Abstract
This study investigates transformations of a pre-mechanically activated saponite-containing material with subsequent high-temperature treatment. The thermogravimetric analysis confirmed that the mechanical activation of saponite leads to the destruction of its layered structure, accompanied by the release of silicon dioxide and magnesium oxide in free form. The values of surface activity for mechanically activated saponite-containing material are also calculated. It is shown that when mechanically activated saponite-containing material is mixed with water, minerals of the serpentine group are formed, and further high-temperature treatment leads to the formation of minerals of the olivine group. It is experimentally shown that high-temperature treatment leads to the creation of a more durable structure of the saponite-containing material. This is due to decreased porosity and pore size, and sorption of moisture from the environment is also reduced. The study showed that saponite-containing waste materials can be effectively treated to create composite materials based on magnesia binders. Thus, with this method, the waste is effectively recycled into various green building material and can be used as supplementary cementitious material or fine aggregate replacement in concrete.
1. Introduction
The significant urbanization of modern territories, regardless of their location, leads to an increase in anthropogenic stress on the environment. This poses a challenge for reducing the environmental impact on the ecosphere, and there is a need to develop ways to dispose of various types of waste generated in significant volumes because of various chemical processes [1,2]. The mining industry is one such industry where many necessary and valuable metals and minerals are extracted from their respective mine ores. However, this process leaves a significant amount of residue, and this additional overburden is usually stored in or dumped into large areas [3]. According to Adiansyah et al. [4], approximately 97–99% by weight of mine ore ends up being residue or waste. This waste is generally known as mine tailings. Recent estimates have indicated that globally, approximately 20–25 billion metric tonnes of mine waste are produced, out of which 7–14 billion tonnes are the mine tailings [5]. With the ever-growing demand for metals for technological advancement, the mining industry’s production is also growing, and thus waste production will grow in direct correlation. These mine tailings are transported in slurry form, as fine particles, to large impoundments, and continuous dumping results in the development of large dams of mine tailings. Approximately 4 × 105 km2 of land is occupied by territories of the mineral industry, including places for storing rock refuse [5]. Such a large accumulation of these mineral rocks in the tailings’ storage area changes the land’s topography and causes damage to hydrogeological and environmental conditions due to their different chemical properties from the local soil [6].
In the Arkhangelsk region, huge amounts of saponite-containing material (SCM) [7] and cyberlite ore dressing are dumped at the diamond mining plant of the open joint stock company (OJSC) “Severalmaz”. The Arkhangelsk pipe of the diamond deposit, named after M.V. Lomonosov, began to be developed 100 km to the northeast of Arkhangelsk in 2002. The uniqueness of the Lomonosov deposit was noted as early as the mid-1980s due to the high quality of diamond raw materials on the one hand and the almost complete substitution of deposit pipe rocks with clay minerals (mainly saponite) on the other, as opposed to solid massive rocks such as in the pipes of the diamond-bearing Yakutsk province [8].
As a result of the development of the Lomonosov diamond deposit, saponite-containing tailings are found in aqueous media in the form of a suspension, making it difficult to use the process water in a recirculated water system. This is due to the low rate of clarification of recycled water as the tailing dump is rapidly filled with tailings. As a result of kimberlite ore dressing, sand and clay rocks in a wet state are sent to the tailing dump, where up to 1 million tons of tailings are stored annually. Tailings transported by hydraulic means are accumulated in special tailing storage tanks to form tailing deposits. The most finely dispersed fraction is formed in a pond area at the tailing dump. The main component of tailing deposits at the Lomonosov deposit is saponite (60–70%), with the following minerals also contained in the solid phase: quartz, montmorillonite, palygorskite, phlogopite, clinochlorite, talc, and dolomite. The chemical composition of SCM in terms of oxides is represented by SiO2 (52%), MgO (19%), Al2O3 (10%), and CaO (4%). The remaining components are found in trace amounts.
To reduce the environmental stress of SCM on the ecosphere, researchers have proposed various ways to use these tailings in the building materials industry [9,10,11,12]. It is known that the pre-activated saponite-containing material is an active component in binding composite systems. It is used as a modifier in concrete mixes and as a component for strengthening soils and can act as an alternative to the phenol-formaldehyde binder of mineral wool materials. At the same time, saponite is classified as a bentonite clay [13] (it has a layered crystal lattice structure, Figure 1) that significantly changes in terms of physical and mechanical properties during heat treatment by synthesizing formations from pre-mechanically activated raw materials. The scheme of the saponite mechanical activation process [14] can be represented by the structural changes shown in Figure 1. However, it should be noted that the modern literature contains very few results of studies into the transformations of a mechanically pre-activated saponite-containing material (SCM) with subsequent high-temperature modification. Therefore, the purpose of this research is to obtain experimental results that allow us to obtain primary data in this area.
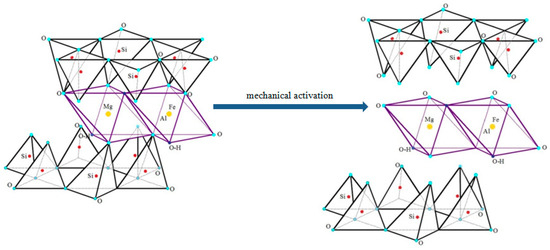
Figure 1.
Crystal lattice of saponite before and after mechanical activation.
At the same time, amorphous phase formation in rocks during their mechanical activation to the ultra- and nano-scale states is an important component of increasing the reactivity of a highly dispersed material. For such systems, this capability is determined by the surface activity. This criterion quantitatively describes the transition of potential energy accumulated by the rock during its genesis to free surface energy due to the raw material’s surface activation [15].
Surface activity (ks) is a thermodynamic criterion for evaluating the surface properties of raw materials and is calculated using the following equation:
where Es is free surface energy, J/kg, and Em is specific mass atomization energy, J/kg (the ratio of the atomization energy (Ea) of the system to the sum of the molar masses of substances (M) that make up the system under consideration [16]).
ks = Es/Em
The atomization energy is a macro parameter of the system and is calculated based on the sample chemical composition by the enthalpy values of its main constituent oxide formation [17]. The parameter of the finely dispersed state of a substance is the free surface energy (Es), which is numerically equal to the product of the specific surface energy of a highly fragmented sample (critical surface tension, σk) and its specific surface area (Sspec) [15]
Es = σk·Sspec
Frolova et al. [18] have worked out methodological techniques to determine the critical surface tension of fine powders based on determining their contact angles of wetting with a reference liquid (G.A. Zisman method).
2. Materials and Methods
For research purposes, the saponite-containing material was separated by electrolyte coagulation from recycled water suspension obtained when dressing kimberlite ores from the Lomonosov diamond deposit [19]. Then, the SCM sample was preliminarily brought to a constant mass at 100 °C. Then, it was mechanically activated by grinding to a highly dispersed state using the Retsch PM100 planetary ball mill for 15 and 45 min with a dry mechanical dispersion method and a carbide-tungsten milling bowl. The specific surface area and pore structure properties were studied with nitrogen sorption using the AUTOSORB-iQ-MP specific surface area and pore size analyzer. The thermogravimetric analysis of experimental samples (synchronous thermogravimetric and differential thermal analysis) was performed at the SDT Q650 thermogravimetric analyzer in a nitrogen atmosphere (50 mL/min flow rate) at a heating rate of 20 °C/min. The temperature range was 30–1150 °C.
3. Results and Discussion
Thermal analysis results for the SCM, mechanically activated SCM and quartz sand (non-mechanically activated as a reference sample) are shown in Figure 2. When analyzing the obtained graphical data, attention should be paid to the temperature range in a region of 576 °C. Thus, Thermogram 1, which corresponds to the SCM sample without preliminary mechanical activation, shows no thermal effects in this temperature region, and Thermogram 2 (mechanically activated SCM) clearly indicates an endothermic peak at this temperature, which corresponds to quartz modification transformations (α → β).
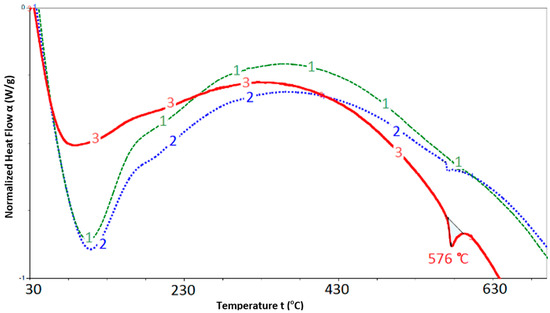
Figure 2.
Thermal analysis of samples: 1—SCM; 2—mechanically activated SCM; 3—quartz sand (98% SiO2).
This can be explained by the fact that the layered structure of saponite is destroyed during its mechanical activation, and this process is accompanied by silicon dioxide release in the free form (Figure 2). This fact is illustrated by the thermal effect at 576 °C in Thermogram 3.
Morozova [14] found that SCM mechanical activation increases the amorphous phase content, and an increase in the amount of the amorphous phase component in the mechanically activated saponite-containing material leads to an increase in the highly dispersed system activity.
The calculated values of ks for highly dispersed SCM samples (Table 1) also confirm that mechanical activation (an increase in the specific surface area) leads to an increase in the material reactivity and, consequently, an increase in the binding properties. The thermal analysis of the mechanically activated SCM mixed with water (Figure 3) shows the presence of an exothermic effect at 850 °C, which is specific to the process of serpentine group mineral formation [20,21].

Table 1.
Properties of SCM samples studied.
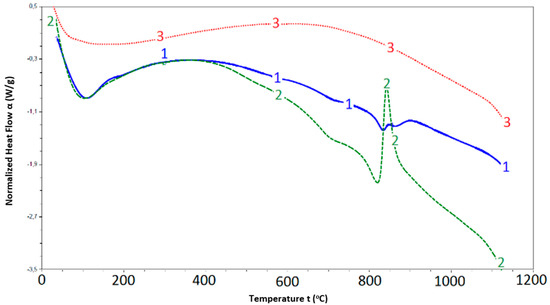
Figure 3.
Thermal analysis of samples: 1—mechanically activated SCM; 2—mechanically activated SCM mixed with water; 3—mechanically activated and thermo-modified (850 °C) SCM.
This can be explained by the fact that SCM’s mechanical activation leads to the release of free silicon and magnesium oxides that can react with each other to form serpentine minerals in the presence of water. Serpentine dehydration effects are present at a treatment temperature of 650–750 °C, and the meta serpentine structure is ordered with the release of forsterite (olivine group) at 850 °C [21,22].
Thermal analysis of the SCM mechanically pre-activated and thermo-modified at 850 °C shows the absence of temperature effects in the thermogram curve, which may indicate that all structural and chemical transformations in the mechanically activated SCM mixed with water are completed at this temperature. New structure generation from a mechanically activated sample can be represented by the following scheme in Equation (3):
MgO + SiO2 → Mg6(OH)8[Si4O10] → Mg2SiO4 + H2O 850 °C
The experiments were followed by evaluating the specific surface area, porosity, and pore size of samples of mechanically activated SCM mixed with water before and after thermal modification. The results of the experiment are presented in Table 2.

Table 2.
SCM specific surface area, porosity, and pore size.
According to the research results, the thermal modification of the mechanically activated SCM mixed with water significantly reduces the specific surface area (up to 50 times), porosity (up to 50 times), and pore diameter (up to 20 times). This indicates that thermal modification leads to a stronger SCM structure formed, and due to reduced porosity and pore size, to a decrease in the moisture sorption from the environment.
4. Conclusions
This paper discusses the environmental effects of waste materials containing saponite and cyberlite ore dressing due to mining activities in the region of Arkhangelsk. Additionally, it proposes the treatment of such waste to reduce the environmental stress. There are various ways to treat such wastes to convert them into materials suitable for use in construction and buildings. In this paper, high-temperature treatment of pre-mechanically activated saponite-containing material is investigated, and the properties of the resulting material after treatment are also investigated. From the study, the following conclusions are made.
- -
- SCM mechanical activation destroys its layered crystal lattice with the release of silicon and magnesium oxides that can interact with each other to form minerals of the serpentine group. This transformation improves the mechanical properties of these waste materials and makes them suitable for applications in ceramics for civil construction projects such as building tiles.
- -
- The thermal modification of the mechanically activated SCM mixed with water contributes to a stronger structure of the saponite-containing material generated due to the formation of olivine group minerals. This fact can be effectively used in the creation of composite materials based on magnesia binders.
- -
- Serpentinites are also used as a thermal and electrical insulator in industrial applications.
Author Contributions
Conceptualization, T.D., M.F. and R.K.C.; methodology, T.D. and R.K.C.; validation, A.A., M.F. and R.K.C.; formal analysis, A.A.; investigation, T.D.; writing—original draft preparation, T.D. and A.A. writing—review and editing, R.K.C. and A.A.J.; visualization, T.D., A.A. and A.A.J.; supervision, M.F., A.A. and R.K.C.; funding acquisition, R.K.C. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by DIKU project CPRU-2018/10013 “Collaborative Educational Programs for Project based Learning in Composite Green Building Materials”.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to acknowledge and appreciate the Department of the Composite Materials and Environmental Engineering, Northern (Arctic) Federal University named after M.V. Lomonosov (NArFU) for allowing the use of unique scientific equipment “Physical Chemistry of Surfaces of Nano-Dispersed Systems” during this experimental work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jhatial, A.A.; Goh, W.I.; Sohu, S.; Mangi, S.A.; Mastoi, A.K. Preliminary investigation of thermal behavior of lightweight foamed concrete incorporating palm oil fuel ash and eggshell powder. Period. Polytech. Civ. Eng. 2021, 65, 168–180. [Google Scholar] [CrossRef]
- Jhatial, A.A.; Goh, W.I.; Mastoi, A.K.; Traore, A.F.; Oad, M. Environmental assessment and mechanical properties of Polypropylene fibres reinforced ternary binder foamed concrete. Environ. Sci. Pollut. Res. 2021, 29, 2985–3007. [Google Scholar] [CrossRef] [PubMed]
- Muther, T.; Qureshi, H.A.; Syed, F.I.; Aziz, H.; Siyal, A.; Dahaghi, A.K.; Negahban, S. Unconventional hydrocarbon resources: Geological statistics, petrophysical characterization, and field development strategies. J. Pet. Explor. Prod. Technol. 2021, 12, 1463–1488. [Google Scholar] [CrossRef]
- Adiansyah, J.S.; Rosano, M.; Vink, S.; Keir, G. A framework for a sustainable approach to mine tailings management: Disposal strategies. J. Clean. Prod. 2015, 108, 1050–1062. [Google Scholar] [CrossRef] [Green Version]
- Hooke, R.L.; Martín-Duque, J.F. Land transformation by humans: A review. GSA Today 2012, 12, 4–10. [Google Scholar] [CrossRef]
- Lazorenko, G.; Kasprzhitskii, A.; Shaikh, F.; Krishna, R.S.; Mishra, J. Utilization potential of mine tailings in geopolymers: Physicochemical and environmental aspects. Process Saf. Environ. Prot. 2021, 147, 559–577. [Google Scholar] [CrossRef]
- Chanturiya, V.A.; Minenko, V.G.; Makarov, D.V.; Suvorova, O.V.; Selivanova, E.A. Recycling prospects for saponite-containing water at diamond processing plants in Arkhangelsk Region, Russia. Preprints 2018, 2018100463. [Google Scholar] [CrossRef]
- Posukhova, T.V.; Dorofeev, S.A.; Garanin, K.V.; Siaoin, G. Diamond industry wastes: Mineral composition and recycling. Mosc. Univ. Geol. Bull. 2013, 68, 96–107. [Google Scholar] [CrossRef]
- Morozova, M.V.; Ayzenshtadt, A.M.; Makhova, T.A. The use of saponite-containing material for producing frost-resistant concretes. Ind. Civ. Constr. 2015, 1, 28–31. [Google Scholar]
- Oblitsov, A.Y.; Rogalev, V.A. Prospective ways of diamondiferous rock enrichment wastes utilization at M.V. Lomonosov diamond deposit. J. Min. Inst. 2012, 195, 163–167. [Google Scholar]
- Stenin, A.A.; Ayzenshtadt, A.M.; Shinkaruk, A.A.; Demidov, M.L.; Frolova, M.A. A mineral modifier of a surface for protection of wood building materials. Constr. Mater. 2014, 10, 51–54. [Google Scholar]
- Drozdyuk, T.A.; Ayzenshtadt, A.M.; Tutygin, A.S.; Frolova, M.A. Inorganic binding agents for mineral wool heat insulation. Stroit. Mater. [Constr. Mater.] 2015, 5, 86–88. [Google Scholar] [CrossRef]
- Apollonov, V.N.; Verzhak, V.V.; Garanin, K.V.; Garanin, V.K.; Kudryavtseva, G.P.; Shlykov, V.G. Saponite from the Lomonosov diamond deposit. J. Geol. Explor. 2003, 3, 20–37. [Google Scholar]
- Morozova, M.V. Surface activity of highly dispersed systems based on saponite-containing waste from diamond mining industry. Bull. Belgorod State Technol. Univ. Named After V.G. Shukhov 2018, 2, 5–9. [Google Scholar] [CrossRef]
- Veshnyakova, L.A.; Drozdyuk, T.A.; Aizenshtadt, A.M.; Frolova, M.A.; Tutygin, A.S. Surface activity of siliconcontaining rocks. Mater. Sci. 2016, 5, 45–48. [Google Scholar]
- Zuev, V.V.; Potselueva, L.N.; Goncharov Yu, D. Crystal Energy as a Basis for Evaluating the Magnesian Properties of Solid Materials (Including Magnesia Cements); Alfopol: Saint Petersburg, FL, USA, 2009; p. 139. [Google Scholar]
- Abramovskaya, I.R.; Ayzenshtadt, A.M.; Veshnyakova, L.A.; Frolova, M.A.; Lesovik, V.S.; Kazlitin, S.A. Calculation of the energy intensity of rock as raw material to produce building materials. J. Ind. Civ. Eng. 2012, 10, 23–25. [Google Scholar]
- Frolova, M.A.; Tutygin, A.S.; Ayzenshtadt, A.M.; Lesovik, V.S.; Makhova, T.A.; Pospelova, T.A. Evaluation criteria of energy properties of surface of nanomaterials. Nanosyst. Phys. Chem. Math. 2011, 2, 120–125. [Google Scholar]
- Tutygin, A.S.; Shinkaruk, A.A.; Ayzenshtadt, A.M.; Frolova, M.A.; Makhova, T.A. Clarification of a saponite-containing suspension by the method of electron coagulation. J. Water Chem. Ecol. 2013, 5, 93–99. [Google Scholar]
- Viti, C. Serpentine minerals discrimination by thermal analysis. Am. Mineral. 2010, 95, 631–638. [Google Scholar] [CrossRef]
- Dlugogorski, B.Z.; Balucan, R.D. Dehydroxylation of serpentine minerals: Implications for mineral carbonation. Renew. Sustain. Energy Rev. 2014, 31, 353–367. [Google Scholar] [CrossRef] [Green Version]
- Guryeva, V.A.; Ilyina, A.A. Bulletin of the volgograd state university of architecture and civil engineering. Ser. Constr. Archit. 2020, 78, 142–148. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).