Abstract
The thyroid biokinetic model of radioactive I-131 was re-evaluated using a refined nine-compartmental model and applied to twelve thyroid cancer patients. In contrast to the simplified four-compartmental model regulated by the ICRP-56 report, the revised model included nine compartments specified in the ICRP-128 report, namely, oral, stomach, body fluid, thyroid, whole body, liver, kidney, bladder, and remainder (i.e., the whole body minus kidney and bladder). A self-developed program run in MATLAB was designed to solve the nine first-order simultaneous linear differential equations. The model was realized in standard and simplified versions. The latter neglected two feedback paths (body fluid to oral, i31, and kidney to the whole body, i87) to reduce computations. Accordingly, the biological half-lives for the major compartments (thyroid and body fluid + whole body) were 36.00 ± 15.01, 15.04 ± 5.63, 34.33 ± 15.42, and 14.83 ± 5.91 of standard and simplified version. The correlations between theoretical and empirical data for each patient were quantified by the dimensionless AT (agreement) index and, the ATtot index integrated each individual AT of a specific organ of one patient. Since small AT values indicated a closer correlation, the obtained range of ATtot (0.048 ± 0.019) proved the standard model’s reliability and high accuracy, while the simplified one yielded slightly higher ATtot (0.058 ± 0.023). The detailed outcomes among various compartments of twelve patients were calculated and compared with other researchers’ work. The correlation results on radioactive I-131 evolution in thyroid cancer patients’ bodies are instrumental in viewpoint of radioactive protection of patients and radiological personnel.
1. Introduction
In this paper, the thyroid biokinetic model of radioactive I-131 was re-evaluated using a refined nine-compartmental model and applied to twelve thyroid cancer patients. The actual time-dependent radioactive iodine concentration distribution among the human organs is vital for nuclear medicine purposes, from the treatment, routine examination, and radiation safety standpoints. In practice, patients who undergo iodine administration after thyroid ablation are strongly recommended to stay in an isolated chamber for at least 72 h according to the standard operation process in Taiwan. Although these precautions may be too conservative, an accurate estimation of radioactive iodine concentration decline in specific human organs is quite problematic and requires substantiated biokinetic models of the human body [1,2]. The thyroid iodine model recommended by the ICRP-56 is reduced to the main four compartments of the human body, namely, stomach, thyroid, whole body, and body fluid [3]. Yet some researchers still prefer to adopt the two-compartmental model for obtaining an instant and rapid solution [4].
Although the above model treated whole body (WB) and body fluid (BF) as separate independent compartments, they cannot be separated, like stomach or thyroid, from the pathological standpoint, which biases the model predictions. This deficiency is aggravated in the case of thyroid cancer patients who need the thyroid dose administration after thyroid ablation: Their residual thyroid gland tissue can barely hold the radioactive iodine solution and, thus, the high concentration of radioactive I-131 is widely distributed via the common metabolic mechanism, and its decline is slower than the theoretical estimation via the ICRP-56 report-inspired models. Many researchers attempted to mitigate this problem theoretically or practically, but most of them used four or even fewer compartments in their simplified models [5,6,7,8].
Given this, it was considered expedient to develop and validate the integrity of the thyroid iodine biokinetic model with the nine-compartmental (9C) structure recommended by the ICRP-128 report [9]. Although the report prescribes a general-purpose biokinetic model, we slightly revised it to address the special need to re-evaluate the above thyroid iodine model. In doing so, we defined a new 9C model, and the correlated preset values were re-considered to match the revised scenario in providing a more accurate solution than previous studies. The newly explored data we used in this 9C model were categorized into sections to elaborate their suitability compared with the previous four-compartmental (4C) one. The presetting of initial values in optimizing a reasonable solution from MATLAB was also discussed. The results obtained contribute to the elaboration of a clinical database and to standard protocols of thyroid cancer patients from the viewpoint of their radioactive protection and safety of radiologists.
2. Materials and Methods
2.1. Biokinetic Model of Radioactive Iodine in the Thyroid
In addition to the simplified four-compartmental (4C) model regulated by the ICRP-56 report, this study applied the general-purpose multicompartment model specified by the ICRP-128 report, which we revised into a standard 9C model with various feedback paths to imply the real human body, namely, (1) oral, (2) stomach, (3) body fluid, (4) liver, (5) thyroid, (6) remainder (whole body minus kidney and bladder), (7) whole body, (8) kidney, (9) bladder, and an added (10) excretion (required for closing the circulation loop). This subdivision is schematically presented in Figure 1. To derive the time-dependent correlation of radioactive I-131 for each compartment, the following nine first-order simultaneous differential equations need to be solved:
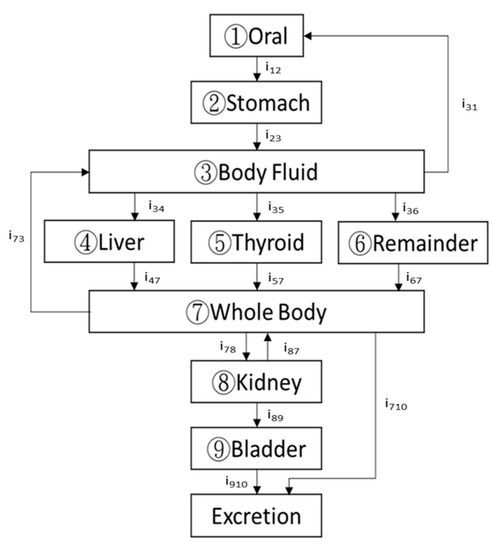
Figure 1.
A typical human body can be subdivided into nine major compartments in the biokinetic model of iodine: (1) stomach, (2) body fluid, (3) thyroid, (4) the whole body, and (5) oral, (6) liver, (7) remainder (whole body minus kidney and bladder), (8) kidney, (9) bladder, according to the ICRP-128 report.
Here qi is the time-dependent activity of the specific radionuclide (radioactive I-131 in this work); λij is the biological decay constant (λij = iij·ln2/Ti(1/2)(bio)); iij is the branching ratio of the infused radioactive solution from the ith compartment to the jth one; Ti(1/2)(bio) is the biological half-life of the ith compartment; and λR is the radiological decay constant of the specific radionuclide (namely, radioiodine, Ln 2/T1/2(radiological)). More detail can be found elsewhere [1,2,10].
Equations (1)–(9) were solved using a self-developed program run in MATLAB [11]. In doing so, Equations (1)–(9) had to be reformatted into a data matrix form presented in Equation (10) and analyzed for the optimal solution:
The loss function in solving the inverse matrix problem was defined to have a minimal difference between empirical data and theoretical estimates. The empirical data concluding each derived time-dependent dataset of nine compartments were collected from in vivo gamma camera scans of the patients taken 1, 4, 24, 48, 72, and 168 h after the radionuclide administration. Thus, 54 (6 × 9 = 54) data points were preset to confine the MATLAB calculation until the minimal loss function was reached.
2.2. A Simplified 9C Model
Before realizing the above 9C model, we tested its simplified version, where two paths in Figure 1, namely, i31 (body fluid to oral) and i87 (kidney to the whole body), were removed to simplify the numerical solution and assess its accuracy. This simplification was based on the assumption of only minor contributions of these two paths so that the loss function could be optimized by the simplified loop of computational matrix analysis. Thus, the simplified 9c model implied the following revised data matrix form:
2.3. AT Examination
A dimensionless index AT (agreement) was adopted as a quantification tool in inspecting the MATLAB output. The empirical data of each patient were collected through the multiple in vivo gamma camera scanning and converted into count × pixel−1 × s−1, then normalized and assigned as the objective expectation for optimization of the program prediction. The AT index earlier introduced and applied by the authors in [1,2,12] was used in this work to assess the similarities between the empirical data and the respective optimal predictions made via the self-developed program. This AT-based approach was beneficial for evaluating the fluctuation among large datasets because a quantified AT was focused not only on the deviations among particular empirical and theoretical results but also on integrating all data for individual patients:
where Yi (nor.) and Yi (MATLAB) are the normalized intensities from each ROI determined from the nth set of empirically obtained data, which are computed using the self-developed program run in MATLAB. The value of N = 6 was adopted since the empirical data were collected in six independent scan protocols. A zero value of AT implies perfect agreement between the theoretical and empirical results, while AT < 0.05 indicates excellent consistency between them, meaning a slight fluctuation of the empirical data from the predicted results [1,2,12]. Furthermore, each obtained AT was calculated from a point-by-point comparison of theoretical estimations with empirical data for each ROI, and the total AT (ATtot) was defined as
where the total AT (ATtot) was calculated as the mean square root of AT of all M compartments (M = 9 in this work). Similarly, a low ATtot represented excellent fit of the theoretical estimation with the empirical dataset of the specific patient.
2.4. Patients’ Characteristics
Twelve thyroid cancer patients (three males and nine females 42~67 years of age) underwent post-thyroid cancer remnant ablation one month after the surgical operation. Each patient was subjected to consecutive weekly whole-body scanning by gamma camera after the administering of I-131. Noteworthy is a special case of female patient No. 8, whose period between administering I-131 and surgery was nearly 12 years because she was diagnosed as having a relapse of hyperthyroidism syndrome after partial thyroid cancer in 2007. However, the correlated derivations of case 8 were still adopted to test the program’s computational ability. The patients’ characteristics are listed in Table 1.

Table 1.
Characteristics of twelve patients with a papillary thyroid cancer diagnosis who underwent the whole-body scanning for further analysis of the iodine biokinetic model. * Case No. 8 was diagnosed as having a relapse of hyperthyroidism after partial ablation of the thyroid gland 12 years before.
2.5. Whole-Body Scanning of Patients
Each patient was given an 1110 MBq I-131 solution one hour before the in vivo gamma camera scanning, and the schedule of 1, 4, 24, 48, 72, and 168 elapsed hours were preset to obtain six data groups for each patient. The preset protocol of the gamma camera for data collection was ±10% peak width of the I-131 main 364 keV peak, high-energy general-purpose (HEGP) collimator, FOV of 40 × 54 cm2, and 256 × 1024 matrix size. Each patient underwent a 20–22 min scan at 10 cm/min scan speed. Obtaining high-quality images is of top priority for nuclear medicine [13], being instrumental for diagnosing the injured regions. This study was approved by the IRB Committee of the Far Eastern Memorial Hospital, New Taipei City, Taiwan, with the credential No. FEMH 106132-F. Figure 2 illustrates the original plots from the gamma camera of patients No. 2 and 8 for comparison. The six plots were consecutively downloaded from the facility after the gamma scanning. The marked ROIs were assigned as oral, thyroid, liver, stomach, kidney-R, kidney-L (right and left), and bladder, whole body (BF + WB), and BKG (background, i.e., part of thigh) to derive the count × pixel−1 × s−1 for further analysis. In addition, the BKG values were subtracted from all net count rates at various ROIs to eliminate overcounting in the analysis.
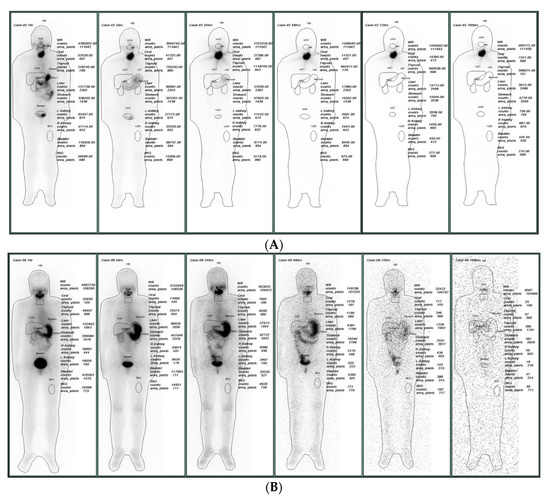
Figure 2.
The obtained results from (A) cases 2 and (B) case 8 with the zero initial values for kidney and bladder; the outcome differs from the experimental data from in vivo gamma camera scanning for comparison. The six plots were consecutively downloaded from the facility after the gamma scanning after 1, 4, 24, 48, 72, and 168 elapsed hours. The marked ROIs were assigned as St (stomach), WB (body fluid + whole body), Th (thyroid), Bl (bladder), and BKG (background) to derive the count × pixel−1 × s−1 for further analysis.
3. Results
3.1. MATLAB Calculations
Figure 3 depicts the normalized empirical data obtained via the standard 9C model for twelve patients and the theoretical estimates from the MATLAB program. The former are illustrated by dots with error bars, and the optimal estimates are plotted with six continuous curves. In contrast, the numeric calculation was finalized in each specific case to ensure that only a minimal ATtot was reached, and then each curve was plotted. Thus, the lines corresponded to stable and converged solutions derived via multiple iterations of the Gaussian elimination according to the inverse problem algorithm [14]. Table 2 and Table 3 summarize the derived biological half-lives and branching ratios for the nine first-order simultaneous differential Equations (1)–(9) adopted in this work. In addition, calculations via the 4C model according to the ICRP-56 report and the simplified 9C model were integrated for comparison. As shown in Table 2 and Table 3, no significant discrepancies between the standard and simplified 9C models were observed. Thus, it was acceptable to ignore the feed paths from body fluid to oral, i31, and kidney to the whole body, i87, which simplified the computation.
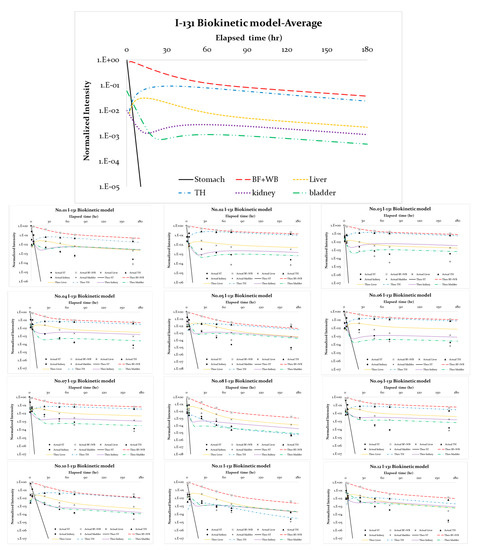
Figure 3.
The normalized empirical data for patients and theoretical estimates via MATLAB. The former are illustrated by dots with error bars, and the optimal estimates are plotted with three continuous lines.

Table 2.
The derived biological half-life values and averages for simultaneous time-dependent differential equations (cf. Equations (1)–(9)) as adopted in this work. The calculation results are theoretical estimations of the time-dependent quantity of I-131 in various compartments for the twelve patients according to the MATLAB calculations. * Case no. 8 was excluded from the calculation of average values.

Table 3.
The derived branching ratios and averages for the simultaneous time-dependent differential equations (cf. Equations (1)–(9)) as adopted in this work. * Case no. 8 was excluded from the calculations of average values.
3.2. AT Inspection
Table 4 shows the ATs of the derived compartments for the twelve patients. AT indicates the curve-fitting agreement between the theoretical estimates and empirical measurements for each specific compartment of the patient’s body. As clearly demonstrated, the ATtot fluctuations predicted by the 4C, standard 9C, and simplified 9C models were 0.046 ± 0.014, 0.048 ± 0.019, and 0.058 ± 0.023, respectively. This implies that the simplified 9C model was inferior to the standard 9C one (according to the ATtot difference of 0.01). Possibly, a smaller number of preset paths obtained by removing i31 and i87 restrained MATLAB from deriving an advanced solution. Quite surprisingly, the 9C model provided a slightly higher ATtot, which differed from the previous 4C model by only 0.002.

Table 4.
The AT values of the derived compartments for twelve patients. AT indicates the curve-fitting agreement between the theoretical estimation and the empirical measurement for each specific compartment of the patient’s body. * Case no. 8 was excluded from the calculations of average values.
4. Discussion
4.1. Comparative Analysis against Other Models
Several researchers used the two-compartmental (2C) model, which contained only thyroidal and extrathyroidal (remainder) compartments, for solving the radioactive I-131 concentration evolution in the human body and predicting its residual values [7,15]. Alternatively, Rabbe adopted the three-compartmental model (thyroid, transfer, and other organs) to predict the same mechanism in the human body [16], although the predicted results were quite inaccurate. Therefore, other researchers have extended the Rabbe model to four compartments (stomach, thyroid body fluid, and the whole body) with one excretion compartment to maintain the close realm of the simultaneous differential equations and easier convergence of the numeric results [5,9,17]. In this work, the number of compartments was increased to nine, the additional five being oral, liver, remainder, kidney, and bladder. The radioactive I-131 could be easily detected in the oral and liver compartments, whereas the kidney and bladder were the major excreting organs reducing the liquid I-131 concentration. Thus, it was essential to adopt the revised 9C model to interpret the real metabolic mechanism of radioactive I-131 in the human body. The numeric analysis was performed via the self-developed program run in MATLAB. The low ATtot of 0.048 ± 0.019 proved the feasibility of the proposed approach in finding the optimal solution (ref. Table 4).
A more detailed investigation of the I-131 concentration evolution in each compartment revealed that oral and ST compartments exhibited simple decay with their effective half-lives (1/Teff = 1/Tbio + 1/Trad). The biological half-lives of oral and ST were 0.15 ± 0.12 and 0.63 ± 0.32, h (ref. Table 2). This implied that the contributions of both the oral and the ST compartments were negligible in the group. In contrast, the biological half-lives of liver, kidney, and bladder, 5.40 ± 1.77, 3.55 ± 1.74, and 2.65 ± 1.34 h, shifted more toward transient equilibrium in the radionuclides’ concentration change with BF + WB, whereas the BF + WB in reality had a biological half-life 15.04 ± 5.63 h (ref. Figure 3). Noteworthy is that the larger the biological half-life of the receiving compartment (i.e., liver, kidney, or bladder in this work) the longer it takes to reach its maximal concentration. However, each compartment possessed its unique time-dependent I-131 concentration curve, which could not be simply interpreted as transient equilibrium in radionuclide chain decay. The real metabolic mechanism was quite complicated and could be assessed by solving simultaneous differential equations (ref. Equations (1)–(9)). It is executable to further explore the excretion from the kidney or bladder by collecting time-dependent concentration of I-131 in urine since the quantified biological half-life and branching ratios are instrumental in the correlated analysis. Table 5 shows further the biological half-lives in the major compartments, whole body (BF + WB), and thyroid calculated in this and previous studies to solidify the comparison among different approaches. The results obtained via different practical methods and theoretical simulations are quite controversial. Thus, the internal doses for thyroidectomy patients have to be specifically considered for accurate estimation in clinical surveys.

Table 5.
The biological half-lives in the major compartments, whole body (BF + WB), and thyroid calculated in this and other studies. The calculations via different practical methods and simulations are quite controversial.
4.2. Removing Feedback Path
The difference between the standard and simplified 9C models was the presence or absence of two paths, namely, i31 (body fluid to oral) and i87 (kidney to the whole body). In contrast, both the ICRP-56 and ICRP-128 reports strongly recommended the i73 path (whole body to body fluid). The path i31 supports the detected I-131 concentration in the oral cavity, although its effect on the total evolution is negligible. Noteworthy is that path i87 dramatically changes the route from the kidney to the bladder, i89. The original value of 0.55 is forced to be 1.00 in the simplified model (ref. Table 3), increasing ATbladder from 0.006 ± 0.007 to 0.007 ± 0.005 (ref. Table 4). Therefore, it is not recommended to omit the above two paths (i31 and i87) in the proposed 9C biokinetic model because this would deteriorate the correlated concentration changes among the nine compartments (ref. Table 2 and Table 3).
4.3. Adjusting the Preset Initial Values
Although the initial values incorporated into the self-developed program run in MATLAB could also be adjusted to comply with the clinical conditions of the in-vivo scanning procedure, zero values in the beginning of computation were used to simplify the procedure. Patients’ kidneys and bladders always have I-131 counts even at the first trial of scan in the practical data survey (e.g., in Figure 2, the preset elapsed time was 1 h). Thus, the I-131 concentration in the kidney or bladder that presumably existed at the initial time can be interpreted as a nonzero initial value in the computation program. If the initial values of either kidney or bladder are reset to zero and the MATLAB program is re-run, the results change significantly. As illustrated in Figure 4, in cases 2 and 8, the initial values for kidney and bladder were reset to 0.0 and 0.0. Then, both the theoretical concentrations decrease in the initial time compared with the real outcome from the in vivo gamma camera scanning. Specifically, ATkidney for cases 2 and 8 increased from 0.002 and 0.002 to 0.010 and 0.004, while ATbladder increased from 0.001, 0.019 to 0.010, 0.036 (ref. Table 4). In addition, the average initial values for kidney and bladder were 0.028 ± 0.022 and 0.025 ± 0.026, respectively. The original preset biokinetic model could not account for the radioactive I-131 concentrations that already existed in the kidney and the bladder. However, changing the initial values in running the computational program could simulate the respective physical phenomena in the numeric analysis.
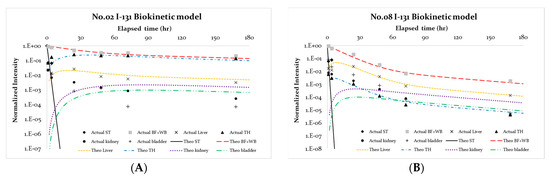
Figure 4.
Cases 2 and 8 with the zero initial values for kidney and bladder; the outcome differs from the experimental data from in vivo gamma camera scanning. (A) Cases 2 and (B) case 8 with the zero initial values for kidney and bladder; the outcome differs from the experi-mental data from in vivo gamma camera scanning.
5. Conclusions
The thyroid biokinetic model of radioactive I-131 in twelve thyroid cancer patients was re-evaluated using a refined nine-compartment model recommended by the ICRP-128 report. The proposed biokinetic model was realized in standard and simplified forms to simulate the real human metabolic mechanism. The simplified version neglected two paths, from body fluid to oral (i31) and from kidney to whole body (i87), to simplify the calculations and obtain an instant solution, slightly deviating the estimates. The self-developed program run in MATLAB was designed to solve the nine first-order simultaneous differential equations. The numeric results were compared with empirical data, and their correlations were assessed via a dimensionless quantified index AT (agreement). The simplified 9C model was inferior to the standard 9C one, although the calculation was comparatively simple. The initial values of the kidney and bladder in the program also could be adjusted to comply with the true in vivo scanning cases. The nonzero initial values properly interpreted the existing I-131 concentrations at the start of the elapsed time calculation in the gamma camera scanning.
Author Contributions
Conceptualization, L.-F.P. and L.-K.P.; Data curation, C.-C.H. and H.-T.K.; Formal analysis, C.-Y.C. and B.-R.P.; Methodology, C.-F.C. and L.-K.P.; Software, C.-C.H., H.-T.K., B.-R.P. and L.-K.P. All authors have read and agreed to the published version of the manuscript.
Funding
The authors highly appreciate the financial support of this study by the Ministry of Health and Welfare of the Republic of China under contract no. MOHW 11116 and Feng Yuan Hospital, Ministry of Health and Welfare of the Republic of China under contract number FYH-111-01.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Far Eastern Memorial Hospital (protocol code FEMH 106132-F, approved 2017-Sep-01).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huang, C.-C.; Lin, Y.-H.; Kittipayak, S.; Hwua, Y.-S.; Wang, S.-Y.; Pan, L.-K. Biokinetic model of radioiodine I-131 in nine thyroid cancer patients subjected to in-vivo gamma camera scanning: A simplified five-compartmental model. PLoS ONE 2020, 15, e0232480. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Chang, P.-J.; ChangLai, S.-P.; Pan, L.-K. Effective half life of iodine for five thyroidectomy patients using an in vivo gamma camera approach. J. Radiat. Res. 2007, 48, 485–493. [Google Scholar] [CrossRef] [PubMed]
- ICRP-56, Age-Dependent Doses to Members of the Public from Intake of Radionuclides: Part 1; Technical Report ICRP-56; International Commission on Radiation Protection; Pergamon Press: Oxford, UK, 1989.
- Di Martino, F.; Traino, A.C.; Brill, A.B.; Stabin, M.G.; Lazzeri, M. A theoretical model for prescription of the patient-specific therapeutic activity for radioiodine therapy of Graves’ disease. Phys. Med. Biol. 2002, 47, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Torres-Garcia, E.; Ferro-Flores, G.; Arteaga de Murphy, C.; Correa-Gonzalez, L.; Pichardo-Romero, P.A. Biokinetics and dosimetry of 188Re-anti-CD20 in patients with non-Hodgkin’s Lymphoma: Preliminary experience. Arch. Med. Res. 2008, 39, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Melendez-Alafort, L.; Nadali, A.; Zangoni, E.; Banzato, A.; Rondina, M.; Rosato, A.; Mazzi, U. Biokinetic and dosimetric studies of 188Re-hyaluronic acid: A new radiopharmaceutical for treatment of hepatocellular carcinoma. Nucl. Med. Biol. 2009, 36, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.M.; Kim, C.-Y.; Son, S.H.; Jung, J.-H.; Lee, C.-H.; Jeong, J.H.; Jeong, S.Y.; Lee, S.-W.; Lee, J.; Ahn, B.-C. I-131 biokinetics of remnant normal thyroid tissue and residual thyroid cancer in patients with differentiated thyroid cancer: Comparison between recombinant human TSH administration and thyroid hormone withdrawal. Ann. Nucl. Med. 2017, 31, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Barquero, R.; Basurto, F.; Vega-Carrillo, H.R.; Iñiguez, M.P.; Ferrer, N.; Esteban, R. Correlation between external exposure and activity in patients undergoing 131i thyroid cancer therapy. Health Phys. 2008, 95, 227–233. [Google Scholar] [CrossRef] [PubMed]
- ICRP-128 Radiation Dose to Patients from Radiopharmaceuticals: A Compendium of Current Information Related to Frequently Used Substances; Technical Report ICRP-128; International Commission on Radiation Protection; Pergamon Press: Oxford, UK, 2015; Volume 44, pp. 7–321.
- Chiang, F.-T.; Li, P.-J.; Chung, S.-P.; Pan, L.-F.; Pan, L.-K. Quantitative analysis of multiple biokinetic models using a dynamic water phantom: A feasibility study. Bioengineered 2016, 7, 304–313. [Google Scholar] [CrossRef] [PubMed][Green Version]
- MATLAB, V7.0.1.24704 (R14); Matrix Laboratory Developed by MathWorks: Natick, MA, USA, 2004.
- Yeh, D.-M.; Chen, C.-Y.; Tang, J.-F.; Pan, L.-K. A quantitative evaluation of multiple biokinetic models using an assembled water phantom: A feasibility study. PLoS ONE 2017, 12, e0189244. [Google Scholar] [CrossRef]
- Mukherjee, S.; Cheng, I.; Miller, S.; Guo, T.; Chau, V.; Basu, A. A fast segmentation-free fully automated approach to white matter injury detection in preterm infants. Med Biol. Eng. Comput. 2018, 57, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.E. Atoms, Radiation, and Radiation Protection, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2000; ISBN 978-3-527-40606-7. [Google Scholar]
- NRC 8.39, Regulatory Guide 8.39, Office of Nuclear Regulatory Research; US Nuclear Regulatory Commission: Washington, DC, USA, 1997.
- Rabbe, O.G. Internal Radiation Dosimetry, Health Physics Society; Medical Physics Publishing: Madison, WI, USA, 1994. [Google Scholar]
- Chen, C.-F.; Chuang, C.-H.; Tang, P.-C.; Tseng, N.-C.; Pan, L.-F.; Pan, L.-K. Biokinetic model development of radioiodine-131 via in vivo gamma camera/8-slice ct technique: Case-control study of feline hyperthyroidism. J. Mech. Med. Biol. 2018, 18, 1840035. [Google Scholar] [CrossRef]
- Willegaignon, J.; Ribeiro, V.P.B.; Sapienza, M.; Ono, C.; Watanabe, T.; Buchpiguel, C. Is it necessary to reduce the radioiodine dose in patients with thyroid cancer and renal failure? Arq. Bras. Endocrinol. Metab. 2015, 54, 413–418. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, B.; Peng, W.; Huang, R.; Tian, R.; Zeng, Y.; Kuang, A. Thyroid Cancer: Radiation Safety Precautions in131I Therapy Based on Actual Biokinetic Measurements. Radiology 2014, 273, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Abuqbeitah, M.; Demir, M.; Kabasakal, L.; Çavdar, I.; Uslu-Beşli, L.; Yeyin, N.; Razavikhosroshahi, S.; Sönmezoğlu, K. Indirect assessment of the maximum empirical activity (250 mCi) with respect to dosimetry concepts in radioiodine therapy of metastatic differentiated thyroid cancer. Nucl. Med. Commun. 2018, 39, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Guiu-Souto, J.; Neira-Castro, S.; Sanchez-Garcia, M.; Pouso, O.L.; Pombar-Camean, M.; Pardo-Montero, J. Adaptive biokinetic modelig of iodie-131 in thyroid cancer treatments: Implications on individualized internal dosimetry. J. Radiol. Prot. 2018, 38, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Taprogge, J.; Carnegie-Peake, L.; Murray, I.; Gear, J.I.; Flux, G.D. Adjustment of the iodine ICRP population pharmacokinetic model for the use in thyroid cancer patients after thyroidectomy. J. Radiol. Prot. 2021, 41, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).