Increased Inflammatory Markers at AMPH-Addicts Are Related to Neurodegenerative Conditions: Alzheimer’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Questionnaire Design
2.3. Inflammatory Biomarker Assessment (ACT, PEDF, and MIP-4)
2.4. Measuring the Level of Liver Transaminases (ALT and AST)
2.5. Statistical Analysis
3. Results
3.1. Sociodemographic Data of AMPH-add Participants
3.2. Biomarkers and Proinflammatory Cytokines Levels in AMPH-adds’ and Control Subjects Sera
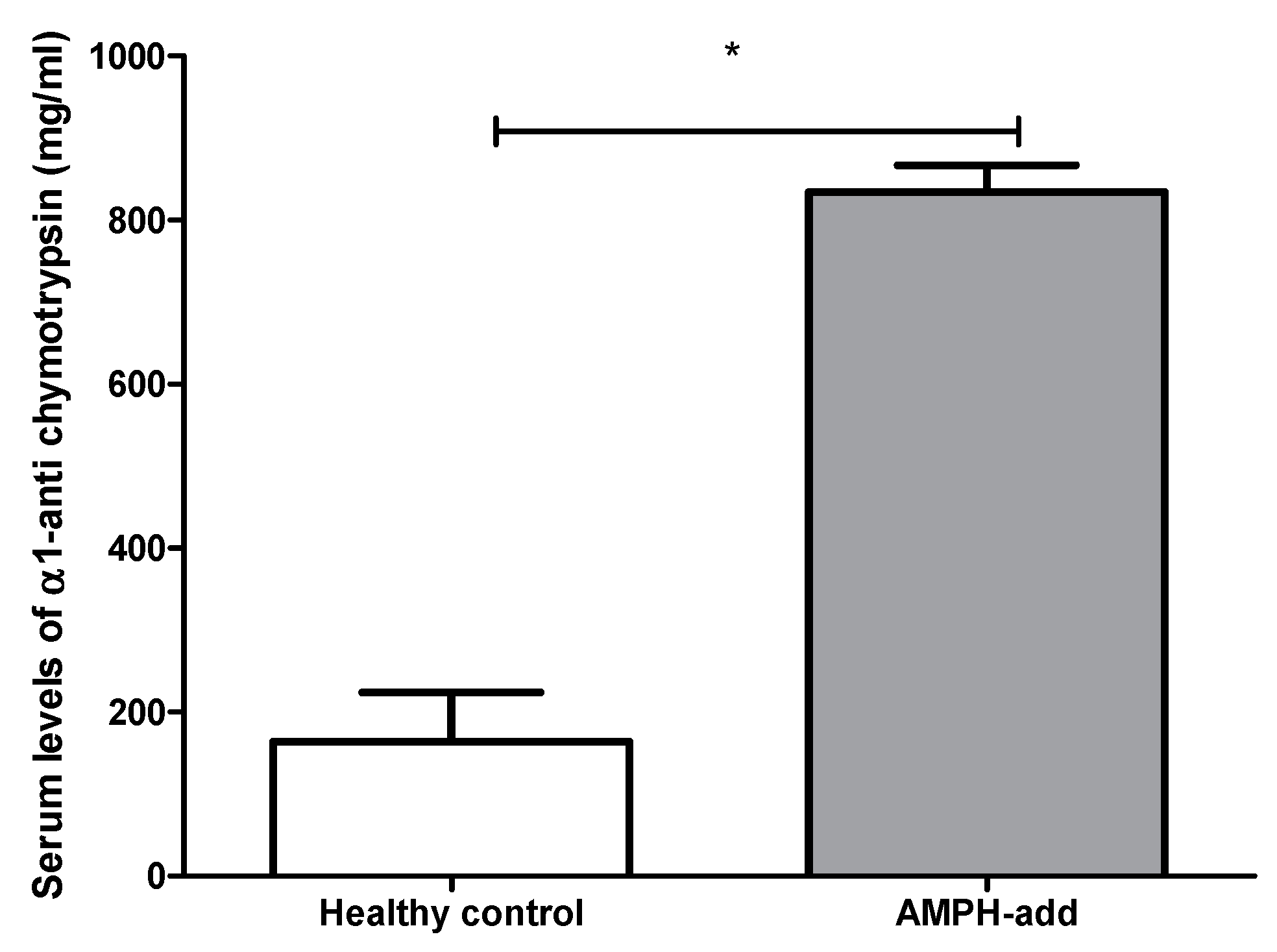
3.2.1. α1-Anti-Chymotrypsin (ACT) Level
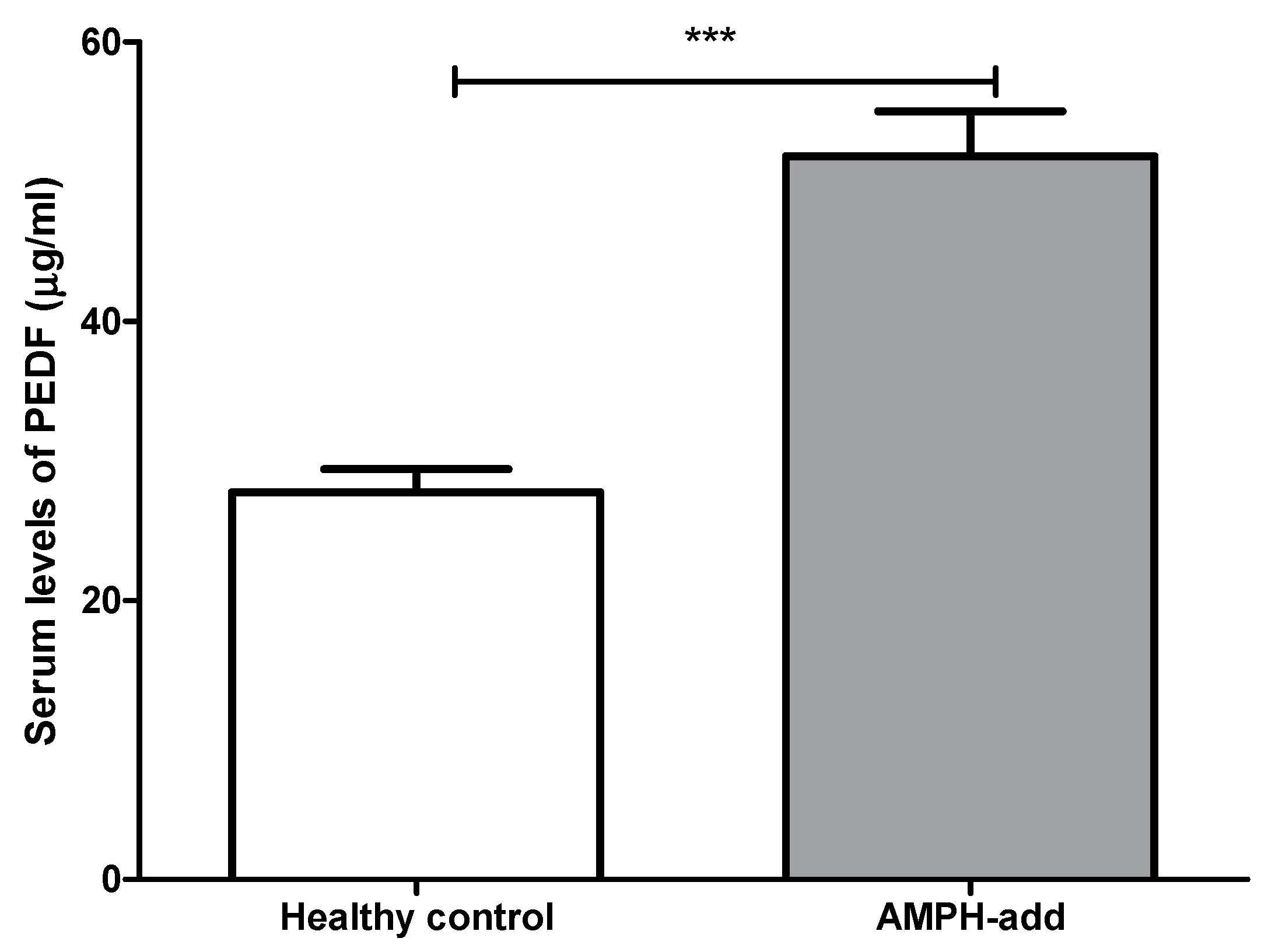
3.2.2. Pigment Epithelium-Derived Factor (PEDF) Level
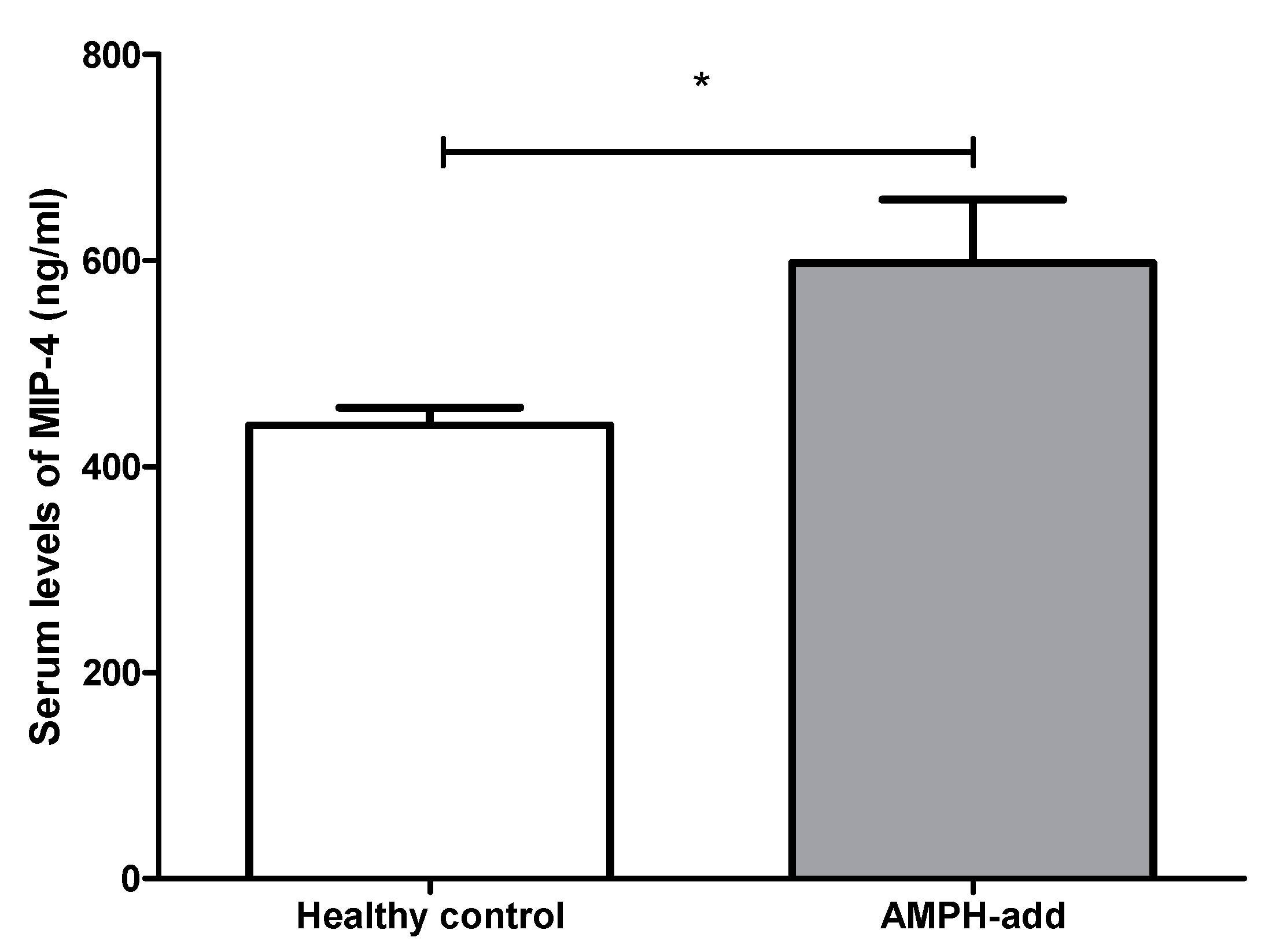
3.2.3. Macrophage Inflammatory Protein 4 (MIP-4) Level
3.3. Comparison of Liver Function Enzymes among Serum of AMPH-adds’ and Control Subjects
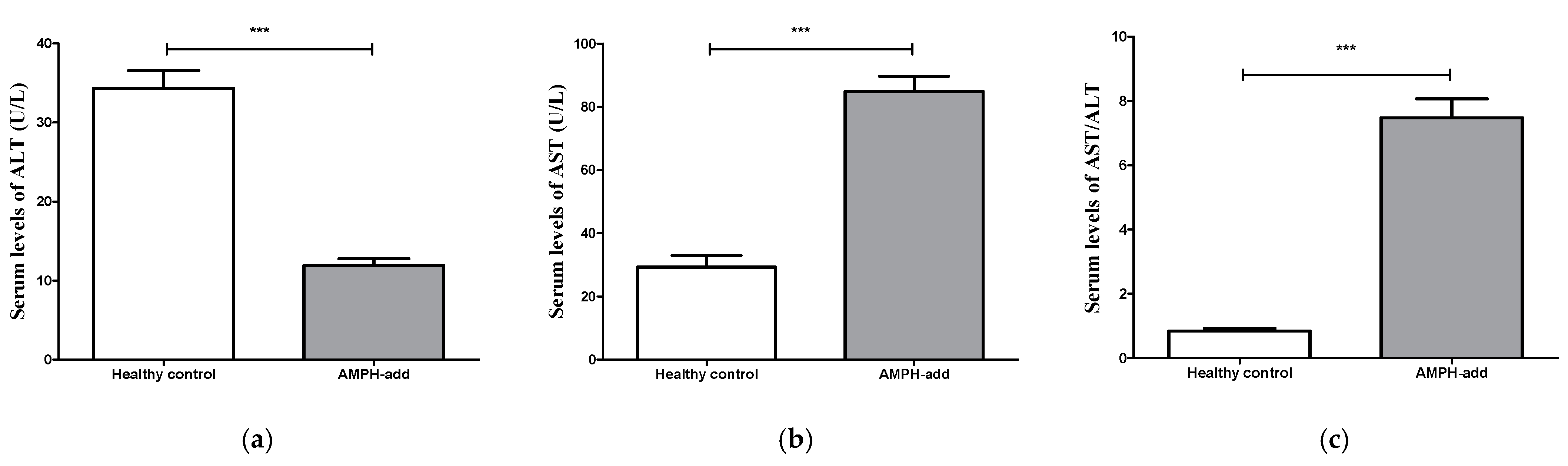
3.3.1. Alanine Aminotransferase (ALT) Level
3.3.2. Aspartate Aminotransferase (AST) Level
3.3.3. AST to ALT Ratio
3.4. Pearson’s Correlation between the Biomarkers and Proinflammatory Cytokines and Liver Transaminases (ALT and AST) in Both AMPH-adds’ Serum and Control Subjects
4. Discussion
5. Limitation and Recommendation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meghraoui, D.; Boudraa, B.; Djeddou, M.; Meksen, T.M.; Boudraa, M. Healthy and Parkinson voices discrimination based on compensation/normalization cepstral features. In Proceedings of the 2018 International Conference on Applied Smart Systems (ICASS), Médéa, Algeria, 24–25 November 2018; pp. 1–5. [Google Scholar]
- Thies, W.; Bleiler, L.; Alzheimer’s Association. 2013 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2013, 9, 208–245. [Google Scholar]
- Padmanabhan, J.; Levy, M.; Dickson, D.W.; Potter, H. Alpha1-antichymotrypsin, an inflammatory protein overexpressed in Alzheimer’s disease brain, induces tau phosphorylation in neurons. Brain 2006, 129, 3020–3034. [Google Scholar] [CrossRef] [PubMed]
- Mielke, M.M.; Vemuri, P.; Rocca, W.A. Clinical Epidemiology of Alzheimer’s Disease: Assessing Sex and Gender Differences. Clin. Epidemiol. 2014, 6, 37–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soleimani, S.M.A.; Ekhtiari, H.; Cadet, J.L. Drug-induced neurotoxicity in addiction medicine: From prevention to harm reduction. Prog. Brain Res. 2016, 223, 19–41. [Google Scholar]
- Alshmrani, S. 7% of Saudis Are Drug Users [Internet]. Saudi Arabia: Al-Hayat; Newspaper 2017. Available online: http://www.alhayat.com/article/812946/ (accessed on 15 September 2019).
- Bazmi, E.; Mousavi, F.; Giahchin, L.; Mokhtari, T.; Behnoush, B. Cardiovascular complications of acute amphetamine abuse: Cross-sectional study. Sultan Qaboos Univ. Med. J. 2017, 17, e31. [Google Scholar] [CrossRef]
- Greene, S.L.; Kerr, F.; Braitberg, G. Review article: Amphetamines and related drugs of abuse. Emerg. Med. Australas. 2008, 20, 391–402. [Google Scholar] [CrossRef]
- Rusyniak, D.E. Neurologic Manifestations of Chronic Methamphetamine Abuse. Neurol. Clin. 2011, 29, 641–655. [Google Scholar] [CrossRef] [Green Version]
- Costa, L.G.; Giordano, G.; Guizzetti, M. In vitro approaches to developmental neurotoxicity. In Reproductive and Developmental Toxicology; Academic Press: Cambridge, MA, USA, 2011; pp. 159–166. [Google Scholar]
- Alibrahim, O.; Elawad, N.; Misau, Y.A.; Shaikh, T.M.; Allam, N. Drug dependence and psychotic symptoms: A retrospective study of adolescents who abuse drugs at Al-Amal Hospital in Jeddah, Saudi Arabia. J. Public Health Afr. 2012, 3, e5. [Google Scholar] [CrossRef]
- Deik, A.; Saunders-Pullman, R.; Luciano, M.S. Substance Abuse and Movement Disorders: Complex Interactions and Comorbidities. Curr. Drug Abus. Rev. 2012, 5, 243–253. [Google Scholar] [CrossRef]
- Steinkellner, T.; Freissmuth, M.; Sitte, H.H.; Montgomery, T. The ugly side of amphetamines: Short- and long-term toxicity of 3,4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’), methamphetamine and d-amphetamine. Biol. Chem. 2011, 392, 103–115. [Google Scholar] [CrossRef] [Green Version]
- da Hage-Melim, L.I.S.; Ferreira, J.V.; de Oliveira, N.K.; Correia, L.C.; Almeida, M.R.; Poiani, J.G.; Taft, C.A.; de Paula da Silva, C.H. The Impact of Natural Compounds on the Treatment of Neurodegenerative Diseases. Curr. Org. Chem. 2019, 23, 335–360. [Google Scholar] [CrossRef]
- Tonda-Turo, C.; Origlia, N.; Mattu, C.; Accorroni, A.; Chiono, V. Current Limitations in the Treatment of Parkinson’s and Alzheimer’s Diseases: State-of-the-Art and Future Perspective of Polymeric Carriers. Curr. Med. Chem. 2019, 25, 5755–5771. [Google Scholar] [CrossRef] [PubMed]
- Alrafiah, A.; Alofi, E.; Almohaya, Y.; Hamami, A.; Qadah, T.; Almaghrabi, S.; Hakami, N.; Alrawaili, M.S.; Tayeb, H.O. Angiogenesis Biomarkers in Ischemic Stroke Patients. J. Inflamm. Research. 2021, 14, p. 4893. Available online: https://www.dovepress.com/by168.149.12.66 (accessed on 22 September 2021).
- Vicente-Rodríguez, M.; Fernández-Calle, R.; Gramage, E.; Pérez-García, C.; Ramos, M.P.; Herradón, G. Midkine Is a Novel Regulator of Amphetamine-Induced Striatal Gliosis and Cognitive Impairment: Evidence for a Stimulus-Dependent Regulation of Neuroinflammation by Midkine. Mediat. Inflamm. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Al-Rafiah, A.; Magadmi, R.; Al-Kaabi, A.; Alsomali, N. Parkinson’s Disease-Related Biomarkers That May Appear in Amphetamine Abusers. BioMed Res. Int. 2021, 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xu, E.; Liu, J.; Liu, H.; Wang, X.; Xiong, H. Inflammasome Activation by Methamphetamine Potentiates Lipopolysaccharide Stimulation of IL-1β Production in Microglia. J. Neuroimmune Pharmacol. 2018, 13, 237–253. [Google Scholar] [CrossRef]
- Tyagi, E.; Fiorelli, T.; Norden, M.; Padmanabhan, J. Alpha 1-Antichymotrypsin, an Inflammatory Protein Overexpressed in the Brains of Patients with Alzheimer’s Disease, Induces Tau Hyperphosphorylation through c-Jun N-Terminal Kinase Activation. Int. J. Alzheimer’s Dis. 2013, 2013, 606083. [Google Scholar] [CrossRef] [Green Version]
- Nilsson LN, G.; Bales, K.R.; DiCarlo, G.; Gordon, M.N.; Morgan, D.; Paul, S.M.; Potter, H. α-1-Antichymotrypsin promotes β-sheet amyloid plaque deposition in a transgenic mouse model of Alzheimer’s disease. J. Neurosci. 2001, 21, 1444–1451. [Google Scholar] [CrossRef] [Green Version]
- Hickman, S.E.; Allison, E.K.; El Khoury, J. Microglial dysfunction and defective β-amyloid clearance pathways in aging Alzheimer’s disease mice. J. Neurosci. 2008, 28, 8354–8360. [Google Scholar] [CrossRef]
- Sogawa, K.; Kodera, Y.; Satoh, M.; Kawashima, Y.; Umemura, H.; Maruyama, K.; Takizawa, H.; Yokosuka, O.; Nomura, F. Increased Serum Levels of Pigment Epithelium-Derived Factor by Excessive Alcohol Consumption-Detection and Identification by a Three-Step Serum Proteome Analysis. Alcohol. Clin. Exp. Res. 2010, 35, 211–217. [Google Scholar] [CrossRef]
- Arina, R.; Marietta, Z.; Menderes, Y.T.; Ryan, C.; Melina, N.-K.K.; Ana, L.P. Pigment Epithelium-Derived Factor Improves Paracellular Blood-Brain Barrier Integrity in the Normal and Ischemic Mouse Brain. Cell. Mol. Neurobiol. 2020, 15, 728–741. [Google Scholar] [CrossRef]
- Sanagi, T.; Yabe, T.; Yamada, H. Gene transfer of PEDF attenuates ischemic brain damage in the rat middle cerebral artery occlusion model. J. Neurochem. 2008, 106, 1841–1854. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Inagaki, Y.; Takeuchi, M.; Sasaki, N. Is pigment epithelium-derived factor level in cerebrospinal fluid a promising biomarker for early diagnosis of Alzheimer’s disease? Med. Hypotheses 2004, 63, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Lang, V.; Zille, M.; Infante-Duarte, C.; Jarius, S.; Jahn, H.; Paul, F.; Ruprecht, K.; Pina, A.L. Alzheimer’s disease: Elevated pigment epithelium-derived factor in the cerebrospinal fluid is mostly of systemic origin. J. Neurol. Sci. 2017, 375, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Zhang, X.; Chen, W.W. Association between alcohol and Alzheimer’s disease. Exp. Ther. Med. 2016, 12, 1247–1250. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.; Qi, W.; Fang, S.; Jiang, P.; Yang, C.; Mo, Y.; Dong, C.; Li, Y.; Zhong, J.; Cai, W.; et al. Pigment Epithelium-Derived Factor Plays a Role in Alzheimer’s Disease by Negatively Regulating Aβ42. Neurotherapeutics 2018, 15, 728–741. [Google Scholar] [CrossRef] [Green Version]
- Abraham, J.-D.; Calvayrac-Pawlowski, S.; Cobo, S.; Salvetat, N.; Vicat, G.; Molina, L.; Touchon, J.; Michel, B.-F.; Molina, F.; Verdier, J.-M.; et al. Combined measurement of PEDF, haptoglobin and tau in cerebrospinal fluid improves the diagnostic discrimination between alzheimer’s disease and other dementias. Biomarkers 2011, 16, 161–171. [Google Scholar] [CrossRef]
- Roher, A.E.; Maarouf, C.L.; Sue, L.I.; Hu, Y.; Wilson, J.; Beach, T.G. Proteomics-derived cerebrospinal fluid markers of autopsy-confirmed Alzheimer’s disease. Biomarkers 2009, 14, 493–501. [Google Scholar] [CrossRef] [Green Version]
- Ziliotto, N.; Bernardi, F.; Jakimovski, D.; Baroni, M.; Bergsland, N.; Ramasamy, D.P.; Weinstock-Guttman, B.; Zamboni, P.; Marchetti, G.; Zivadinov, R.; et al. Increased CCL18 plasma levels are associated with neurodegenerative MRI outcomes in multiple sclerosis patients. Mult. Scler. Relat. Disord. 2018, 25, 37–42. [Google Scholar] [CrossRef]
- McDonnell-Dowling, K. The Role of Oxidative Stress in Methamphetamine-induced Toxicity and Sources of Variation in the Design of Animal Studies. Curr. Neuropharmacol. 2017, 15, 300–314. [Google Scholar] [CrossRef] [Green Version]
- Tripathy, D.; Thirumangalakudi, L.; Grammas, P. Expression of macrophage inflammatory protein 1-α is elevated in Alzheimer’s vessels and is regulated by oxidative stress. J. Alzheimer’s Dis. 2007, 11, 447–455. [Google Scholar] [CrossRef]
- Liu, C.; Cui, G.; Zhu, M.; Kang, X.; Guo, H. Neuroinflammation in Alzheimer’s disease: Chemokines produced by astrocytes and chemokine receptors. Int. J. Clin. Exp. Pathol. 2014, 7, 8342. [Google Scholar] [PubMed]
- Bennett, S.; Grant, M.; Creese, A.J.; Mangialasche, F.; Cecchetti, R.; Cooper, H.J.; Aldred, S. Plasma levels of complement 4a protein are increased in Alzheimer’s disease. Alzheimer Dis. Assoc. Disord. 2012, 26, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Zuena, A.R.; Casolini, P.; Lattanzi, R.; Maftei, D. Chemokines in alzheimer’s disease: New insights into prokineticins, chemokine-like proteins. Front. Pharm. 2019, 10, 622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nho, K.; Kueider-Paisley, A.; Ahmad, S.; Mahmoudiandehkordi, S.; Arnold, M.; Risacher, S.L.; Louie, G.; Blach, C.; Baillie, R.; Han, X.; et al. Association of Altered Liver Enzymes With Alzheimer Disease Diagnosis, Cognition, Neuroimaging Measures, and Cerebrospinal Fluid Biomarkers. JAMA Netw. Open 2019, 2, e197978. [Google Scholar] [CrossRef]
- Giambattistelli, F.; Bucossi, S.; Salustri, C.; Panetta, V.; Mariani, S.; Siotto, M.; Cassetta, E. Effects of hemochromatosis and transferrin gene mutations on iron dyshomeostasis, liver dysfunction and on the risk of Alzheimer’s disease. Neurobiol. Aging 2012, 33, 1633–1641. [Google Scholar] [CrossRef]
- Varma, V.R.; Oommen, A.M.; Varma, S.; Casanova, R.; An, Y.; Andrews, R.M.; Toledo, J. Brain and blood metabolite signatures of pathology and progression in Alzheimer disease: A targeted metabolomics study. PLoS Med. 2018, 15, e1002482. [Google Scholar] [CrossRef]
- Sugimoto, K.; Takei, Y. Pathogenesis of alcoholic liver disease. Hepatol. Res. 2017, 47, 70–79. [Google Scholar] [CrossRef]
- He, R.; Yan, X.; Guo, J.; Xu, Q.; Tang, B.; Sun, Q. Recent Advances in Biomarkers for Parkinson’s Disease. Front. Aging Neurosci. 2018, 10, 305. [Google Scholar] [CrossRef]
- He, X.; Cheng, R.; Benyajati, S.; Ma, J. PEDF and its roles in physiological and pathological conditions: Implication in diabetic and hypoxia-induced angiogenic diseases. Clin. Sci. 2015, 128, 805–823. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.; Yin, J.; Yuan, H.; Jin, C.; Zhang, F.; Wang, Z.; Xiao, S. Blood-Derived Plasma Protein Biomarkers for Alzheimer’s Disease in Han Chinese. Front. Aging Neurosci. 2018, 10, 414. [Google Scholar] [CrossRef] [Green Version]
| Parameters | Control (n = 19) | AMPH-add (n = 17) | p-Value |
|---|---|---|---|
| Age (years) | 31.56 ± 5.04 | 32.23 ± 7.09 | 0.901 |
| Male gender | 19 (100%) | 17 (100%) | 1.000 |
| Education | |||
| University | 4 (21%) | 3 (17.6%) | 0.820 |
| Intermediate | 5 (26.4%) | 4 (23.5%) | |
| Secondary | 10 (52.6%) | 10 (58.8%) | |
| Smoking | 18 (94.1%) | 16 (94.1%) | 0.798 |
| Toombak | 12 (64.7%) | - | - |
| Parameters | Control (n = 19) | AMPH-add (n = 17) | p-Value |
|---|---|---|---|
| ACT (ng/mL) | 163,406.31 ± 60,769.13 | 834,027.99 ± 329,296.57 | 0.037 * |
| PEDF (ng/mL) | 27,709.64 ± 1672.57 | 51,804.41 ± 3205.92 | 0.001 *** |
| MIP-4 (ng/mL) | 439.84 ± 17.19 | 597.59 ± 61.31 | 0.012 * |
| Parameters | ALT | AST | AST/ALT Ratio | ACT | PEDF |
|---|---|---|---|---|---|
| AST (r) | −0.617 *** | - | - | - | - |
| Sig. | 0.000 | ||||
| AST/ALT ratio (r) | −0.800 *** | 0.915 *** | - | - | - |
| Sig. | 0.000 | 0.000 | |||
| ACT (r) | −0.296 | 0.183 | 0.250 | - | - |
| Sig. | 0.080 | 0.285 | 0.142 | ||
| PEDF (r) | −0.618 *** | 0.551 *** | 0.651 *** | 0.237 | - |
| Sig. | 0.000 | 0.000 | 0.000 | 0.164 | |
| MIP4 (r) | −0.052 | 0.069 | 0.077 | 0.078 | 0.181 |
| Sig. | 0.772 | 0.699 | 0.663 | 0.661 | 0.306 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrafiah, A.R.; Abu-Illah, M.M.; Magadmi, R.M.; Aqeel, A.; Najmi, A.; Jaddoh, S. Increased Inflammatory Markers at AMPH-Addicts Are Related to Neurodegenerative Conditions: Alzheimer’s Disease. Appl. Sci. 2022, 12, 5536. https://doi.org/10.3390/app12115536
Alrafiah AR, Abu-Illah MM, Magadmi RM, Aqeel A, Najmi A, Jaddoh S. Increased Inflammatory Markers at AMPH-Addicts Are Related to Neurodegenerative Conditions: Alzheimer’s Disease. Applied Sciences. 2022; 12(11):5536. https://doi.org/10.3390/app12115536
Chicago/Turabian StyleAlrafiah, Aziza R., Mohammed M. Abu-Illah, Rania M. Magadmi, Aqeel Aqeel, Abdulmuttaleb Najmi, and Sattam Jaddoh. 2022. "Increased Inflammatory Markers at AMPH-Addicts Are Related to Neurodegenerative Conditions: Alzheimer’s Disease" Applied Sciences 12, no. 11: 5536. https://doi.org/10.3390/app12115536
APA StyleAlrafiah, A. R., Abu-Illah, M. M., Magadmi, R. M., Aqeel, A., Najmi, A., & Jaddoh, S. (2022). Increased Inflammatory Markers at AMPH-Addicts Are Related to Neurodegenerative Conditions: Alzheimer’s Disease. Applied Sciences, 12(11), 5536. https://doi.org/10.3390/app12115536






