants of emerging concern in liquid effluents: A Review. Molecules 2021, 26, 5363. [Google Scholar] [CrossRef]
- Studart, A.R.; Gonzenbach, U.T.; Tervoort, E.; Gauckler, L.J. Processing Routes to Macroporous Ceramics: A Review. J. Am. Ceram. Soc. 2006, 89, 1771–1789. [Google Scholar] [CrossRef]
- Yildiz, T.; Yatmaz, H.C.; Öztürk, K. Anatase TiO2 powder immobilized on reticulated Al2O3 ceramics as a photocatalyst for degradation of RO16 azo dye. Ceram. Int. 2020, 46, 8651–8657. [Google Scholar] [CrossRef]
- Obregon, S.; Gonzalez, V.R. Photocatalytic TiO2 thin films and coating prepared sol-gel processing: A brief review. J. Sol-Gel Sci. Technol. 2021, 102, 125–141. [Google Scholar] [CrossRef]
- Pedrini, L.F.K.; Escaliante, L.C.; Scalvi, L.V.A. Deposition of TiO2 thin films by dip coating technique from a two-phase solution method and application to photocatalysis. Mater. Res. 2021, 24 (Suppl. S1), e20210007. [Google Scholar] [CrossRef]
- Zrinka, S.; Vilko, M.; Lidija., C.; Martina, B.; Irena, A.; Mattia., G. Titania-Coated Alumina foam Photocatalyst for Memantine Degradation Derived by Replica Method and Sol-Gel Reaction. Materials 2020, 13, 227. [Google Scholar]
- Hu, L.; Yoko, T.; Kozuka, H.; Sakka, S. Effects of solvent on properties of sol–gel-derived TiO2 coating films. Thin Solid Films 1992, 219, 18–23. [Google Scholar] [CrossRef]
- Yu, J.G.; Yu, H.Y.; Cheng, B.; Zhao, X.J.; Yu, J.C.; Ho, W.K. The effect of Calcinationn Temperature on the Surface Microstructure and Photocatalytic Activity of TiO2 Thin Films Prepared by Liquid Phase Deposition. J. Phys. Chem. B 2003, 107, 13871–13879. [Google Scholar] [CrossRef]
- Omotoso, T.; Boonyasuwat, S.; Crossley, S.P. Understanding the role of TiO2 crystal structure on the enhanced activity and stability of Ru/TiO2 catalysts for the conversion of lignin-derived oxygenates. Green Chem. 2014, 16, 645–652. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, B.; Chen, J. Effects of Calcination Temperature on Preparation of Boron-Doped TiO2 by Sol-Gel Method. Int. J. Photoenergy 2012, 2012, 528637. [Google Scholar] [CrossRef] [Green Version]
- Yoko, T.; Hu, L.; Kozuka, H.; Sakka, S. Photoelectrochemical properties of TiO2 coating films prepared using different solvents by the sol-gel method. Thin Solid Films 1996, 283, 188–195. [Google Scholar] [CrossRef]
- Kandiel, T.A.; Robben, L.; Alkaim, A.; Bahnemann, D. Brookite versus anatase TiO2 photocatalysts: Phase transformations and photocatalytic activities. Photochem. Photobiol. Sci. 2013, 12, 602–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pengyi, Z.; Fuyan, L.; Gang, Y.; Qing, C.; Wanpeng, Z. A Comparative Study on decomposition of gaseous toluene by O3/UV, TiO2/UV and O3/TiO2/UV. J. Photchem. Photobiol. A Chem. 2003, 156, 189–194. [Google Scholar] [CrossRef]
- Avasarala, B.K.; Tirukkovalluri, S.R.; Bojja, S. Photocatalytic degradation of monocrotophos pesticide—An endocrine disruptor by magnesium doped titania. J. Hazard. Mater. 2011, 186, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.; Ma, C.M.; Shen, Y.S. Decomposition of gaseous trichloroethylene in a photoreactor with TiO2-coated nonwoven fiber textile. Appl. Catal. B Environ. 2001, 34, 181–190. [Google Scholar] [CrossRef]
- Chuang, Y.H.; Hong, G.B.; Chang, C.T. Study on particulates and volatile organic compounds removal with TiO2 nonwoven filter prepared by electrospinning. J. Air Waste Manag. Assoc. 2014, 64, 738–742. [Google Scholar] [CrossRef]
- Sopyan, I.; Watanabe, M.; Murasawa, S.; Hashimoto, K.; Fujishima, A. An efficient TiO2 thin film photocatalyst: Photocatalytic properties in gas phase acetaldehyde degradation. J. Photochem. Photobiol. A Chem. 1996, 98, 79–86. [Google Scholar] [CrossRef]
- Guo, M.Z.; Ling, T.C.; Poon, C.S. Photocatalytic NOx degradation of concrete surface layers intermixed and spray-coated with nano-TiO2: Influence of experimental factors. Cem. Concr. Compos. 2017, 83, 279–289. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.J.; Kim, E.M.; Jeon, H.K.; Kale, S.B.; Choi, J.Y.; Kim, J.H. Photoreactor-Initiated Acetaldehyde Conversion Rate of a TiO2-Surface-Treated Alumina Photocatalyst Prepared Using the Sol–Gel Method. Appl. Sci. 2022, 12, 5796. https://doi.org/10.3390/app12125796
Kim SJ, Kim EM, Jeon HK, Kale SB, Choi JY, Kim JH. Photoreactor-Initiated Acetaldehyde Conversion Rate of a TiO2-Surface-Treated Alumina Photocatalyst Prepared Using the Sol–Gel Method. Applied Sciences. 2022; 12(12):5796. https://doi.org/10.3390/app12125796
Chicago/Turabian StyleKim, Su Jin, Eun Mi Kim, Hee Kyung Jeon, Shital Bhaskar Kale, Jeong Yeon Choi, and Jin Hyeok Kim. 2022. "Photoreactor-Initiated Acetaldehyde Conversion Rate of a TiO2-Surface-Treated Alumina Photocatalyst Prepared Using the Sol–Gel Method" Applied Sciences 12, no. 12: 5796. https://doi.org/10.3390/app12125796
APA StyleKim, S. J., Kim, E. M., Jeon, H. K., Kale, S. B., Choi, J. Y., & Kim, J. H. (2022). Photoreactor-Initiated Acetaldehyde Conversion Rate of a TiO2-Surface-Treated Alumina Photocatalyst Prepared Using the Sol–Gel Method. Applied Sciences, 12(12), 5796. https://doi.org/10.3390/app12125796





