Geophagic Materials Characterization and Potential Impact on Human Health: The Case Study of Maputo City (Mozambique)
Abstract
1. Introduction
2. Materials and Methods
2.1. Geophagic Material Sources Characterization
2.2. Geophagic Material Sold in Markets
2.3. Samples Preparation and Physical Parameters
2.4. Mineralogical, Chemical, and Morphological Analysis
3. Results and Discussion
Risk Assessment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| EC | Electrical conductivity |
| HI | hazard index |
| HQ | hazard quotient |
| OM | organic matter |
| PTEs | potential toxic elements |
| Qa | alluvial deposits |
| Qdi | eolian sands |
| RDD | Recommended daily dose |
| XRD | X-ray diffraction |
| XRF | X-ray fluorescence |
References
- Wilson, M.J. Clay Mineralogical and Related Characteristics of Geophagic Materials. J. Chem. Ecol. 2003, 29, 1525–1547. [Google Scholar] [CrossRef] [PubMed]
- Abdul, R.M.; Arhin, E.; Arhin Jnr, A.A. Mineralogy and geochemistry of geophagic materials at Mfensi-Adankwame in the Ashanti region of Ghana and possible health implications the Ashanti region of Ghana and possible health implications. Geol. Ecol. Landsc. 2021, 1–12. [Google Scholar] [CrossRef]
- Borruso, L.; Checcucci, A.; Torti, V.; Correa, F.; Sandri, C.; Luise, D.; Cavani, L.; Modesto, M.; Spiezo, C.; Mimmo, T.; et al. I Like the Way You Eat It: Lemur (Indri indri) Gut Mycobiome and Geophagy. Microb. Ecol. 2021, 82, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Halsted, J.A. Geophagia in man: Its nature and nutritional effects. Am. J. Clin. Nutr. 1968, 21, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- Mashao, U.; Ekosse, G.; Odiyo, J.; Bukalo, N. Geophagic practice in Mashau Village, Limpopo Province, South Africa. Heliyon 2021, 7, e06497. [Google Scholar] [CrossRef]
- Kwong, L.H.; Ercumen, A.; Pickering, A.J.; Unicomb, L.; Davis, J.; Leckie, J.O.; Luby, S.P. Soil ingestion among young children in rural Bangladesh. J. Expo. Sci. Environ. Epidemiol. 2021, 31, 82–93. [Google Scholar] [CrossRef]
- Ekosse, G.; Nkeng, G.E.; Bukalo, N.; Oyebanjo, O. Geophagic Clays from Cameroon: Provenance, Metal Contamination and Health Risk Assessment. Int. J. Environ. Res. Public Health 2021, 18, 8315. [Google Scholar] [CrossRef]
- Macheka, L.R.; Olowoyo, J.O.; Matsela, L.; Khine, A.A. Prevalence of geophagia and its contributing factors among pregnant women at Dr. George Mukhari Academic Hospital, Pretoria. Afr. Health Sci. 2016, 16, 972–978. [Google Scholar] [CrossRef]
- Kambunga, S.N.; Candeias, C.; Hasheela, I.; Mouri, H. Review of the nature of some geophagic materials and their potential health effects on pregnant women: Some examples from Africa. Environ. Geochem. Health 2019, 41, 2949–2975. [Google Scholar] [CrossRef]
- Mahaney, W.C.; Milner, M.W.; Mulyono, H.; Hancock, R.G.V.; Aufreiter, S.; Reich, M.; Wink, M. Mineral and chemical analyses of soils eaten by humans in Indonesia. Int. J. Environ. Res. Public Health 2000, 10, 93–109. [Google Scholar] [CrossRef]
- Hooda, P.S.; Henry, C.J.K.; Seyoum, T.A.; Armstrong, L.D.M.; Fowler, M.B. The potential impact of geophagia on the bioavailability of iron, zinc and calcium in human nutrition. Environ. Geochem. Health 2002, 24, 305–319. [Google Scholar] [CrossRef]
- Cerqueira, Â.; Costa, C.; Rocha, F. Sedimentary and residual clays from Santiago, Boavista e Fogo (Cape Verde): Assessment of their properties as geophagic materials. Arab. J. Geosci. 2019, 12, 510. [Google Scholar] [CrossRef]
- Kambunga, S.N.; Candeias, C.; Hasheela, I.; Mouri, H. The geochemistry of geophagic material consumed in Onangama Village, Northern Namibia: A potential health hazard for pregnant women in the area. Environ. Geochem. Health 2019, 41, 1987–2009. [Google Scholar] [CrossRef] [PubMed]
- Kortei, N.K.; Koryo-dabrah, A.; Akonor, P.T.; Yaw, N.; Manaphraim, B.; Ayim-akonor, M.; Tettey, C.; Boadi, N.O.; Essuman, E.K.; Tettey, C. Potential health risk assessment of toxic metals contamination in clay eaten as pica (geophagia) among pregnant women of Ho in the Volta Region of Ghana. BMC Pregnancy Childbirth 2020, 4, 160. [Google Scholar] [CrossRef]
- Nematollahi, M.J.; Keshavarzi, B.; Zaremoaiedi, F.; Rajabzadeh, M.A.; Moore, F. Ecological-health risk assessment and bioavailability of potentially toxic elements (PTEs) in soil and plant around a copper smelter. Environ. Monit. Assess. 2020, 192, 639. [Google Scholar] [CrossRef]
- Flaxman, S.M.; Sherman, P.W. Morning sickness: A mechanism for protecting mother and embryo. Q. Rev. Biol. 2000, 75, 113–148. [Google Scholar] [CrossRef]
- Mestawet, G.; Getachew, M.; Yeshigeta, R.; Tiruneh, A.; Dereje, E.; Mekonnen, Z. Soil-Transmitted Helminthic Infections and Geophagia among Pregnant Women in Jimma Town Health Institutions, Southwest Ethiopia. Ethiop. J. Health Sci. 2021, 31, 1033. [Google Scholar] [CrossRef]
- Orisakwe, O.E.; Udowelle, N.A.; Azuonwu, O.; Nkeiruka, I.Z.; Nkereuwem, U.A.; Frazzoli, C. Cadmium and lead in geophagic clay consumed in Southern Nigeria: Health risk from such traditional nutraceutical. Environ. Geochem. Health 2020, 42, 3865–3875. [Google Scholar] [CrossRef]
- Njiru, H.; Elchalal, U.; Paltiel, O. Geophagy during pregnancy in Africa: A literature review. Obstet. Gynecol. Surv. 2011, 66, 452–459. [Google Scholar] [CrossRef]
- Miller, J.D.; Collins, S.M.; Omotayo, M. Geophagic earths consumed by women in western Kenya contain dangerous levels of lead, arsenic, and iron. Am. J. Hum. Biol. 2018, 30, e23130. [Google Scholar] [CrossRef]
- Woode, A.; Hackman-Duncan, S.F. Risks Associated with Geophagia in Ghana. Can. J. Pure Appl. Sci. 2014, 8, 2789–2794. Available online: http://www.cjpas.net/wp-content/uploads/pdfs/8/1/Paper (accessed on 10 December 2021).
- Momoh, A.; Akinsola, H.A.; Nengovhela, M.; Akinyemi, S.A.; Ojo, O.J. Geophagic Practice in Vhembe District, Limpopo Province, South Africa. J. Hum. Ecol. 2015, 51, 273–278. [Google Scholar] [CrossRef]
- Gevera, P.K.; Mouri, H. Geochemical and mineralogical composition of geophagic materials from Baringo town, Kenyan Rift Valley and their possible health effects on the consumers. Environ. Geochem. Health 2021, 43, 4831–4846. [Google Scholar] [CrossRef] [PubMed]
- Shinondo, C.; Mwikuma, G. Geophagy as a Risk Factor for Helminth Infections in Pregnant Women in Lusaka, Zambia. Med. J. Zambia 2009, 35, 48–52. [Google Scholar] [CrossRef]
- Odongo, A.O.; Moturi, W.N.; Mbuthia, E.K. Heavy metals and parasitic geohelminths toxicity among geophagous pregnant women: A case study of Nakuru Municipality, Kenya. Environ. Geochem. Health 2016, 38, 123–131. [Google Scholar] [CrossRef]
- Amiri, H.; Daneshvar, E.; Azadi, S.; Azadi, S. Contamination level and risk assessment of heavy metals in the topsoil around cement factory: A case study. Environ. Eng. Res. 2021, 27, 210313. [Google Scholar] [CrossRef]
- Candeias, C.; Vicente, E.; Rocha, F.; Paula Ávila, A.C. Geochemical, Mineralogical and Morphological Characterization of Road Dust and Associated Health Risks. Int. J. Environ. Res. Public Health 2020, 17, 1563. [Google Scholar] [CrossRef]
- Candeias, C.; Ávila, P.F.; Sequeira, C.; Manuel, A.; Rocha, F. Potentially toxic elements dynamics in the soil rhizospheric-plant system in the active volcano of Fogo (Cape Verde) and interactions with human health. Catena 2022, 209, 105843. [Google Scholar] [CrossRef]
- Bai, B.; Long, F.; Rao, D.; Xu, T. The effect of temperature on the seepage transport of suspended particles in a porous medium. Hydrol. Processes 2017, 31, 382–393. [Google Scholar] [CrossRef]
- Dreyer, M.J.; Chaushev, P.G.; Gledhill, R.F. Biochemical investigations in geophagia. J. R. Soc. Med. 2004, 97, 48. [Google Scholar] [CrossRef]
- Harvey, P.W.; Dexter, P.B.; Darnton-Hill, I. The impact of consuming iron from non-food sources on iron status in developing countries. Public Health Nutr. 2000, 3, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.M. Macroterme Geophagy and Pregnancy Clays in Southern Africa. J. Cult. Geogr. 1993, 14, 69–92. [Google Scholar] [CrossRef]
- Abrahams, P.W.; Parsons, J.A. Geophagy in the tropics: An appraisal of three geophagical materials. Environ. Geochem. Health 1997, 19, 19–22. [Google Scholar] [CrossRef]
- Ngole, V.M.; Ekosse, G.E. Nutrient bioaccessibility of geophagic soils from Eastern Cape, South Africa. Sci. Res. Essays 2012, 7, 1319–1331. [Google Scholar] [CrossRef]
- Cerqueira, Â.; Costa, C.; Terroso, D.; Sequeira, C.; Rocha, F. Assessment of clayey materials from Santa Maria (Azores, Portugal) for preparation of peloids. Clay Miner. 2019, 54, 299–307. [Google Scholar] [CrossRef]
- Savana. Areia Que dá Dinheiro e Doenças. Maputo. 2010. Available online: https://macua.blogs.com/moambique_para_todos/2010/06/areia-que-d%C3%A1-dinheiro-e-doen%C3%A7as.html (accessed on 10 November 2021).
- MISAU. Prevenção da Anemia Por Deficiência de Ferro Nas Mulheres—Material de Apoio Para o Aconselhamento nas Unidades Sanitárias e Comunidades. Ministério de Saúde de Moçambique. Available online: https://www.mcsprogram.org/wp-content/uploads/dlm_uploads/2019/02/Mozambique-Flip-Chart-Anemia-2.pdf (accessed on 10 November 2021).
- Capone, D.; Bivins, A.; Knee, J.; Cumming, O.; Nalá, R.; Brown, J. Quantitative Microbial Risk Assessment of Pediatric Infections Attributable to Ingestion of Fecally Contaminated Domestic Soils in Low-Income Urban Maputo, Mozambique. Environ. Sci. Technol. 2021, 55, 1941–1952. [Google Scholar] [CrossRef]
- INE. Estatísticas do Distrito Marracuene. Available online: http://www.ine.gov.mz/estatisticas/estatisticas-territorias-distritais/maputo-provincia/marco-de-2012/distrito-de-marracuene.pdf/view (accessed on 1 February 2022).
- CIAT. Climate-Smart Agriculture in Mozambique. In CSA Country Profiles for Africa Series; International Center for Tropical Agriculture, World Bank: Washington, DC, USA, 2017; 25p. [Google Scholar]
- Muchangos, A.D. Paisagens e Regiões Naturais de Moçambique; Editora Escolar: Maputo, Mozambique, 1999. [Google Scholar]
- Vicente, E.M. Aspects of the Engineering Geologic of Maputo City. Ph.D. Thesis, School of Geological Sciences, University of KwaZulu-Natal-Durban, Durban, South Africa, 2011. Available online: http://hdl.handle.net/10413/8078 (accessed on 7 December 2021).
- Momade, F.J.; Ferrara, M.; Oliveira, J.T. Notícia Explicativa da Carta Geológica 2532 Maputo (Escala 1:50,000); Direção Nacional de Geologia: Maputo, Mozambique, 1996. (In Portuguese) [Google Scholar]
- Munsell Color. Munsell Soil Color Book; Color Charts; Munsell Colour Company. Inc.: Newburgh, NY, USA, 2009. [Google Scholar]
- USDA. Soil Quality Indicators: pH. Managing Soils and Terrestrial Systems. United States Department of Agriculture. Available online: http://soils.usda.gov (accessed on 1 December 2021).
- Ávila, P.A.; Ferreira da Silva, E.; Candeias, C. Health risk assessment through consumption of vegetables rich in heavy metals: The case study of the surrounding villages from Panasqueira mine, Central Portugal. Environ. Geochem. Health 2017, 39, 565–589. [Google Scholar] [CrossRef]
- Ngole, V.M.; Ekosse, G.E.; de Jager, L.; Songca, S.P. Physicochemical characteristics of geophagic clayey soils from South Africa and Swaziland. Afr. J. Biotech. 2010, 9, 5929–5937. [Google Scholar] [CrossRef]
- Nkansah, M.A.; Korankye, M.; Darko, G.; Dodd, M. Heavy metal content and potential health risk of geophagic white clay from the Kumasi Metropolis in Ghana. Toxicol. Rep. 2016, 3, 644–651. [Google Scholar] [CrossRef]
- Akah, P.A.; Zeigbo, T.O.; Oforkansi, M.N.; Onyeto, C.A. Effect of Kaolin Consumption on Serum Heavy Metal Levels of Pregnant Women. Int. J. Sci. 2020, 9, 28–32. [Google Scholar] [CrossRef]
- Diko, M.L.; Siewe épse Diko, C.N. Physico-chemistry of geophagic soils ingested to relief nausea and vomiting during pregnancy. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Badu, J.K.; Nude, P.M.; Dodor, D.E.; Nartey, E.K.; Adjadeh, T.A. Characterization of the Geophagic Materials and Their Associated Rocks and Soils from Anfoega, Ghana. Ghana J. Sci. 2021, 61, 133–155. [Google Scholar] [CrossRef]
- Lakudzala, D.D.; Khonje, J.J. Nutritive potential of some “edible” soils in Blantyre city, Malawi. Malawi Med. J. 2011, 23, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Pebsworth, P.A.; Huffman, M.A.; Lambert, J.E.; Young, S.L. Geophagy among nonhuman primates: A systematic review of current knowledge and suggestions for future directions. Am. J. Phys. Anthropol. 2019, 168, 164–194. [Google Scholar] [CrossRef] [PubMed]
- Pentoś, K.; Pieczarka, K.; Serwata, K. The relationship between soil electrical parameters and compaction of sandy clay loam soil. Agriculture 2021, 11, 114. [Google Scholar] [CrossRef]
- Uchenna, O.G.; Vuyo, M.; Antoine, M.F. Mineralogical Profile of Geophagic Clayey Soils Sold in Selected South African Informal Markets. Trans. R. Soc. S. Afr. 2016, 73, 79–85. [Google Scholar] [CrossRef]
- Elvis Duplex, K.K.; Sylvain Ludovic, W.A.; Daniel, N.; Ivo, E.G. Physico-chemical Characterization of Clayey Materials Consumed by Geophagism in Locality of Sabga (North-western Cameroon): Health Implications. Int. J. Environ. Res. Public Health 2018, 8, 57–68. [Google Scholar] [CrossRef]
- Ngole, V.M.; Ekosse, G.E. A comparative analysis of granulometry, mineral composition and major and trace element concentrations in soils commonly ingested by humans. Int. J. Environ. Res. Public Health 2015, 12, 8933–8955. [Google Scholar] [CrossRef]
- Droy-Lefaix, M.T.; Tateo, F. Clays and Clay Minerals as Drugs. Dev. Clay Sci. 2006, 1, 743–752. [Google Scholar] [CrossRef]
- Linares, C.F.; Afonso, L.; Brussin, M.R. Modified Venezuelan Kaolin as Possible Antacid Drug. J. Appl. Sci. 2004, 4, 472–476. [Google Scholar] [CrossRef]
- Carretero, M.I.; Gomes, C.S.F.; Tateo, F. Chapter 11.5 Clays and Human Health. In Developments in Clay Science V.1; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 717–741. [Google Scholar]
- Awad, M.E.; López-Galindo, A.; Setti, M.; El-Rahmany, M.M.; Iborra, C.V. Kaolinite in pharmaceutics and biomedicine. Int. J. Pharm. 2017, 533, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Enoh, B.S.; Okeowo, K.I.; Udeme, J.D. Characterization Adsorption and Antibacterial Properties of Silver-Modified Kaolinite Clay from Kwi, Plateau State Nigeria. Chem. Mater. Res. 2021, 13, 27–35. [Google Scholar] [CrossRef]
- Asowata, I.T. Geophagic clay around Uteh-Uzalla near Benin: Mineral and trace elements compositions and possible health implications. SN Appl. Sci. 2021, 3, 569. [Google Scholar] [CrossRef]
- Huebl, L.; Leick, S.; Guettl, L.; Akello, G.; Kutalek, R. Geophagy in Northern Uganda: Perspectives from Consumers and Clinicians. Am. J. Trop. Med. Hyg. 2016, 95, 1440–1449. [Google Scholar] [CrossRef]
- Oyebanjo, O.; Ekosse, G. Health Risk Evaluation of Trace Elements in Geophagic Kaolinitic Clays within Eastern Dahomey and Niger Delta Basins, Nigeria. Int. J. Environ. Res. Public Health 2020, 17, 4813. [Google Scholar] [CrossRef]
- Tishin, A.N.; Krut, U.A.; Tishina, O.M.; Beskhmelnitsyna, E.A.; Yakushev, V.I. Physico-chemical properties of montmorillonite clays and their application in clinical practice. Pharm. Clin. Pharmacol. 2017, 3, 119–128. [Google Scholar] [CrossRef]
- Bukhanov, V.D.; Vezentsev, A.I.; Filippova, O.V.; Nadezhdin, S.V.; Pankova, O.N.; Firsova, T.I.; Tishin, A.N. The influence of the concentration of montmorillonite containing sorbent and pH of the culture medium on the antibiotic sensitivity of Escherichia coli, as well as the effect of ground on growth of Escherichia. Pharm. Clin. Pharm. 2017, 3, 97–104. [Google Scholar] [CrossRef]
- Mujawar, Q.M.; Naganoor, R.; Ali, M.D.; Malagi, N.; Thobbi, A.N. Efficacy of Dioctahedral Smectite in Acute Watery Diarrhea in Indian Children: A randomized clinical trial. J. Trop. Pediatr. 2012, 58, 63–67. [Google Scholar] [CrossRef][Green Version]
- Prince, R.J.; Luoba, A.I.; Adhiambo, P.; Ng’uono, J.; Geissler, P.W. Geophagy is common among Luo women in western Kenya. Trans. R. Soc. Trop. Med. Hyg. 1999, 93, 515–516. [Google Scholar] [CrossRef]
- Nyanza, E.C.; Joseph, M.; Premji, S.S.; Thomas, D.S.; Mannion, C. Geophagy practices and the content of chemical elements in the soil eaten by pregnant women in artisanal and small-scale gold mining communities in Tanzania. BMC Pregnancy Childbirth 2014, 14, 144. [Google Scholar] [CrossRef]
- Young, S.L.; Miller, J.D. Medicine Beneath Your Feet: A Biocultural Examination of the Risks and Benefits of Geophagy. Clays Miner. 2019, 67, 81–90. [Google Scholar] [CrossRef]
- Cormick, G.; Belizán, J.M. Calcium Intake and Health. Nutrients 2019, 11, 1606. [Google Scholar] [CrossRef] [PubMed]
- WHO. Calcium Supplementation in Pregnant Women; World Health Organization: Geneva, Switzerland; Available online: http://apps.who.int/iris/bitstream/handle/10665/85120/9789241505376_eng.pdf (accessed on 2 December 2021).
- WHO. Potassium Intake for Adults and Children; World Health Organization: Geneva, Switzerland; Available online: https://www.who.int/publications/i/item/9789241504829 (accessed on 1 December 2021).
- EFSA. Tolerable upper Intake Level on Vitamins and Minerals. European Food Safety Authority. Available online: https://www.efsa.europa.eu/sites/default/files/efsa_rep/blobserver_assets/ndatolerableuil.pdf (accessed on 2 December 2021).
- WHO. Sodium Intake for Adults and Children; World Health Organization: Geneva, Switzerland; Available online: https://www.who.int/publications/i/item/9789241504836 (accessed on 2 December 2021).
- WHO. Daily Iron Supplementation in Infants and Children, 44; World Health Organization: Geneva, Switzerland; Available online: https://apps.who.int/iris/bitstream/handle/10665/204761/9789241510196_eng.pdf (accessed on 2 December 2021).
- Abdul, R.M.; Arhin, E. Mineralogy and Geochemistry of Geophagic Soils in Ghana: A Review. Eur. J. Environ. Earth Sci. 2020, 1. [Google Scholar] [CrossRef]
- Plum, L.M.; Rink, L.; Hajo, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef] [PubMed]
- NIH. Zinc. National Institutes of Health. Available online: https://ods.od.nih.gov/factsheets/Zinc-HealthProfessional/#h4 (accessed on 12 February 2022).
- Blachier, F.; Andriamihaja, M.; Blais, A. Sulfur-Containing Amino Acids and Lipid Metabolism. J. Nutr. 2020, 150, 2524S–2531S. [Google Scholar] [CrossRef]
- WHO. Ten Chemicals of Major Public Health; World Health Organization: Geneva, Switzerland; Available online: https://www.who.int/news-room/photo-story/photo-story-detail/10-chemicals-of-public-health-concern (accessed on 12 February 2022).
- Braga, J.O.; Santos, D.M.; Cotting, F.; Lins, V.C.; Leão, N.M.; Soares, D.F.; Mazzer, E.M.; Houmard, M.; Figueiredo, R.B.; Nunes, E.M. Surface modification of magnesium with a novel composite coating for application in bone tissue engineering. Surface Coat. Technol. 2022, 433, 128078. [Google Scholar] [CrossRef]
- NIH. Magnesium. National Institutes of Health. Available online: https://ods.od.nih.gov/factsheets/Magnesium-HealthProfessional/#change (accessed on 2 December 2021).
- Barker, D. Tooth wear as a result of pica. Br. Dent. J. 2005, 199, 271–273. [Google Scholar] [CrossRef]
- Jugdaohsingh, R.; Anderson, S.H.C.; Tucker, K.L.; Elliott, H.; Kiel, D.P.; Thompson, R.P.H.; Powell, J.J. Dietary silicon intake and absorption. Am. J. Clin. Nutr. 2002, 75, 887–893. [Google Scholar] [CrossRef]
- Krewski, D.; Yokel, R.A.; Nieboer, E.; Borchelt, D.; Cohen, J.; Harry, J.; Kacew, S.; Lindsay, J.; Mahfouz, A.M.; Rondeau, V. Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J. Toxicol. Environ. Health 2007, 1, 1–269. [Google Scholar] [CrossRef]
- Mehri, A. Trace Elements in Human Nutrition (II)–An Update. Int. J. Prev. Med. 2020, 11, 2. [Google Scholar] [CrossRef]
- WHO. Barium and Barium Compounds; World Health Organization: Geneva, Switzerland; Available online: https://apps.who.int/iris/bitstream/handle/10665/42398/9241530332.pdf;jsessionid=FD320B2DB2001150448F4403E41C0E98?sequence=1 (accessed on 6 December 2021).
- Moffet, D.; Smith, C.; Stevens, Y.; Ingerman, L.; Swarts, S.; Chappell, L. Toxicological Profile for Barium and Barium Compounds; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2007. [Google Scholar]
- Peana, M.; Medici, S.; Dadar, M.; Zoroddu, M.A.; Pelucelli, A.; Chasapis, C.T.; Bjørklund, G. Environmental barium: Potential exposure and health-hazards. Arch. Toxicol. 2021, 95, 2605–2612. [Google Scholar] [CrossRef] [PubMed]
- CCME. Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health-Nickel. Canadian Council of Ministers of the Environment. Available online: https://ccme.ca/en/res/nickel-canadian-soil-quality-guidelines-for-the-protection-of-environmental-and-human-health-en.pdf (accessed on 15 February 2022).
- Wilk, A.; Szypulska-Koziarska, D.; Wiszniewska, B. The toxicity of vanadium on gastrointestinal, urinary, and reproductive system, and its influence on fertility and fetuses malformations. Postepy Hig. Med. Dosw. 2017, 71, 850–859. [Google Scholar] [CrossRef] [PubMed]
- WHO. Vanadium; World Health Organization: Geneva, Switzerland; Available online: https://www.euro.who.int/__data/assets/pdf_file/0016/123082/AQG2ndEd_6_12vanadium (accessed on 1 December 2021).
- Bai, B.; Nie, Q.; Zhang, Y.; Wang, X.; Hu, W. Cotransport of heavy metals and SiO2 particles at different temperatures by seepage. J. Hydrol. 2021, 597, 125771. [Google Scholar] [CrossRef]
- Jones, J.V.; Piatak, N.M.; Bedinger, G.M. Zirconium and hafnium, Chap. V. In Critical Mineral Resources of the United States—Economic and Environ Mental Geology and Prospects for Future Supply; Schulz, K.J., DeYoung, J.H., Jr., Seal, R.R., Bradley, D.C., Eds.; U.S. Geological Survey Professional: St. Petersburg, FL, USA, 2017; p. 26. [Google Scholar] [CrossRef]
- Nthenya, D.S.; Simiyu, G.M.; Munyao, T.M. Temporal lead contamination and health risks of geophagia in Eldoret Municipality, Kenya. Int. J. Biol. Chem. Sci. 2010, 4, 1056–1064. [Google Scholar] [CrossRef]
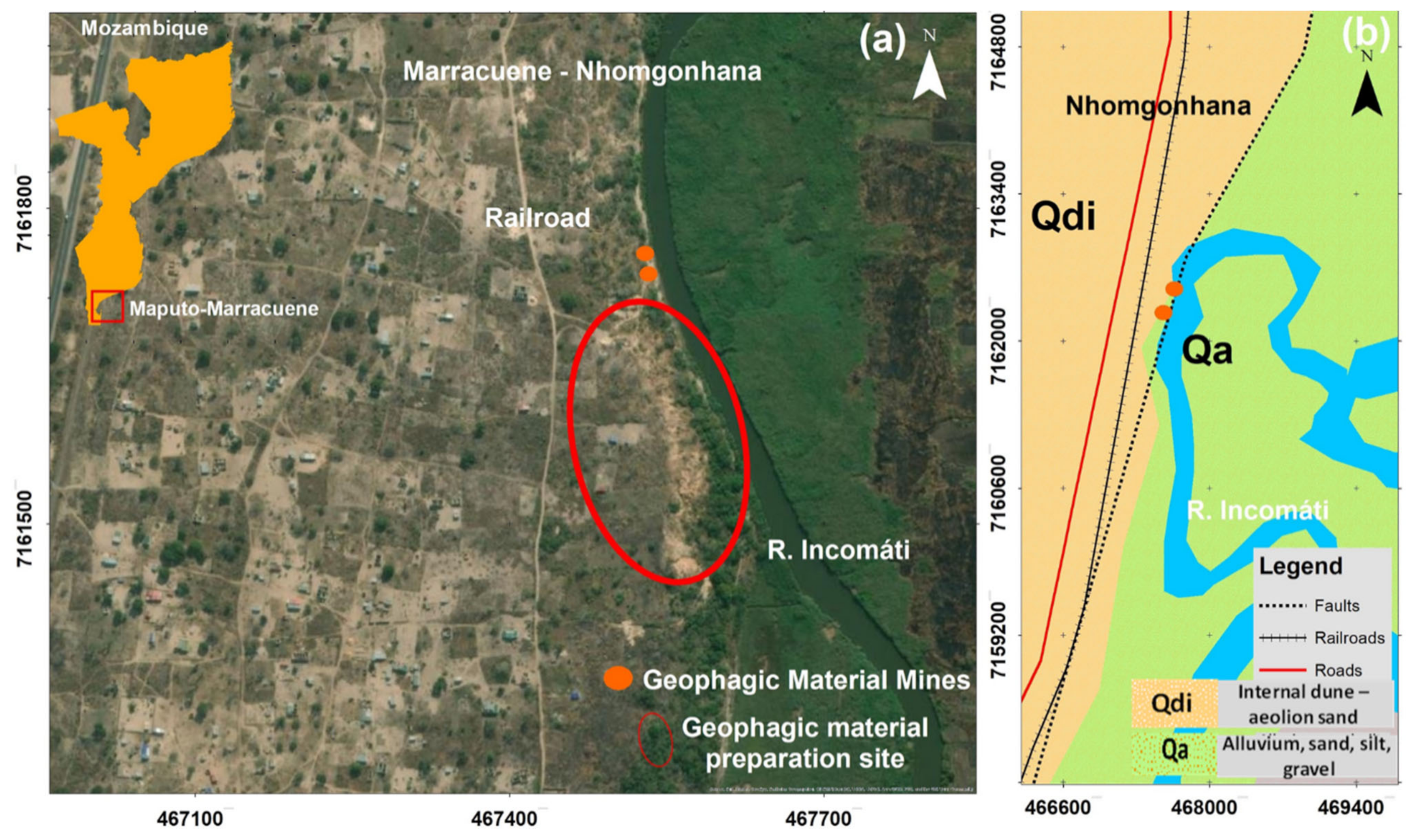


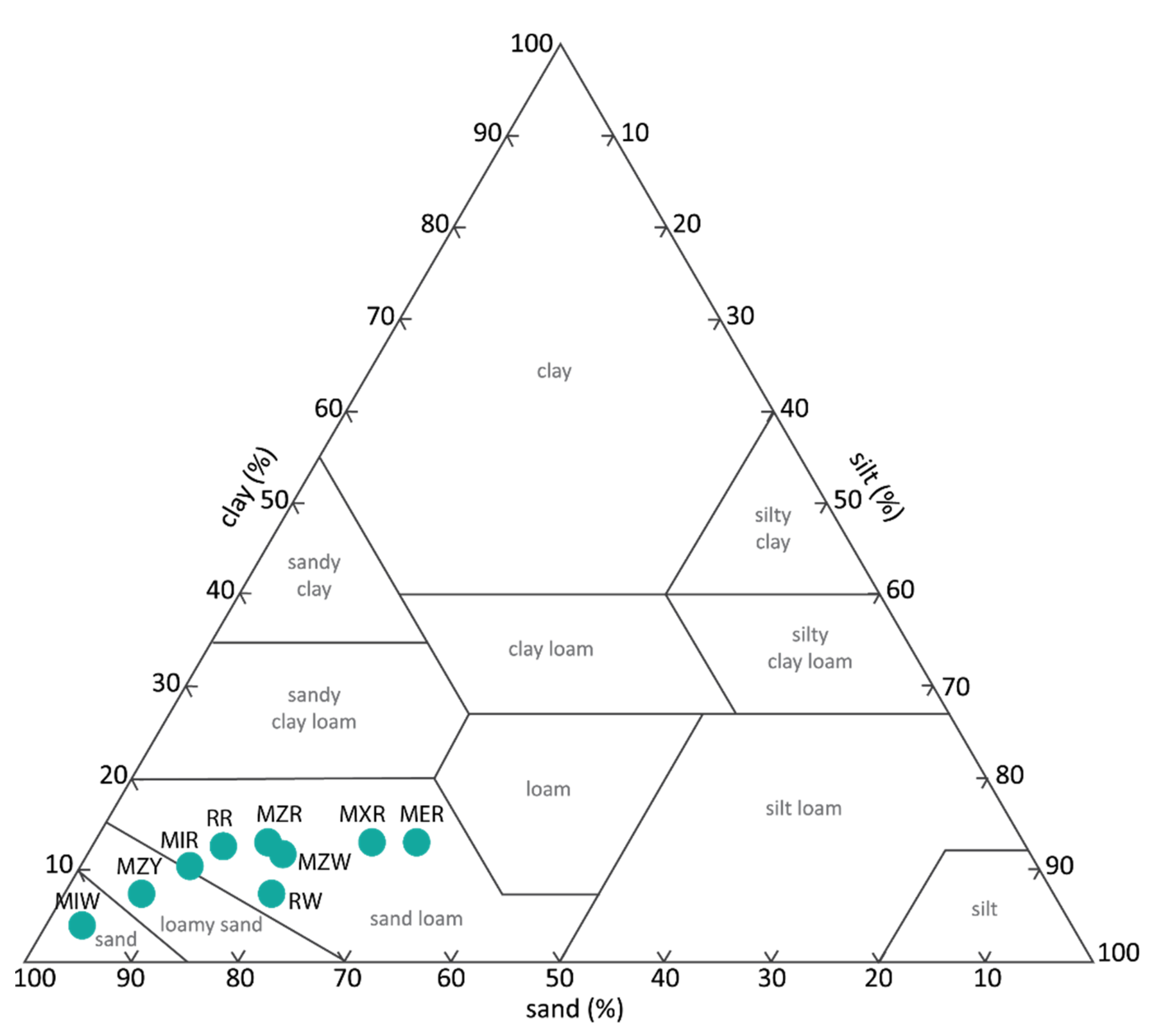
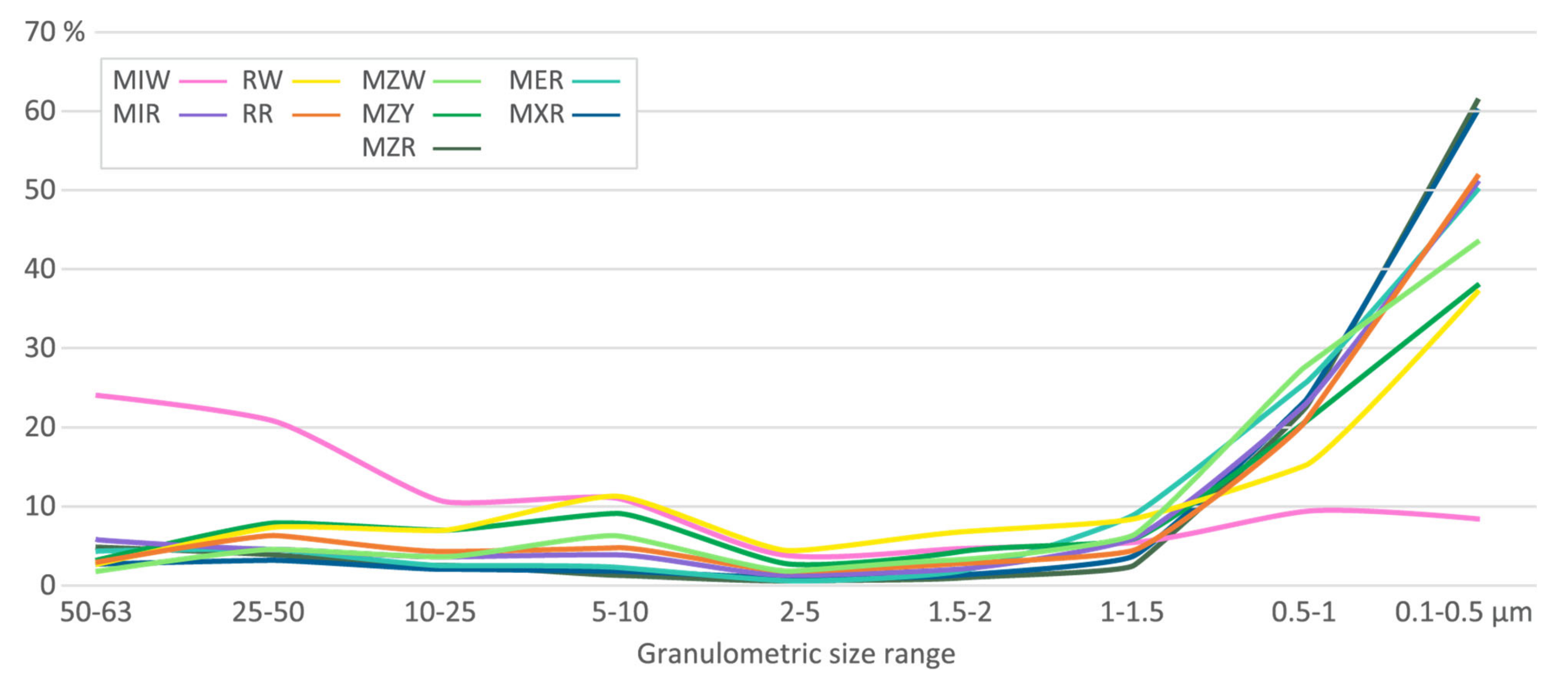
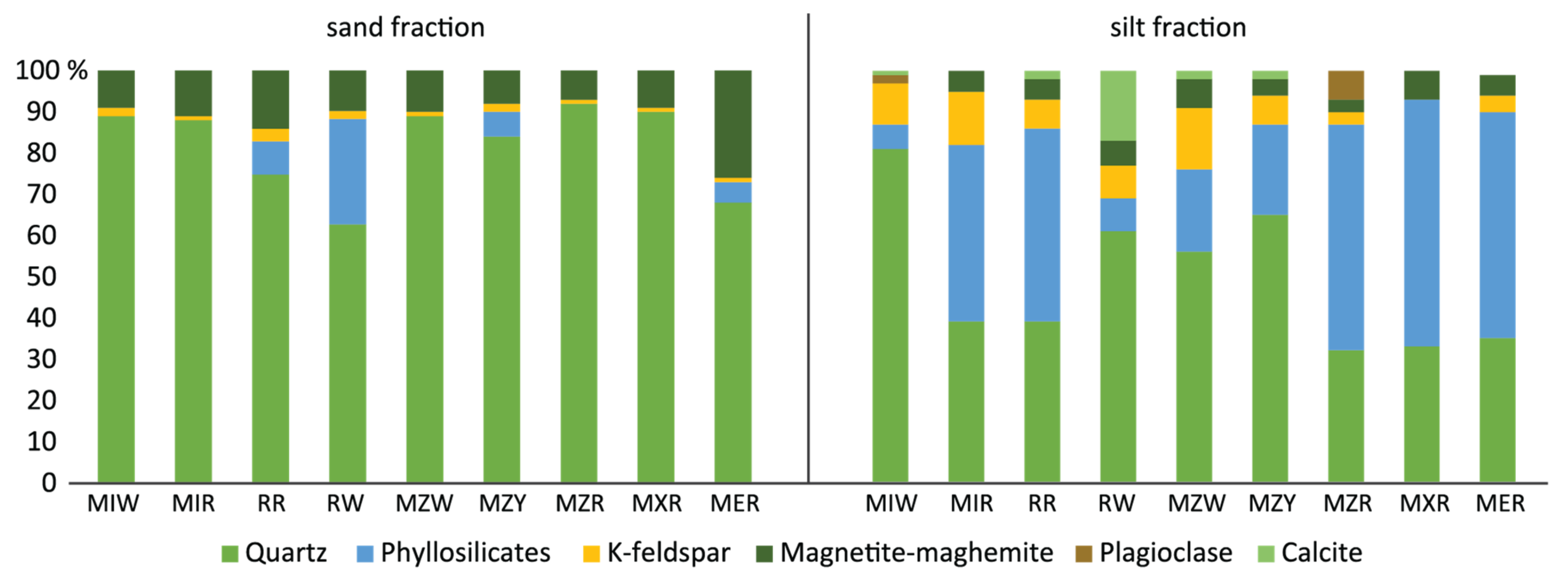


| Var | Unprepared Samples | Prepared Samples | Markets Samples | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MIW | MIR | RW | RR | MZW | MZY | MZR | MER | MXR | |
| Color | Pinkish white 7.5YR 8/2 | Pale red 2.5YR 7/4 | White 2.5YR 8/1 | Pale red 2.5YR 7/4 | White 2.5YR 8/1 | Pale yellow 5Y 8/2 | Pale red 7/4 2.5YR | Pale red 7/4 2.5YR | Pale red 7/4 2.5YR |
| pH | 7.22 | 6.98 | 7.52 | 7.18 | 7.18 | 7.51 | 6.2 | 6.09 | 6.17 |
| EC | 13 | 25 | 47 | 28 | 301 | 465 | 264 | 401 | 269 |
| OM | 1.35 | 1.45 | 8.14 | 1.53 | 1.21 | 1.35 | 2.7 | 2 | 2.76 |
| ID | Kaolinite | Illite | Smectite | Corrensite | Halloysite |
|---|---|---|---|---|---|
| MIW | tr | +++ | |||
| MIR | +++ | tr | |||
| RW | +++ | + | tr | ||
| RR | +++ | + | tr | ||
| MZW | +++ | + | tr | ||
| MZY | +++ | tr | |||
| MZR | +++ | tr | |||
| MXR | +++ | ||||
| MER | +++ | tr |
| Var | Sand | Silt | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | SD | Sk | Min | Max | Mean | SD | Sk | |
| Al | 14,632 | 120,889 | 77,615 | 32,160 | −0.626 | 57,111 | 191,537 | 164,576 | 41,851 | −2.591 |
| Ba | 3.5 | 339 | 66.5 | 128 | 1.795 | 3.5 | 946 | 491 | 314 | −0.584 |
| Br | 0.4 | 21.3 | 7.5 | 8.7 | 0.534 | 0.4 | 35.5 | 16.5 | 13.1 | 0.189 |
| Ca | 2145 | 3032 | 2414 | 269 | 1.64 | 1979 | 2795 | 2235 | 249 | 1.469 |
| Cr | 48.4 | 139.5 | 110.0 | 28.6 | −1.304 | 172 | 269 | 230 | 29.2 | −0.756 |
| Cu | 1.5 | 87.0 | 33.2 | 31.8 | 1.17 | 26.6 | 96.9 | 44.7 | 21.1 | 2.245 |
| Fe | 1541 | 12,717 | 7877 | 3085 | −0.725 | 10,333 | 33,040 | 27,633 | 6800 | −2.497 |
| K | 9701 | 16,517 | 12,436 | 2278 | 0.499 | 10,321 | 31,934 | 15,122 | 6544 | 2.591 |
| Mg | 766 | 2429 | 1744 | 460 | −0.974 | 1228 | 3782 | 2837 | 876 | −0.833 |
| Mn | 3.0 | 164.1 | 86.8 | 51.8 | −0.357 | 114 | 410 | 186 | 103 | 1.654 |
| Na | 100 | 7116.9 | 3675 | 2912 | −0.302 | 100 | 5263 | 3238 | 1553 | −1.089 |
| Ni | 1.0 | 46.1 | 31.2 | 14.0 | −1.279 | 41.3 | 92.3 | 69.1 | 18.5 | −0.243 |
| P | 1907 | 3969 | 2503 | 630 | 1.704 | 1409 | 1912 | 1583 | 141 | 1.645 |
| Pb | 1.0 | 42.1 | 9.6 | 17.1 | 1.641 | 30.8 | 53.5 | 45.4 | 7.7 | −0.675 |
| S | 100 | 343 | 221 | 85.0 | −0.161 | 170 | 1887 | 395 | 561 | 2.977 |
| Si | 227,241 | 526,562 | 456,435 | 94,067 | −2.201 | 281,203 | 439,925 | 318,063 | 48,084 | 2.468 |
| Sr | 14.2 | 32.8 | 22.6 | 6.5 | 0.796 | 32.8 | 75.3 | 47.4 | 12.8 | 1.425 |
| Ti | 1920 | 7711 | 4091 | 1926 | 1.199 | 5845 | 8050 | 6642 | 634 | 1.336 |
| V | 1.5 | 104 | 37.2 | 38.3 | 0.556 | 50.2 | 255 | 195 | 59.1 | −2.12 |
| Zn | 9.1 | 23.3 | 15.9 | 4.5 | 0.149 | 27.9 | 66.0 | 53.2 | 12.3 | −1.215 |
| Zr | 101 | 440 | 209 | 121 | 1.108 | 176 | 531 | 258 | 108 | 2.477 |
| Ca | Cl | Cu | Fe | K | Mg | Mn | Na | P | S | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca | 1 | 0.6 | 0.750 * | 0.117 | 0.117 | 0.067 | 0.234 | 0.627 | −0.350 | −0.059 | −0.100 |
| Cl | 0.167 | 1 | 0.350 | 0.583 | −0.117 | 0.450 | −0.142 | 0.780 * | −0.167 | 0.326 | −0.167 |
| Cu | 0.281 | 0.434 | 1 | 0.017 | −0.033 | 0.100 | 0.276 | 0.254 | −0.383 | −0.109 | 0.017 |
| Fe | −0.267 | 0.517 | −0.136 | 1 | −0.450 | 0.517 | −0.251 | 0.441 | −0.417 | 0.686 * | 0.383 |
| K | −0.283 | −0.133 | −0.443 | 0.483 | 1 | −0.167 | 0.385 | 0.051 | −0.283 | 0.033 | −0.833 ** |
| Mg | −0.350 | 0.133 | −0.196 | 0.2 | 0.650 | 1 | −0.092 | 0.678 * | 0.133 | 0.820 ** | −0.117 |
| Mn | −0.267 | 0.117 | −0.170 | 0.700 * | 0.883 ** | 0.650 | 1 | 0.043 | −0.226 | −0.084 | −0.075 |
| Na | 0.792 * | 0.366 | 0.548 | −0.034 | −0.460 | −0.366 | −0.187 | 1 | 0.017 | 0.562 | −0.288 |
| P | 0.600 | −0.083 | 0.366 | −0.817 ** | −0.717 * | −0.417 | −0.783 * | 0.468 | 1 | −0.226 | −0.050 |
| S | 0.367 | 0.817 ** | 0.068 | 0.367 | 0.117 | 0.317 | 0.167 | 0.221 | −0.033 | 1 | −0.151 |
| Zn | 0.237 | 0.627 | 0.199 | 0.373 | 0.390 | 0.339 | 0.356 | 0.009 | −0.254 | 0.797 * | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernardo, B.; Candeias, C.; Rocha, F. Geophagic Materials Characterization and Potential Impact on Human Health: The Case Study of Maputo City (Mozambique). Appl. Sci. 2022, 12, 4832. https://doi.org/10.3390/app12104832
Bernardo B, Candeias C, Rocha F. Geophagic Materials Characterization and Potential Impact on Human Health: The Case Study of Maputo City (Mozambique). Applied Sciences. 2022; 12(10):4832. https://doi.org/10.3390/app12104832
Chicago/Turabian StyleBernardo, Bernardino, Carla Candeias, and Fernando Rocha. 2022. "Geophagic Materials Characterization and Potential Impact on Human Health: The Case Study of Maputo City (Mozambique)" Applied Sciences 12, no. 10: 4832. https://doi.org/10.3390/app12104832
APA StyleBernardo, B., Candeias, C., & Rocha, F. (2022). Geophagic Materials Characterization and Potential Impact on Human Health: The Case Study of Maputo City (Mozambique). Applied Sciences, 12(10), 4832. https://doi.org/10.3390/app12104832








