Abstract
Drug testing, either on animals or on 2D cell cultures, has its limitations due to inaccurate mimicking of human pathophysiology. The liver, as one of the key organs that filters and detoxifies the blood, is susceptible to drug-induced injuries. Integrating 3D bioprinting with microfluidic chips to fabricate organ-on-chip platforms for 3D liver cell cultures with continuous perfusion can offer a more physiologically relevant liver-mimetic platform for screening drugs and studying liver function. The development of organ-on-chip platforms may ultimately contribute to personalized medicine as well as body-on-chip technology that can test drug responses and organ–organ interactions on a single or linked chip model.
1. Introduction
Approximately 10 years and $2.6 billion are required to bring a drug to market [1], while nearly 90% of proposed drugs fail to acquire clinical approval [2,3]. Even after attaining clinical approval, adverse drug reactions/events (ADRs/ADEs) are prevalent. ADR/ADE refers to allergic reactions, medication errors, and overdoses. The National Medical Products Administration (NMPA) of China received 1.514 million reports of ADRs/ADEs in 2019 alone [4]. In the United States, ADEs account for virtually one-third of hospital adverse events (1 million emergency department visits per year) and prolongs hospital stays by 1.7 to 4.6 days [5]. Animal testing raises moral concerns and is not accurate in predicting the pathophysiology of human disease [6,7]—roughly half of the preclinically approved drugs turn out to be toxic to humans [8], highlighting the importance of conducting drug testing directly on human cells [9]. Despite being widely accepted and useful, two-dimensional (2D) cell cultures face challenges in emulating in vivo-like cell bioactivity (e.g., cell morphology and cell–cell and cell–matrix interactions) [10,11]. Sandwich culture and micro-patterning are two common 2D cell culture methods [12]. On the other hand, compared to 2D cell cultures, three-dimensional (3D) cell cultures recapitulate in vivo function, structure, and the reaction of human tissue to drugs more effectively [13]. Moreover, the 3D extracellular matrix (ECM) enhances mechano-responses, proliferation, survival, and differentiation of cells. Spheroid culture, biopolymer and prefabricated scaffolds, hydrogels, and cell sheets are the most commonly used 3D cell culture techniques [12,14,15].
Microfluidics allows for multiplexing biotechnological techniques and enabling applications ranging from single-cell analysis [16,17,18,19,20] to on-chip applications [21,22]. Polymeric microfluidic chips (particularly polydimethylsiloxane (PDMS) as the most widely used material for microfluidic chip fabrication) can serve as bioreactors for 3D cell culture due to biocompatibility as well as the inertness of PDMS to aqueous solutions, temperature, and pressure encountered in cell culture [12]. Organ-on-chip (OOC) technology, the intersection of microfluidics and tissue engineering, refers to the extraction and culture of specific organ cells on a continuously perfused microfluidic chip to create cellular microenvironments in vitro with desired structural, mechanical, and fluidic properties [7,23,24]. Many of the OOC designs enable real-time imaging and analysis of metabolic responses of living cells in a functional organ context by mimicking (i) systematic organ interaction (e.g., drug distribution and absorption) [25,26], (ii) tissue-level organization of parenchymal cells [27,28], and (iii) epithelial and multicellular vascular interfaces of organs (e.g., a network of blood vessels) [6,29,30]. In 2004, the first report on the study of human disease or physiology at the organ level using a microfluidic chip was published [31]. This was a system for capturing the systemic interaction between the liver and lungs on a very small (one square inch) silicon chip known as a cell culture analogue (CCA) [6]. The concept of OOC was introduced in 2010 as a means to study lung function [32]. Culturing on microfluidic chips offers the possibility of programmed circulation of fluids and reagents directly on the chip to nourish the cells (e.g., oxygen) and remove waste material, which promotes the proliferation of cultured cells during long culture periods [6]. Integration of OOC platforms with sensors allows real-time monitoring of fluid pressure, cell migration, and tissue barrier integrity, as well as glucose, lactate, oxygen, and pH levels [33,34]. OOC methods can also mimic the interaction of multiple organs on a single chip by culturing different cell types in adjacent channels separated by a porous membrane (e.g., the blood–brain barrier) [35]. Linking multiple organ models via their vascular channels also creates human body-on-a-chip systems that enable studies of drug pharmacokinetics (PK) and pharmacodynamics (PD) in vitro, providing advantageous alternatives to animal models [36,37,38]. By using flexible side chambers, OOC is also able to simulate cyclic strains, fluid shear stresses, and mechanical compression that cells experience in vivo during heartbeat, peristalsis, and respiration [33,39].
Three-dimensional bioprinting (a subset of 3D printing [40,41]) is a promising method to produce 3D biomimetic cell cultures on microfluidic chips by the precise layer-by-layer placement of single/multiple cell type(s) [42,43,44,45,46]. After bioprinting, the printed material should be able to effectively adhere to the microfluidic substrate and maintain its mechanical properties and structure (either using an external scaffold or by establishing its own extracellular matrix) during the incubation period under culture conditions (e.g., the temperature and pressure required for culture) [47]. Three common approaches for 3D bioprinting are (Figure 1A): light-induced printing (e.g., stereolithography, a high-resolution (10 μm), non-contact printing method), inkjet-based printing (a fast multi-material printing method with higher cell viability compared to extrusion-based methods due to lower shear stress on the cells during printing [48]), and extrusion-based printing (where multiple print heads can be used to print the bioink and microfluidic chip simultaneously at 100 μm resolution) [47,49,50]. While light-induced methods are costly, slow, and cause thermal damage to the cells, inkjet printing suffers from poor functionality for vertical printing causing clogging of the nuzzle, while extrusion-based bioprinting supports limited biomaterial (high-viscosity material with shear-thinning capability is required) (Table 1) [48]. Fabrications methods of liver-on-chip platforms do not limit to 3D bioprinted methods, e.g., photolithography and micromolding [51]. Although these methods are well-established approaches with high resolution, they usually need a cleanroom for production and high skills in micromanufacturing. However, a discussion of the advantages and limitations of these methods, as well as materials used for these fabrication methods, are out of the scope of this perspective paper. Further information can be found elsewhere [51,52,53].

Table 1.
Summary of properties of commonly used 3D bioprinting methods for OOC applications. Reproduced with permission from [54], 2022, the authors, under the terms of the Creative Commons CC BY 4.0.
The liver is the main organ for filtering, detoxification, biotransformation of drugs, bile acid production, and protein synthesis in the body [71,72]. Ingested drugs hit the liver first after entering the bloodstream. Since drug-induced damage to the liver is ubiquitous, there is an urgent need for the development of in vitro liver models for preclinical and clinical testing. Liver-on-chip methods are beneficial for monitoring drug efficacy/toxicity, screening foodborne pathogens/diseases, inspecting the effects of cosmetics and dietary supplements on human organs, and understanding liver function and disease impairment (Table 2) [73,74]. Although the liver from human biopsies has a high regenerative and proliferative capacity, in conventional 2D and 3D cultures, in vitro hepatocyte functionality (e.g., albumin secretion and enzymatic activity) diminishes after being removed from the natural environment of the liver (within one week), which is due to the lack of an efficient perfusion system for proper nutrient delivery and waste product removal [6]. However, liver-on-chip platforms provide continuous perfusion through microfluidic channels, allowing a long-term culture of liver cells [75]. Liver cells for OOC cultures can be derived from primary human hepatocytes (PHHs) (they possess intrinsic properties of the liver but are difficult to isolate, costly, and unsuitable for long-term culture), stem cell-derived hepatocytes (a stable source of hepatocytes, but they are difficult to manipulate and require specific induce factors), and hepatic-derived cell lines (which have an indefinite lifespan and are easy to manipulate, but suffer from inaccuracy in drug response and rapid loss of expression of liver-specific enzymes/transporters) [48,73,76].

Table 2.
Three-dimensional-bioprinted liver-on-chip platforms.
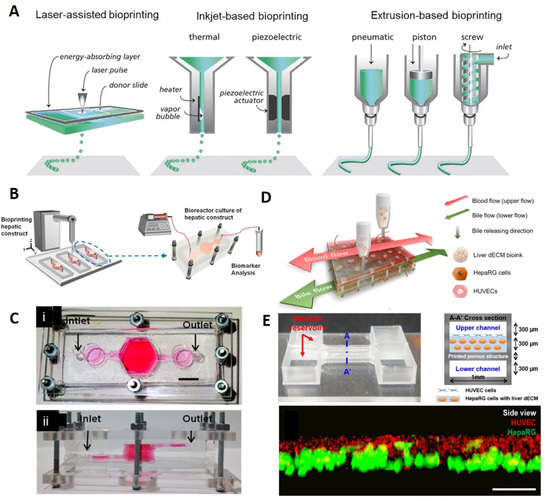
Figure 1.
Three-dimensional bioprinting. (A) Different 3D bioprinting methods. Reproduced with permission from [8]. 2016, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (B) Schematic representation of liver-on-chip 3D bioprinting to form a bioreactor with continuous perfusion for long-term cell culture. (C) Implemented liver chip comprised of PDMS and PMMA layers, compressed by nuts and screws to enable disassembly of the setup for examining cell culture anytime [75]. (B,C) Reproduced with permission from [75]. 2016, IOP publishing Ltd. (D) Schematic view of a single-step 3D bioprinting of a liver-on-chip platform with vascular/biliary channels to supply nutrients and remove waste products to enhance cell proliferation and survival. (E) (Top) View of the microfluidic chip and its cross-section. The chip contained two upper and lower channels with a 3D bioprinted microporous membrane. HepaRG liver decellularized extracellular matrix (dECM) bioink was printed on the micromembrane. (Bottom) The evaluation of cell positioning after cell printing on the microporous membrane, demonstrating a successful bioink printing (scale bar: 100 μm) [71]. (D,E) Reproduced with permission from [71]. 2019, IOP publishing Ltd.
2. Material
Bioinks: The most commonly used natural polymeric hydrogel-based bioinks for 3D bioprinting are dECM, agar (or agarose), alginate, silk, gelatin, collagen, hyaluronan, fibrinogen, and chitosan [79]. The bioink should be selected based on the proposed 3D bioprinting method (e.g., photo-curable bioink for light-induced printing and bioink with shear-thinning ability for extrusion-based bioprinting). Hybrid bioinks are introduced to modify the mechanical properties of current bioinks. For example, pure alginate, although widely used, biocompatible, and readily processable, has poor pattern fidelity and printability. However, the combination of it with cellulose nanocrystals (CNCs) (whisker/rod-shaped nanoparticles obtained from the crystalline regions of cellulose fibers and characterized by high mechanical strength, renewability, low cytotoxicity, and low density) results in a strengthened bioink with shear-thinning capability, which can be printed through a 100 μm nuzzle without clogging [49]. Using this hybrid bioink, a liver-memetic construct was printed with high cell viability, demonstrating the superior protection ability of hybrid bioinks to protect encapsulated cells from the mechanical stress of the extrusion process [49].
Microfluidic chips: PDMS is an intriguing candidate for the fabrication of microfluidic chips. PDMS can be plasma-treated, which allows manipulation of surface properties as well as the interconnection of multiple layers to fabricate complex 3D microfluidic structures suitable for OOC applications. In addition, PDMS is deformable, which enables programmed transport of fluids (e.g., reagents) through pneumatically controllable deformation of microchannels [6]. Moreover, as a consequence of the elasticity of PDMS, the natural cyclic stretching of organs (e.g., lungs and heart) can be mechanically mimicked on PDMS microchannels to create more realistic models of organs on a chip. In addition to the transparency of PDMS, which enables optical microscopy, PDMS is biocompatible and gas-permeable (e.g., oxygen), allowing biological experiments to be performed. However, the flexibility of PDMS may lead to undesirable distortions, while the gas permeability can lead to bubble formation in channels and evaporation of fluids due to the high surface-to-volume ratio. Furthermore, PDMS can absorb and leach hydrophobic drugs/compounds used in the culture chamber. Polyurethane elastomers, thermoplastics, and styrene–ethylene/butylene–styrene are alternatives to PDMS for OOC applications that are not widely used, although they are castable, flexible, and drug-inert [80]. Moreover, coating PDMS with paraffin or parylene can attenuate the drug absorption of PDMS while compromising cell attachment [6].
Poly(ethylene glycol) (PEG) is a widely used ink for the construction of spatially heterogeneous and complex structures such as hollow-shaped frameworks, as it is a tunable material for biomolecules besides being biocompatible and water-soluble. Poly(ethylene glycol) diacrylate (PEGDA), a conjugated PEG, can be used as a cell-encapsulating ink to improve stiffness, cell adhesion, cellular interactions, bioactivity, cell viability, and proliferation [81,82]. For 3D-bioprinted porous scaffolds, PEGDA is a good alternative to other biocompatible and non-immunogenic materials (e.g., PDMS) owing to its photopolymerizability and resistivity to protein adsorption. Photopolymerizable acrylate groups provide the unique ability to perform photolithographical patterning of biochemical and biomechanical architectures by allowing the matrix to be covalently cross-linked by UV light after printing [83,84]. Confocal microscopy images show that structures bioprinted with PEGDA form homogeneous and high-density cellular encapsulation throughout the structure [82].
PCL is a promising biocompatible platform with less protein absorption than PDMS, increasing the sensitivity of the metabolism measurements and drug screening [78]. Additionally, a PCL-based platform was developed for mimicking liver functions during drug administration, showing higher cell viability compared to other biocompatible platforms since it has a relatively low melting point (less thermal stress for cells in the printing process). However, this platform faced challenges in imaging due to its low optical transparency (compared to PDMS) [78].
3. Three-Dimensional-Bioprinted Liver-on-Chip Platforms
Bhise et al. generated a 3D-bioprinted, syringe-pump driven liver-on-chip setup with continuous perfusable bioreactor for long-term culture (30 days) and analysis of human HepG2/C3A spheroids for drug toxicity (Figure 1B,C) [75]. The bioreactor was comprised of multiple PDMS and poly(methyl methacrylate) (PMMA) layers (PDMS was cast around laser cut PMMA molds) to form three chambers and a set of channels with a total volume of 2.4 mL and flow rate of 200 μL.h−1. While most PDMS-based microfluidic chips are permanently sealed using plasma, in this setup, layers were compressed using nuts and screws, preventing leakage of compounds, so that the setup can be disassembled in any stage of the experiment for direct scrutiny of cell culture conditions. The bioprinting bioink was composed of HepG2/C3A spheroids (191 ± 10 μm) and gelatin methacryloyl (GelMA), crosslinked using ultraviolet (UV) light (850 mW for 15 s). The drug simulation performance was analyzed using 15mm acetaminophen to trigger a toxic response in the hepatic culture, reporting analogous performance to animal tests. During the 30-day culture period, cell number increased tenfold, which is a typical cell density range for hydrogel encapsulation. The hepatic function of the proposed setups was examined by measuring the secretion of biomarkers (albumin, ceruloplasmin, A1AT, and transferrin). The proposed setup was able to efficiently emulate key hepatic tissue cell types, such as Kupffer, stellate, hepatocytes, and endothelial cells, distribution of different ECM-like biomaterials, and their ratios [75].
Lee et al. developed a pumpless (gravity-induced), single-step 3D-bioprinted liver-on-chip setup (0.03 × 0.1 × 1 mm3) with a 3D liver decellularized extracellular matrix (dECM) environment for cells, two reservoirs to ensure a continuous supply of nutrients through channels, and vascular/biliary channels for supplying nutrients (upper vascular channel) and removing waste products (lower biliary channel) (flow rate in the 3D liver-on-a-chip was 25 μL min−1) (Figure 1D,E) [71]. Sterilized transparent PMMA and poly(ethylene/vinyl acetate) were used as printing substrates and structural materials for the 3D liver-on-a-chip, respectively. Cell printing was performed using liver dECM bioinks and gelatin. The setup was comprised of 2 channels: a lower channel, which was printed firstly, followed by the printing of a microporous structure above the lower channel (200 μm × 500 μm pore size was selected for maximum bioink retention and waste product removal). Subsequently, a HepaRG-liver dECM bioink mixture was printed on the microporous structure. Finally, after printing the second channel and reservoir, a human umbilical vein endothelial cells (HUVEC)–gelatin bioink mixture was used to fill the gaps. The structural printing was conducted under 110 °C and 660 kPa conditions. The accuracy of cell positioning was examined by obtaining images of the cell layer, demonstrating successful delivery of HUVECs above the HepaRG cell-laden liver dECM bioink (Figure 1E, green-labeled cells are HepaRG and red-labeled cells are HUVECs). In order to evaluate the basic function of the proposed 3D-bioprinted platform, albumin/urea secretion was compared to that of a 2D culture, pointing out a higher level of secreted albumin and urea in the 3D-bioprinted model, whereas the secretion level decreased overtime continuously in the 2D model. Moreover, the drug response of the model was examined by applying 5 mm of acetaminophen (APAP) and measuring albumin secretion, highlighting the higher drug responsiveness and sensitivity of the 3D-bioprinted liver-on-chip setup compared to the 2D model. The presence of biliary channels and multiple cell types, as well as simulating a liver-like microenvironment, were the main aspects that enhanced the functionality of this setup [71].
In spite of the substantial attention given to OOC platforms, this technology still suffers from poor selectivity of different cell types in the presence of spatial heterogeneity while facing challenges in forming different types of ECM environments for cell–ECM interactions and protein absorption, primarily owing to conventional fabrication methods (e.g., micromolding, microcontact printing, and soft lithography) [78]. To address these pitfalls, PCL can be a viable candidate. PCL is a biocompatible, biodegradable, and non-toxic polyester with a low melting point (~60 °C) that produces less cell damage during the bioprinting process (i.e., higher cell viability) [78]. Moreover, co-bioprinting enables simultaneous printing of the microchip and cells with no need for a low-throughput secondary cell seeding step. In the co-printing method, fabrication by multiple printing materials enables the embedding of microfluidic structures and self-healing materials into the structure, facilitating tissue engineering of organs containing multiple vasculatures, ECM, and cells [85]. As one of the first attempts to co-print liver tissue on a chip, a single-step co-3D-bioprinting approach (without a secondary cell seeding process) was developed to fabricate a PCL-based 3D-printed microchip for perfused liver-on-a-chip applications [78]. PCL (to produce microchannels) and hydrogels (containing encapsulated HUVEC and HepG2 cell lines) were printed by a pneumatic 3D bioprinter with a minimum line width of 175 μm and channel size of 15 mm × 1.5 mm × 1.5 mm. The protein absorption rates of the PCL-based bioprinted 3D channels were compared with those of soft lithographic PDMS-based channels), confirming the better performance of PCL-based channels on heterotypic cell types and biomaterials for more accurate drug screening (protein absorption depth in the PCL-based channel wall was 50 μm, while absorption depth in the PDMS-based channel wall was 400 μm). Nevertheless, PCL-based microfluidic channels are less optically transparent compared to PDMS channels, confining the assessment of the cell proliferation/viability rate and real-time analysis of cell interactions as well as drug responses [78].
The advent of coaxial printing and sacrificial printing allowed the fabrication of vascularized liver-on-chip platforms, enabling the study and real-time analysis of liver-specific phenomena (e.g., zonation) [77,86]. Blood flow carries nutrients and oxygen to liver tissue, during which the partial pressure of oxygen drops as a result of progressing across the liver sinusoid (i.e., the functional unit of the liver from periportal to perivenous hepatocytes) [87]. The difference between oxygen levels in the liver tissue (known as zonation) plays a role in regulating functionality and the response of the liver to metabolic and toxic stimuli by facilitating differential metabolism. Non-perfused cell cultures suffer from oxygen scarcity since proximate cells consume a considerable portion of available oxygen, reducing the adequate presence of oxygen in cell populations away from the oxygen source [87]. Nonetheless, perfused on-chip platforms not only can distribute oxygen to different culture sites on the chip to sustain cell proliferation, but also can enable the creation of oxygen zones with different saturation levels by intentional manipulation of flow rate and oxygen levels to study the oxygen sensitivity of liver tissue [53].
In addition, liver fibrosis is a pathophysiological process caused by different insults (e.g., viral infection, alcoholism, metabolic disease, drugs, and chemical exposure), resulting in complicated HC–NPC interactions that can bring about liver stiffening, cirrhosis, and failure. However, it is difficult to reproduce a liver fibrosis model in vitro following standard approaches due to the complex factors involved in liver fibrosis. Three-dimensional bioprinting is a promising method to fabricate liver fibrosis models for drug evaluation and therapy owing to its multimaterial spatial distribution ability [86].
4. Future Perspective
Future liver-on-chip studies may potentially use hepatocytes derived from induced pluripotent stem cells (iPSCs) to cover greater population variability, which would ultimately improve the thoroughness of drug screening tests (e.g., idiosyncratic drug-induced liver injury is difficult to predict with current clinical trials) [3]. Although the immune system plays a critical role in liver infections, drug-induced hepatotoxicity, and disease progression by altering the secretion of cytokines and histamine from immune cells in the liver, there is almost no liver chip that takes into account the immune system [6,73]. A liver immuno-chip could contribute significantly to a more accurate examination of the liver. Furthermore, because the liver is closely associated with lungs, skin, and kidneys, combining the liver with other organs on a chip can be used to monitor drug effects on multiple organs [73]. A variety of multi-organ-on-a-chip technologies can be used for in vitro drug toxicity assessment; examples include a lung/liver-on-a-chip platform to study the toxicity of inhaled aerosols [88] and a kidney/liver-on-a-chip model to study the toxicity of drug metabolites [89]. An ultimate goal of OOCs is the implementation of body-on-a-chip platforms that facilitates patient-specific drug screening and disease investigation considering all organs. However, the perfusion media used on OOC platforms are mostly optimized for a specific organ. Thus, finding a single culture medium (i.e., an alternative for blood) that is compatible with multiple organs would be an intriguing area of research for the future.
Perfusion is imperative to improve cell proliferation and survival. However, the use of micropumps for simultaneous perfusion of multiple cell cultures increases the complexity and overall cost of the chip. Passive, gravity-driven perfusion methods based on differential fluid pressure in the reservoirs can be a simple, low-cost alternative without the need for expert users to perform the cell culture [6]. Paper provides a biocompatible, perfusable, and porous natural support medium that physically emulates the cellular microenvironment for cell culturing [90,91,92]. Future studies can develop paper-based 3D-bioprinted platforms for cell cultures, benefiting from the advantages of both 3D bioprinting and paper substrates (e.g., biocompatibility, low cost, disposability, modifiable pore size, and passive fluid transportation). Moreover, external fields such as magnetic or acoustic fields can play a role in convenient cell separation/isolation/characterization in microfluidic channels at the outlet of chips to enable an analysis of the subtle changes in the concentration of elements in the perfusion medium [93,94,95]. Because OOC platforms consist of a small number of cells, detection and sampling of small secreted cellular products are challenging [96]. Increasing the length of the culture channel, decreasing the perfusion flow rate, and using multiple culture chambers in parallel can be a number of possible solutions to obtain a sufficient amount of secreted products.
Furthermore, the lack of a stable readout and uniform testing standards are two of the reasons why almost none of the liver chips have received approval from the Food and Drug Administration (FDA) [73]. Integration of molecular reporters and nanoscale biosensors with liver-on-chip platforms enables dynamic real-time screening/imaging of multiple physiological processes on a chip to enable stable, high-throughput readout [33,73]. Since contemporary microscopes can provide a large amount of readout data (images/videos), interpretation of acquired data (e.g., detection and quantification of target cells such as cancer cells, as well as classification of cells based on their morphology) outpaces the ability of human experts. Therefore, OOC platforms would benefit significantly from the integration of electronic microscopy and machine learning (ML) techniques to automatically classify and decode available data with minimal human intervention [97,98,99].
Microfluidic devices have enabled a wide range of biochemical and clinical applications, such as cancer screening, high-throughput drug testing, point-of-care diagnostics, and OOC platforms, owing to advantages such as requiring a low volume of samples and costly reagents, disposability, and enabling the realization of chips with complex 3D structures for simultaneous multi-analyte analysis [100,101,102]. However, translating microfluidic devices from bench to bedside necessitates scaling up current manufacturing procedures [103]. New investors in the microfluidic domain should consider mass-production with a competitive cost in the shortest possible design to end-product time, demonstrating a dire need for a change from more labor-intensive processes to information-technology-enabled manufacturing processes [103]. Digital manufacture (DM) is a general term referring to a manufacturing system wherein the entire design, supply chain, and fabrication process can be simulated in advance to figure out deficiencies as well as feasible optimization, followed by a fully- or semi-operated/controlled fabrication line with the help of high-performance computers (e.g., for big data analysis and artificial intelligence (AI)), simulation software (e.g., computer-aided design (CAD) software), sensors, actuators, and interconnected network (e.g., cloud-based servers and internet-of-things) [103,104,105]. While the CAD software linked to AI can optimize the design parameters (e.g., dimensions), suggest suitable material for desired end application, assist in the selection of fabrication methods for faster and more cost-efficient production (either subtractive or additive method), and predict the final quality, sensors/actuators can acquire data and cooperate with AI to detect deviations from the design process and optimize fabrication parameters in real-time. Furthermore, DM can actively play a role in obtaining data regarding demand in the market and user expectations/feedback to facilitate a user-centered design process, ultimately resulting in better user acceptance and commercialization.
5. Concluding Remarks
The liver is the central hub of xenobiotic metabolism, filtering, and detoxification, and is susceptible to drug- and cosmetic-induced toxicity. Microfluidic chips, as high-throughput platforms, enable the culture of multiple cells on a single chip, facilitating the study of cell–cell interactions and their reactions for applied medicine (e.g., modeling of drug absorption and immune response in an interconnected network of liver–gut–kidney [87]). Three-dimensional bioprinting can contribute to multi-cell cultures using multi-nozzle configurations to print different cells and microfluidic chips simultaneously with no need for a secondary cell-seeding process. Moreover, the advent of coaxial printing and sacrificial printing allowed the fabrication of vascularized liver-on-chip platforms, enabling the study and real-time analysis of liver-specific phenomena (e.g., zonation) [86].
Three-dimensional bioprinting offers precision in fabrication and versatility in design, ultimately facilitating the manufacture of intricate 3D organ-mimicking cell structures on chips. Nonetheless, none of the available 3D bioprinting techniques is the best. Even though light-induced methods can produce complex 3D structures with high resolutions, scalability, limited material selection, and the high cost are still challenging. Furthermore, additional studies are necessary to determine the possible photo-induced damage to cells in the short and long term [54]. Nozzle-based bioprinters, on the other hand, are readily available and cost-effective methodologies. However, moderate resolution and shear stress (leading to cell damage and low cell viability) are their major challenges [54]. Future studies can aim to integrate multiple 3D bioprinting methods to obtain a better resolution and high throughput. In addition, the development of human-on-chip platforms can substantially increase the capability of OOC platforms to achieve a multi-aspect study of diseases and drug responses.
Author Contributions
Conceptualization, S.R.D. and S.T.; writing—original draft preparation, S.R.D. and B.O.; writing—review and editing, N.M. and S.T.; supervision, S.T.; project administration, S.T.; funding acquisition, S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This article was funded by Tubitak 2232 International Fellowship for the Outstanding Researchers Award (118C391), Alexander von Humboldt Research Fellowship for Experienced Researchers, Marie Skłodowska-Curie Individual Fellowship (101003361), and Royal Academy Newton-Katip Çelebi Transforming Systems Through Partnership award (120N019).
Institutional Review Board Statement
Not applicable as this article does not involve any human/animal tests.
Informed Consent Statement
Not applicable as this article does not involve any human/animal tests.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Acknowledgments
S.T. acknowledges the Tubitak 2232 International Fellowship for the Outstanding Researchers Award (118C391), Alexander von Humboldt Research Fellowship for Experienced Researchers, Marie Skłodowska-Curie Individual Fellowship (101003361), and Royal Academy Newton-Katip Çelebi Transforming Systems Through Partnership award (120N019) for financial support of this research. Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the TÜBİTAK. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thakkar, S.; Li, T.; Liu, Z.; Wu, L.; Roberts, R.; Tong, W. Drug-induced liver injury severity and toxicity (DILIst): Binary classification of 1279 drugs by human hepatotoxicity. Drug Discov. Today 2020, 25, 201–208. [Google Scholar] [CrossRef] [PubMed]
- DiMasi, J.A.; Grabowski, H.G.; Hansen, R.W. The cost of drug development. N. Engl. J. Med. 2015, 372, 1972. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, S.; Tasoglu, S. A bioprinted liver-on-a-chip for drug screening applications. Trends Biotechnol. 2016, 34, 681–682. [Google Scholar] [CrossRef] [PubMed]
- Annual Report for National Adverse Drug Reaction Monitoring (2019) Released. Available online: http://english.nmpa.gov.cn/2020-04/10/c_500154.htm#:~:text=In%202019%2C%20the%20National%20ADR%20Monitoring%20Network%20received%20156%2C000%20reports,same%20period%20(Figure%202) (accessed on 10 January 2020).
- Adverse Drug Events. Available online: https://health.gov/our-work/health-care-quality/adverse-drug-events (accessed on 10 January 2020).
- Zhang, B.; Korolj, A.; Lai, B.F.L.; Radisic, M. Advances in organ-on-a-chip engineering. Nat. Rev. Mater. 2018, 3, 257–278. [Google Scholar] [CrossRef]
- Ustun, M.; Rahmani Dabbagh, S.; Ilci, I.S.; Bagci-Onder, T.; Tasoglu, S. Glioma-on-a-Chip Models. Micromachines 2021, 12, 490. [Google Scholar] [CrossRef]
- Pati, F.; Gantelius, J.; Svahn, H.A. 3D bioprinting of tissue/organ models. Angew. Chem. Int. Ed. 2016, 55, 4650–4665. [Google Scholar] [CrossRef]
- Ingber, D.E. Is it Time for Reviewer 3 to Request Human Organ Chip Experiments Instead of Animal Validation Studies? Adv. Sci. 2020, 7, 2002030. [Google Scholar] [CrossRef]
- Bikmulina, P.; Kosheleva, N.; Efremov, Y.; Antoshin, A.; Heydari, Z.; Kapustina, V.; Royuk, V.; Mikhaylov, V.; Fomin, V.; Vosough, M. 3D or not 3D: A guide to assess cell viability in 3D cell systems. Soft Matter 2022, 18, 2222–2233. [Google Scholar] [CrossRef]
- Yoon, J.-Y. Cell Culture. In Tissue Eng; Springer: Berlin/Heidelberg, Germany, 2022; pp. 13–32. [Google Scholar]
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling physiological events in 2D vs. 3D cell culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Bilirgen, A.C.; Toker, M.; Odabas, S.; Yetisen, A.K.; Garipcan, B.; Tasoglu, S. Plant-based scaffolds in tissue engineering. ACS Biomater. Sci. Eng. 2021, 7, 926–938. [Google Scholar] [CrossRef]
- Knowlton, S.; Cho, Y.; Li, X.-J.; Khademhosseini, A.; Tasoglu, S. Utilizing stem cells for three-dimensional neural tissue engineering. Biomater. Sci. 2016, 4, 768–784. [Google Scholar] [CrossRef] [PubMed]
- Yenilmez, B.; Temirel, M.; Knowlton, S.; Lepowsky, E.; Tasoglu, S. Development and characterization of a low-cost 3D bioprinter. Bioprinting 2019, 13, e00044. [Google Scholar] [CrossRef]
- Fung, C.W.; Chan, S.N.; Wu, A.R. Microfluidic single-cell analysis—Toward integration and total on-chip analysis. Biomicrofluidics 2020, 14, 021502. [Google Scholar] [CrossRef] [PubMed]
- Matuła, K.; Rivello, F.; Huck, W.T. Single-cell analysis using droplet microfluidics. Adv. Biosyst. 2020, 4, 1900188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozdalgic, B.; Ustun, M.; Dabbagh, S.R.; Haznedaroglu, B.Z.; Kiraz, A.; Tasoglu, S. Microfluidics for Microalgal Biotechnology. Biotechnol. Bioeng. 2021, 118, 1716–1734. [Google Scholar] [CrossRef] [PubMed]
- Tasoglu, S. Toilet-based continuous health monitoring using urine. Nat. Rev. Urol. 2022, 19, 219–230. [Google Scholar] [CrossRef]
- Amin, R.; Knowlton, S.; Yenilmez, B.; Hart, A.; Joshi, A.; Tasoglu, S. Smart-phone attachable, flow-assisted magnetic focusing device. RSC Adv. 2016, 6, 93922–93931. [Google Scholar] [CrossRef]
- Horejs, C. Organ chips, organoids and the animal testing conundrum. Nat. Rev. Mater. 2021, 6, 372–373. [Google Scholar] [CrossRef]
- Imparato, G.; Urciuolo, F.; Netti, P.A. Organ on Chip Technology to Model Cancer Growth and Metastasis. Bioengineering 2022, 9, 28. [Google Scholar] [CrossRef]
- Dornhof, J.; Kieninger, J.; Muralidharan, H.; Maurer, J.; Urban, G.A.; Weltin, A. Microfluidic organ-on-chip system for multi-analyte monitoring of metabolites in 3D cell cultures. Lab. Chip. 2022, 22, 225–239. [Google Scholar] [CrossRef]
- Sart, S.; Ronteix, G.; Jain, S.; Amselem, G.; Baroud, C.N. Cell Culture in Microfluidic Droplets. Chem. Rev. 2022, 122, 7061–7096. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.G.; Shuler, M.L. Design and demonstration of a pumpless 14 compartment microphysiological system. Biotechnol. Bioeng. 2016, 113, 2213–2227. [Google Scholar] [CrossRef] [PubMed]
- Oleaga, C.; Bernabini, C.; Smith, A.S.; Srinivasan, B.; Jackson, M.; McLamb, W.; Platt, V.; Bridges, R.; Cai, Y.; Santhanam, N. Multi-Organ toxicity demonstration in a functional human in vitro system composed of four organs. Sci. Rep. 2016, 6, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Khetani, S.R.; Bhatia, S.N. Microscale culture of human liver cells for drug development. Nat. Biotechnol. 2008, 26, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Frey, O.; Misun, P.M.; Fluri, D.A.; Hengstler, J.G.; Hierlemann, A. Reconfigurable microfluidic hanging drop network for multi-tissue interaction and analysis. Nat. Commun. 2014, 5, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.J.; Huh, D.; Hamilton, G.; Ingber, D.E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 2012, 12, 2165–2174. [Google Scholar] [CrossRef]
- Benam, K.H.; Villenave, R.; Lucchesi, C.; Varone, A.; Hubeau, C.; Lee, H.-H.; Alves, S.E.; Salmon, M.; Ferrante, T.C.; Weaver, J.C. Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro. Nat. Methods 2016, 13, 151–157. [Google Scholar] [CrossRef]
- Viravaidya, K.; Sin, A.; Shuler, M.L. Development of a microscale cell culture analog to probe naphthalene toxicity. Biotechnol. Prog. 2004, 20, 316–323. [Google Scholar] [CrossRef]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Clarke, G.A.; Hartse, B.X.; Niaraki Asli, A.E.; Taghavimehr, M.; Hashemi, N.; Abbasi Shirsavar, M.; Montazami, R.; Alimoradi, N.; Nasirian, V.; Ouedraogo, L.J. Advancement of Sensor Integrated Organ-on-Chip Devices. Sensors 2021, 21, 1367. [Google Scholar] [CrossRef] [PubMed]
- Park, T.-E.; Mustafaoglu, N.; Herland, A.; Hasselkus, R.; Mannix, R.; FitzGerald, E.A.; Prantil-Baun, R.; Watters, A.; Henry, O.; Benz, M. Hypoxia-enhanced Blood-Brain Barrier Chip recapitulates human barrier function and shuttling of drugs and antibodies. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Novak, R.; Ingram, M.; Marquez, S.; Das, D.; Delahanty, A.; Herland, A.; Maoz, B.M.; Jeanty, S.S.; Somayaji, M.R.; Burt, M. Robotic fluidic coupling and interrogation of multiple vascularized organ chips. Nat. Biomed. Eng. 2020, 4, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Herland, A.; Maoz, B.M.; Das, D.; Somayaji, M.R.; Prantil-Baun, R.; Novak, R.; Cronce, M.; Huffstater, T.; Jeanty, S.S.; Ingram, M. Quantitative prediction of human pharmacokinetic responses to drugs via fluidically coupled vascularized organ chips. Nat. Biomed. Eng. 2020, 4, 421–436. [Google Scholar] [CrossRef]
- Zuchowska, A.; Skorupska, S. Multi-organ-on-chip approach in cancer research. Organs-on-a-Chip 2022, 4, 100014. [Google Scholar] [CrossRef]
- Ingber, D.E. From mechanobiology to developmentally inspired engineering. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170323. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, V.; Gonçalves, I.; Lage, T.; Rodrigues, R.O.; Minas, G.; Teixeira, S.F.; Moita, A.S.; Hori, T.; Kaji, H.; Lima, R.A. 3D Printing Techniques and Their Applications to Organ-on-a-Chip Platforms: A Systematic Review. Sensors 2021, 21, 3304. [Google Scholar] [CrossRef]
- Dabbagh, S.R.; Sarabi, M.R.; Rahbarghazi, R.; Sokullu, E.; Yetisen, A.K.; Tasoglu, S. 3D-Printed Microneedles in Biomedical Applications. iScience 2020, 24, 102012. [Google Scholar] [CrossRef]
- Xu, H.; Liu, J.; Zhang, Z.; Xu, C. Cell sedimentation during 3D bioprinting: A mini review. Bio-Des. Manuf. 2022, 1–10. [Google Scholar] [CrossRef]
- Nooranidoost, M.; Izbassarov, D.; Tasoglu, S.; Muradoglu, M. A computational study of droplet-based bioprinting: Effects of viscoelasticity. Phys. Fluids 2019, 31, 081901. [Google Scholar] [CrossRef] [Green Version]
- Knowlton, S.; Anand, S.; Shah, T.; Tasoglu, S. Bioprinting for neural tissue engineering. Trends Neurosci. 2018, 41, 31–46. [Google Scholar] [CrossRef]
- Knowlton, S.; Onal, S.; Yu, C.H.; Zhao, J.J.; Tasoglu, S. Bioprinting for cancer research. Trends Biotechnol. 2015, 33, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Tasoglu, S.; Demirci, U. Bioprinting for stem cell research. Trends Biotechnol. 2013, 31, 10–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knowlton, S.; Yenilmez, B.; Tasoglu, S. Towards single-step biofabrication of organs on a chip via 3D printing. Trends Biotechnol. 2016, 34, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Kryou, C.; Leva, V.; Chatzipetrou, M.; Zergioti, I. Bioprinting for liver transplantation. Bioengineering 2019, 6, 95. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Lin, Z.Y.W.; Wenger, A.C.; Tam, K.C.; Tang, X.S. 3D bioprinting of liver-mimetic construct with alginate/cellulose nanocrystal hybrid bioink. Bioprinting 2018, 9, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Ye, W.; Yan, Y. Advances in Photocrosslinkable Materials for 3D Bioprinting. Adv. Eng. Mater. 2022, 24, 2100663. [Google Scholar] [CrossRef]
- Kasi, D.G.; de Graaf, M.N.; Motreuil-Ragot, P.A.; Frimat, J.-P.M.; Ferrari, M.D.; Sarro, P.M.; Mastrangeli, M.; van den Maagdenberg, A.M.; Mummery, C.L.; Orlova, V.V. Rapid Prototyping of Organ-on-a-Chip Devices Using Maskless Photolithography. Micromachines 2021, 13, 49. [Google Scholar] [CrossRef]
- Ferrari, E.; Nebuloni, F.; Rasponi, M.; Occhetta, P. Photo and Soft Lithography for Organ-on-Chip Applications. In Organ-on-a-Chip; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–19. [Google Scholar]
- Low, L.A.; Mummery, C.; Berridge, B.R.; Austin, C.P.; Tagle, D.A. Organs-on-chips: Into the next decade. Nat. Rev. Drug Discov. 2021, 20, 345–361. [Google Scholar] [CrossRef]
- Rahmani Dabbagh, S.; Rezapour Sarabi, M.; Birtek, M.T.; Mustafaoglu, N.; Zhang, Y.S.; Tasoglu, S. 3D bioprinted organ-on-chips. Aggregate 2022, e197. [Google Scholar] [CrossRef]
- Tang, M.; Rich, J.N.; Chen, S. Biomaterials and 3D Bioprinting Strategies to Model Glioblastoma and the Blood–Brain Barrier. Adv. Mater. 2021, 33, 2004776. [Google Scholar] [CrossRef] [PubMed]
- Hoch, E.; Schuh, C.; Hirth, T.; Tovar, G.E.; Borchers, K. Stiff gelatin hydrogels can be photo-chemically synthesized from low viscous gelatin solutions using molecularly functionalized gelatin with a high degree of methacrylation. J. Mater. Sci. Mater. Med. 2012, 23, 2607–2617. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S.; Yan, W.-C.; Lu, W.F.; Wang, C.-H.; Fuh, J.Y.H. 3D bioprinting of tissues and organs for regenerative medicine. Adv. Drug Deliv. Rev. 2018, 132, 296–332. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Holzl, K.; Lin, S.; Tytgat, L.; Van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef]
- Homan, K.A.; Kolesky, D.B.; Skylar-Scott, M.A.; Herrmann, J.; Obuobi, H.; Moisan, A.; Lewis, J.A. Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef]
- Kačarević, Ž.; Rider, P.; Alkildani, S.; Retnasingh, S.; Smeets, R.; Jung, O.; Ivanišević, Z.; Barbeck, M. An Introduction to 3D Bioprinting: Possibilities, Challenges and Future Aspects. Materials 2018, 11, 2199. [Google Scholar] [CrossRef] [Green Version]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D printing and customized additive manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Pages, E.; Ducom, A.; Fontaine, A.; Guillemot, F. Controlling laser-induced jet formation for bioprinting mesenchymal stem cells with high viability and high resolution. Biofabrication 2014, 6, 045001. [Google Scholar] [CrossRef]
- Dubbin, K.; Tabet, A.; Heilshorn, S.C. Quantitative criteria to benchmark new and existing bio-inks for cell compatibility. Biofabrication 2017, 9, 044102. [Google Scholar] [CrossRef] [PubMed]
- Tse, C.; Whiteley, R.; Yu, T.; Stringer, J.; MacNeil, S.; Haycock, J.W.; Smith, P.J. Inkjet printing Schwann cells and neuronal analogue NG108-15 cells. Biofabrication 2016, 8, 015017. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yu, C.; Wang, P.; Xu, W.; Wan, X.; Lai, C.S.E.; Liu, J.; Koroleva-Maharajh, A.; Chen, S. Rapid 3D bioprinting of decellularized extracellular matrix with regionally varied mechanical properties and biomimetic microarchitecture. Biomaterials 2018, 185, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Zuev, D.M.; Nguyen, A.K.; Putlyaev, V.I.; Narayan, R.J. 3D printing and bioprinting using multiphoton lithography. Bioprinting 2020, 20, e00090. [Google Scholar] [CrossRef]
- Koch, L.; Deiwick, A.; Franke, A.; Schwanke, K.; Haverich, A.; Zweigerdt, R.; Chichkov, B. Laser bioprinting of human induced pluripotent stem cells—the effect of printing and biomaterials on cell survival, pluripotency, and differentiation. Biofabrication 2018, 10, 035005. [Google Scholar] [CrossRef]
- Hakobyan, D.; Médina, C.; Dusserre, N.; Stachowicz, M.-L.; Handschin, C.; Fricain, J.-C.; Guillermet-Guibert, J.; Oliveira, H. Laser-assisted 3D bioprinting of exocrine pancreas spheroid models for cancer initiation study. Biofabrication 2020, 12, 035001. [Google Scholar] [CrossRef]
- Lee, H.; Chae, S.; Kim, J.Y.; Han, W.; Kim, J.; Choi, Y.; Cho, D.-W. Cell-printed 3D liver-on-a-chip possessing a liver microenvironment and biliary system. Biofabrication 2019, 11, 025001. [Google Scholar] [CrossRef]
- Kanabekova, P.; Kadyrova, A.; Kulsharova, G. Microfluidic Organ-on-a-Chip Devices for Liver Disease Modeling In Vitro. Micromachines 2022, 13, 428. [Google Scholar] [CrossRef]
- Deng, J.; Wei, W.; Chen, Z.; Lin, B.; Zhao, W.; Luo, Y.; Zhang, X. Engineered liver-on-a-chip platform to mimic liver functions and its biomedical applications: A review. Micromachines 2019, 10, 676. [Google Scholar] [CrossRef] [Green Version]
- Fetah, K.; Tebon, P.; Goudie, M.J.; Eichenbaum, J.; Ren, L.; Barros, N.; Nasiri, R.; Ahadian, S.; Ashammakhi, N.; Dokmeci, M.R. The emergence of 3D bioprinting in organ-on-chip systems. Prog. Biomed. Eng. 2019, 1, 012001. [Google Scholar] [CrossRef]
- Bhise, N.S.; Manoharan, V.; Massa, S.; Tamayol, A.; Ghaderi, M.; Miscuglio, M.; Lang, Q.; Zhang, Y.S.; Shin, S.R.; Calzone, G. A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication 2016, 8, 014101. [Google Scholar] [CrossRef] [PubMed]
- Matsusaki, M.; Sakaue, K.; Kadowaki, K.; Akashi, M. Three-Dimensional Human Tissue Chips Fabricated by Rapid and Automatic Inkjet Cell Printing. Adv. Healthc. Mater. 2013, 2, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, B.; Paulsen, S.J.; Corbett, D.C.; Sazer, D.W.; Fortin, C.L.; Zaita, A.J.; Greenfield, P.T.; Calafat, N.J.; Gounley, J.P.; Ta, A.H.; et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 2019, 364, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Cho, D.-W. One-step fabrication of an organ-on-a-chip with spatial heterogeneity using a 3D bioprinting technology. Lab Chip 2016, 16, 2618–2625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Chen, Q.; Liu, C.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Wang, X. Natural polymers for organ 3D bioprinting. Polymers 2018, 10, 1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, S.B.; Wu, Q.; Yazbeck, J.; Liu, C.; Okhovatian, S.; Radisic, M. Beyond Polydimethylsiloxane: Alternative Materials for Fabrication of Organ-on-a-Chip Devices and Microphysiological Systems. ACS Biomater. Sci. Eng. 2020, 7, 2880–2899. [Google Scholar] [CrossRef]
- Yi, H.-G.; Kim, H.; Kwon, J.; Choi, Y.-J.; Jang, J.; Cho, D.-W. Application of 3D bioprinting in the prevention and the therapy for human diseases. Signal Transduct. Target. Ther. 2021, 6, 177. [Google Scholar] [CrossRef]
- Zhu, W.; Cui, H.; Boualam, B.; Masood, F.; Flynn, E.; Rao, R.D.; Zhang, Z.-Y.; Zhang, L.G. 3D bioprinting mesenchymal stem cell-laden construct with core–shell nanospheres for cartilage tissue engineering. Nanotechnology 2018, 29, 185101. [Google Scholar] [CrossRef]
- Cuchiara, M.P.; Allen, A.C.B.; Chen, T.M.; Miller, J.S.; West, J.L. Multilayer microfluidic PEGDA hydrogels. Biomaterials 2010, 31, 5491–5497. [Google Scholar] [CrossRef]
- Mu, X.; Bertron, T.; Dunn, C.; Qiao, H.; Wu, J.; Zhao, Z.; Saldana, C.; Qi, H. Porous polymeric materials by 3D printing of photocurable resin. Mater. Horiz. 2017, 4, 442–449. [Google Scholar] [CrossRef]
- Kolesky, D.B.; Truby, R.L.; Gladman, A.S.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 2014, 26, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wu, Y.; Li, Y.; Aazmi, A.; Zhou, H.; Zhang, B.; Yang, H. Current Advances on 3D-Bioprinted Liver Tissue Models. Adv. Healthc. Mater. 2020, 9, 2001517. [Google Scholar] [CrossRef] [PubMed]
- Beckwitt, C.H.; Clark, A.M.; Wheeler, S.; Taylor, D.L.; Stolz, D.B.; Griffith, L.; Wells, A. Liver ‘organ on a chip’. Exp. Cell Res. 2018, 363, 15–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bovard, D.; Sandoz, A.; Luettich, K.; Frentzel, S.; Iskandar, A.; Marescotti, D.; Trivedi, K.; Guedj, E.; Dutertre, Q.; Peitsch, M.C. A lung/liver-on-a-chip platform for acute and chronic toxicity studies. Lab Chip 2018, 18, 3814–3829. [Google Scholar] [CrossRef] [Green Version]
- Theobald, J.; Ghanem, A.; Wallisch, P.; Banaeiyan, A.A.; Andrade-Navarro, M.A.; Taškova, K.; Haltmeier, M.; Kurtz, A.; Becker, H.; Reuter, S. Liver-kidney-on-chip to study toxicity of drug metabolites. ACS Biomater. Sci. Eng. 2018, 4, 78–89. [Google Scholar] [CrossRef]
- Temirel, M.; Dabbagh, S.R.; Tasoglu, S. Hemp-Based Microfluidics. Micromachines 2021, 12, 182. [Google Scholar] [CrossRef]
- Dabbagh, S.R.; Becher, E.; Ghaderinezhad, F.; Havlucu, H.; Ozcan, O.; Ozkan, M.; Yetisen, A.K.; Tasoglu, S. Increasing the packing density of assays in paper-based microfluidic devices. Biomicrofluidics 2021, 15, 011502. [Google Scholar] [CrossRef]
- Ghaderinezhad, F.; Koydemir, H.C.; Tseng, D.; Karinca, D.; Liang, K.; Ozcan, A.; Tasoglu, S. Sensing of electrolytes in urine using a miniaturized paper-based device. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Alseed, M.M.; Dabbagh, S.R.; Zhao, P.; Ozcan, O.; Tasoglu, S. Portable magnetic levitation technologies. Adv. Opt. Technol. 2021, 10, 109–121. [Google Scholar] [CrossRef]
- Gao, Q.-H.; Wen, B.; Kang, Y.; Zhang, W.-M. Pump-free microfluidic magnetic levitation approach for density-based cell characterization. Biosens. Bioelectron. 2022, 204, 114052. [Google Scholar] [CrossRef]
- Dabbagh, S.R.; Alseed, M.M.; Saadat, M.; Sitti, M.; Tasoglu, S. Biomedical Applications of Magnetic Levitation. Adv. NanoBiomed Res. 2021, 2, 2100103. [Google Scholar] [CrossRef]
- Huh, D.; Kim, H.J.; Fraser, J.P.; Shea, D.E.; Khan, M.; Bahinski, A.; Hamilton, G.A.; Ingber, D.E. Microfabrication of human organs-on-chips. Nat. Protoc. 2013, 8, 2135–2157. [Google Scholar] [CrossRef] [PubMed]
- Dabbagh, S.R.; Rabbi, F.; Doğan, Z.; Yetisen, A.K.; Tasoglu, S. Machine learning-enabled multiplexed microfluidic sensors. Biomicrofluidics 2020, 14, 061506. [Google Scholar] [CrossRef] [PubMed]
- Strzelecki, M.; Badura, P. Machine Learning for Biomedical Application. Appl. Sci. 2022, 12, 2022. [Google Scholar] [CrossRef]
- Rabbi, F.; Dabbagh, S.R.; Angin, P.; Yetisen, A.K.; Tasoglu, S. Deep Learning-Enabled Technologies for Bioimage Analysis. Micromachines 2022, 13, 260. [Google Scholar] [CrossRef] [PubMed]
- Volpatti, L.R.; Yetisen, A.K. Commercialization of microfluidic devices. Trends Biotechnol. 2014, 32, 347–350. [Google Scholar] [CrossRef]
- Sochol, R.D.; Sweet, E.; Glick, C.C.; Wu, S.-Y.; Yang, C.; Restaino, M.; Lin, L. 3D printed microfluidics and microelectronics. Microelectron. Eng. 2018, 189, 52–68. [Google Scholar] [CrossRef]
- Amin, R.; Knowlton, S.; Hart, A.; Yenilmez, B.; Ghaderinezhad, F.; Katebifar, S.; Messina, M.; Khademhosseini, A.; Tasoglu, S. 3D-printed microfluidic devices. Biofabrication 2016, 8, 022001. [Google Scholar] [CrossRef]
- Naderi, A.; Bhattacharjee, N.; Folch, A. Digital manufacturing for microfluidics. Annu. Rev. Biomed. Eng. 2019, 21, 325–364. [Google Scholar] [CrossRef]
- Chong, L.; Ramakrishna, S.; Singh, S. A review of digital manufacturing-based hybrid additive manufacturing processes. Int. J. Adv. Manuf. Technol. 2018, 95, 2281–2300. [Google Scholar] [CrossRef]
- Paritala, P.K.; Manchikatla, S.; Yarlagadda, P.K. Digital manufacturing-applications past, current, and future trends. Procedia Eng. 2017, 174, 982–991. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).