Abstract: Background
The effect of laser radiation has never been tested on the antimicrobial activity of cyanobacterial extracts. In order to investigate this, aqueous extracts from three cyanobacterial strains identified as Thermoleptolyngbya sp., Leptolyngbya sp., and Synechococcus elongatus were exposed to laser radiation. The aqueous extracts both directly exposed to the laser and those derived from pre-exposed biomass were tested for their antimicrobial activity to select the most active extracts under different exposure times and distances from the laser source. Methods: A fixed weight of one-month old cyanobacterial biomass was used in extraction. Another similar biomass was exposed to laser before aqueous extraction. The laser treatment was performed using two distances, 5 and 10 cm, with three exposure times, 4, 16, and 32 min. The antimicrobial assay was performed against the bacterial plant pathogen, whose identity was confirmed by molecular analysis and cell wall structure by a Gram stain. Results: The pathogenic bacterium was identified as Gram-negative Pantoae vagans. The aqueous extract that was not exposed to laser treatment (control) was mostly ineffective against the pathogenic bacterium, whereas a significant increase in the antimicrobial effect was observed for the extract directly exposed to the laser followed by the extract derived from laser-pre-exposed cyanobacterial biomass. In the case of Synechococcus elongatus extracts, the extract that was directly exposed to the laser showed the highest statistically significant antimicrobial activity against Pantoea vagans, with an inhibition zone of 15.5 mm, at 10 cm and 4 min of laser treatment. Conclusions: This is the first report on the effect of laser on enhancing the antimicrobial profile of cyanobacterial extracts. The direct exposure of cyanobacterial extracts to the laser was more effective and biologically safer than exposing the biomass itself prior to extraction. The laser used was a monochromatic red light within the visible range. This radiation increased the antimicrobial activity of cyanobacterial extracts and can be used as an eco-friendly biocontrol strategy.
1. Background
Cyanobacteria are a rich source of bioactive compounds with various antimicrobial activities [1,2,3]. These bioactive compounds are mostly produced during secondary growth. Secondary metabolism is mainly concerned with the production of molecules that have biological effects but are not necessary for growth or reproduction. Instead, they allow cyanobacteria to survive under different stresses. In addition, they play a role in treating and preventing several forms of diseases, including fungal, viral, and bacterial diseases [4]. With regard to the molecular basis of production of bioactive compounds, including antimicrobials, Ehrenreich et al. [5] reported that this is mainly related to genes responsible for the synthesis of the non-ribosomal peptide synthetases (NRPSs) and polyketide synthases (PKSs) but they are not the only genetic systems for bioactive compound production. Strains of Cyanothece sp. strain WH8904 and Lyngbya sp. strain PCC7419, for example, do not have those typical genetic systems but still produce bioactive compounds. The bioactive compounds produced by cyanobacteria depend on the culture conditions and growth phase. Therefore, manipulation of physical and biochemical factors would allow for the production of bioactive compounds from cyanobacteria [6,7]. The most important factors include physical factors, such as the incubation period and the pH of the culture media [8], as well as biochemical factors, such as the culture nutrient composition [9]. Cyanobacterial extracts are known for their antibacterial action against a wide array of bacteria. Extracts of Phormidium species inhibited the growth of pathogenic strains of Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli, and Streptococcus enteritidis [10]. An organic acetone extract of Oscillatoria agardhii was highly active against Salmonella senftenberg [11]. Ethanol extracts of Spirulina platensis demonstrated antimicrobial activity against different species of Gram-negative and Gram-positive bacteria [12]. Ethanol and aqueous extracts of Anabaena circinalis showed antibacterial activity against Serratia marcescens and Escherichia coli. Additionally, Nostoc commune and Nostoc muscorum exhibited antimicrobial activity [13]. Organic extracts of different species of phytoplankton also inhibited the growth of different species of bacteria [14]. Interestingly, the antimicrobial bioactivity of cyanobacterial extracts was increased significantly with the use of UV and gamma irradiation of the cyanobacterial cells [15]. Additionally, the antimicrobial bioactivity of extracts directly exposed to UV irradiation was increased as well [16]. However, the laser irradiation effect on the antimicrobial bioactivity has never been investigated.
Laser is an abbreviation for “light amplification by the stimulated emission of radiation” and is non-ionizing radiation. Laser discharge mainly occurs in the ultraviolet, visible light, and infrared frequencies, depending on the wavelength generated [17]. A laser is a gadget that excites molecules or atoms to release light at specific frequencies and intensify that light, essentially creating an exceptionally focused light emission [18]. In biological cells, the penetration and absorption of laser radiation depends on several factors, such as the distance from the laser beam to the cell, the area of the cell exposed to the laser radiation, laser wavelength, dose, and power 2.5. density. The dose and power density are calculated from the following formulas [19]: dose (J) = mean power × treatment time; and power density for continuous output laser (mW/cm2) = mean power/spot size sample. A low-energy laser has been found to modify several biological processes in cell cultures and animal models [20]. At the cellular level, laser irradiation can cause important biological effects, such as cellular reproduction, collagen synthesis, and emission of growth factor from cells [21]. Laser creates biological modification not only through direct radiation effects but also through thermal effects [22]. A laser can be used in medicine as a mode of therapy. The laser permits surgeons to carry out their work with high precision by targeting a small area, minimizing damage to the surrounding tissue [17]. Patients who undergo laser therapy experience less pain, scarring, and swelling than in traditional surgery. Laser therapy is used to shrink and destroy tumors or precancerous growths, eliminate kidney stones, relieve cancer symptoms, repair detached retinas, treat hair loss due to aging or alopecia, and improve vision [23]. The used laser is a semiconductor-diode laser, which emits light near or in the red visible region after the electric current passes through. The emission happens at the p-n junction [17]. Red laser light was found to have a typical penetration depth of nearly 1 mm (a depth that reduces the incident energy to 37%), while infrared laser light has a penetration depth of 3 mm [24]. Since irradiation can result in an increase in the antimicrobial bioactivity of cyanobacterial extracts, as found by El Semary [15] using UV and gamma radiation, the laser irradiation effect on cyanobacterial extracts should be tested as well. The literature on the topic is scare; the only available report is the use of laser on cyanobacterial biomass to isolate lipids [25]. Therefore, the objectives of the present study were the selection of conditions and parameters that result in the enhancement of antimicrobial activity of the cyanobacterial extracts by laser treatment. In order to reach our goals, we first had to identify the most appropriate time for laser treatment. Thus, aqueous extracts both directly exposed to laser and those derived from pre-exposed biomass were tested for their antimicrobial activity to select the most appropriate laser treatment condition. Moreover, laser treatment is affected by the exposure time and distance from the source. Therefore, those parameters were tested to select the ones that resulted in the highest antimicrobial bioactivity of cyanobacterial extracts.
2. Methods
2.1. Isolation of Tomato Pathogenic Bacterium and Initial Detection via Gram Stain
Bacterial swap from infected tomato fruit was spread on nutrient agar plates (HIMEDIA) and incubated at 30 °C for 24 h. One colony of bacteria was picked and re-streaked on a nutrient agar plate and incubated at 30 °C for 24 h. Pure colonies of bacteria samples were inoculated on 70% broth media (HIMEDIA) and 30% glycerol (v/v) and incubated at 30 °C for 24 h, and then were kept in a fridge at −4 °C for use in future experiments. All bacterial isolates were subjected to Gram staining according to Claus [26].
2.2. Cyanobacterial Samples
Cyanobacterial samples were isolated from several freshwater locations. The samples were collected from sulfur spring water and a Hassawi rice field in the Al-Ahsa region in May 2019. All samples were collected in sterile plastic containers and filtered with filter paper. Cyanobacterial samples were isolated by centrifuging at 3000 rpm for 30 min (HERMLE-Z200A, Wehingen, Germany). The biomass was harvested and inoculated in the BG-11 growth medium. The freshwater samples were streaked on solid BG-11 culture medium [27,28]. Single filaments or colonies were selected and placed in vials containing liquid growth medium. Cultures were morphologically identified under light microscope according to Rippka et al. [29]. Three cyanobacterial cultures were established: Thermoleptolyngbya sp., Leptolyngbya sp., and Synechococcus elongatus. They were examined by light microscopy using OLYMPUS-Cx23 (Japan) with 40× and 100× oil immersion lenses. Thermoleptolyngbya sp. was isolated from sulfur spring water, and Leptolyngbya and Synechococcus elongatus were isolated from the Hassawi rice field.
2.3. DNA Extraction of Bacterial Colonies
Genomic bacterial DNA was initially extracted by boiling at 95 °C for 5 min to release DNA from the lysed cells. The bacterial pellets were briefly suspended in 200 µL of TE buffer (10 mM Tris-HCl and 1 mM EDTA) at pH 8.0. Immediately after boiling, the mixture was transferred to Eppendorf tubes in an ice bath for 15 min, and then centrifuged at 14,000 rpm for 5 min and transferred into clean Eppendorf tubes and stored at −20 °C [30].
2.4. PCR Amplification of DNA from Pathogenic Bacteria
Amplification of the 16S_rRNA gene from bacterial isolate was carried out using the universal primers 27F (5′AGA GTT TGA TCM TGG CTC AG’3) and 1492R (5′TAC GGY TAC CTT GTT ACG ACTT’3).
The main PCR steps were programmed as follows:
- Initial denaturation at 94 °C for 2 min.
- Thirty cycles where in each cycle the following stages were run:
Denaturation at 94 °C for 45 s.
Annealing at 55 °C for 60 s.
Extension 72 °C for 90 s.
- 3.
- Final extension step at 72 °C for 10 min.
The PCR mixture volume was 25 µL. One µL of DNA extract, 2 mM MgCl2, 2.5 µL of 10× PCR buffer, 1.5 µL of 10 M of each primer, 2.5 µL of 10 mM dNTPs, and 0.3 µL of 5 Units of Taq DNA polymerase were all inoculated in the mixture. The mixture was completed with 25 µL of nuclease-free water. PCR products were purified with the QIAquick R PCR Purification Kit (Cat. No. 28106) according to the manufacturer’s procedures. The purified PCR products were sequenced by Macrogen Inc., (Seoul, Korea), and sequencing of the purified isolates was performed in both directions using the specific primer pair. The sequence retrieved was subjected to BLASTn (nucleotide search) to retrieve the closely related sequences. A neighbor-joining phylogenetic tree was constructed using the option of phylogenetic tree in the Blast tool. The neighbor-joining method algorithm is the algorithm dependent on the distance between each pair of sequences to form the tree.
2.5. Cyanobacterial Biomass and Cyanobacterial Extract Preparation for Laser Treatment
One-month-old cyanobacterial cultures were centrifuged at 3000 rpm for 30 min. This was repeated three times. Each time, the supernatant was discarded, and the biomass was resuspended in water and centrifuged. About 0.1 gm of freshwater/1 mL of distilled water (w/v) was used for each sample with replicates.
To measure the effect of different laser treatments, three sets were prepared:
- Cyanobacterial extract not exposed to laser (control).
- Cyanobacterial extract derived from biomass pre-exposed to laser radiation.
- Cyanobacterial extract directly exposed to laser radiation.
All extracts were incubated at 37 °C for 72 h before antibiotic bioassay. Factors affecting laser dosage were tested, namely: exposure time and laser distance. The effects of these were assayed on laser-treated cyanobacterial biomass and cyanobacterial extract. In biological cells, the penetration depth and absorption of laser radiation received depend on several factors: the distance from the laser beam to the cell and the laser wavelength, dose, and power densities. The dose and power density can be calculated from the following formulas: dose (J) = mean power x treatment time where power density for continuous output laser (mW/cm2) = mean power/spot size on biological cell.
The diode laser (635 nm) (red laser) was used. Different doses were introduced by changing exposure time in combination with distance from the laser source. The distances used were 5 cm and 10 cm, and the exposure times were 4 min, 16 min, and 32 min.
The diode laser used had a probe tip 3 mm in diameter and power density of <1 mW in continuous irradiation mode. The beam diameter and laser output were obtained from manuals. Laser treatment was conducted over exposure times of 4, 16, and 32 min in combination with distances of 5 cm and 10 cm from the source of the laser. Both cyanobacterial biomass and extracts of cyanobacteria were exposed to laser radiation directly for periods of 4, 16, and 32 min at distances of 5 cm and 10 cm from the laser source. The control extract was not subjected to laser radiation.
2.6. Antimicrobial Bioassay
The bacterial strain was activated by culturing on agar plate and incubating it overnight at 37 °C in Mueller–Hinton agar. One colony of bacteria was inoculated in 10 mL of Luria–Bertani broth medium and incubated at 30 °C overnight. Then, they were mixed with 100 mL of sterilized liquid nutrient agar. Finally, the agar plates were poured and left to solidify. The disc diffusion method was used to assess the antimicrobial activity of cyanobacterial biomass and cyanobacterial extracts. Sterilized diffusion discs 6 mm in diameter were used in the susceptibility test modified by Kirby–Bauer [31]. Diffusion discs were saturated with 20 µL of cyanobacterial aqueous extracts and placed on the agar plates inoculated with bacterial strain according to [32,33]. The agar plates were then incubated at 37 °C overnight.
2.7. Statistical Analysis Using Split Plot -ANOVA
The Split Plot-ANOVA is a statistical test used to determine if two or more repeated measures from two or more groups are significantly different from each other with regard to the variable of interest. The variable of interest must be continuous, normally distributed, and have a similar spread across groups. Replicate measures from the same units of observation must be recorded, and there must be enough data (https://www.statstest.com/split-plot-anova/ (accessed on 1 March 2021)). The experimental design was split into two plots: biomass pre-exposed to laser and extract directly exposed to laser, which represented the main two plots while distance was presented as sub-plots and exposure time sub-sub plots. Data were statistically analyzed with the ANOVA of Statistics 6 software from the StatSoft company (Statsoft, 2001), and the significance of differences among means was calculated with the least significant difference test (LSD) at p = 0.05.
3. Results
3.1. Characterization of the Plant Pathogenic Bacterial Strain Isolated from Tomato
The bacterial strain isolated from tomato was identified as a Gram-negative bacillus bacterium whose colonies are yellow in color with undulating margins and convex elevation (Figure 1). The molecular characterization revealed its identity as Pantoea vegans. A neighbor-joining phylogenetic tree for Pantoea vegans was constructed using the closely related 16S_rDNA sequences (Figure 2).
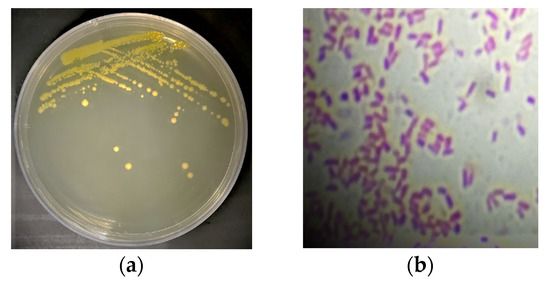
Figure 1.
(a) A morphological characterization of Pantoea vagans colony growth, (b) a light micrograph of Gram stain of Pantoea vagans showing Gram-negative bacilli bacteria. The micrograph was taken using 100× objective lens, total magnification of 1000×.
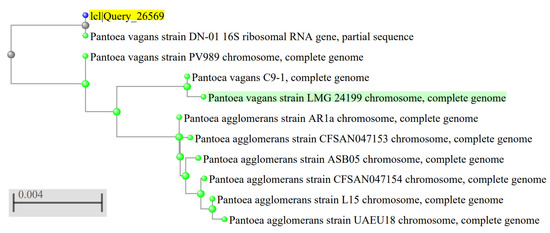
Figure 2.
A phylogenetic tree based on neighbor-joining method. Created by phylogenetic tree function in BLAST tool. The sequence of the bacterial strain was designated IcI Query_ 26569 (denoted by blue circle and highlighted in yellow), The best match Pantoe vagans strain LMG 24199 (denoted by green circle and highlighted in green) using phylogenetic tree in BLASTn tool.
3.2. Laser Treatments
With regard to laser treatments, three time intervals were used: 4 min, 16 min, and 32 min at both 5 cm and 10 cm between the laser source and the sample. It was evident that Thermoleptolyngbya sp., Leptolyngbya sp., and Synechococcus elongatus extracts derived from cyanobacterial biomass pre-exposed to the laser and extracts directly exposed to 635 nm diode laser irradiation had enhanced antimicrobial effects against Pantoea vagans. Direct laser treatment of extracts resulted in a much higher inhibitory effect than that of the untreated cyanobacterial extract used as a negative control (Table 1).

Table 1.
Effect of interaction between distance and time intervals as functions of laser dose treatment on the antimicrobial effect of cyanobacterial extracts derived from cyanobacterial biomass pre-exposed to laser and extracts directly exposed to laser. Experiments were carried out in triplicate.
3.3. Effect of Laser Treatment on Antimicrobial Activity of Thermoleptolyngbya sp. Biomass Pre-Exposed to Laser and Directly Exposed Extracts against Pantoea vagnas
The extract derived from cyanobacterial biomass pre-exposed to the laser of Thermoleptolyngbya sp. and its aqueous extract, which was directly exposed to the laser, showed some antimicrobial action against Pantoea vagnas (Table 1). Thermoleptolyngbya sp. extracts derived from cyanobacterial biomass pre-exposed to the laser and extracts directly exposed to the laser were tested against Pantoea vagans. The inhibition zone for the negative control was 9.5 mm. At a treatment distance of 5 cm, no enhancing effect of the laser treatment was observed at any time interval, as shown in (Table 1). Similarly, the effect of extracts directly exposed to laser at 5 cm against Pantoea vagans was not statistically significant. Extracts directly exposed to the laser for different time intervals did not exhibit enhanced antimicrobial action. At a treatment distance of 10 cm, the Thermoleptolyngbya sp. extract derived from biomass pre-exposed to the laser against Pantoea vagans exhibited similar results to those at the 5 cm distance. In contrast, the Thermoleptolyngbya sp. extract directly exposed to the laser exhibited higher inhibition zone diameters for most exposure times: 15 mm for both 4 min and 32 min, more than that of the control, as shown in (Table 1).
3.4. Effect of Laser Treatment on Antimicrobial Activity of Leptolyngbya sp. Biomass Pre-Exposed to Laser and Extracts Directly Exposed against Pantoea vagnas
The extract derived from Leptolyngbya sp. biomass pre-exposed to the laser and the extract directly exposed to the laser showed dissimilar antimicrobial effects against Pantoea vagnas (Table 1). At a treatment distance of 5 cm, only the Leptolyngbya sp. extract directly exposed to the laser showed enhanced antimicrobial activity with increasing time intervals against Pantoea vagans (Table 1). At a treatment distance of 10 cm, only the Leptolyngbya sp. extract directly exposed to the laser showed enhanced antimicrobial activity against Pantoea vagans with 32 min exposure (Table 1).
3.5. Effect of Laser Treatment on Antimicrobial Activity of Synechococcus elongatus Biomass Pre-Exposed to Laser and Extracts Directly Exposed against Pantoea vagnas
The LASER treatment of distance 5 cm and time interval of 32 min significantly enhanced the antimicrobial activity of the Synechococcus elongatus extract directly exposed to the laser against Pantoea vagans. The extract derived from biomass pre-exposed to the laser showed increasing antimicrobial activity with increased time, reaching a maximum at 32 min (Table 1).
LASER treatment of distance 10 cm, a highly significant enhancement of antimicrobial activity was obtained for the Synechococcus elongatus extract directly exposed to the laser against Pantoea vagans, with an exposure time of 32 min (Figure 3).
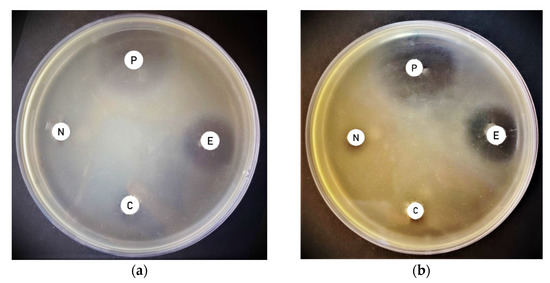
Figure 3.
An agar plate showing the greatest inhibition zone of extract derived from biomass pre-exposed to laser and extract directly exposed to laser and extract directly exposed to laser of Synechococcus elongatus against Pantoea vagans at 10 cm. (a) Effect of laser treatment for different exposure times with 4 min intervals at a distance of 10 cm. (b) Effect of laser treatment for different exposure times with 16 min intervals at a distance of 10 cm. (P) positive control—(N) negative control—(C) an extract derived from cyanobacterial biomass pre-exposed to laser—(E) an extract directly exposed to laser.
4. Discussion
To investigate the effect of radiation doses on the antimicrobial activity, different time intervals in combination with different distances from the laser source were used. This is a novel approach to enhancing the antimicrobial activity of cyanobacterial extracts by applying non-ionizing visible radiation to avoid the hazards that result from ionizing radiation. Hence, we used the red-visible-light 635 nm diode laser beam to ensure that the cyanobacteria were not subjected to any genetic change. Thus, the risks were minimized, and the effectiveness increased. We also exposed cyanobacterial biomass to compare the antimicrobial action of their extracts to extracts directly exposed to the laser in order to select the best treatment in terms of biosafety, efficacy, and reproducibility. Although aqueous extracts’ effectiveness may be below that of organic extracts [34] but they are eco-friendlier and more cost-effective. To concentrate bioactive compounds in aqueous extracts as well as to enhance antimicrobial activity, aqueous extracts were incubated at 37 °C for 72 h after laser treatment. This step increased the inhibition zone diameter considerably. Incubation allowed excess water to evaporate, thus increasing the concentration of the bioactive compounds, as Monserrat et al. [35] described for concentrating aqueous extracts of Anabaena spiroides at 40 °C. The split-plot ANOVA provided an insight into the significance or non-significance of the variables tested across groups. Here, we used two main groups: the extract derived from biomass pre-exposed to the laser and the extract directly exposed to the laser, and we tested their interaction with exposure time and distance to show which treatment resulted in the highest significant change from the control.
Cyanobacteria contain bioactive antimicrobial agents. Some of these agents are hydrophilic molecules that dissolve in water. In this study, the aqueous extracts of cyanobacteria contained polar molecules and ions in addition to water-soluble pigments. Certain pigments are specifically found in cyanobacteria, and they do have antimicrobial activity. Phycobiliproteins (phycocyanin, allophycocyanin, and phycoerythrin) are a family of water-soluble photosynthetic accessory pigments which all possess antimicrobial activity. Phycobiliproteins are water-soluble proteinaceous pigments [36]. Interestingly, Refs. [37,38] showed that phycocyanin pigment of Spirulina platensis and Westiellopsis sp. has a potent antimicrobial effect against Gram-positive and Gram-negative bacteria. In addition, Afreen and Fatma [39] reported that phycoerythrin of Microchaete has antibacterial activity against pathogenic bacteria.
Both light intensity and quality can impact photosynthetic pigments in cyanobacteria. The control of light conditions by synthetic light can be sufficient to affect cyanobacterial pigment production. This in agreement with Kim et al. [40], who reported that the artificial light LED is a suitable economic energy source that increases the cyanobacterial pigment content. An LED is a red-light, light-emitting diode [41], specific monochromatic light can affect photosynthesis and pigment production [42]. Indeed, the quality of light can influence the photosynthetic pigments’ quantity. For example, Ref. [43] proved that cyanobacteria that grow in red light contain a higher concentration of phycocyanin than cyanobacteria grown in white light. In our case, it is most likely that exposure of cyanobacteria to a red-light laser resulted in an increase in the phycocyanin content. This particular pigment has antimicrobial activity against pathogenic bacteria [43]. This may account for the increase in the antimicrobial activity after exposure to a red-light laser.
Synechococcus elongatus was isolated from rice field water. S. elongatus is a unicellular rod-shaped cyanobacterium [44]. S. elongatus forms biofilms with extracellular polymeric materials, and these give S. elongatus the ability to resist environmental stress and antimicrobial drugs [45]. Extracts of S. elongatus showed antimicrobial effects before and after laser treatment. Similarly, Ref. [46] proved the Synechococcus genus can be considered as a source of bioactive compounds against bacteria. Methanolic extracts of S. elongatus showed antimicrobial activity against several strains of bacterial species [14]. With regard to the molecular basis of bioactive compound production from cyanobacteria in general and Syenchococcus sp. in particular, Strieth et al. [47] recommended the use of in silico alongside in vivo screening for antimicrobial compounds from cyanobacteria. Indeed, Konstantinou et al. [48] screened marine cyanobacteria associated with sponge and found that three marine Syechococcus sp. have polyketide synthase genetic systems. Nonetheless, Ehrenreich et al. [5] reported that strains of Synechococcu sp. lack non-ribosomal peptide synthetase and polyketide synthase genetic systems. This suggests that the presence or absence of those systems is strain-dependent. Strains of the same species differ in the presence or absence of those systems, and this may explain the ability of some strains of the same species to have antimicrobial activity while others do not. Indeed, Konstantinou et al. [48] showed that Synechococcus sp. 0715 had no antimicrobial activity against Straphylococcus aureus, whereas strain 0815 of the same species had antimicrobial activity. Moreover, Konstantinou et al. [48] also showed that the Leptothoe spongobia1115 strain had both a non-ribosomal peptide synthetase system as well as a polyketide synthase genetic system and was effective against Staphylococcus aureus. On the other hand, Leptothoe spongobia1105 only had a polyketide synthase genetic system and did not show antimicrobial bioactivity against Staphylococcus aureus. Therefore, more studies need to be carried out to further explore the molecular machinery of antimicrobial biosynthesis within cyanobacteria. No studies are available on the effect of laser on those systems. Nonetheless, with regard to radiation in general, a study that involved Synechococcus elongatus, according to (https://www.osti.gov/servlets/purl/1367105, accessed 20 April 2022), showed that cyanobacteria may undergo changes to resist ionizing radiation, such as an increase in genome copy number (being polyploidy, overexpression of reactive oxygen species’ degradation enzymatic system and condensed nucleotide structure). A study on Synechococcus lividus demonstrated that low doses of ionizing radiation stimulated cell proliferation as a result of peroxide-degrading enzymes and glucose catabolism [49]. Xue et al. [50] showed that nitric oxide alleviates oxidative damage induced by enhanced ultraviolet-B radiation in a cyanobacterium. Rastogi et al. [51] reviewed the effects of UV-B radiation on cyanobacteria; it can induce damage on cellular DNA and directly interacts with certain biomolecules that absorb in the UV range. It can also cause indirect effects through inducing oxidative stress exerted by the reactive oxygen species generated. Rastogi et al. [51] also reviewed the several lines of defense and tolerance mechanisms, including antioxidant production, protein resynthesis, DNA repair, apoptosis, and the synthesis of UV-protecting compounds, including mycosporine-like amino acids and scytonemin.
Although the laser used here was in the visible range, a speculation can be made that some intracellular changes may occur in case of cyanobacterial biomass exposed to a laser, especially for molecules absorbing light in the red region, such as the pigment phycocyanin. Those changes may affect the bioactive compounds’ metabolism intracellularly and possibly induce mutagenic changes as well. On contrary, extracts directly exposed to radiation were derived from cyanobacterial cell that were not exposed to radiation beforehand. Therefore, there were no possible cellular alterations or “mutagenesis”. This is a much more eco-friendly, and bio-secure approach.
In general, extracts derived from cyanobacterial biomass pre-exposed to a laser at a distance of 5 cm showed a negative effect, where the inhibition zone was smaller than that of the control. However, at a longer treatment distance 10 cm, cyanobacteria seemed to better “adapt” to laser stress and started to give positive results. However, the extracts directly exposed to laser gave better results than the extracts derived from the cyanobacterial biomass pre-exposed to the laser possibly due to direct effect on the chemical entities found in the aqueous extract. Our study, hopefully, opens the door for future investigations.
5. Conclusions
To the best of our knowledge, this is the first study on the effect of different laser treatment doses on the antimicrobial bioactivity of cyanobacteria against Pantoea vagnas. The exposure was performed directly on cyanobacterial cells followed by aqueous extraction as well as cyanobacterial extracts to compare their antimicrobial effects. However, extracts from cyanobacteria exposed directly to the laser were more effective and safer to use, lacking presumably mutagenic cyanobacteria. Therefore, the use of aqueous extracts directly exposed to a laser is highly recommended. Using a diode laser at 635 nm and controlling distance from source and exposure time significantly enhanced antimicrobial activity. The application of a red-light laser significantly enhanced the antimicrobial bioactivity of cyanobacterial extracts, which can be used in the biocontrol of plant pathogens instead of harmful pesticides.
Author Contributions
N.E.S. was responsible for conceptualization of the initial research idea, designing experiments, supervising isolation, culturing and characterization of cyanobacterium, antimicrobial bioassay, supplying resources, interpretation of data, and the writing up of the manuscript and applying for the chair fund in addition to two complete rounds of rewritings of the manuscript in addition to final revision of the manuscript to meet the requests of the kind reviewers. H.A.N. was responsible for conducting experiments on isolation and characterization of bacteria and cyanobacteria, antimicrobial bioassay with and without laser exposure, interpretation of data, and writing. M.F.A. was responsible for advising on the antimicrobial bioassay. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Al Bilad Bank Scholarly Chair for Food Security in Saudi Arabia, The Deanship of Scientific Research, The Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, and Al Ahsa, Saudi Arabia under grant chair number 44.
Institutional Review Board Statement
Not applicable (No human materials, data, or participants were involved).
Informed Consent Statement
All authors agreed to publication. No written permission is needed from a third party.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
The authors express their deepest gratitude to al Bilad Bank Scholarly Chair for Food Security in Saudi Arabia, The Deanship of Scientific Research, The Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, and Al Ahsa, Saudi Arabia, grant chair number 44. The authors are grateful for financial and moral support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- El Semary, N.A. The polyphasic description of a Phormidium-like cyanobacterium from Egypt with antimicrobial activity of its methanolic extract. Nova Hedwig. Ger. 2011, 92, 377–390. [Google Scholar] [CrossRef]
- El Semary, N.A. The characterisation of bioactive compounds from an Egyptian Leptolyngbya sp. strain. Ann. Microbiol. 2012, 62, 55–59. [Google Scholar] [CrossRef]
- El Semary, N.A. The antimicrobial profile of extracts of a Phormidium-like cyanobacterium changes with phosphate levels. World J. Microbiol. Biotechnol. 2012, 28, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Rath, J. Secondary metabolites of cyanobacteria and drug development. In Extremophilic Cyanobacteria for Novel Drug Development; Springer: Berlin, Germany, 2015; pp. 23–43. [Google Scholar]
- Ehrenreich, I.M.; Waterbury, J.B.; Webb, E.A. Distribution and diversity of natural product genes in marine and freshwater cyanobacterial cultures and genomes. Appl. Environ. Microbiol. 2005, 71, 7401–7413. [Google Scholar] [CrossRef] [Green Version]
- Borowitzka, M.A. Microalgae as sources of pharmaceuticals and other biologically active compounds. J. Appl. Phycol. 1995, 7, 3–15. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Osman, M.E.; Dyab, M.A.; Amer, M.S. Production and characterization of antimicrobial active substance from the cyanobacterium nostoc muscorum. Environ. Toxicol. Pharmacol. 2006, 21, 42–50. [Google Scholar] [CrossRef]
- Gupta, V.; Ratha, S.K.; Sood, A.; Chaudhary, V.; Prasanna, R. New insights into the biodiversity and applications of cyanobacteria (blue-green algae)—Prospects and challenges. Algal Res. 2013, 2, 79–97. [Google Scholar] [CrossRef]
- Noaman, N.H.; Fattah, A.; Khaleafa, M.; Zaky, S.H. Factors affecting antimicrobial activity of Synechococcus leopoliensis. Microbiol. Res. 2004, 159, 395–402. [Google Scholar] [CrossRef]
- Thummajitsakul, S.; Silprasit, K.; Sittipraneed, S. Antibacterial activity of crude extracts of Cyanobacteria Phormidium and Microcoleus species. Afr. J. Microbiol. Res. 2012, 6, 2574–2579. [Google Scholar] [CrossRef]
- Abd El-Aty, A.M.; Mohamed, A.A.; Samhan, F.A. In vitro antioxidant and antibacterial activities of two fresh water cyanobacterial species, Oscillatoria agardhii and Anabaena sphaerica. J. Appl. Pharm. Sci. 2014, 4, 69. [Google Scholar]
- Ozdemir, G.; Ulku Karabay, N.; Dalay, M.C.; Pazarbasi, B. Antibacterial activity of volatile component and various extracts of Spirulina platensis. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2004, 18, 754–757. [Google Scholar]
- Shaieb, F.A.; Issa, A.A.; Meragaa, A. Antimicrobial activity of crude extracts of Cyanobacteria nostoc commune and spirulina platensis. Arch. Biomed. Sci. 2014, 2, 34–41. [Google Scholar]
- Del Pilar Sánchez-Saavedra, M.; Licea-Navarro, A.; Bernáldez-Sarabia, J. Evaluation of the antibacterial activity of different species of phytoplankton. Rev. Biol. Mar. Oceanogr. 2010, 45, 531–536. [Google Scholar] [CrossRef]
- El Semary, N.A.; Osman, M.E.; Ahmed, A.S.; Botros, H.W.; Farag, A.T. Antibiosis towards human and plant pathogens via radiation-induced Synechococcus elongatus. Egypt. J. Biol. Pest Control 2015, 25, 393–399. [Google Scholar]
- Al Dayel, M.; El Semary, N.A. UV irradiation promoting effect on the antibacterial activity of cyanobacterial extracts against plant pathogens: A first record. Egyptian J. Biol. Pest Control. 2020, 30, 132. [Google Scholar] [CrossRef]
- Najeeb, S.; Khurshid, Z.; Zafar, M.S.; Ajlal, S. Applications of light amplification by stimulated emission of radiation (LASERs) for restorative dentistry. Med. Princ. Pract. 2016, 25, 201–211. [Google Scholar] [CrossRef]
- Rethfeld, B.; Ivanov, D.S.; Garcia, M.E.; Anisimov, S.I. Modelling ultrafast LASER ablation. J. Phys. D Appl. Phys. 2017, 50, 193001. [Google Scholar] [CrossRef]
- Bjordal, J.M.; Couppé, C.; Chow, R.T.; Tunér, J.; Ljunggren, E.A. A systematic review of low level LASER therapy with location-specific doses for pain from chronic joint disorders. Aust. J. Physiother. 2003, 49, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Oron, U.; Yaakobi, T.; Oron, A.; Mordechovitz, D.; Shofti, R.; Hayam, G.; Dror, U.; Gepstein, L.; Wolf, T.; Haudenschild, C.; et al. Low-Energy Laser Irradiation Reduces Formation of Scar Tissue After Myocardial Infarction in Rats and Dogs. Circulation 2001, 103, 296–301. [Google Scholar] [CrossRef] [Green Version]
- Sommer, A.P.; Pinheiro, A.L.; Mester, A.R.; Franke, R.-P.; Whelan, H.T. Biostimulatory windows in low-intensity laser activation: Lasers, scanners, and nasa’s light-emitting diode array system. J. Clin. Laser Med. Surg. 2001, 19, 29–33. [Google Scholar] [CrossRef]
- Hu, W.P.; Wang, J.J.; Yu, C.L.; Lan CC, E.; Chen, G.S.; Yu, H.S. Helium–neon laser irradiation stimulates cell proliferation through photostimulatory effects in mitochondria. J. Investig. Dermatol. 2007, 127, 2048–2057. [Google Scholar] [CrossRef] [Green Version]
- Belykh, E.; Yagmurlu, K.; Martirosyan, N.L.; Lei, T.; Izadyyazdanabadi, M.; Malik, K.M.; Byvaltsev, V.A.; Nakaji, P.; Preul, M.C. Laser application in neurosurgery. Surg. Neurol. Int. 2017, 8, 274. [Google Scholar] [PubMed]
- Kolari, P.; Airaksinen, O. Penetration of infra-red and helium-neon LASER light into the tissue. Acupunct. Electro Ther. Res. J. 1988, 13, 232–233. [Google Scholar]
- Nykyforov, V.; Maznytska, O.; Novokhatko, O.; Pasenko, A.; Malovanyy, M.; Tymchuk, I. LAER pretreating of cyanobacteria biomass to produce lipids as a renewable energy source. Environ. Eng. Manag. J. 2021, 20, 1255–1262. [Google Scholar]
- Claus, D. A standardized gram staining procedure. World J. Microbiol. Biotechnol. 1992, 8, 451–452. [Google Scholar] [CrossRef] [Green Version]
- Allen, M.M. Simple conditions for growth of unicellular blue-green algae on plates. J. Phycol. 1968, 4, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.M.; Stanier, R.Y. Selective isolation of blue-green algae from water and soil. Microbiology 1968, 51, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of Cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef] [Green Version]
- Junior, J.C.R.; Tamanini, R.; Soares, B.F.; de Oliveira, A.M.; de Godoi Silva, F.; da Silva, F.F.; Augusto, N.A.; Beloti, V. Efficiency of boiling and four other methods for genomic DNA extraction of deteriorating spore-forming bacteria from milk. Semin. Ciências Agrárias 2016, 37, 3069–3078. [Google Scholar] [CrossRef]
- Bauer, A.W. Antibiotic susceptibility testing by a standardized single disc method. Am. J. Clin. Pathol. 1966, 45, 149–158. [Google Scholar] [CrossRef]
- Al Dayel, M.; El Semary, N.; Al Amer, K.; Al Ali, K. Investigating the applications of Chlorella vulgaris in agriculture and nanosilver production. J. Environ. Biol. 2020, 41, 1099–1104. [Google Scholar] [CrossRef]
- Heidari, F.; Riahi, H.; Yousefzadi, M.; Asadi, M. Antimicrobial activity of Cyanobacteria isolated from hot spring of geno. Middle-East J. Sci. Res. 2012, 12, 336–339. [Google Scholar]
- Gerez-Martel, I.; García-Poza, S.; Rodríguez-Martel, G.; Rico, M.; Afonso-Olivares, C.; Gómez-Pinchetti, J.L. Phenolic profile and antioxidant activity of crude extracts from microalgae and Cyanobacteria strains. J. Food Qual. 2017, 2017, 2924508. [Google Scholar] [CrossRef] [Green Version]
- Monserrat, J.M.; Yunes, J.S.; Bianchini, A. Effects of Anabaena spiroides (Cyanobacteria) aqueous extracts on the acetylcholinesterase activity of aquatic species. Environ. Toxicol. Chem. Int. J. 2001, 20, 1228–1235. [Google Scholar] [CrossRef]
- Sonani, R.R.; Rastogi, R.P.; Patel, R.; Madamwar, D. Recent advances in production, purification and applications of phycobiliproteins. World J. Biol. Chem. 2016, 7, 100. [Google Scholar] [CrossRef]
- Najdenski, H.M.; Gigova, L.G.; Iliev, I.I.; Pilarski, P.S.; Lukavský, J.; Tsvetkova, I.V.; Ninova, M.S.; Kussovski, V.K. Antibacterial and antifungal activities of selected microalgae and Cyanobacteria. Int. J. Food Sci. Technol. 2013, 48, 1533–1540. [Google Scholar] [CrossRef]
- Sabarinathan, K.; Ganesan, G. Antibacterial and toxicity evaluation of C-phycocyanin and cell extract of filamentous freshwater cyanobacterium–. Eur. Rev. Med. Pharmacol. Sci. 2008, 12, 79–82. [Google Scholar]
- Afreen, S.; Fatma, T. Extraction, purification and characterization of phycoerythrin from Michrochaete and its biological activities. Biocatal. Agric. Biotechnol. 2018, 13, 84–89. [Google Scholar]
- Kim, N.N.; Shin, H.S.; Park, H.G.; Lee, J.; Kil, G.S.; Choi, C.Y. Profiles of photosynthetic pigment accumulation and expression of photosynthesis-related genes in the marine cyanobacteria Synechococcus sp.: Effects of LED wavelengths. Biotechnol. Bioprocess Eng. 2014, 19, 250–256. [Google Scholar] [CrossRef]
- Li, W.T.; Leu, Y.C.; Wu, J.L. Red-light light-emitting diode irradiation increases the proliferation and osteogenic differentiation of rat bone marrow mesenchymal stem cells. Photomed. LASER Surg. 2010, 28, S-157. [Google Scholar] [CrossRef]
- Chen, H.W.; Yang, T.S.; Chen, M.J.; Chang, Y.C.; Wang, E.I.C.; Ho, C.L.; Lai, Y.J.; Yu, C.C.; Chou, J.C.; Chao, L.K.P.; et al. Purification and immunomodulating activity of C-phycocyanin from Spirulina platensis cultured using power plant flue gas. Process Biochem. 2014, 49, 1337–1344. [Google Scholar] [CrossRef] [Green Version]
- Sivasankari, S.; Vinoth, M.; Ravindran, D.; Baskar, K.; Alqarawi, A.A.; Abd_Allah, E.F. Efficacy of red light for enhanced cell disruption and fluorescence intensity of phycocyanin. Bioprocess Biosyst. Eng. 2021, 44, 141–150. [Google Scholar] [CrossRef]
- Sugita, C.; Ogata, K.; Shikata, M.; Jikuya, H.; Takano, J.; Furumichi, M.; Kanehisa, M.; Omata, T.; Sugiura, M.; Sugita, M. Complete nucleotide sequence of the freshwater unicellular cyanobacterium Synechococcus elongatus PCC 6301 chromosome: Gene content and organization. Photosynth. Res. 2007, 93, 55–67. [Google Scholar] [CrossRef]
- Tan, L.R.; Xia, P.F.; Sun, X.F.; Guo, N.; Song, C.; Li, Q.; Wang, S.G. Ecological insights into low-level antibiotics interfered biofilms of Synechococcus elongatus. RSC Adv. 2016, 6, 78132–78135. [Google Scholar] [CrossRef]
- Martins, R.F.; Ramos, M.F.; Herfindal, L.; Sousa, J.A.; Skærven, K.; Vasconcelos, V.M. Antimicrobial and cytotoxic assessment of marine Cyanobacteria-Synechocystis and Synechococcus. Mar. Drugs 2008, 6, 1–11. [Google Scholar] [CrossRef]
- Strieth, D.; Lenz, S.; Ulber, R. In vivo and in silico screening for antimicrobial compounds from cyanobacteria. MicrobiologyOpen 2022, 11, e1268. [Google Scholar] [CrossRef]
- Konstantinou, D.; Mavrogonatou, E.; Zervou, S.K.; Giannogonas, P.; Gkelis, S. Bioprospecting sponge-associated marine cyanobacteria to produce bioactive compounds. Toxins 2020, 12, 73. [Google Scholar] [CrossRef]
- Conter, A.; Dupouy, D.; Delteil, C.; Planel, H. Influence of very low doses of ionizing radiation on Synechococcus lividus metabolism during the initial growth phase. Arch. Microbiol. 1986, 144, 286–290. [Google Scholar] [CrossRef]
- Xue, L.; Li, S.; Sheng, H.; Feng, H.; Xu, S.; An, L. Nitric oxide alleviates oxidative damage induced by enhanced ultraviolet-B radiation in cyanobacterium. Curr. Microbiol. 2007, 55, 294–301. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Sinha, R.P.; Moh, S.H.; Lee, T.K.; Kottuparambil, S.; Kim, Y.J.; Rhee, J.S.; Choi, E.M.; Brown, M.T.; Häder, D.P.; et al. Ultraviolet radiation and cyanobacteria. J. Photochem. Photobiol. B Biol. 2014, 141, 154–169. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).