Hydrodynamic Fingering Induced by Gel Film Formation in Miscible Fluid Systems: An Experimental and Mathematical Study
Abstract
:1. Introduction
2. Methods
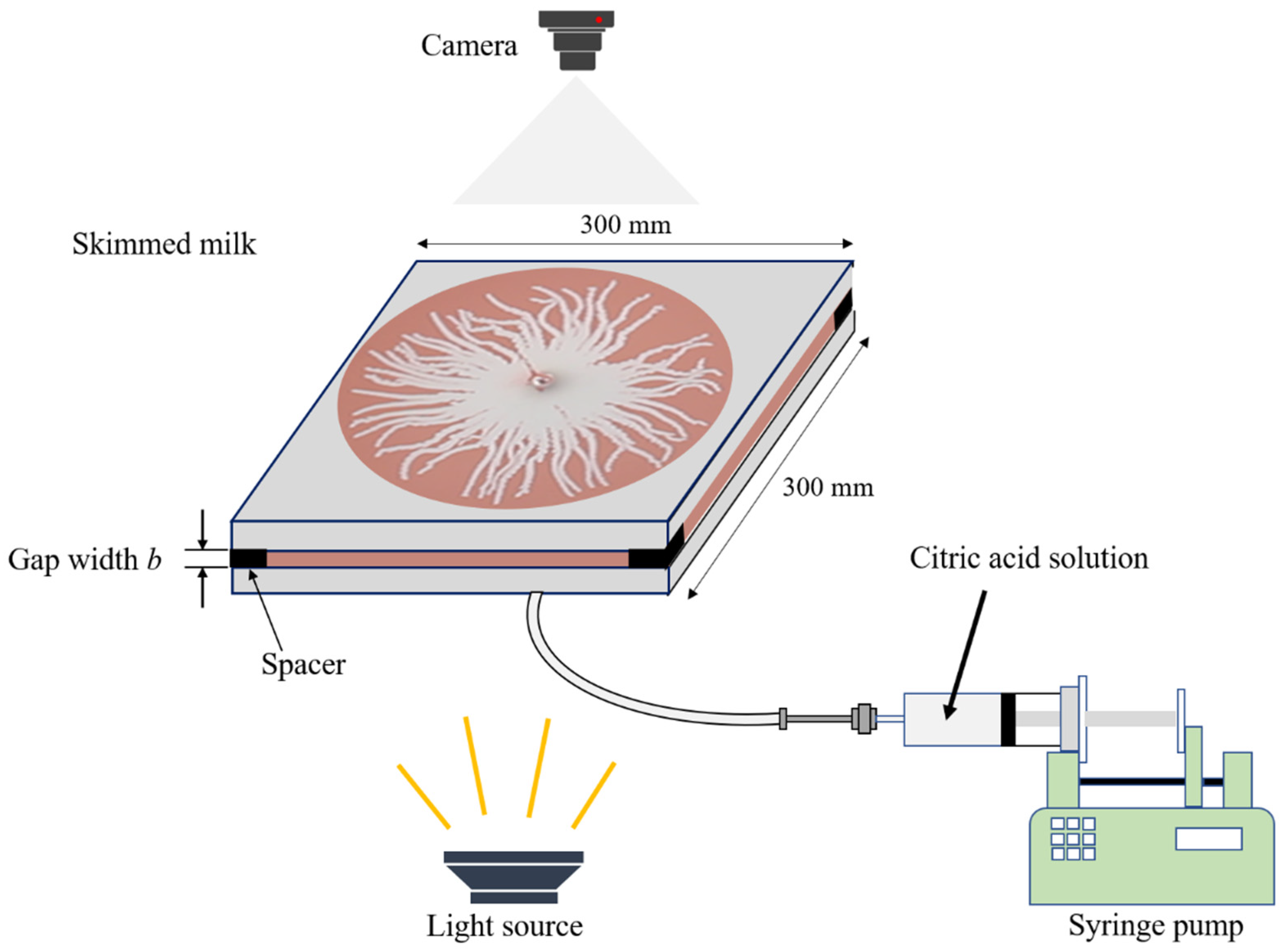
2.1. Experimental Setup
2.2. Fluid Pair
2.3. Experimental Procedures
3. Results and Discussion
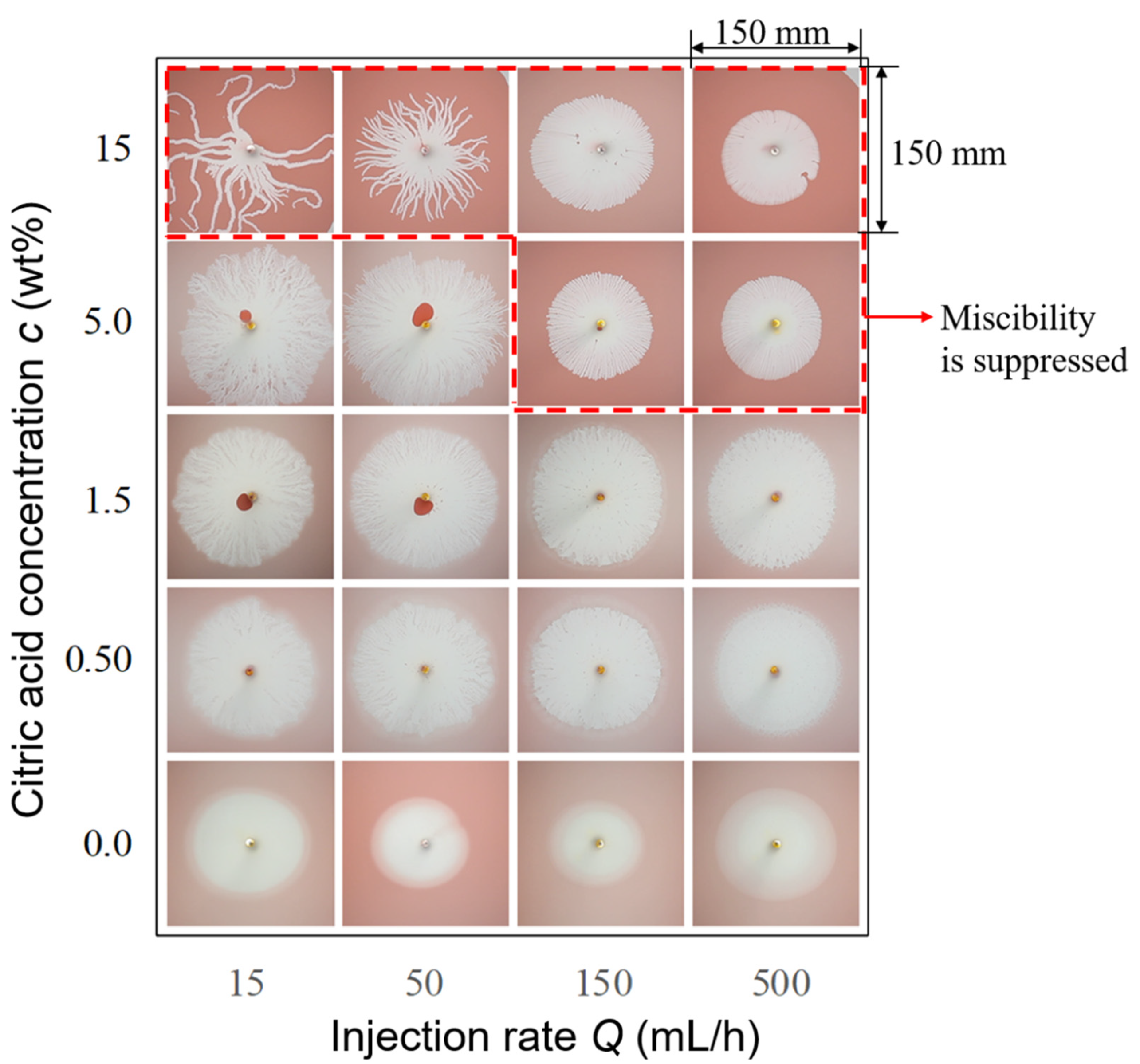
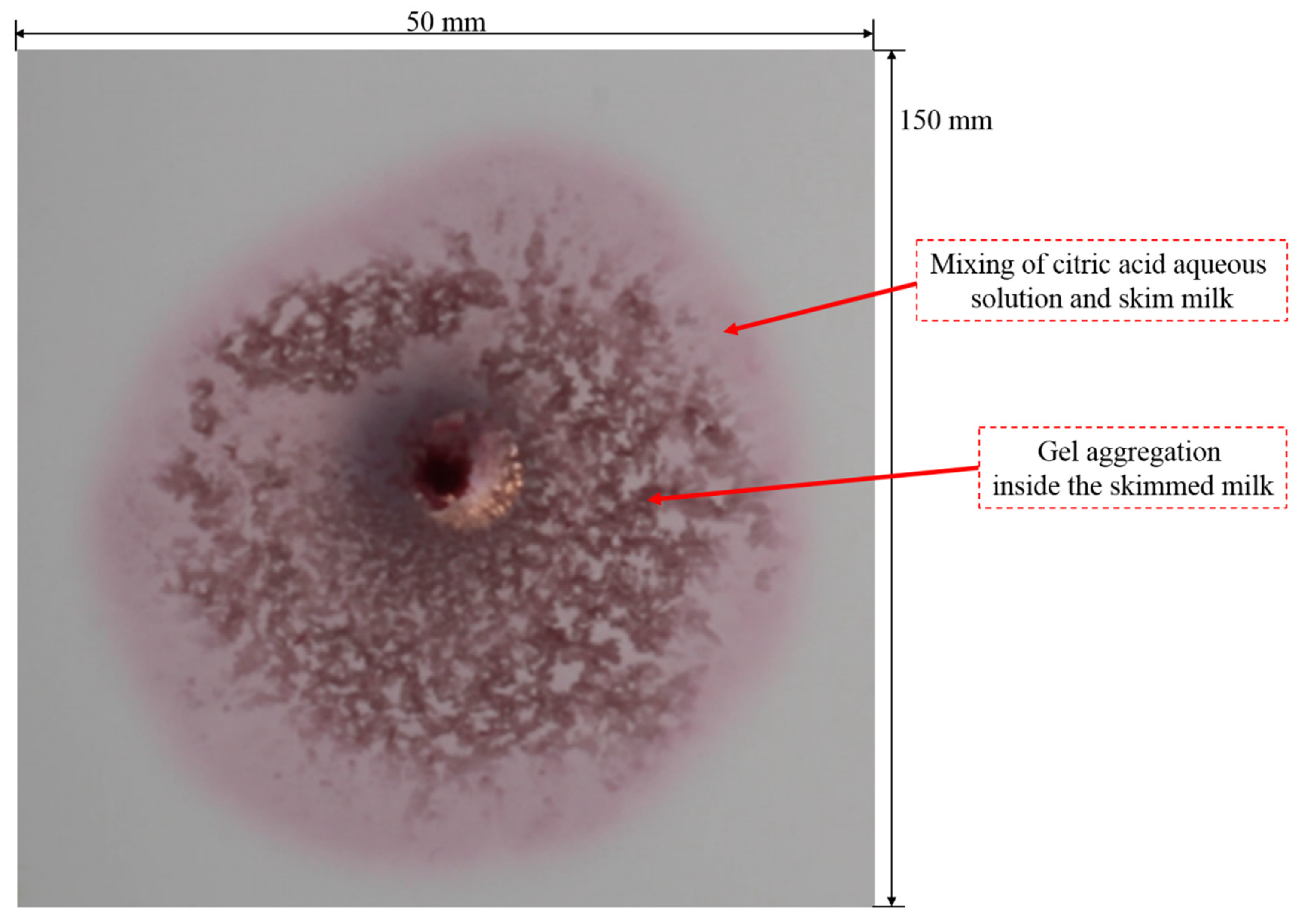
3.1. Typical Fingering Structure Induced by Gel Formation
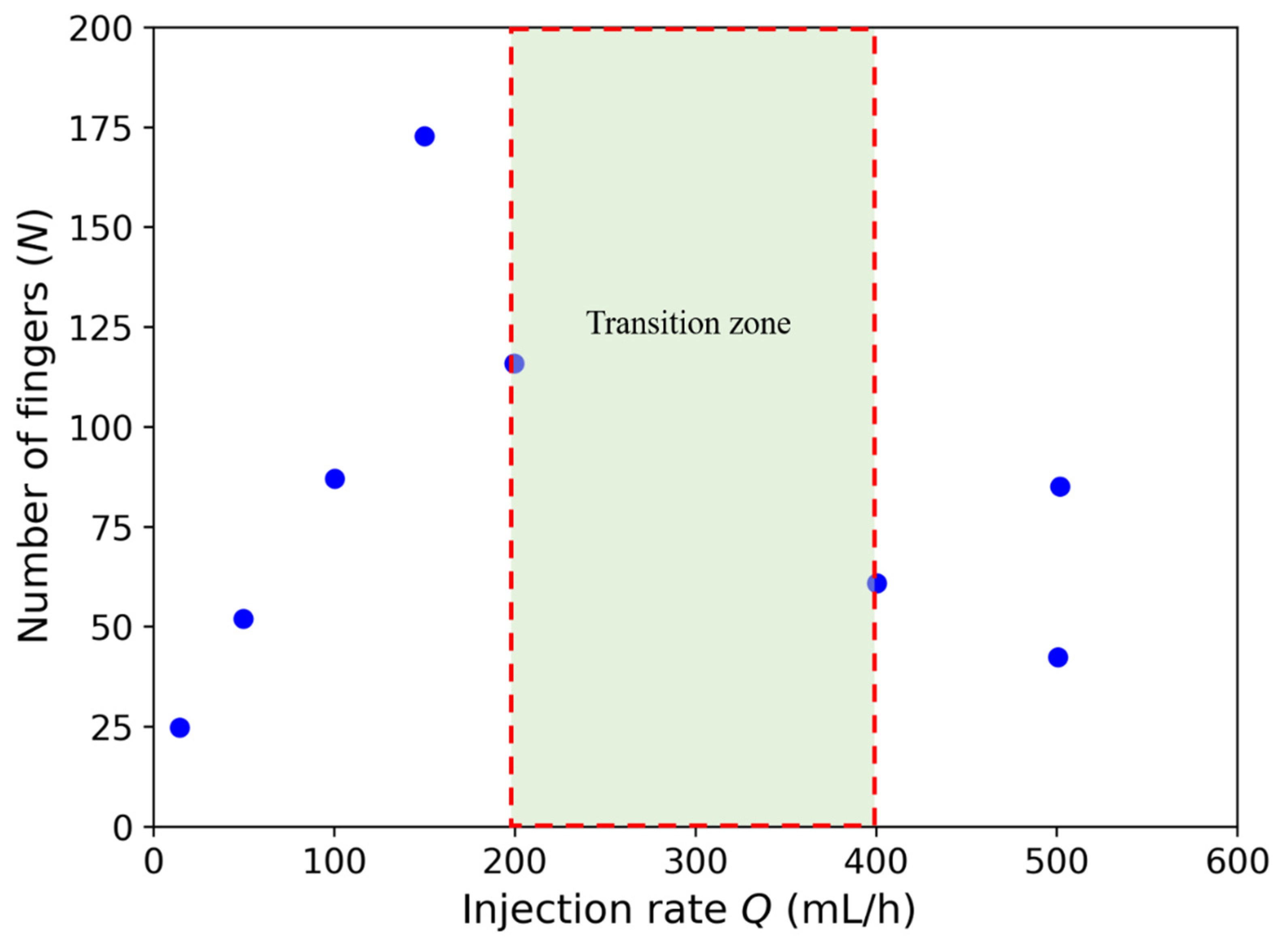
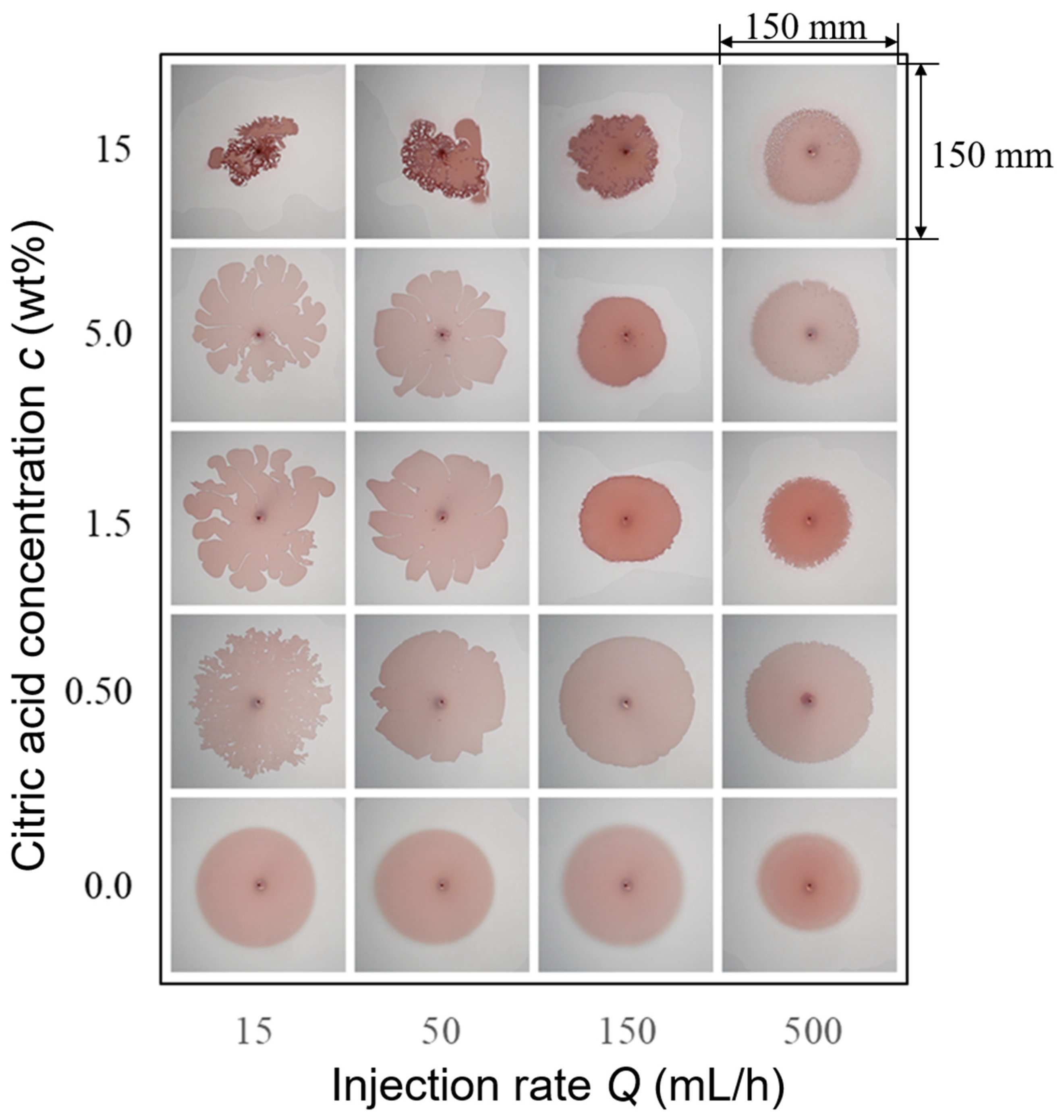
3.2. Effects of Injection Rate and Concentration of the Citric Acid on Displacement Pattern
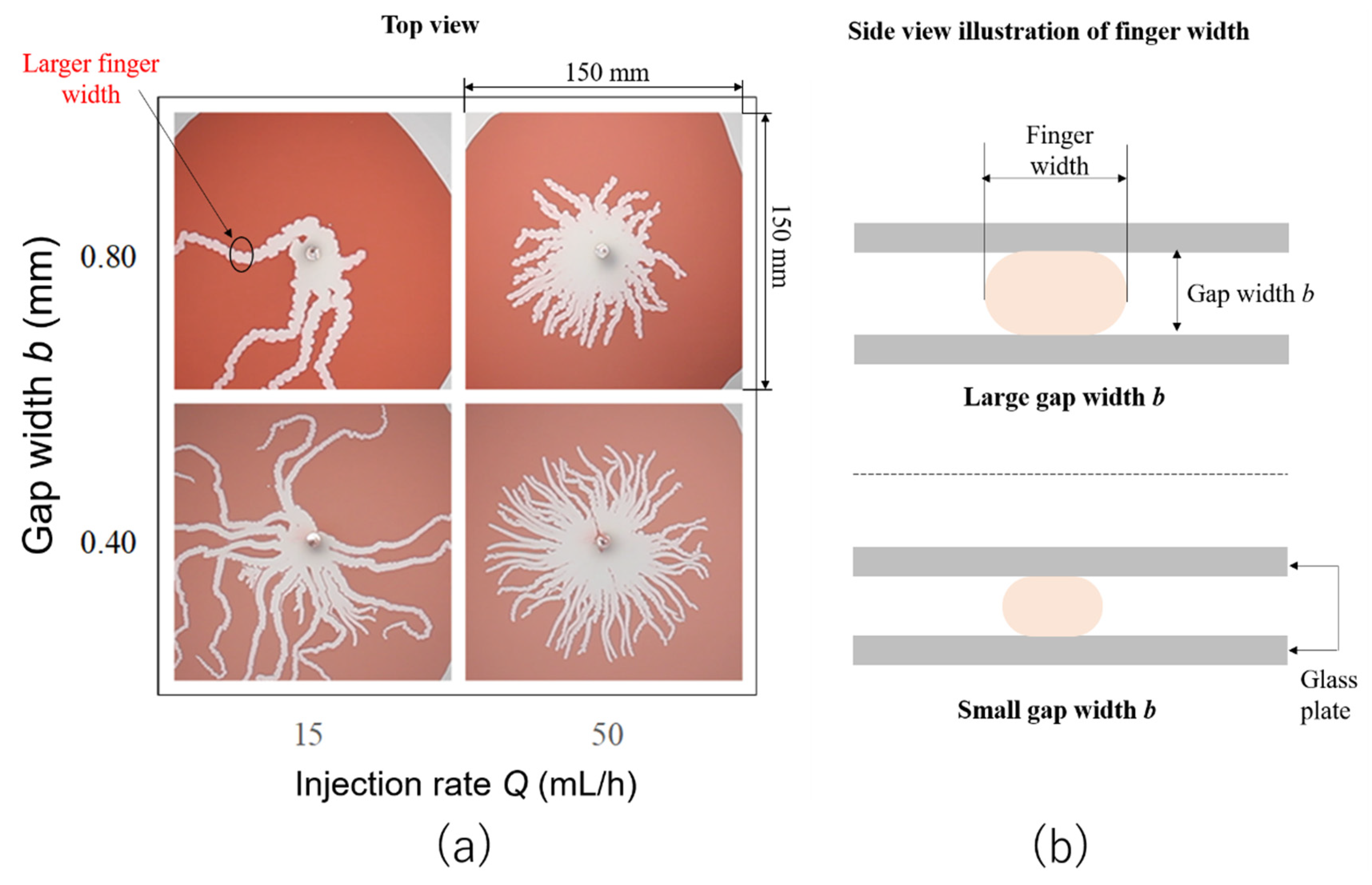
3.3. Gap Width Effects on the Finger Width
3.4. Reverse Injection Displacement Pattern (Skim Milk Injected into Citric Acid)
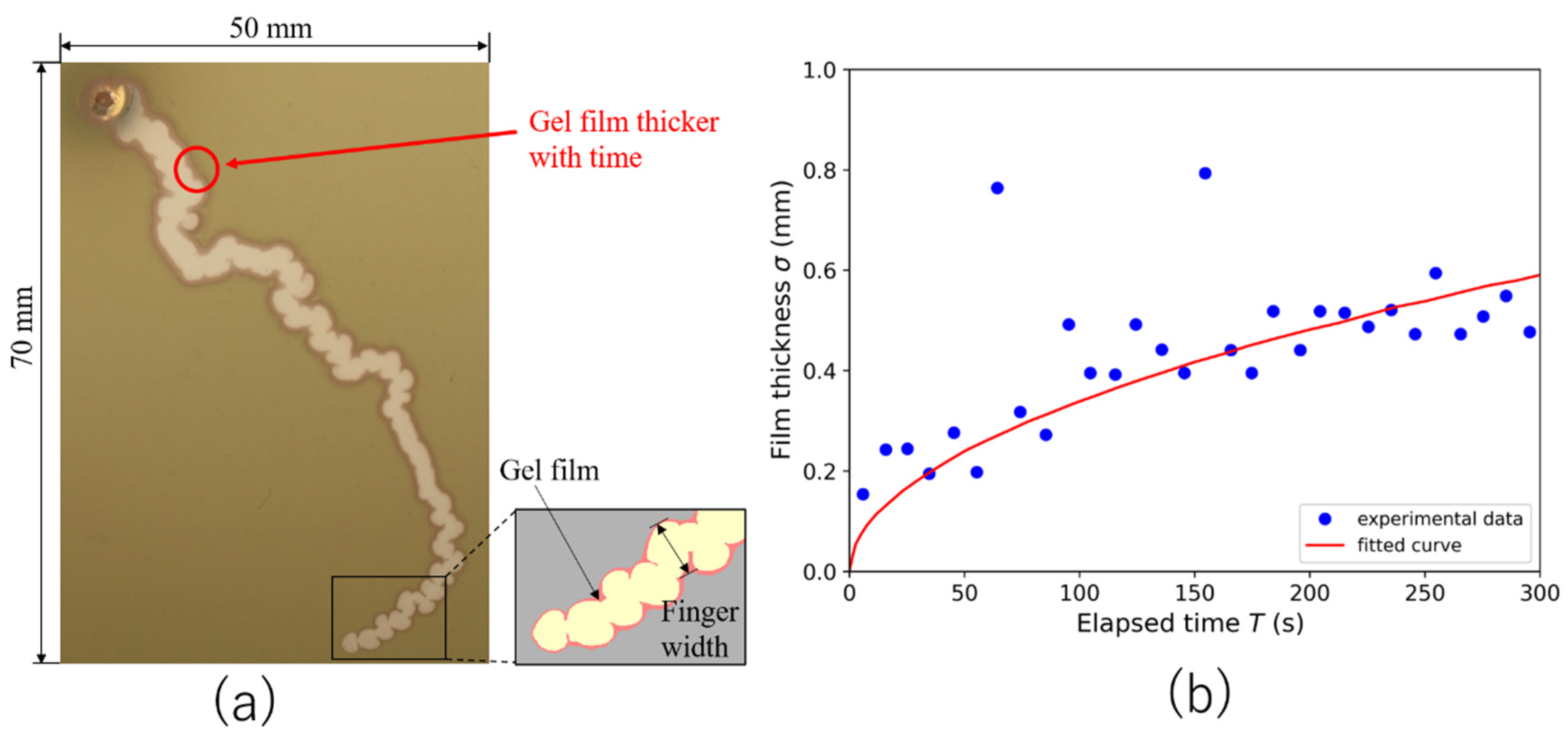
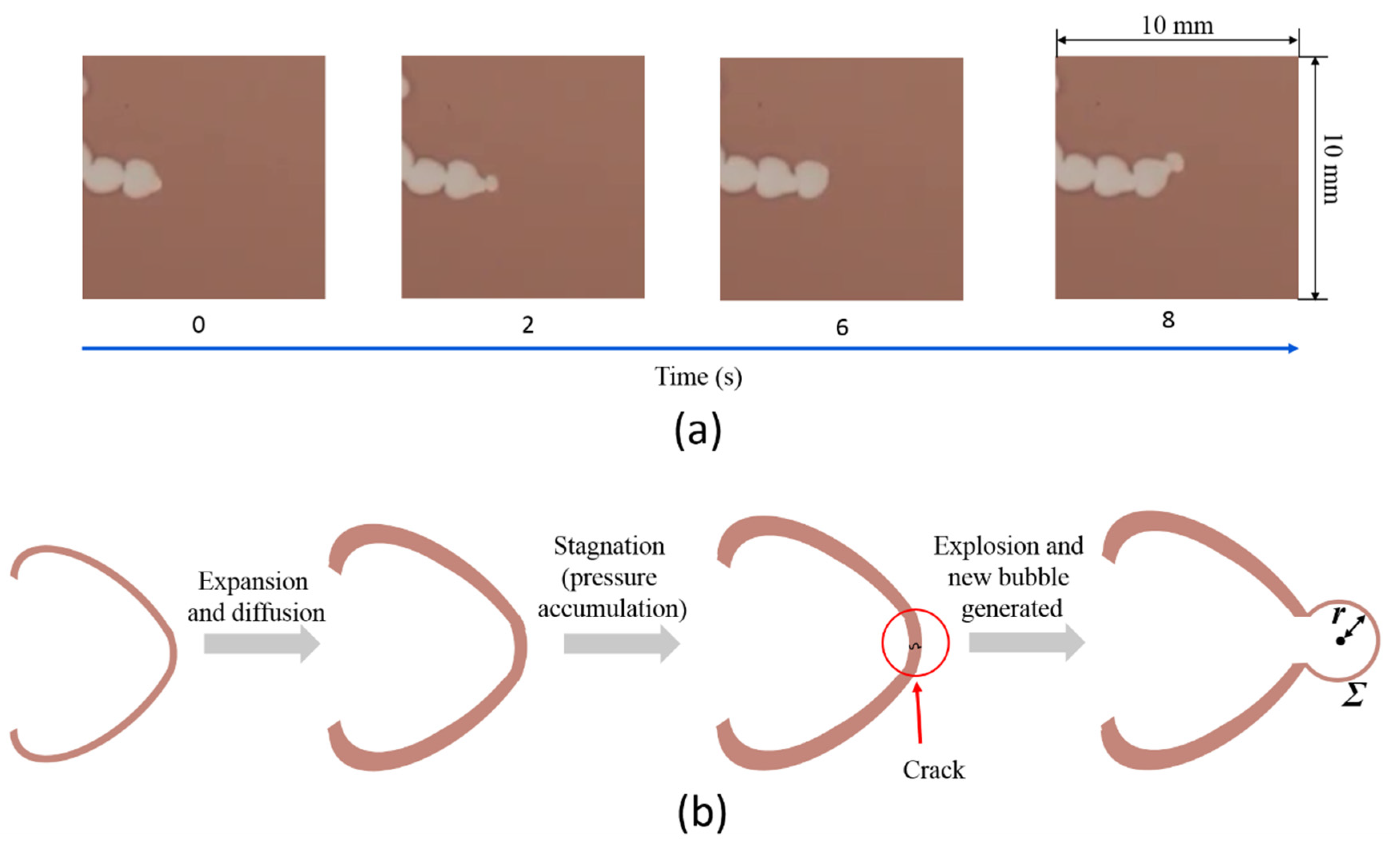
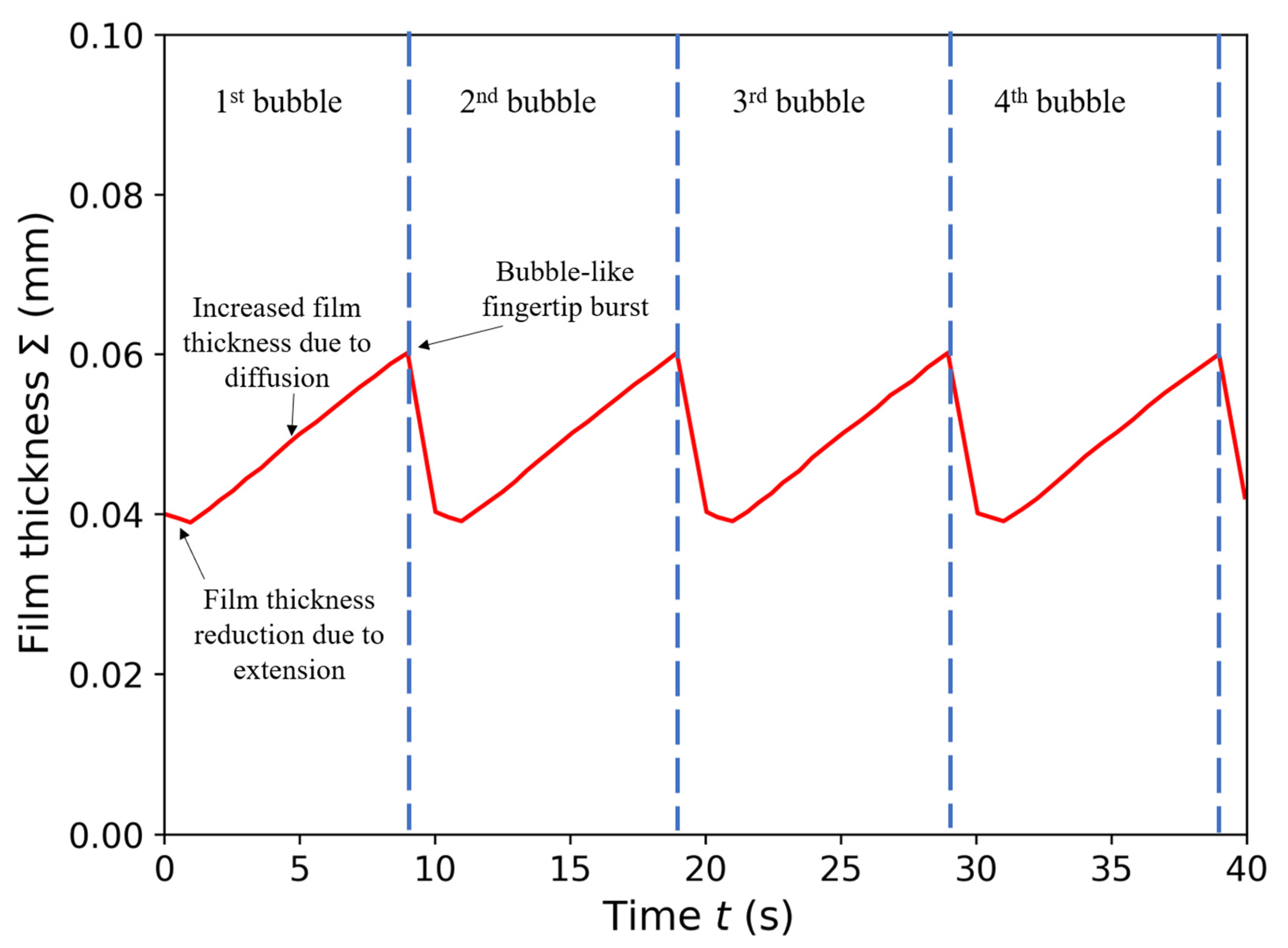
3.5. Mathematical Model of Sequential Growth of Gel Film Thickness at the Fingertip
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghatak, A.; Chaudhury, M.K.; Shenoy, V.; Sharma, A. Meniscus Instability in a Thin Elastic Film. Phys. Rev. Lett. 2000, 85, 4329–4332. [Google Scholar] [CrossRef] [Green Version]
- Sandnes, B.; Flekkoy, E.G.; Knudsen, H.A.; Maloy, K.J.; See, H. Patterns and flow in frictional fluid dynamics. Nat. Commun. 2011, 2, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhury, M.K.; Chakrabarti, A.; Ghatak, A. Adhesion-induced instabilities and pattern formation in thin films of elastomers and gels. Eur. Phys. J. E 2015, 38, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, S.; Nakanishi, Y.; Hyodo, A.; Wang, L.; Suekane, T. Three-dimensional fingering structure associated with gravitationally unstable mixing of miscible fluids in porous media. Heat Transf. Res. 2018, 49, 1023–1039. [Google Scholar] [CrossRef]
- Nagatsu, Y.; Ishii, Y.; Tada, Y.; De Wit, A. Hydrodynamic Fingering Instability Induced by a Precipitation Reaction. Phys. Rev. Lett. 2014, 113, 024502. [Google Scholar] [CrossRef] [Green Version]
- Patmonoaji, A.; Hu, Y.; Nasir, M.; Zhang, C.; Suekane, T. Effects of Dissolution Fingering on Mass Transfer Rate in Three-Dimensional Porous Media. Water Resour. Res. 2021, 57, e2020WR029353. [Google Scholar] [CrossRef]
- Saffman, P.G. Viscous fingering in Hele-Shaw cells. J. Fluid Mech. 1986, 173, 73–94. [Google Scholar] [CrossRef] [Green Version]
- Stewart, S.; Marin, D.; Tullier, M.; Pojman, J.; Meiburg, E.; Bunton, P. Stabilization of miscible viscous fingering by a step growth polymerization reaction. Exp. Fluids 2018, 59, 114. [Google Scholar] [CrossRef]
- Sin, S.; Suekane, T.; Nagatsu, Y.; Patmonoaji, A. Three-dimensional visualization of viscous fingering for non-Newtonian fluids with chemical reactions that change viscosity. Phys. Rev. Fluids 2019, 4, 054502. [Google Scholar] [CrossRef]
- Nagatsu, Y.; Kondo, Y.; Kato, Y.; Tada, Y. Effects of moderate Damköhler number on miscible viscous fingering involving viscosity decrease due to a chemical reaction. J. Fluid Mech. 2009, 625, 97–124. [Google Scholar] [CrossRef]
- Sorbie, K.S.; Al Ghafri, A.Y.; Skauge, A.; Mackay, E.J. On the Modelling of Immiscible Viscous Fingering in Two-Phase Flow in Porous Media. Transp. Porous Media 2020, 135, 331–359. [Google Scholar] [CrossRef]
- Hewitt, D.R.; Neufeld, J.A.; Lister, J.R. Convective shutdown in a porous medium at high Rayleigh number. J. Fluid Mech. 2013, 719, 551–586. [Google Scholar] [CrossRef] [Green Version]
- Forbes, L.K.; Browne, C.A.; Walters, S.J. The Rayleigh–Taylor instability in a porous medium. SN Appl. Sci. 2021, 3, 188. [Google Scholar] [CrossRef]
- Livescu, D.; Wei, T.; Brady, P. Rayleigh–Taylor instability with gravity reversal. Phys. D Nonlinear Phenom. 2021, 417, 132832. [Google Scholar] [CrossRef]
- Wang, T.-S.; Shi, W.-Y. Marangoni instability induced by evaporation in well-defined non-spherical sessile droplet. Int. J. Heat Mass Transf. 2019, 141, 168–179. [Google Scholar] [CrossRef]
- Li, Y.; Meijer, J.G.; Lohse, D. Marangoni instabilities of drops of different viscosities in stratified liquids. J. Fluid Mech. 2021, 932. [Google Scholar] [CrossRef]
- Li, Y.; Diddens, C.; Prosperetti, A.; Lohse, D. Marangoni Instability of a Drop in a Stably Stratified Liquid. Phys. Rev. Lett. 2021, 126, 124502. [Google Scholar] [CrossRef]
- Podgorski, T.; Sostarecz, M.C.; Zorman, S.; Belmonte, A. Fingering instabilities of a reactive micellar interface. Phys. Rev. E-Stat. Nonlinear Soft Matter Phys. 2007, 76, 016202. [Google Scholar] [CrossRef] [Green Version]
- Goriely, A.; Tabor, M. Self-Similar Tip Growth in Filamentary Organisms. Phys. Rev. Lett. 2003, 90, 108101. [Google Scholar] [CrossRef] [Green Version]
- Tronnolone, H.; Tam, A.; Szenczi, Z.; Green, J.E.F.; Balasuriya, S.; Tek, E.L.; Gardner, J.M.; Sundstrom, J.F.; Jiranek, V.; Oliver, S.; et al. Diffusion-Limited Growth of Microbial Colonies. Sci. Rep. 2018, 8, 5992. [Google Scholar] [CrossRef] [Green Version]
- Matsushita, M.; Fujikawa, H. Diffusion-limited growth in bacterial colony formation. Phys. A Stat. Mech. Its Appl. 1990, 168, 498–506. [Google Scholar] [CrossRef]
- Schwarcz, D.; Levine, H.; Ben-Jacob, E.; Ariel, G. Uniform modeling of bacterial colony patterns with varying nutrient and substrate. Phys. D Nonlinear Phenom. 2016, 318–319, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Mimura, M.; Sakaguchi, H.; Matsushita, M. Reaction–diffusion modelling of bacterial colony patterns. Phys. A Stat. Mech. Its Appl. 2000, 282, 283–303. [Google Scholar] [CrossRef]
- Haudin, F.; Cartwright, J.H.E.; Brau, F.; De Wit, A.; Takayama, K.; Morisaki, Y.; Kuno, S.; Nagamoto, Y.; Harada, K.; Furukawa, N.; et al. Spiral precipitation patterns in confined chemical gardens. Proc. Natl. Acad. Sci. USA 2014, 111, 17363–17367. [Google Scholar] [CrossRef] [Green Version]
- Haudin, F.; Cartwright, J.H.E.; De Wit, A. Direct and Reverse Chemical Garden Patterns Grown upon Injection in Confined Geometries. J. Phys. Chem. C 2015, 119, 15067–15076. [Google Scholar] [CrossRef]
- Haudin, F.; Brasiliense, V.; Cartwright, J.H.E.; Brau, F.; De Wit, A. Genericity of confined chemical garden patterns with regard to changes in the reactants. Phys. Chem. Chem. Phys. 2015, 17, 12804–12811. [Google Scholar] [CrossRef] [Green Version]
- Syed, A.; Pantin, B.; Durucan, S.; Korre, A.; Shi, J.Q. The use of polymer-gel solutions for remediation of potential CO2 leakage from storage reservoirs. Energy Procedia 2014, 63, 4638–4645. [Google Scholar] [CrossRef] [Green Version]
- Ito, T.; Xu, T.; Tanaka, H.; Taniuchi, Y.; Okamoto, A. Possibility to remedy CO2 leakage from geological reservoir using CO2 reactive grout. Int. J. Greenh. Gas Control 2014, 20, 310–323. [Google Scholar] [CrossRef]
- Mahardika, M.A.; She, Y.; Shori, F.; Patmonoaji, A.; Matsushita, S.; Suekane, T.; Nagatsu, Y. Enhanced Heavy Oil Recovery by Calcium Hydroxide Flooding with the Production of Viscoelastic Materials: Study with 3-D X-Ray Tomography and 2-D Glass Micromodels. Energy Fuels 2021, 35, 11210–11222. [Google Scholar] [CrossRef]
- Nagatsu, Y.; Abe, K.; Konmoto, K.; Omori, K. Chemical Flooding for Enhanced Heavy Oil Recovery via Chemical-Reaction-Producing Viscoelastic Material. Energy Fuels 2020, 34, 10655–10665. [Google Scholar] [CrossRef]
- Coutinho, M.; Rocha, F.M.; Miranda, J.A. Viscous normal stresses and fingertip tripling in radial Hele-Shaw flows. Phys. Rev. E 2021, 104, 045106. [Google Scholar] [CrossRef]
- Suzuki, R.X.; Kobayashi, S.; Nagatsu, Y.; Ban, T. Tunable Hydrodynamic Interfacial Instability by Controlling a Thermodynamic Parameter of Liquid–Liquid Phase Separation. J. Phys. Chem. B 2021, 125, 7508–7514. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, Y.; Eckert, K.; Schwarzenberger, K. Entrance effects in a radial Hele-Shaw cell: Numerical and experimental study. Chem. Eng. J. 2022, 428, 131146. [Google Scholar] [CrossRef]
- Cieślik, A.; Akkermans, R.; Kamp, L.; Clercx, H.J.; van Heijst, G. Dipole-wall collision in a shallow fluid. Eur. J. Mech.-B/Fluids 2009, 28, 397–404. [Google Scholar] [CrossRef]
- Lee, W.; Lucey, J. Structure and Physical Properties of Yogurt Gels: Effect of Inoculation Rate and Incubation Temperature. J. Dairy Sci. 2004, 87, 3153–3164. [Google Scholar] [CrossRef]
- Bönisch, M.P.; Huss, M.; Lauber, S.; Kulozik, U. Yoghurt gel formation by means of enzymatic protein cross-linking during microbial fermentation. Food Hydrocoll. 2007, 21, 585–595. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, C.; Patmonoaji, A.; Hu, Y.; Matsushita, S.; Suekane, T.; Nagatsu, Y. Effect of gas generation by chemical reaction on viscous fingering in a Hele–Shaw cell. Phys. Fluids 2021, 33, 093104. [Google Scholar] [CrossRef]
- Zittle, C. Precipitation of Casein from Acidic Solutions by Divalent Anions. J. Dairy Sci. 1966, 49, 361–364. [Google Scholar] [CrossRef]
- Etrati, A.; Alba, K.; Frigaard, I.A. Two-layer displacement flow of miscible fluids with viscosity ratio: Experiments. Phys. Fluids 2018, 30, 052103. [Google Scholar] [CrossRef]
- Chairunisa, W.; Imawan, C. The effect of pH on the characteristics of the methyl red solution as a gamma-ray dosimeter. J. Phys. Conf. Ser. 2019, 1321, 022015. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Nakanishi, Y.; Teston, A.D.; Suekane, T. Effect of diffusing layer thickness on the density-driven natural convection of miscible fluids in porous media: Modeling of mass transport. J. Fluid Sci. Technol. 2018, 13, JFST0002. [Google Scholar] [CrossRef] [Green Version]
- Muhr, A.H.; Blanshard, J.M. Diffusion in gels. Polymer 1982, 23, 1012–1026. [Google Scholar] [CrossRef]
- Patmonoaji, A.; Mahardika, M.A.; Nasir, M.; She, Y.; Wang, W.; Muflikhun, M.A.; Suekane, T. Stereolithography 3D Printer for Micromodel Fabrications with Comprehensive Accuracy Evaluation by Using Microtomography. Geosciences 2022, 12, 183. [Google Scholar] [CrossRef]
| [wt%] | [kg/m3] | [mPa·s] | [°C] |
|---|---|---|---|
| 0.0 | 1.00 | 1.17 | 18.8 |
| 0.5 | 0.99 | 1.04 | 21.8 |
| 1.5 | 0.99 | 1.12 | 21.1 |
| 5.0 | 1.00 | 1.18 | 20.7 |
| 15 | 1.05 | 1.59 | 22.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasir, M.; Yamaguchi, R.; She, Y.; Patmonoaji, A.; Mahardika, M.A.; Wang, W.; Li, Z.; Matsushita, S.; Suekane, T. Hydrodynamic Fingering Induced by Gel Film Formation in Miscible Fluid Systems: An Experimental and Mathematical Study. Appl. Sci. 2022, 12, 5043. https://doi.org/10.3390/app12105043
Nasir M, Yamaguchi R, She Y, Patmonoaji A, Mahardika MA, Wang W, Li Z, Matsushita S, Suekane T. Hydrodynamic Fingering Induced by Gel Film Formation in Miscible Fluid Systems: An Experimental and Mathematical Study. Applied Sciences. 2022; 12(10):5043. https://doi.org/10.3390/app12105043
Chicago/Turabian StyleNasir, Muhammad, Ryuhei Yamaguchi, Yun She, Anindityo Patmonoaji, Mohammad Azis Mahardika, Weicen Wang, Zijing Li, Shintaro Matsushita, and Tetsuya Suekane. 2022. "Hydrodynamic Fingering Induced by Gel Film Formation in Miscible Fluid Systems: An Experimental and Mathematical Study" Applied Sciences 12, no. 10: 5043. https://doi.org/10.3390/app12105043
APA StyleNasir, M., Yamaguchi, R., She, Y., Patmonoaji, A., Mahardika, M. A., Wang, W., Li, Z., Matsushita, S., & Suekane, T. (2022). Hydrodynamic Fingering Induced by Gel Film Formation in Miscible Fluid Systems: An Experimental and Mathematical Study. Applied Sciences, 12(10), 5043. https://doi.org/10.3390/app12105043







