Abstract
Pulmonary arterial hypertension is a severe, progressive disease in children, that causes right ventricular dysfunction over time. Tissue motion annular displacement is a novel speckle-tracking derived echocardiographic parameter used in assessing ventricular function. The aim of our study was to determine the prognostic value of this echocardiographic parameter in children with pulmonary arterial hypertension. We conducted a case-control study by assessing twenty children with pulmonary arterial hypertension (idiopathic or secondary) and twenty age- and sex-matched controls, using clinical (WHO functional class, 6-min walking test), laboratory (brain natriuretic peptide level) and echocardiographic parameters (conventional and speckle-tracking derived tissue motion annular displacement) at enrolment and after one year of follow-up. According to their WHO functional class altering after one year, the pulmonary arterial hypertension patients were divided into two groups: non-worsening (eleven) and worsening (nine). The conventional echocardiographic parameters and all measured tricuspid tissue motion annular displacement indices (lateral, septal, midpoint and midpoint fractional displacement—TMADm%) were significantly lower in both pulmonary arterial hypertension groups (non-worsening and worsening) compared to controls. Comparing the worsening and non-worsening groups, only the TMADm% and brain natriuretic peptide level was significantly lower in worsening in comparison with non-worsening pulmonary arterial hypertension children (p = 0.010 and p = 0.018, respectively). In receiver-operating characteristic curve analysis, we found a cut-off value of 16.15% for TMADm% and a cut-off value of 34.35 pg/mL for the brain natriuretic peptide level that can predict worsening in pulmonary arterial hypertension children. In conclusion, tricuspid annulus midpoint fractional displacement, an angle-dependent speckle-tracking derived parameter, could be a good additional parameter in the assessment of the longitudinal right ventricular systolic function and in prediction of clinical worsening in children with pulmonary arterial hypertension.
1. Introduction
Pulmonary arterial hypertension (PAH), included in the first group of pulmonary hypertensions (Nice, 2018) [1], is a severe, progressive disease in children, that causes pulmonary vascular remodeling and right ventricular (RV) dysfunction over time.
Transthoracic echocardiography is the most accessible, non-invasive diagnostic tool for the initial assessment and clinical follow-up of children with PAH [2,3,4]. In the current guidelines, the importance of the multiparametric approach in the echocardiographic assessment of these children is emphasized [3].
According to the risk stratification model implemented by the 6th World Symposium on Pulmonary Hypertension—Pediatric Task Force in 2018 (Nice), and by the updated consensus document of the “European Pediatric Pulmonary Vascular Disease Network”, some echocardiographic parameters have been suggested as risk factors and predictors of outcome such as right atrial (RA) and right ventricular (RV) enlargement, reduced left ventricular (LV) size, increased RV/LV ratio, reduced tricuspid annular plane systolic excursion (TAPSE), increased systolic/diastolic (S/D) ratio, pulmonary artery acceleration time, low RV fractional area changes (FAC) and pericardial effusion [3,4]. However, because these parameters have only level of evidence C (due to lack of pediatric data) and because of the significant operator and interpretation variability of some echocardiographic parameters, the need for identification of more valid, easy-to-determine predictors and treatment goals in pediatric patients with PAH is emphasized [3].
Tissue motion annular displacement (TMAD) is a speckle-tracking derived echocardiographic parameter in which the annular tissue is tracked toward the ventricular apex. This angle-independent echocardiographic parameter has been used in assessing left (TMAD of the mitral valve) [5,6,7,8] and right (TMAD of the tricuspid valve) [9,10,11] ventricular function. However, its usefulness in the assessment of the right ventricular function in PAH children has not been studied so far.
The aim of our study was to determine the prognostic value of TMAD of the tricuspid valve in children with PAH.
2. Materials and Methods
2.1. Study Population
We have performed a clinical observational study on forty patients: twenty children with PAH (five idiopathic PAH, twelve PAH secondary to ventricular septal defect and three PAH secondary to truncus arteriosus communis) and twenty age- and sex-matched healthy children as a control group. After enrolment, all forty patients have undergone complete physical examination including 6 min walking test, standard and advanced echocardiographic examination (speckle tracking derived TMAD) and blood sampling for brain natriuretic peptide and were repeated after one year of follow-up. According to their WHO functional class altering after one year, the patients were divided into two groups: worsening and non-worsening. The patients were recruited at the same time from baseline and all the patients have the same 1-year follow-up. There was not any follow-up lost. We followed the methods of Muntean et al. [12]. The general characteristics of the patients involved in the present study are presented in Table 1.

Table 1.
General characteristics of the children from the study groups (control, non-worsening PAH children and worsening PAH children).
The PAH was stated according to the NICE 2018 definition [4], and all PAH children were on pulmonary vasodilator medication (mono- or combined drug). In children from the control group, who were investigated for fatigability or chest pain, any cardiac disease was ruled out.
Exclusion criteria included patients with other causes of PAH. Patients older than 18 years or those with arrhythmia were excluded from the present study.
2.2. Standard Echocardiographic Examination
The echocardiographic examination was performed by an iE33 (Philips Medical Systems, Best, The Netherlands) ultrasound system using an S5-1 transducer, by a single sonographer and minimum three beats were stored. Depth, width and frame rate (60–100/s) were adjusted for an accurate speckle-tracking postprocessing.
During the echocardiographic examination we have measured the following parameters TAPSE, RV-FAC, RV-S/D ratio, RV-MPI (myocardial performance index), RA area, tissue Doppler derived systolic myocardial velocity (S’), left ventricular eccentricity index (LV-EI) as recommended by the current guidelines [2,13,14].
2.3. TMAD Data Acquisition and Analysis
TMAD of the tricuspid valve was measured in the RV focused apical four-chamber view, using the 2-dimensional speckle tracking technique. The speckle tracking analysis was performed offline using the QLAB software (QLAB 15.0, Cardiac motion quantification software, Philips Medical Systems, Best, The Netherlands). Three points were selected in a diastolic frame: insertion of the anterior leaflet and the septal leaflet, respectively, into the tricuspid annulus and the right ventricular apex. Further, the lateral (TMADlat) and septal (TMADsept) tricuspid annular points longitudinal displacement were tracked during the cardiac cycle. Midpoint displacement (TMADmid) of the tricuspid valve annulus was also computed by the software. Further, tricuspid annulus midpoint fractional displacement (TMADm%) also known as right ventricular longitudinal shortening fraction (RV-LSF) was calculated automatically by the software, as the maximum displacement of the midpoint throughout the cardiac cycle, as follows: (end diastolic RV length − end systolic RV length) × 100/(end diastolic RV length) (Figure 1) [15].
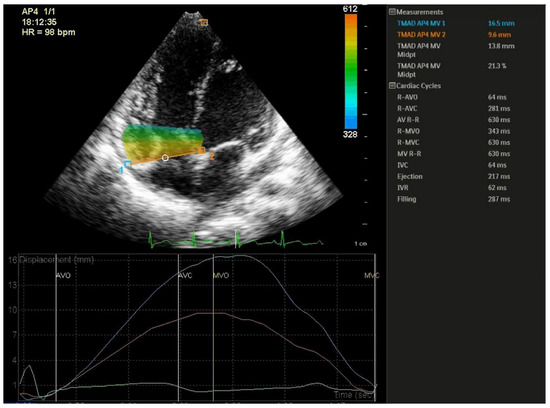
Figure 1.
Speckle-tracking derived tissue annular motion displacement of the tricuspide valve assessment. Three points were selected: insertion of the anterior tricuspid leaflet (1), the septal tricuspid leaflet (2), and the right ventricular apex.
2.4. Statistical Analysis
Statistical analysis was performed using SPSS version 20 (IBM SPSS STATISTICS 20). Data were categorized as nominal or quantitative variables. Nominal variables were expressed as numbers or percentages. Quantitative variables were expressed by mean ± standard deviation or median and percentiles (25; 75%), whenever appropriate. A Kolmogorov–Smirnov test was used in order to test for normality of distribution of quantitative variables. Differences between the mean or median between two groups were analyzed using the t-test, or Mann–Whitney test when appropriate. Differences between three groups with normally distributed variables were analyzed using general linear model test with Bonferroni post-hoc processing. Receiver-operating characteristic (ROC) curves were constructed, and areas under curve were calculated. Sensitivities and specificities were determined for the ability to identify worsening PAH children. A p-value of <0.05 was considered statistically significant.
3. Results
3.1. General Information
A total of forty children were included in the present study, twenty with PAH (eleven in the non-worsening group and nine in the worsening group) and twenty control children. As summarized in Table 1, there were no statistically significant differences between the three groups regarding age, sex, height, weight and body surface area.
Regarding 6MWD, we found a progressive decrease in this parameter in the three groups, with the largest value in the control group and the lowest in the worsening PAH group. Further, we observed that the BNP level was higher in both PAH groups in comparison to the control group, with the largest value in the worsening group. We found a statistically significant difference between worsening group and the two other groups.
3.2. Conventional Echocardiographic Parameters
Table 2 shows comparison of echocardiographic parameters between the three study groups (control, non-worsening PAH children, worsening PAH children).

Table 2.
Comparison of echocardiographic parameters between the study groups (control, non-worsening PAH children and worsening PAH children).
Regarding conventional echocardiographic parameters, significant differences were observed in RV-FAC, RV-MPI, RV S/D, S’, LV-EI between non-worsening PAH children and control group. We performed the receiver-operator characteristic analysis for all these parameters, and the reference group was the non-worsening PAH group. The ROC curves are presented in Figure 2.
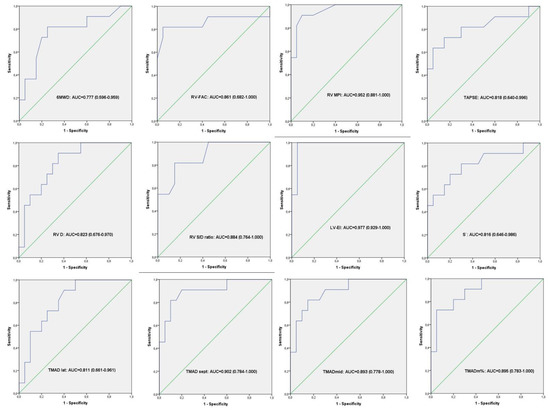
Figure 2.
Receiver-operator characteristic analysis. ROC curves result from all the variables (echocardiography and 6MWD) that showed significant difference between controls and non-worsening PAH group.
The same significant differences were observed in RV-FAC, RV-MPI, RV S/D, S’, LV-EI between the worsening group and control group. In addition, comparing these two last groups, we also found a statistical difference in the indexed RV and RA area, explained by the fact that in the worsening group, the right ventricle was more enlarged than in the non-worsening group (Table 2). We have also performed the receiver-operator characteristic analysis for all these parameters, and the reference group was the worsening PAH group. The ROC curves are presented in Figure 3.
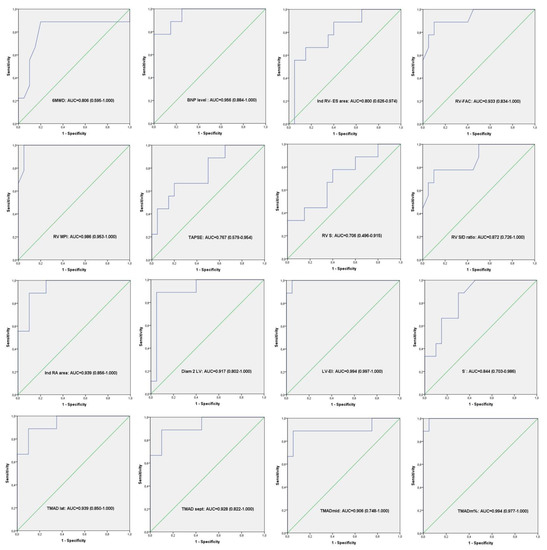
Figure 3.
Receiver-operator characteristic analysis. ROC curves result from all the variables (echocardiography, BNP level and 6MWD) that showed significant difference between controls and worsening PAH group.
However, comparing the worsening and non-worsening PAH groups, we found no statistically significant differences regarding conventional echocardiographic parameters (Table 2).
3.3. TMAD Analysis
Considering the speckle-tracking derived TMAD of tricuspid valve, all measured TMAD indices (TMADlat, TMADsept, TMADmid and TMADm%) were significantly decreased in the non-worsening group as well as in the worsening group in comparison with the control group. Additionally, in all three groups, we found a descendent distribution of the TMAD values from the lateral to mid and to the septal point (Figure 4).
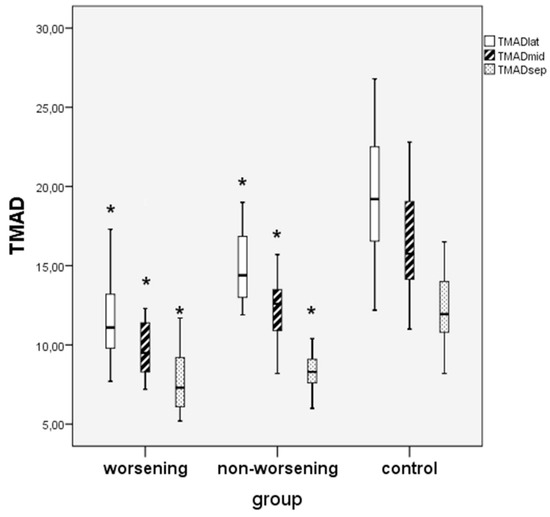
Figure 4.
Comparison between worsening, non-worsening PAH children and controls regarding TMAD of tricuspid valve values in three points: lateral, midpoint and septal. * worsening or non-worsening PAH vs. control group, p < 0.05.
Furthermore, we compared the TMAD indices between the two PAH groups. Although all were lower in the worsening group compared to the non-worsening group, only the TMADm% was significantly decreased in worsening PAH children (p = 0.010) (Table 2, Figure 5).
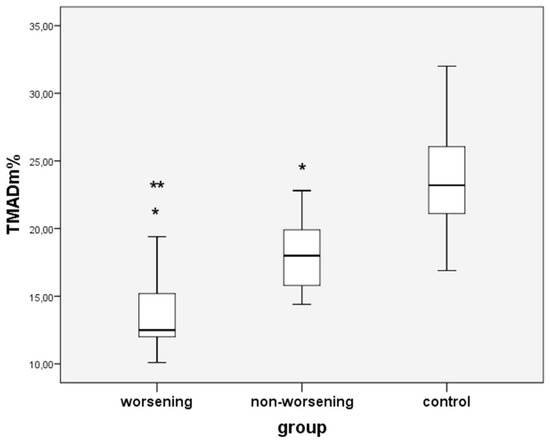
Figure 5.
Comparison between worsening, non-worsening PAH children and controls regarding TMADm%; * non-worsening or worsening PAH vs. controls; ** worsening vs. non-worsening PAH group; p < 0.05.
We have performed ROC curve analysis for TMADm% and BNP level that showed significant difference between non-worsening and worsening PAH groups. We found a cut-off value of 16.15% for TMADm% that can predict worsening in PAH children, with a sensitivity and specificity of 88.90% and 72.70%, respectively (Figure 6).
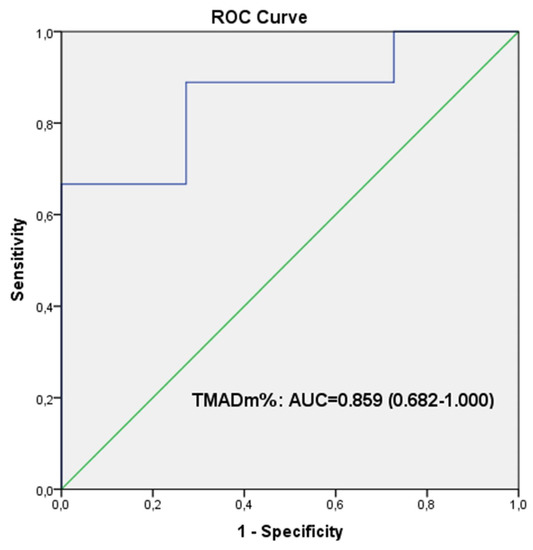
Figure 6.
Receiver-operator characteristic analysis. ROC curves result from TMADm% that showed significant difference between non-worsening and worsening PAH groups. The optimum cut-off value for TMADm% to predict clinical worsening was 16.15%; the sensitivity and specificity were 88.90% and 72.70%, respectively.
Additionally, we found a cut-off value of 34.35 pg/mL for BNP level that can predict worsening in PAH children, with a sensitivity and specificity of 77.80% and 72.70%, respectively (Figure 7).
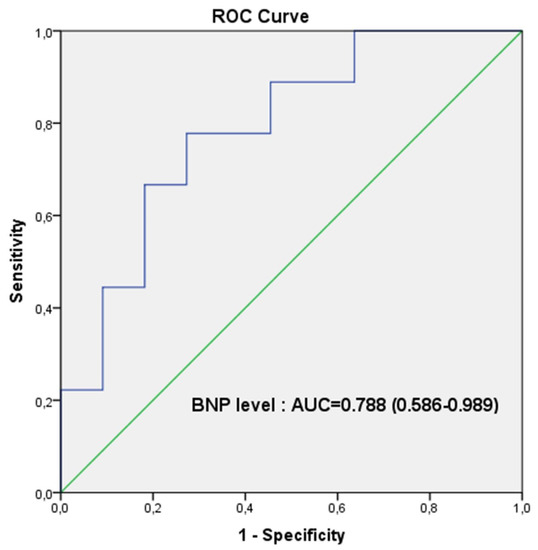
Figure 7.
Receiver-operator characteristic analysis. ROC curves result from BNP level that showed significant difference between non-worsening and worsening PAH groups. The optimum cut-off value for BNP level to predict clinical worsening was 34.35 pg/mL; the sensitivity and specificity were 77.80% and 72.70%, respectively.
4. Discussion. This Study Emphasizes the Prognostic Value of TMAD of the Tricuspid Valve in PAH Children
4.1. Parameters for Assessing RV Function
Evaluation of the right ventricle is paramount in PAH patients, considering that right ventricular function determines the prognosis and the treatment responsiveness in these patients [16].
Some conventional echocardiographic parameters such as RA dimension, RV dimension, TAPSE, S/D ratio, FAC, pulmonary artery acceleration time, have been suggested as predictors of outcome in PAH patients (Nice, 2018) [3,4]; however, the need for identification of more valid, easy-to-determine predictors and treatment goals in pediatric patients with PAH is emphasized [3].
In our study, we found a significant decrease in the following conventional parameters RV-FAC, TAPSE, S’ or increase in the following conventional parameters RV-MPI, RV S/D ratio, LV-EI between non-worsening PAH children compared to controls and between worsening PAH children compared to controls. In addition, these parameters were more affected in the worsening group in comparison with the non-worsening group, however, in our study, there was no significant difference between the two groups. We speculate that this could be explained by the fact that in our study, the majority of patients in the worsening group has a baseline WHO functional class ≤ 2, so in that phase of the disease, the conventional echocardiographic parameters are not sensitive enough to predict worsening.
4.2. RV Longitudinal Function and TMAD of the Tricuspid Valve
Anatomical studies have shown that the longitudinal fibers are predominant in the RV, localized in the subendocardial layer, whereas less circumferential fibers are found in the superficial layer in healthy children. This explains why the longitudinal shortening is a greater contributor to RV stroke volume [17,18,19].
Echocardiographic assessment of RV longitudinal function, according to the present guidelines [3,13,14], includes the following parameters: FAC, TAPSE and RV strain measurements.
The TMAD is a novel parameter derived from speckle-tracking echocardiography and is based on tracking bright echoes within the myocardium [15]. Although it was determined offline in our study with specialized software, the new echo machines allow an easy way to obtain this parameter.
The TMAD of tricuspid valve is a method to evaluate the longitudinal motion of the tricuspid annulus in different points (lateral, medial and midpoint). It was validated against magnetic resonance imaging (MRI) derived RV ejection fraction (EF) in assessing RV function [10,20]. Although tricuspid annular plane systolic excursion (TAPSE) using M-mode is a widely used parameter for assessing RV function, it evaluates only the motion of the tricuspid annulus. We speculate that TMAD, especially TMADm%, are more reliable parameters as they consider the complete longitudinal contraction (from base to apex), so is factoring the RV length from end-diastole to end-systole.
Our study demonstrated that TMADlat was higher than TMADmid and TMADsept, so the values differed based on the site of measurements. Li, Y. et al. found the same distribution of the different TMAD values [20]. According to these values, the displacement was higher at the level of the RV free wall than the septal wall, likely explained by the interventricular mechanical coupling between the two ventricles through the interventricular septum. This finding is consistent with prior studies demonstrating the ventricular interdependence [20,21].
Moreover, tricuspid annulus midpoint fractional displacement (TMADm%) also known as right ventricular longitudinal shortening fraction (RV-LSF), calculated as the maximum displacement of the tricuspid midpoint throughout the cardiac cycle, is a more complex parameter that factors the RV length throughout the cardiac cycle. The TMADm% has been studied as a marker of the RV dysfunction. Maniwa et al. found that a poor RV systolic function, expressed by 3-dimensional echocardiography—measured RVEF < 45%—was detected by a TMADm% < 14.7% with a high sensitivity and specificity of 93% and 95%, respectively, followed by RV free wall longitudinal strain [22]. Li et al. found that TMADm% is the best predictor for RV dysfunction expressed by MRI-derived RVEF in PAH patients, with an optimal cut-off value of 16.05% [20]. In a study conducted in infants (age ranged from 3 to 11 months) with dominant right ventricular physiology who underwent bidirectional Glenn anastomosis, Penk et al. demonstrated that TMAD of the tricuspid valve is an independent predictor of midterm mortality or heart transplantation in these children, with a mean value of 10% for non-survivors or heart transplanted [23]. Beyls et al. found that TMADm% (named RV longitudinal shortening fraction in their study) was markedly decreased in COVID-19 patients with acute cor pulmonale, and a cut-off value of 17% is able to identify RV dysfunction in these patients, with high specificity and sensibility [24].
4.3. Prognostic Value of TMAD in PAH Children
The prognostic value of TMAD of the tricuspid valve in PAH children was not studied so far. We found that, although all TMAD parameters are lower in worsening group as compared to non-worsening group, only TMADm% is significantly lower (p < 0.05). Furthermore, we showed that a cut-off value of 16.15% for TMADm% is associated with clinical worsening in PAH children.
In addition to TMADm%, BNP level is significantly lower in worsening group as compared to non-worsening group (p < 0.05). However, comparing the ROC curves for the two parameters (TMADm% and BNP level), we observed a better sensitivity of TMADm% to predict worsening, expressed by the area under curve: AUC = 0.859 (0.682–1.000) for the TMADm% in comparison to AUC = 0.788 (0.586–0.989) for the BNP level.
According to our knowledge, this is the first study that describes the predictive value of TMAD in children with PAH.
5. Conclusions
Tricuspid annulus midpoint fractional displacement, namely TMADm%, an angle-dependent speckle-tracking derived parameter, could be a good additional parameter in the assessment of the longitudinal RV systolic function. Since TMADm% is significantly lower in worsening PAH group, it can be considered a good parameter in predicting clinical worsening in children with PAH.
6. Study Limitations
The first limitation of our study is that we have not compared TMAD with MRI which is currently the gold standard for RV ejection fraction assessment. However, several studies have already demonstrated good correlation between TMAD of the tricuspide valve measured by 2D-speckle tracking and MRI-derived RV ejection fraction [10,20,21]. The small number of cases included in the present study is another limitation, so we consider that our findings need to be confirmed by further studies with a larger number of cases.
Author Contributions
Conceptualization, I.M., C.C.Ș. and R.T.; Methodology, I.M., C.C.Ș. and M.M.; Formal Analysis, I.M.; Investigation, I.M., M.M. and C.C.Ș.; Resources, I.M., A.F., M.M. and R.T.; Data Curation, I.M.; Writing—Original Draft Preparation, I.M., C.C.Ș. and A.F.; Writing—Review & Editing, I.M., A.F. and R.T.; Supervision, R.T.; Project Administration, I.M. and A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the University of Medicine, Pharmacy, Science and Technology “George Emil Palade” of Târgu Mureș Research Grant number 511/5/17 January 2022.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of University of Medicine, Pharmacy, Science and Technology “George Emil Palade” of Târgu Mureș (Approval Number: 1596/2 February 2022).
Informed Consent Statement
Written informed consent was obtained from all the patients’ parents/legal guardian included in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef] [PubMed]
- Koestenberger, M.; Apitz, C.; Abdul-Khaliq, H.; Hansmann, G. Transthoracic echocardiography for the evaluation of children and adolescents with suspected or confirmed pulmonary hypertension. Expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension. The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and DGPK. Heart 2016, 102 (Suppl. 2), ii14–ii22. [Google Scholar] [PubMed]
- Hansmann, G.; Koestenberger, M.; Alastalo, T.P.; Apitz, C.; Austin, E.D.; Bonnet, D.; Budts, W.; D’Alto, M.; Gatzoulis, M.A.; Hasan, B.S.; et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: The European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J. Heart Lung Transplant. 2019, 38, 879–901. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, E.B.; Abman, S.H.; Adatia, I.; Beghetti, M.; Bonnet, D.; Haworth, S.; Ivy, D.D.; Berger, R.M. Paediatric pulmonary arterial hypertension: Updates on definition, classification, diagnostics and management. Eur. Respir. J. 2019, 53, 1801916. [Google Scholar] [CrossRef] [PubMed]
- Asada, D.; Okumura, K.; Ikeda, K.; Itoi, T. Tissue motion annular displacement of the mitral valve can be a useful index for the evaluation of left ventricular systolic function by echocardiography in normal children. Pediatr. Cardiol. 2018, 39, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Black, D.E.; Bryant, J.; Peebles, C.; Godfrey, K.M.; Hanson, M.; Vettukattil, J.J. Tissue motion annular displacement of the mitral valve using two-dimensional speckle tracking echocardiography predicts the left ventricular ejection fraction in normal children. Cardiol. Young 2014, 24, 640–648. [Google Scholar] [CrossRef][Green Version]
- Liu, L.; Tuo, S.; Zhang, J.; Zuo, L.; Liu, F.; Hao, L.; Sun, Y.; Yang, L.; Shao, H.; Qi, W.; et al. Reduction of left ventricular longitudinal global and segmental systolic functions in patients with hypertrophic cardiomyopathy: Study of two-dimensional tissue motion annular displacement. Exp. Ther. Med. 2014, 7, 1457–1464. [Google Scholar] [CrossRef]
- Liu, S.; Ren, W.; Zhang, J.; Ma, C.; Yang, J.; Zhang, Y.; Guan, Z. Incremental value of the tissue motion of annular displacement derived from speckle-tracking echocardiographu for differentiating chronic constrictive pericarditis from restrictive cardiomyoptahy. J. Ultrasound Med. 2018, 37, 2637–2645. [Google Scholar] [CrossRef]
- Alonso, P.; Andres, A.; Miro, V.; Igual, B.; Sánchez, I.; Salvador, A. Diagnostic power of echocardiographic speckle tracking of the tricuspid annular motion to assess right ventricular dysfunction. Int. J. Cardiol. 2014, 172, e218–e219. [Google Scholar] [CrossRef]
- Ahmad, H.; Mor-Avi, V.; Lang, R.M.; Nesser, H.J.; Weinert, L.; Tsang, W.; Steringer-Mascherbauer, R.; Niel, J.; Salgo, I.S.; Sugeng, L. Assessment of right ventricular function using echocardiographic speckle tracking of the tricuspid annular motion: Comparison with cardiac magnetic resonance. Echocardiography 2012, 29, 19–24. [Google Scholar] [CrossRef]
- Yang, H.S.; Kim, T.Y. Intraoperative transesophageal echocardiography in tricuspid valve surgery. Clin. Ultrasound 2019, 4, 67–77. [Google Scholar] [CrossRef]
- Muntean, I.; Benedek, T.; Melinte, M.; Suteu, C.; Toganel, R. Deformation pattern and predictive value of right ventricular longitudinal strain in children with pulmonary arterial hypertension. Cardiovasc. Ultrasound 2016, 14, 27. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lopez, L.; Colan, S.D.; Frommelt, P.C.; Ensing, G.J.; Kendall, K.; Younoszai, A.K.; Lai, W.W.; Geva, T. Recommendations for Quantification Methods During the Performance of a Pediatric Echocardiogram: A Report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J. Am. Soc. Echocardiogr. 2010, 23, 465–495. [Google Scholar] [CrossRef] [PubMed]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [PubMed]
- Geyer, H.; Caracciolo, G.; Abe, H.; Wilansky, S.; Carerj, S.; Gentile, F.; Nesser, H.J.; Khandheria, B.; Narula, J.; Sengupta, P.P. Assessment of myocardial mechanics using speckle tracking echocardiography: Fundamentals and clinical applications. J. Am. Soc. Echocardiogr. 2010, 23, 351–369. [Google Scholar] [CrossRef]
- Condon, D.F.; Nickel, N.P.; Anderson, R.; Mirza, S.; de Jesus Perez, V.A. The 6th World Symposium on Pulmonary Hypertension: What’s old is new. F1000Research 2019, 8, 888. [Google Scholar] [CrossRef]
- Ho, S.H.; Nihoyannopoulos, P. Anatomy, echocardiography and normal right ventricular dimensions. Heart 2006, 92 (Suppl. 1), i2–i13. [Google Scholar] [CrossRef]
- Carlsson, M.; Ugander, M.; Heiberg, E.; Arheden, H. The quantitative relationship between longitudinal and radial function in left, right, and total heart pumping in humans. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H636–H644. [Google Scholar] [CrossRef]
- Rushmer, R.F.; Crystal, D.K.; Wagner, C. The functional anatomy of ventricular contraction. Circ. Res. 1953, 1, 162–170. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Yang, Y.; Liu, M.; Meng, X.; Shi, Y.; Zhu, W.; Lu, X. Tricuspid annular displacement measured by 2-dimensional speckle tracking echocardiography for predicting right ventricular function in pulmonary hypertension. Medicine 2018, 97, e11710. [Google Scholar] [CrossRef]
- Li, Y.D.; Wang, Y.D.; Zhai, Z.G.; Guo, X.J.; Wu, Y.F.; Yang, Y.H.; Lu, X.Z. Relationship between echocardiographic and cardiac magnetic resonance imaging-derived measures of right ventricular function in patients with chronic thromboembolic pulmonary hypertension. Thromb. Res. 2015, 135, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Maniwa, N.; Hozumi, T.; Takemoto, K.; Wada, T.; Kashiwagi, M.; Shimamura, K.; Shiono, Y.; Kuroi, A.; Matsuo, Y.; Ino, Y.; et al. Value of tissue-tracking tricuspid annular plane by speckle-tracking echiocardiography for the assessment of right ventricular systolic dysfunction. Echocardiography 2018, 36, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Penk, J.S.; Zaidi, S.J.H.; Lefaiver, C.A.; Muangmingsuk, S.; Cui, V.W.; Roberson, D.A. Tissue motion annulat displacement predicts mortality/transplant after the bidirectional Glenn. World J. Pediatr. Congenit. Heart Surg. 2018, 9, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Beyls, C.; Bohbot, Y.; Huette, P.; Booz, T.; Daumin, C.; Abou-Arab, O.; Mahjoub, Y. Usefulness of Right Ventricular Longitudinal Shortening Fraction to Detect Right Ventricular Dysfunction in Acute Cor Pulmonale Related to COVID-19. J. Cardiothorac. Vasc. Anesth. 2021, 35, 3594–3603. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).