Abstract
Tissue engineering or tissue reconstruction/repair/regeneration may be considered as a guiding strategy in oral and maxillofacial surgery, as well as in endodontics, orthodontics, periodontics, and daily clinical practice. A wide range of techniques has been developed over the past years, from tissue grafts to the more recent and innovative regenerative procedures. Continuous research in the field of natural and artificial materials and biomaterials, as well as in advanced scaffold design strategies has been carried out. The focus has also been on various growth factors involved in dental tissue repair or reconstruction. Benefiting from the recent literature, this review paper illustrates current innovative strategies and technological approaches in oral and maxillofacial tissue engineering, trying to offer some information regarding the available scientific data and practical applications. After introducing tissue engineering aspects, an overview on additive manufacturing technologies will be provided, with a focus on the applications of superparamagnetic iron oxide nanoparticles in the biomedical field. The potential applications of magnetic fields and magnetic devices on the acceleration of orthodontic tooth movement will be analysed.
1. Introduction
Various dental diseases including congenital defects, tooth loss, or trauma affect more than 3.9 billion people [1], making necessary intervention related to tissue repair or reconstruction to restore oral or maxillofacial functionalities [2,3].
Sustainable approaches must be developed to improve oral health and to re-direct oral health research towards a “social determinants” model, as evidenced by the studies conducted by Sheiham et al. [4,5], and for a closer collaboration–integration between general- and dental health research [6].
According to the World Health Organization, 5–10% of healthcare budgets are represented by dental treatment costs and other costs are related to absences from work [7].
The molecular control over cells behaviour during embryonic tooth development has gained great attention, thus generating improved awareness regarding the genesis of dental tissues as well as on teeth regeneration [8].
Tissue engineering or tissue reconstruction/repair/regeneration represents a major interest in periodontics, oral and maxillofacial surgery, endodontics, orthodontics, and even daily clinical practice. A vast array of techniques has been studied and developed over the years, starting from tissue transplants to more innovative and recent regenerative practices involved in the concept of tissue engineering. Recently, continuous research has been performed in the field of biomaterials and scaffolds, artificial and natural materials, genes, stem cells from dental follicle, deciduous teeth, dental pulp, periodontal ligament, salivary glands, and adipose tissue. The attention has also been focused on various growth factors involved in dental tissue repair or reconstruction [8,9].
Some recent studies evidenced a strong correlation between oral bacteria and neurodegenerative disorders (i.e., Alzheimer’s disease (AD)). Pathogenic bacteria present in dental plaque could enter into the bloodstream and their metabolites/derived molecules pass through the blood–brain barrier (BBB), after negatively affecting its permeability, and reach the brain, promoting an increase in the levels of inflammatory cytokines, and cell and vascular adhesion molecules [10].
In this scenario, an intriguing strategy towards the development of 3D multifunctional hybrid structure combined with active/antibacterial systems and magnetic stimulation could improve periodontal tissue prevention and treatment or repair.
This review paper reports current innovative strategies and technological approaches in oral and maxillofacial tissue engineering. Notions dealing with the available scientific data and practical applications are reported. Starting from an introducing to tissue engineering, an overview on additive manufacturing technologies is provided. The applications of superparamagnetic iron oxide nanoparticles (SPIONs) in the biomedical field are also analysed, with a particular focus on bone and maxillofacial tissue repair. We look towards magnetic fields effects and potential applications on the acceleration of orthodontic tooth movement. In this scenario, an important thought towards the development of 3D multifunctional hybrid structure combined with active/antibacterial systems and magnetic stimulation could improve periodontal tissue prevention and treatment or repair.
2. Tissue Engineering
Aspects related to the engineering and manufacturing of “replacement tissue” are often identified with the term “tissue engineering” (TE), thus providing organ analogues, which are obtained from patients’ own cells, and functional tissues, offering an alternative method to grafts or transplants [9]. This method became increasingly important in dental and maxillofacial regenerative medicine, providing novel opportunities for the reconstruction of periodontium, teeth, bones, oral mucosa, skin, conjunctiva, temporomandibular joint, cartilage, bone, nerves, muscles, tendons, and blood vessels in the oral/maxillofacial area [9].
TE represents a valuable alternative for specific tissue defect reconstruction, because of the available bioengineered resources. Current treatments recognized as gold standard are often related to many disadvantages for patients, such as loss of motor and sensorial functionalities of craniofacial structures, high risk of inflammation and infection, or unpredictable compatibility for autologous grafts [11].
Three pillars support tissue engineering: (i) Stem cells/progenitor cells, which are responsible for the synthesis and deposition of new extracellular matrix (ECM); (ii) signalling/growth factors for promotion of functionalities; (iii) scaffolds, as a support for cell adhesion, proliferation, and differentiation, as well as for the aspects related to ECM biosynthesis (Figure 1).
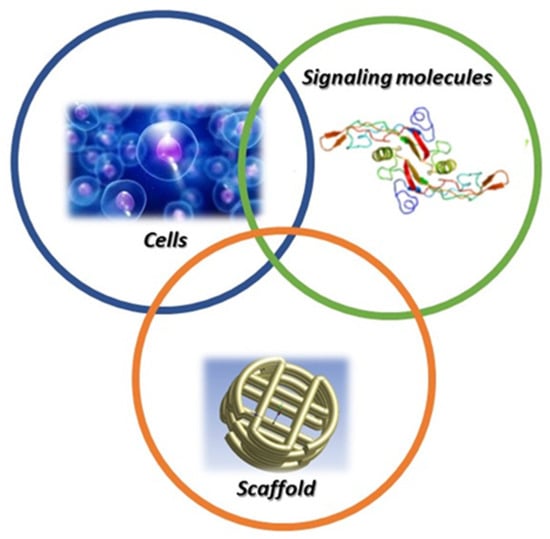
Figure 1.
Tissue engineering paradigm.
Cells communicate with each other and with their environment, being influenced by different aspects concerning scaffold biomaterials, surface chemistry, topography, and roughness [12,13]. The combination of cells, scaffold and signalling pathway is responsible for correct damaged tissue replacement, with similar function to the original tissue or with the capability of stimulating the regeneration of the damaged tissue [14,15]. Stem cells, chondrocytes, fibroblasts, and keratinocytes derived from human or animal origin have been adopted in clinical studies towards tissue engineering and regenerative medicine [16].
Scaffolds and biomaterials represent crucial elements in dental tissue engineering, since they can be adopted as: (i) adhesion sites for surrounding tissues cells during the regeneration, (ii) template for tissue regeneration, (iii) source of implantable odontogenic cells which are capable to differentiate into required cell type, and (iv) carrier for bioactive molecules, such as growth factors, which may improve regenerative potential [17,18].
Tissue engineering approach involves cells and biomaterials, adopting an array of scaffold design and optimization strategies.
A wide variety of biomaterials is widely employed in oral and maxillofacial TE strategies. Examples are natural organic, synthetic organic, or even inorganic materials.
Natural organic materials include peptides (e.g., gelatin and collagen) and polysaccharides (e.g., agarose, alginate, chitosan). Synthetic organic materials which are commonly used are poly(lactic acid) (PLA), poly(caprolactone) (PCL), poly(lactic-co-glycolic acid) (PLGA), and poly(glycolic acid) (PGA) [19].
On the other hand, examples of the most used inorganic materials in bone replacement are glasses or calcium phosphates (e.g., β-tricalciumphosphate, hydroxyapatite,) and cementitious complexes of calcium silicate or calcium phosphate [20].
2.1. Scaffold Design Strategies
Among all the scaffold design strategies, additive manufacturing methodologies have emerged as powerful tools for obtaining polymeric and/or micro- nano-composite structures with an appropriate and controlled morphology and porosity. The chemical structure of the scaffold material and its internal architecture (filaments and walls diameter, and porosity) control and modulate the biological performances of the cells [21].
Cell behaviour over time is strictly connected to pores morphology and dimensions. Furthermore, a fully interconnected porosity is crucial for the ingrowth of surrounding tissues [22]. An interconnected and open porosity enhances mass transport properties and the elimination of waste generated by cellular metabolism [23]. Furthermore, the enzymatical or hydrolytic degradation of polymers represents an added value. Natural polymers such as chitosan, alginate, collagen, and gelatin are frequently used as bioinks and subject to enzymatic degradation, due to the presence of microorganisms in the biological environment [24]. The availability and concentration of respective enzymes is strictly connected with the rate of enzymatic degradation.
Hydrolytic degradation of synthetic polymers is related to the splitting of hydrolytically sensitive bonds, resulting in bulk or surface erosion, which is crucial for the determination of the best choice for different applications [25]. Surface erosion implies the preservation of mechanical integrity, also enhancing bone ingrowth, and ensuring gradually scaffold substitution by new bone tissue [26].
Generally, polymers can be processed in order to obtain porous structures which are capable of facilitating the transport of growth factors, nutrients, anabolites and catabolites, and controllable degradation [27]. Furthermore, composite materials may properly be adopted because of their versatility resulting from the combination of organic and inorganic phases. Recent studies on this subject have highlighted the innovation of targeted and scaffold-assisted regeneration of enamel, dentin, and cementum [28].
Biocompatible, biodegradable, porous, and without toxic metabolites materials adopted as scaffold fabrication materials for dental regeneration approaches must possess other peculiar characteristics suitable for the specific environment and that make them applicable to the oral cavity: pH, temperature, microorganisms’ interaction, and mastication forces. In order to achieve these properties, most scaffolds mimic the structure of the natural ECM, thus avoiding inflammatory responses. In this context, the development of smart immunomodulatory biomaterials which prevent foreign body reaction on the patients and regulate the immunological microenvironments to ensure cell survival is crucial [29,30].
Biomimetic porous PLGA microspheres coupled with peptides have been prepared to mimic natural tissues in terms of chemical composition and structure and adopted as biomimetic and bionic smart scaffolds [31].
MSCs, bone marrow, and macrophages behaviour over time has been analysed in conjunction with amino-functionalized bioactive glass scaffold [32]. β-tricalcium phosphate coatings on Mg scaffolds have been also analysed to modulate its harmful osteoimmunomodulatory properties [33].
Shape-memory porous smart scaffolds for bone repair defects have been also proposed. In particular, bone morphogenetic protein2-loaded shape-memory porous nanocomposite scaffolds have been developed adopting nanoparticles of poly(ε-caprolactone) and hydroxyapatite with shape-memory recovery which were chemically crosslinked [34]. Furthermore, 3D flexible piezoelectric poly(vinylidene fluoride-trifluoroethylene) (PVDF-TrFE) fibrous scaffolds were obtained and adopted for MSCs differentiation and novel tissue formation [35], whilst an electrospun PVDF-TrFE fibre scaffold containing zinc oxide nanoparticles was highlighted to promote human MSCs performances in terms of adhesion and proliferation and also to enhance the blood vessel formation [35].
Interesting approaches involve the use polymers and hydrogels of materials that can modify their behaviour as response to an endogenous and/or exogenous stimulus, at the same time allowing the delivery of the required amount of drug on-demand [36,37,38]. Oral controlled-release drug delivery carrier has been developed adopting pH-responsive bacterial cellulose-g-poly(acrylic acidco-acrylamide) hydrogel [38], or a poly(ethylene glycol) hydrogel loaded with drugs by β-eliminative linkers [39]. PH-responsive drug release capability has been also demonstrated for farnesol-loaded nanoparticles, composed of 2-(dimethylamino)ethyl methacrylate, butyl methacrylate, and 2-propylacrylic acid [40].
Multifunctional nanoparticle-based drug delivery systems represent another intriguing strategy in bone repair. For example, mesoporous silica nanoparticles have been decorated with a bone-forming peptide and encapsulated into arginine–glycine–aspartic acid-treated alginate hydrogel [41].
Biomimetic drug delivery systems have been also obtained employing dendrimers, hydrogels, liposomes, micelles, polymeric carriers, and their nanostructures [42,43].
Dental and periodontal regeneration has been promoted employing smart biomaterials and constructs, such as multi-layered structures. In this context, bilayered PLGA/calcium phosphate structures or tri-layered nanocomposite scaffolds based on PLGA reinforced with nanobioactive glass ceramic and/or growth factors have been developed [44,45].
PH responsive smart dental resins have been adopted in tooth structures preservation and protection, by analysing, i.e., dental composites containing nanoparticles of amorphous calcium phosphate and tetracalcium phosphate [46].
PH-sensitive materials are characterized by a selective inhibition of acid-producing bacteria. Examples are cationic poly(phenylene vinylene) derivative, and pH-sensitive quaternary pyridinium salts, for which the antibacterial potency can be controlled by varying the pH [47,48].
Furthermore, oral biofilm composition could be modulated by advanced and smart resins, at the same time avoiding drug resistance. Dental resins containing quaternary ammonium methacrylates such as 12-methacryloyloxy dodecyl pyridinium bromide, methacryloxylethyl cetyl dimethyl ammonium chloride, quaternary ammonium polyethylenimine, and dimethylaminododecyl methacrylate showed the ability to decrease the biofilm viability and lactic acid production [49,50,51]. Antibacterial-coated material to enhance wound healing and tissue regeneration have been developed adopting different absorbable or non-resorbable, natural/synthetic materials, also assessing mechanical properties, and bacterial adherence [52]. It is worth noting that the oral cavity hosts a plethora of tissues with diverse functions. This complex environment hosts cells, bacteria, viruses, and fungi [53]. Several studies highlighted potential correlation between dental procedures (i.e., surgical and non-surgical), periodontal disease, systemic, and/or cardiological diseases (i.e., infective endocarditis) [54]. Cardiovascular and neurological disorders should be correlated with low-grade chronic inflammation by periodontal pathogens [54]. This complexity of factors offers the healing particularities and challenges for further studies in the oral cavity treatments [53].
2.2. Additive Manufacturing Technologies for Oral Tissue Engineering
Recent advancements in process and manufacturing technologies open novel perspectives in multifunctional scaffolds design with physical–chemical features similar to the ECM ones, thus acting as a microenvironment for cell adhesion, proliferation, and differentiation [55,56].
Additive manufacturing (3D printing) of biomaterials offers promising perspectives in the field of bioengineering [57], especially towards tailorable clinical applications or personalized medicine (e.g., patient-specific devices).
Techniques involving extrusion of living cells along with other materials (3D bioprinting) [58], and non-cellular manufacturing methods can be included among additive manufacturing techniques for medical and tissue engineering applications.
3D bioprinting consists in the layer-by-layer deposition of specific “bioinks”, biological constituents, biochemicals and/or living cells, also enabling the on-demand “printing” of cells, tissues, and organs [59,60,61]. 3D tissue engineered structures obtained via 3D bioprinting can be optimized and tailored by combining diverse bioprinting techniques [62,63].
Small ink droplets deposition into specific coordinates can be obtained via Inkjet bioprinting driven by thermal or piezoelectric actuation [64].
Pulsed laser source, a ribbon, and a receiving substrate characterize laser-based bioprinting, in which biological material, in liquid form, is irradiated by the laser, evaporates, and reaches the receiving substrate as droplets. High-resolution printing of biological material can be obtained via laser-based bioprinting [65]; however, sometimes resulting in compromised cell viability [66].
Stereolithography bioprinting is characterized by a photo-crosslinking light source which hits a specific bioink from a reservoir with a precise trajectory to obtain the desired pattern onto a movable platform [67].
Biomaterials in form of solutions, pastes or dispersions can be adopted in pressure-assisted bioprinting, in which a filament is pneumatically extruded through a microneedle [68].
Bioink features possess key features in the fabrication process having to be biocompatible and biodegradable, deformable and flowable, at the same time ensuring certain properties and morphology after printing [69,70,71,72].
Polymers, ceramics, hydrogels, and composites, currently used in tissue engineering can be employed as bioinks, with ever new and constantly evolving possibilities. However, it is important to underline that cells fate should be altered during the bioprinting process of cellularized bioinks [73,74,75,76].
To this aim, “non-cellular” additive manufacturing approaches have been adopted in oral and maxillofacial tissue engineering, with their advantages and disadvantages [77,78,79,80,81]. Table 1 reports a summary of the “non-cellular” additive manufacturing techniques adopted in tissue engineering. Obviously, it is constantly evolving and updating.

Table 1.
Additive manufacturing methods for oral tissue engineering. Adapted from [2].
3. SPIONs in the Biomedicine
Advanced strategies to improve hard tissue regeneration consists in the application of a magnetic field and magnetic nanoparticles, which have a strong influence on cell fate.
The use of static and pulsed magnetic field (SFM, PMF) in regenerative medicine has been often associated to increase of bone regeneration, wound healing and also to magnetic resonance technique.
The concept of magnetism in the medical field was firstly introduced in 1970 by Freeman and his research group [82]. Since then, numerous research advancements on different types of magnetic particles and on different ways for their use by optimizing their properties have been performed. Superparamagnetic iron oxide nanoparticles (SPIONs) are synthetic particles made up of maghemite (γ-Fe2O3), magnetite (Fe3O4), and hematite (α-Fe2O3) characterized by a core diameter between 10 and 100 nm and a coating for drug or protein loading, thus making SPIONs able to be carried to a specific tissue through the use of an external magnet. SPIONs are magnetisable up to a saturation value in the presence of an external magnetic field. When the application of the magnetic field is interrupted, the nanoparticles will not show residual magnetisation. This property is a function of the size of the nanoparticles and usually takes over for values of at least 10–20 nm in diameter. Below these values the system does not have the multiple domains that are instead present in all the larger magnets and on the contrary behaves like a “single super spin” with high magnetic susceptibility.
SPIONs, for their physical, chemical, thermal, and magnetic, have been largely employed in different biomedical areas like cell labelling and cell separation; magnetic resonance imaging (MRI); tissue repair; magnetofection; hyperthermia; treatment of arthritis and drug delivery. Cell labelling with iron/paramagnetic represents a method used for in vivo cell separation and to ensure that labelled cells should be identified by MRI. In vivo cell labelling and separation techniques adopts two different approaches: attachment of magnetic nanoparticles on the external cell surface or internalization of biocompatible magnetic nanoparticles through fluid phase endocytosis, receptor mediated endocytosis or phagocytosis [83,84,85,86].
A strategy for effective and specific cell marking with magnetic nanoparticles consists in modifying the surface of the nanoparticles with different kinds of specific ligands, in such a way that the cell does not only recognizes the system but internalizes it through receptor-mediated endocytosis [87]. The SPIONs, suitably functionalized with organic molecules, can act as markers of specific cells in vivo; they can be used, for example, to identify tumour areas especially in the liver and lymph nodes. Another application is to functionalize the SPIONs with specific ligands for some biomolecules to act as a DNA marker to identify “wrong” sequences. Tissue repair involves transplanting stem cells into injured tissues, which growing, proliferating, and differentiating repair tissue damage. In this field the SPIONs have been proposed as tools to support stem cells and allow their delivery to desired sites. If the stem cells are labelled with SPIONs, it makes it easier to trace them inside the body using, for example, MRI. In doing so, in fact, it will be possible to follow the path of stem cells in vivo without using invasive techniques [88,89].
SPIONs are biocompatible magnetic nanoparticles and approved by the Food and Drug Administration (FDA) as contrast agents. The magnetic susceptibility of the nanoparticles makes the whole system a good contrast agent for MRI; moreover, they can undergo superficial modifications allowing different types of stem cells anchoring. Superparamagnetic nanocrystals of iron oxides act as contrast agents in MRI (magnetic resonance imaging), a technique used in the medical field that measures the relaxation of proton nuclear spin in tissues, managing to transform the signals into an image. Micrometric magnetic particles or chelated complexes of magnetic ions injected into tissues act as proton relaxation agents. Magnetic nanocrystals offer the advantage of being able to flow in the blood for a longer time than larger crystals, thus providing capillary walls crossing and reaching the lymph nodes.
The use of hyperthermia for malignant tumours treatment have been widely investigated. The magnetic field cannot be absorbed by living tissues and is applied to deep areas of the body. When magnetic nanoparticles undergo the action of an alternating magnetic field, the magnetic loss of hysteresis generates heat. The amount of generated heat varies according to the nature of the magnetic material and the parameters of the field. The magnetic particles incorporated inside the tumour cells subjected to an oscillating magnetic field, warm up, reaching a different temperature that depends on the strength of the magnetic field, the magnetic properties of the material, the frequency of oscillation and the cooling capacity of the blood flow in the site where the tumour is present. Therefore, as cancer cells are sensitive to high temperatures will be destroyed when the temperature reaches values close to or above 43 °C, while normal cells, more resistant to high temperatures, will survive [90]. In magnetofection, superparamagnetic nanoparticles linked with DNA vectors are transfected inside the cells through the application of the external magnetic field. For this reason, superparamagnetic nanoparticles must be coated with polycations, such as polyethylene (PEI) [91]. SPIONs are able to release the gene of interest into the nucleus, once passed the endosomal barrier. Nanoparticles will be forced to follow the magnetic field by using permanent or alternating magnets, thus avoiding free diffusion. Recently, systems based on SPIONs and corticosteroids, have been shown to be useful in solving the problem related to arthritis therapy. In particular, corticosteroids directly injected directly into the affected joints can cause crystals formation over time and consequent joint infections. By using the external magnetic field, these systems can be retained in the joint capsules avoiding repeated injections and elimination due to macrophage and drainage systems [92]. Finally, an important application of SPIONs provides for the specific distribution of the drug, drug delivery systems (DDS), at the site of action. The functionalization of the surface of the SPIONs makes them excellent drug carriers that are thus directed into the organ target and released there. In fact, by applying the external magnetic field it will be possible to target the drug delivered to a specific site, thus reducing the doses to be administered and the potential harmful side effects [93,94,95]. Properly designed carriers can be applied in numerous pharmaceutical fields, for oral administration, for sustained release, for drug targeting at specific sites, and for parenteral administration of anticancer drugs. For such application, the size, the surface chemistry, and charge of the magnetic particles are very crucial, as they have the effect on the circulation time in the blood and the bioavailability of the particles within the body [96].
The introduction of SPIONs in tissue engineering approaches, in particular for bone regeneration, has been recently implemented. Efficient scaffold fixation could be obtained adopting magnetic scaffolds and magnetic forces, thus providing a smart solution for the treatment of wide bone defects. Conventionally, fixation is ensured by external systems, such as intramedullary nails, plates and screws which require a constant control and multiple surgical interventions. To this purpose, the application of a magnetic scaffold highlighting a saturation value of 17 emu/g represents an innovative magnetic fixation approach [97].
Magnetic force-based tubular and sheet-like structures have been also proposed for tissue engineering purposes adopting magnetite NPs and magnetic force [98,99]. Magnetic force mechanical conditioning bioreactors for tissue engineering have been also suggested [100], highlighting the possibility to have a control on cell function through the application of an external magnetic field by binding MNPs on cells surface [101]. Magnetic scaffolds may produce higher magnetic field gradients, thus providing significant magnetic attractive forces. A superparamagnetic material, used for magnetic scaffold design and development, may reach appropriate magnetisation values for ferrofluid or MNPs adhesion when applying an external magnetic field [102], but it may also be magnetically “turned off” by removing the applied magnetic field, thus providing magnetic gradients suitable for the attraction of cells or other bioagents linked to MNPs.
The possibility of obtaining magnetic nanocomposite scaffolds with the addition of superparamagnetic PVP-coated Fe₃O₄ nanoparticles in a PCL matrix has been also considered [103], also highlighting the possibility of adopting the obtained structures as fixed station, whose magnetisation can be switched on and off by means of an external magnetic field. The long-term effects of iron-oxide-based phases such as magnetite or maghemite in human body remain unclear [103,104]. Surface-modification methods have been adopted for the design of biocompatible layers consisting of on particle surface, thus avoiding eventual toxicity problems [105].
Biocompatible bioresorbable superparamagnetic-like phase (FeHA) has been proposed by doping hydroxyapatite with Fe3+/Fe2+ ions. [106]. PCL/FeHA nanocomposite substrates were characterized and designed using different polymer-to-particle weight ratios, showing that the synergistic contribution of surface chemistry and topography may be responsible for the overall features of the proposed structure: enhancement of the hydrophilic character, cell attachment, and proliferation [107].
However, the different ductility of the polymer matrix and the inorganic nanofillers should be always considered in a scaffold design process since eventual weakness in a biocompatible structure may be the result of a discontinuities in the stress transfer and production of stress concentration at the nanoparticle/matrix interface at specific concentration amount of inorganic nanofillers. It has been already proved that it would be possible to obtain magnetic field gradients capable to attract bioaggregates into the completely biodegradable scaffolds with a fully interconnected pore network just embedding a small amount of superparamagnetic nanoparticles in polymer matrix [103,107,108]. In vivo pilot experiments, carried out in rabbit animal model, suggested that 3D nanocomposite magnetic scaffold could represent a potential innovative option to autologous bone implantation [103,107,108].
Furthermore, magnetic force may induce adaptive changes in microenvironments, including the cell matrix, cell membrane, cytoskeleton, nucleoprotein, and genome. Consequently, signals are transduced to the cell nucleus, regulating and stimulating different biological responses.
The design and development of fully bio-degradable and magnetic nanocomposite substrates and the correlation between the application of a time-dependent magnetic field and hMSCs biological performance has been studied [109]. In particular, Alamar Blue assay, ALP activity normalized for the DNA content (ALP/DNA), CLSM analysis and cell shape factor, p-ERK1/2 expression suggested interesting and, sometimes in many cases, unreported information, on the synergistic combination of PCL/Fe3O4 magnetic nanocomposites with a discontinuous application of the external magnetic field on cell behaviour over time: the use of the time-dependent magnetic field enhanced the activation of the MAPK pathway, as evidenced by an increase of ERK phosphorylation for both PCL and PCL/Fe3O4 scaffolds [109].
On the same way, multifunctional hybrid scaffolds have been proposed to reproduce the different features of periodontal ligament, alveolar bone, and cementum. Specifically, the integration of biomineralization process, electrospinning techniques and tape casting have been brought together, also employing superparamagnetic apatite phase for promoting osteogenesis via remote magnetic field signals. The periodontal scaffold has been obtained by engineering three different layers, reproducing the crucial compositional and microstructural features of the target tissues, into a monolithic multifunctional graded device. Results confirm that the proposed biocompatibility structure exhibits a good mimicry of the periodontal tissue complex, making innovative regenerative applications in dentistry [110].
A preliminary concept of multifunctional CaP-based scaffolds obtained via additive manufacturing from an innovative ink composition, with potential for bone tissue regeneration, drug delivery and cancer treatment by local magnetic hyperthermia platforms has been demonstrated. A highly loaded ink composed by iron-doped hydroxyapatite and β-tricalcium phosphate powders mixed in a chitosan-based solution, in the presence of levofloxacin (LEV) as model drug and magnetic nanoparticles (MNP) have been used, highlighting a synergistic effect between the iron-doped CaP-based powders and the MNP due to ferro/ferrimagnetic interactions. Furthermore, the iron presence enhances human mesenchymal stem cell metabolic activity and proliferation [111].
Additive manufactured magnetic scaffolds have been also proposed as a novel strategy for three-dimensional magnetic patterning of two cell types in vitro, also as a conceptual precursor for the vascularised tissue. The realisation of separate arrangements of vascular and osteoprogenitor cells, labelled with biocompatible magnetic nanoparticles, was established adopting nonhomogeneous magnetic gradients and loading magnetic configuration. It has been shown that scaffold magnetisation was able to amplify the cell guiding effects by an additional trapping of cells due to short range magnetic forces. Thus, the proposed solution should be employed as a compact assembled strategy for adequate arrangement of cellular constructs [112].
More recently, soft intelligent elastomeric structures for application in the field of soft robotics and biomedical devices with the capability to reshape and reconfigure under magnetic field while floating on the surface of water have been developed. These magnetoactive soft actuators have been obtained by embedding carbonyl iron particles in homocomposite silicone capillary ink via 3D printing technologies [113].
The possibility of obtaining soft architectures with isotropic/anisotropic contraction or multiple shape changes or other reconfiguration open novel perspectives for active scaffolds for cell cultures and soft robotics.
In this scenario, the research is currently directed towards 4D printing, in which the fourth dimension (4D) can be obtained by predicting time-dependent part configurations of complex structures characterized by shape-memory functionalities and environmental stimulus adaptation capability (Figure 2).
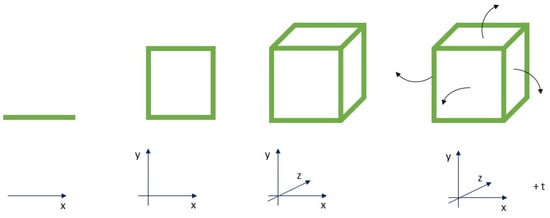
Figure 2.
4D paradigm: complex structures evolving over-time in predictable manner.
3D and 4D printing technologies together with recent advances in the research and development of advanced magnetic materials can open novel perspectives in the field of acceleration of orthodontic tooth movement.
4. Magnetic Fields Effects on the Acceleration of Orthodontic Tooth Movement
Currently, researcher’s attention has been focused on accelerating methods for tooth movement, given the ever-increasing demand for shorter orthodontic treatment time. Several disadvantages (i.e., caries, gingival recession, and root resorption) are often related to long orthodontic treatment time poses [114].
Different strategies have been approached for obtaining quicker results, with many still open questions.
Orthodontic tooth movement occurs because of mechanical stimuli, subsequently followed by alveolar bone and periodontal ligament (PDL) remodelling. Applied force and the biological responses from the PDL modulate orthodontic tooth movement, with blood flow alterations, secretion of inflammatory cytokines, growth factors, neurotransmitters, colony-stimulating factors, and arachidonic acid metabolites [114].
Direct electric currents, pulsed electromagnetic field, static magnetic field, resonance vibration, and low level laser represent some of the strategies adopted in this field, benefiting from “bone bending theory” and bioelectrical potential. It has been shown that vibrations for different duration per day accelerated tooth movements between 15% and 30% in animal experiments [114,115,116]. Cyclical force devices provide from 2 to 3 mm/month of tooth movement, adopting a vibration rate from 20 to 30 Hz, 20 min/day [117].
Direct electric current techniques were tested on animals generating local responses and acceleration of bone remodelling by applying direct current to the anode at the pressure sites and cathode at the tension sites (by 7 V) [118].
Photobiomodulation or Low Level Laser Therapy (LLLT), because of their biostimulatory effect on bone regeneration in the midpalatal suture during rapid palatal expansion or after bone fractures and extraction site [119,120,121] have been recently adopted in the field of acceleration of orthodontic tooth movement. Laser light stimulates osteoclast, osteoblast, and fibroblasts proliferation and hence influence bone remodelling probably by ATP production cytochrome C activation, as well as via receptor activator of NFkappaB-ligand RANK/RANKL and the macrophage colony-stimulating factor [122,123,124].
Recently, magnets and pulsed electric magnetic fields (PEMFs) have been used in orthopaedic applications, i.e., for the treatment of fractures in human long bones. PEMF applications in dentistry have been limited to the ability to increase the rate of orthodontic tooth movement [125].
It has been suggested that PEMF intervenes in the modulation of membrane permeability of intracellular cyclic adenosine monophosphate and cyclic guanosine monophosphate, allowing increased flow of calcium, sodium, and potassium ions across the cell membrane: tooth movement results accelerated due to an increase of active cells. or a static magnetic field have been shown to be successful in increasing the rate of orthodontic tooth movement in guinea pigs [126]. PEMF also induced vibrations that probably should enhance the effect of mechanical and magnetic forces on tooth movement [127]. As a result of exposure to PEMF with differing intensities, statistically significant increase and decrease of osteoclastogenesis and bone resorption areas have been observed and correlated to osteoprotegerin (OPG), RANKL, (macrophage colony-stimulating factor (M-CSF), osteoclast numbers, and bone resorption [128].
Another study showed that the canine exposed to PEMF moved 1.5 mm more than the control canine in 5 ± 0.6 months [125]. Considering that each tooth can be moved 1 mm/month, this treatment resulted more efficient than the normal one.
5. Conclusions
The purpose of this work is to provide an overview of current strategies and innovative technological approaches in tissue engineering, thus trying to offer some information regarding the available scientific data and practical applications in the field of oral and maxillofacial repair strategies. After introducing tissue engineering aspects, an overview on additive manufacturing technologies has been provided. The issue related to the applications of SPIONs in the biomedical field has been addressed. The work also offers a new reading key for the potential application of the magnetic field and magnetic structures on the acceleration of orthodontic tooth movement, due to the ever-increasing demand for shorter orthodontic treatment time. The possibility of combining magnetic scaffolds, with their proven ability to enhance cell-material interaction, especially in bone repair applications, as well as of magnetising 3D structures on demand may open innovative scenario for their application in clinical orthodontic practice. Advancements in developing deformable and shape memory 3D structures together with the emerging 4D printing technology may open fascinating and unexplored scenarios for future researchers. Furthermore, it is important to underline that the mouth/oral microbiome is in continuous communication with the external environment, and that it constitutes the main entry for many microorganisms with relevant consequences for human health. In this context, an integrated design of 3D multifunctional hybrid structure combined with active/antibacterial systems and magnetic stimulation could represent a functional strategy for improving periodontal tissue prevention and treatment or repair, also trying to create novel possibility in prevention of systemic as well as neurodegenerative disorders.
Author Contributions
Conceptualization, T.R. and A.G.; methodology, T.R., V.P., P.F., R.D.S. and A.G.; resources, T.R., R.D.S., A.G.; data curation, T.R., V.P., P.F., R.D.S. and A.G.; writing—original draft preparation, T.R.; writing—review and editing, T.R. and A.G.; visualization, T.R., V.P. and P.F., R.D.S. and A.G.; supervision, T.R., R.D.S. and A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Richards, D. Oral Diseases affect some 3.9 Billion people. Evid. -Based Dent. 2013, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Matichescu, A.; Ardelean, L.C.; Rusu, L.-C.; Craciun, D.; Bratu, E.A.; Babucea, M.; Leretter, M. Advanced Biomaterials and Techniques for Oral Tissue Engineering and Regeneration—A Review. Materials 2020, 13, 5303. [Google Scholar] [CrossRef] [PubMed]
- Rodella, L.F.; Favero, G.; Labanca, M. Biomaterials in Maxillofacial Surgery: Membranes and Grafts. Int. J. Biomed. Sci. IJBS 2011, 7, 81–88. [Google Scholar] [PubMed]
- Sheiham, A.; Alexander, D.; Cohen, L.; Marinho, V.; Moysés, S.; Petersen, P.; Spencer, J.; Watt, R.; Weyant, R. Global oral health inequalities: Task group—Implementation and delivery of oral health strategies. Adv. Dent. Res. 2011, 23, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Sheiham, A. Major changes in strategies are needed to promote oral health worldwide. J. Public Health Dent. 2013, 73, 87–88. [Google Scholar] [CrossRef]
- Watt, R.G. Social determinants of oral health inequalities: Implications for action. Community Dent. Oral Epidemiol. 2012, 40, 44–48. [Google Scholar] [CrossRef]
- Petersen, P.E.; Bourgeois, D.; Ogawa, H.; Estupinan-Day, S.; Ndiaye, C. The global burden of oral diseases and risks to oral health. Bull. World Health Organ. 2005, 83, 661–669. [Google Scholar]
- Battistella, E.; Mele, S.; Rimondini, L. Dental Tissue Engineering: A New Approach to Dental Tissue Reconstruction. In Biomimetics Learning from Nature; InTech: Rijeka, Croatia, 2010. [Google Scholar]
- Rai, R.; Raval, R.; Khandeparker, R.; Chidrawar, S.K.; Khan, A.A.; Ganpat, M.S. Tissue Engineering: Step Ahead in Maxillofacial Reconstruction. J. Int. Oral Health 2015, 7, 138–142. [Google Scholar]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef]
- Borrelli, M.R.; Hu, M.S.; Longaker, M.T.; Lorenz, H.P. Tissue Engineering and Regenerative Medicine in Craniofacial Reconstruction and Facial Aesthetics. J. Craniofacial Surg. 2020, 31, 15–27. [Google Scholar] [CrossRef]
- Upadhyay, R.K. Role of Biological Scaffolds, Hydro Gels and Stem Cells in Tissue Regeneration Therapy. Advances in Tissue Engineering & Regenerative Medicine. Open Access 2017, 2, 121–135. [Google Scholar]
- Zhang, K.; Wang, S.; Zhou, C.; Cheng, L.; Gao, X.; Xie, X.; Sun, J.; Wang, H.; Weir, M.D.; Reynolds, M.A.; et al. Advanced smart biomaterials and constructs for hard tissue engineering and regeneration. Bone Res. 2018, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Avci-Adali, M.; Alarçin, E.; Cheng, H.; Kashaf, S.S.; Li, Y.; Chawla, A.; Jang, H.L.; Khademhosseini, A. Development of hydrogels for regenerative engineering. Biotechnol. J. 2017. Available online: https://pubmed.ncbi.nlm.nih.gov/28220995/ (accessed on 21 November 2021). [CrossRef] [PubMed]
- Gao, Z.H.; Hu, L.; Liu, G.L.; Wei, F.L.; Liu, Y.; Liu, Z.H.; Fan, Z.P.; Zhang, C.M.; Wang, J.S.; Wang, S.L. Bio-Root and Implant-Based Restoration as a Tooth Replacement Alternative. J. Dent. Res. 2016, 95, 642–649. [Google Scholar] [CrossRef]
- Alix, H.; Park, J.C. Dental Stem cells and their applications. Chin. J. Dent. Res. 2015, 18, 207–212. [Google Scholar]
- Tonk, C.H.; Witzler, M.; Schulze, M.; Tobiasch, E. Mesenchymal Stem Cells, in Essential Current Concepts in Stem Cell Biology; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 21–39. [Google Scholar]
- Baranova, J.; Büchner, D.; Götz, W.; Schulze, M.; Tobiasch, E. Tooth Formation: Are the Hardest Tissues of Human Body Hard to Regenerate? Int. J. Mol. Sci. 2020, 21, 4031. [Google Scholar] [CrossRef] [PubMed]
- Marin, E.; Boschetto, F.; Pezzotti, G. Biomaterials and biocompatibility: An historical overview. J. Biomed. Mater. Res. Part A 2020, 108, 1617–1633. [Google Scholar] [CrossRef]
- Ferrage, L.; Bertrand, G.; Lenormand, P.; Grossin, D.; Ben-Nissan, B. A review of the additive manufacturing (3DP) of bioceramics: Alumina, zirconia (PSZ) and hydroxyapatite. J. Aust. Ceram. Soc. 2016, 53, 11–20. [Google Scholar] [CrossRef]
- Derby, B. Printing and Prototyping of Tissues and Scaffolds. Science 2012, 338, 921–926. [Google Scholar] [CrossRef]
- Matsiko, A.; Gleeson, J.P.; O’Brien, F.J. Scaffold Mean Pore Size Influences Mesenchymal Stem Cell Chondrogenic Differentiation and Matrix Deposition. Tissue Eng. Part A 2015, 21, 486–497. [Google Scholar] [CrossRef]
- Banerjee, A.; Chatterjee, K.; Madras, G. Enzymatic degradation of polymers: A brief review. Mater. Sci. Technol. 2014, 30, 567–573. [Google Scholar] [CrossRef]
- Rey-Vinolas, S.; Engel, E.; Mateos-Timoneda, M.A. Polymers for bone repair. In Bone Repair Biomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 179–197. [Google Scholar]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, P.X. Polymeric Scaffolds for Bone Tissue Engineering. Ann. Biomed. Eng. 2004, 32, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Freed, L.E.; Vunjak-Novakovic, G.; Biron, R.J.; Eagles, D.B.; Lesnoy, D.C.; Barlow, S.K.; Langer, R. Biodegradable Polymer Scaffolds for Tissue Engineering. Nat. Biotechnol. 1994, 12, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Young, C.; Terada, S.; Vacanti, J.; Honda, M.; Bartlett, J.; Yelick, P. Tissue Engineering of Complex Tooth Structures on Biodegradable Polymer Scaffolds. J. Dent. Res. 2002, 81, 695–700. [Google Scholar] [CrossRef]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Mittal, A.; Negi, P.; Garkhal, K.; Verma, S.; Kumar, N. Integration of porosity and bio-functionalization to form a 3D scaffold: Cell culture studies and in vitro degradation. Biomed. Mater. 2010, 5, 045001. [Google Scholar] [CrossRef]
- Zeng, D.; Zhang, X.; Wang, X.; Huang, Q.; Wen, J.; Miao, X.; Peng, L.; Li, Y.; Jiang, X. The osteoimmunomodulatory properties of MBG scaffold coated with amino functional groups. Artif. Cells, Nanomed. Biotechnol. 2017, 46, 1425–1435. [Google Scholar] [CrossRef]
- Chen, Z.; Mao, X.; Tan, L.; Friis, T.; Wu, C.; Crawford, R.; Xiao, Y. Osteoimmunomodulatory properties of magnesium scaffolds coated with β-tricalcium phosphate. Biomaterials 2014, 35, 8553–8565. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, K.; Gong, T.; Song, J.; Bao, C.; Luo, E.; Weng, J.; Zhou, S. Delivery of Growth Factors Using a Smart Porous Nanocomposite Scaffold to Repair a Mandibular Bone Defect. Biomacromolecules 2014, 15, 1019–1030. [Google Scholar] [CrossRef]
- Damaraju, S.M.; Shen, Y.; Elele, E.; Khusid, B.; Eshghinejad, A.; Li, J.; Jaffe, M.; Arinzeh, T.L. Three-dimensional piezoelectric fibrous scaffolds selectively promote mesenchymal stem cell differentiation. Biomaterials 2017, 149, 51–62. [Google Scholar] [CrossRef]
- Augustine, R.; Dan, P.; Sosnik, A.; Kalarikkal, N.; Tran, N.; Vincent, B.; Thomas, S.; Menu, P.; Rouxel, D. Electrospun poly(vinylidene fluoride-trifluoroethylene)/zinc oxide nanocomposite tissue engineering scaffolds with enhanced cell adhesion and blood vessel formation. Nano Res. 2017, 10, 3358–3376. [Google Scholar] [CrossRef]
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The Smart Drug Delivery System and Its Clinical Potential. Theranostics 2016, 6, 1306–1323. [Google Scholar] [CrossRef] [PubMed]
- Kondiah, P.J.; Choonara, Y.E.; Kondiah, P.P.D.; Marimuthu, T.; Kumar, P.; du Toit, L.C.; Pillay, V. A Review of Injectable Polymeric Hydrogel Systems for Application in Bone Tissue Engineering. Molecules 2016, 21, 1580. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.C.I.M.; Ahmad, N.; Pandey, M.; Xin, C.J. Stimuli-responsive bacterial cellulose-g-poly(acrylic acid-co-acrylamide) hydrogels for oral controlled release drug delivery. Drug Dev. Ind. Pharm. 2013, 40, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Ashley, G.W.; Henise, J.; Reid, R.; Santi, D.V. Hydrogel drug delivery system with predictable and tunable drug release and degradation rates. Proc. Natl. Acad. Sci. USA 2013, 110, 2318–2323. [Google Scholar] [CrossRef]
- Horev, B.; Klein, M.I.; Hwang, G.; Li, Y.; Kim, D.; Koo, H.; Benoit, D.S.W. pH-activated nanoparticles for controlled topical delivery of farnesol to disrupt oral biofilm virulence. ACS Nano 2015, 9, 2390–2404. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, S.; Pan, J.; Shi, R.; Liu, H.; Lyu, Y.; Han, X.; Li, Y.; Yang, Y.; Xu, Z.; et al. Time-responsive osteogenic niche of stem cells: A sequentially triggered, dual-peptide loaded, alginate hybrid system for promoting cell activity and osteo-differentiation. Biomaterials 2018, 163, 25–42. [Google Scholar] [CrossRef]
- Sant, S.; Hancock, M.J.; Donnelly, J.P.; Iyer, D.; Khademhosseini, A. Biomimetic gradient hydrogels for tissue engineering. Can. J. Chem. Eng. 2010, 88, 899–911. [Google Scholar] [CrossRef]
- Sheikhpour, M.; Barani, L.; Kasaeian, A. Biomimetics in drug delivery systems: A critical review. J. Control. Release 2017, 253, 97–109. [Google Scholar] [CrossRef]
- Reis, E.C.; Borges, A.P.B.; Araújo, M.V.; Mendes, V.C.; Guan, L.; Davies, J.E. Periodontal regeneration using a bilayered PLGA/calcium phosphate construct. Biomaterials 2011, 32, 9244–9253. [Google Scholar] [CrossRef] [PubMed]
- Sowmya, S.; Mony, U.; Jayachandran, P.; Reshma, S.; Kumar, R.A.; Arzate, H.; Nair, S.V.; Jayakumar, R. Tri-Layered Nanocomposite Hydrogel Scaffold for the Concurrent Regeneration of Cementum, Periodontal Ligament, and Alveolar Bone. Adv. Health Mater. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Weir, M.D.; Ruan, J.; Zhang, N.; Chow, L.C.; Zhang, K.; Chang, X.; Bai, Y.; Xu, H.H.K. Effect of calcium phosphate nanocomposite on in vitro remineralization of human dentin lesions. Dent. Mater. 2017, 33, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, J.; Eckert, R.; Yarbrough, D.; Lux, R.; Anderson, M.; Shi, W. Design and Characterization of an Acid-Activated Antimicrobial Peptide. Chem. Biol. Drug Des. 2009, 75, 127–132. [Google Scholar] [CrossRef]
- Yang, Y.; Reipa, V.; Liu, G.; Meng, Y.; Wang, X.; Mineart, K.P.; Prabhu, V.M.; Shi, W.; Lin, N.J.; He, X.; et al. pH-Sensitive Compounds for Selective Inhibition of Acid-Producing Bacteria. ACS Appl. Mater. Interfaces 2018, 10, 8566–8573. [Google Scholar] [CrossRef]
- Cheng, L.; Weir, M.D.; Zhang, K.; Wu, E.J.; Xu, S.M.; Zhou, X.; Xu, H.H. Dental plaque microcosm biofilm behavior on calcium phosphate nanocomposite with quaternary ammonium. Dent. Mater. 2012, 28, 853–862. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H.; Ren, B.; Li, X.; Wang, L.; Zhou, H.; Weir, M.D.; Zhou, X.; Masri, R.M.; Oates, T.W.; et al. Drug resistance of oral bacteria to new antibacterial dental monomer dimethylaminohexadecyl methacrylate. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Li, F.; Weir, M.D.; Xu, H.H.K. Effects of quaternary ammonium chain length on antibacterial bonding agents. J. Dent. Res. 2013, 92, 932–938. [Google Scholar] [CrossRef]
- Maftei, G.A.; Martu, C.M.; Popa, C.; Geletu, G.; Danila, V.; Jelihovschi, I.; Foia, L. The Biomechanical Properties of Suture Materials and Their Relationship to Bacterial Adherence. Mater. Plast. 2019, 56, 980–985. [Google Scholar] [CrossRef]
- Martu, M.-A.; Maftei, G.-A.; Luchian, I.; Popa, C.; Filioreanu, A.-M.; Tatarciuc, D.; Nichitean, G.; Hurjui, L.-L.; Foia, L.-G. Wound healing of periodontal and oral tissues: Part II—Patho-phisiological conditions and metabolic diseases. Rom. J. Oral Rehab. 2020, 12, 30–40. [Google Scholar]
- Del Giudice, C.; Vaia, E.; Liccardo, D.; Marzano, F.; Valletta, A.; Spagnuolo, G.; Ferrara, N.; Rengo, C.; Cannavo, A.; Rengo, G. Infective Endocarditis: A Focus on Oral Microbiota. Microorganisms 2021, 9, 1218. [Google Scholar] [CrossRef]
- Baumgartner, S.; Gmeiner, R.; Schönherr, J.A.; Stampfl, J. Stereolithography-based additive manufacturing of lithium disilicate glass ceramic for dental applications. Mater. Sci. Eng. C 2020, 116, 111180. [Google Scholar] [CrossRef] [PubMed]
- Jasiuk, I.; Abueidda, D.W.; Kozuch, C.; Pang, S.; Su, F.Y.; McKittrick, J. An Overview on Additive Manufacturing of Polymers. JOM 2018, 70, 275–283. [Google Scholar] [CrossRef]
- Mederle, N.; Marin, S.; Marin, M.M.; Danila, E.; Mederle, O.; Kaya, M.G.A.; Ghica, M.V. Innovative Biomaterials Based on Collagen-Hydroxyapatite and Doxycycline for Bone Regeneration. Adv. Mater. Sci. Eng. 2016, 2016, 1–5. [Google Scholar] [CrossRef][Green Version]
- Papaioannou, T.G.; Manolesou, D.; Dimakakos, E.; Tsoucalas, G.; Vavuranakis, M.; Tousoulis, D. 3D Bioprinting Methods and Techniques: Applications on Artificial Blood Vessel Fabrication. Acta Cardiol. Sin. 2019, 35, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Zadpoor, A.A.; Malda, J. Additive Manufacturing of Biomaterials, Tissues, and Organs. Ann. Biomed. Eng. 2016, 45, 1–11. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Wei, Y.; Hou, W.; Tong, H.; Bai, S. 3D Bioprinting Technologies for Hard Tissue and Organ Engineering. Materials 2016, 9, 802. [Google Scholar] [CrossRef]
- Xiongfa, J.; Hao, Z.; Liming, Z.; Jun, X. Recent advances in 3D bioprinting for the regeneration of functional cartilage. Regen. Med. 2018, 13, 73–87. [Google Scholar] [CrossRef]
- Nakamura, M.; Kobayashi, A.; Takagi, F.; Watanabe, A.; Hiruma, Y.; Ohuchi, K.; Iwasaki, Y.; Horie, M.; Morita, I.; Takatani, S. Biocompatible Inkjet Printing Technique for Designed Seeding of Individual Living Cells. Tissue Eng. 2005, 11, 1658–1666. [Google Scholar] [CrossRef]
- Saunders, R.E.; Gough, J.E.; Derby, B. Delivery of human fibroblast cells by piezoelectric drop-on-demand inkjet printing. Biomaterials 2008, 29, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Catros, S.; Fricain, J.-C.; Guillotin, B.; Pippenger, B.; Bareille, R.; Remy, M.; Lebraud, E.; Desbat, B.; Amédée, J.; Guillemot, F. Laser-assisted bioprinting for creating on-demand patterns of human osteoprogenitor cells and nano-hydroxyapatite. Biofabrication 2011, 3, 025001. [Google Scholar] [CrossRef] [PubMed]
- Guillemot, F.; Souquet, A.; Catros, S.; Guillotin, B. Laser-assisted cell printing: Principle, physical parameters versus cell fate and perspectives in tissue engineering. Nanomedicine 2010, 5, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, D.; Alexander, P.G.; Yang, G.; Tan, J.; Cheng, A.W.-M.; Tuan, R.S. Application of visible light-based projection stereolithography for live cell-scaffold fabrication with designed architecture. Biomaterials 2012, 34, 331–339. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Fan, X.; Zhou, H. Recent advances in bioprinting techniques: Approaches, applications and future prospects. J. Transl. Med. 2016, 14, 1–15. [Google Scholar] [CrossRef]
- Theus, A.S.; Ning, L.; Hwang, B.; Gil, C.; Chen, S.; Wombwell, A.; Mehta, R.; Serpooshan, V. Bioprintability: Physiomechanical and Biological Requirements of Materials for 3D Bioprinting Processes. Polymers 2020, 12, 2262. [Google Scholar] [CrossRef] [PubMed]
- Dorishetty, P.; Dutta, N.K.; Choudhury, N.R. Bioprintable tough hydrogels for tissue engineering applications. Adv. Colloid Interface Sci. 2020, 281, 102163. [Google Scholar] [CrossRef]
- Compaan, A.M.; Christensen, K.; Huang, Y. Inkjet Bioprinting of 3D Silk Fibroin Cellular Constructs Using Sacrificial Alginate. ACS Biomater. Sci. Eng. 2016, 3, 1519–1526. [Google Scholar] [CrossRef]
- Jose, R.R.; Rodriguez, M.J.; Dixon, T.A.; Omenetto, F.; Kaplan, D.L. Evolution of Bioinks and Additive Manufacturing Technologies for 3D Bioprinting. ACS Biomater. Sci. Eng. 2016, 2, 1662–1678. [Google Scholar] [CrossRef]
- Nicodemus, G.D.; Bryant, S.J. Cell Encapsulation in Biodegradable Hydrogels for Tissue Engineering Applications. Tissue Eng. Part B Rev. 2008, 14, 149–165. [Google Scholar] [CrossRef]
- Wüst, S.; Godla, M.E.; Müller, R.; Hofmann, S. Tunable hydrogel composite with two-step processing in combination with innovative hardware upgrade for cell-based three-dimensional bioprinting. Acta Biomater. 2014, 10, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Duffy, R.M.; Sun, Y.; Feinberg, A.W. Understanding the Role of ECM Protein Composition and Geometric Micropatterning for Engineering Human Skeletal Muscle. Ann. Biomed. Eng. 2016, 44, 2076–2089. [Google Scholar] [CrossRef] [PubMed]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.-J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Data from: Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef] [PubMed]
- Levato, R.; Visser, J.; Planell, J.A.; Engel, E.; Malda, J.; Timoneda, M.A.M. Biofabrication of tissue constructs by 3D bioprinting of cell-laden microcarriers. Biofabrication 2014, 6, 035020. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.F.D.; Blaeser, A.; Weber, M.; Jäkel, J.; Neuss, S.; Jahnen-Dechent, W.; Fischer, H. Three-dimensional printing of stem cell-laden hydrogels submerged in a hydrophobic high-density fluid. Biofabrication 2012, 5, 015003. [Google Scholar] [CrossRef]
- Gruene, M.; Deiwick, A.; Koch, L.; Schlie, S.; Unger, C.; Hofmann, N.; Bernemann, I.; Glasmacher, B.; Chichkov, B. Laser Printing of Stem Cells for Biofabrication of Scaffold-Free Autologous Grafts. Tissue Eng. Part C Methods 2011, 17, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Zeng, B.; Han, Y.; Dai, H.; Liu, J.; Sun, Y.; Li, F. Preparation and laser powder bed fusion of composite microspheres consisting of poly(lactic acid) and nano-hydroxyapatite. Addit. Manuf. 2020, 34, 101305. [Google Scholar] [CrossRef]
- Mostafaei, A.; Elliott, A.M.; Barnes, J.E.; Li, F.; Tan, W.; Cramer, C.L.; Nandwana, P.; Chmielus, M. Binder jet 3D printing—Process parameters, materials, properties, modeling, and challenges. Prog. Mater. Sci. 2021, 119, 100707. [Google Scholar] [CrossRef]
- Gonzalez, J.A.; Mireles, J.; Lin, Y.; Wicker, R.B. Characterization of ceramic components fabricated using binder jetting additive manufacturing technology. Ceram. Int. 2016, 42, 10559–10564. [Google Scholar] [CrossRef]
- Pu’Ad, N.M.; Haq, R.A.; Noh, H.M.; Abdullah, H.; Idris, M.; Lee, T. Review on the fabrication of fused deposition modelling (FDM) composite filament for biomedical applications. Mater. Today Proc. 2020, 29, 228–232. [Google Scholar] [CrossRef]
- Yap, Y.L.; Wang, C.; Sing, S.L.; Dikshit, V.; Yeong, W.Y.; Wei, J. Material jetting additive manufacturing: An experimental study using designed metrological benchmarks. Precis. Eng. 2017, 50, 275–285. [Google Scholar] [CrossRef]
- Davoudinejad, A.; Diaz-Perez, L.C.; Quagliotti, D.; Pedersen, D.B.; Albajez-García, J.A.; Yagüe-Fabra, J.A.; Tosello, G. Additive manufacturing with vat polymerization method for precision polymer micro components production. Procedia CIRP 2018, 75, 98–102. [Google Scholar] [CrossRef]
- Freeman, M.W.; Arrott, A.; Watson, J.H.L. Magnetism in Medicine. J. Appl. Phys. 1960, 31, S404–S405. [Google Scholar] [CrossRef]
- Handgretinger, R.; Lang, P.; Schumm, M.; Taylor, G.; Neu, S.; Koscielnak, E.; Niethammer, D.; Klingebiel, T. Isolation and transplantation of autologous peripheral CD34+ progenitor cells highly purified by magnetic-activated cell sorting. Bone Marrow Transplant. 1998, 21, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Schoepf, U.; Marecos, E.M.; Melder, R.J.; Jain, R.K.; Weissleder, R. Intracellular Magnetic Labeling of Lymphocytes for In Vivo Trafficking Studies. BioTechniques 1998, 24, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Cuatrecasas, P.; Roth, T.F. Receptor-Mediat. Endocytosis, ed. Series; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Volume 15. [Google Scholar]
- Weissleder, R.; Stark, D.D.; Engelstad, B.L.; Bacon, B.R.; Compton, C.C.; White, D.L.; Jacobs, P.; Lewis, J. Superparamagnetic iron oxide: Pharmacokinetics and toxicity. Am. J. Roentgenol. 1989, 152, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Bilbao, G.; Gómez-Navarro, J.; Curiel, D.T. Targeted Adenoviral Vectors for Cancer Gene Therapy. In Gene Therapy of Cancer; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 1998; Volume 451, pp. 365–374. [Google Scholar]
- Bulte, J.W.; Douglas, T.; Witwer, B.; Zhang, S.-C.; Strable, E.; Lewis, B.K.; Zywicke, H.; Miller, B.; Van Gelderen, P.; Moskowitz, B.M.; et al. Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat. Biotechnol. 2001, 19, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Jendelova, P.; Herynek, V.; DeCroos, J.; Glogarová, K.; Andersson, B.; Hájek, M.; Syková, E. Imaging the fate of implanted bone marrow stromal cells labeled with superparamagnetic nanoparticles. Magn. Reson. Med. 2003, 50, 767–776. [Google Scholar] [CrossRef]
- Gordon, R.T.; Hines, J.R.; Gordon, D. Intracellular hyperthermia a biophysical approach to cancer treatment via intracellular temperature and biophysical alterations. Med Hypotheses 1979, 5, 83–102. [Google Scholar] [CrossRef]
- Scherer, F.; Anton, M.; Schillinger, U.; Henke, J.; Bergemann, C.; Krüger, A.; Gänsbacher, B.; Plank, C. Magnetofection: Enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002, 9, 102–109. [Google Scholar] [CrossRef]
- Russo, A.; Shelyakova, T.; Casino, D.; Lopomo, N.; Strazzari, A.; Ortolani, A.; Visani, A.; Dediu, V.; Marcacci, M. A new approach to scaffold fixation by magnetic forces: Application to large osteochondral defects. Med. Eng. Phys. 2012, 34, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Hibino, E.; Kobayashi, C.; Terasaki, H.; Kagami, H.; Ueda, M.; Kobayashi, T.; Honda, H. Construction and Delivery of Tissue-Engineered Human Retinal Pigment Epithelial Cell Sheets, Using Magnetite Nanoparticles and Magnetic Force. Tissue Eng. 2005, 11, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Ino, K.; Hayashida, M.; Kobayashi, T.; Matsunuma, H.; Kagami, H.; Ueda, M.; Honda, H. Novel Methodology for Fabrication of Tissue-Engineered Tubular Constructs Using Magnetite Nanoparticles and Magnetic Force. Tissue Eng. 2005, 11, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Dobson, J. Magnetic nanoparticles for drug delivery. Drug Dev. Res. 2006, 67, 55–60. [Google Scholar] [CrossRef]
- Dobson, J. Remote control of cellular behaviour with magnetic nanoparticles. Nat. Nanotechnol. 2008, 3, 139–143. [Google Scholar] [CrossRef]
- Bock, N.; Riminucci, A.; Dionigi, C.; Russo, A.; Tampieri, A.; Landi, E.; Goranov, V.A.; Marcacci, M.; Dediu, V. A novel route in bone tissue engineering: Magnetic biomimetic scaffolds. Acta Biomater. 2010, 6, 786–796. [Google Scholar] [CrossRef]
- De Santis, R.; Gloria, A.; Russo, T.; D’Amora, U.; Zeppetelli, S.; Dionigi, C.; Sytcheva, A.; Herrmannsdörfer, T.; Dediu, V.; Ambrosio, L. A basic approach toward the development of nanocomposite magnetic scaffolds for advanced bone tissue engineering. J. Appl. Polym. Sci. 2011, 122, 3599–3605. [Google Scholar] [CrossRef]
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of Nanoparticles. Small 2008, 4, 26–49. [Google Scholar] [CrossRef]
- Gloria, A.; Russo, T.; D’Amora, U.; Zeppetelli, S.; D’Alessandro, T.; Sandri, M.; Bañobre-López, M.; Piñeiro-Redondo, Y.; Uhlarz, M.; Tampieri, A.; et al. Magnetic poly(ε-caprolactone)/iron-doped hydroxyapatite nanocomposite substrates for advanced bone tissue engineering. J. R. Soc. Interface 2013, 10, 20120833. [Google Scholar] [CrossRef]
- Tampieri, A.; D’Alessandro, T.; Sandri, M.; Sprio, S.; Landi, E.; Bertinetti, L.; Panseri, S.; Pepponi, G.; Goettlicher, J.; Bañobre-López, M.; et al. Intrinsic magnetism and hyperthermia in bioactive Fe-doped hydroxyapatite. Acta Biomater. 2012, 8, 843–851. [Google Scholar] [CrossRef]
- De Santis, R.; Russo, A.; Gloria, A.; D’Amora, U.; Russo, T.; Panseri, S.; Sandri, M.; Tampieri, A.; Marcacci, M.; Dediu, V.A.; et al. Towards the Design of 3D Fiber-Deposited Poly( -caprolactone)/Iron-Doped Hydroxyapatite Nanocomposite Magnetic Scaffolds for Bone Regeneration. J. Biomed. Nanotechnol. 2015, 11, 1236–1246. [Google Scholar] [CrossRef]
- Russo, T.; D’Amora, U.; Gloria, A.; Tunesi, M.; Sandri, M.; Rodilossi, S.; Albani, D.; Forloni, G.; Giordano, C.; Cigada, A.; et al. Systematic Analysis of Injectable Materials and 3D Rapid Prototyped Magnetic Scaffolds: From CNS Applications to Soft and Hard Tissue Repair/Regeneration. Procedia Eng. 2013, 59, 233–239. [Google Scholar] [CrossRef]
- Russo, T.; Peluso, V.; Gloria, A.; Oliviero, O.; Rinaldi, L.; Improta, G.; De Santis, R.; D’Antò, V. Combination Design of Time-Dependent Magnetic Field and Magnetic Nanocomposites to Guide Cell Behavior. Nanomaterials 2020, 10, 577. [Google Scholar] [CrossRef] [PubMed]
- Sprio, S.; Campodoni, E.; Sandri, M.; Preti, L.; Keppler, T.; Müller, F.A.; Pugno, N.M.; Tampieri, A. A Graded Multifunctional Hybrid Scaffold with Superparamagnetic Ability for Periodontal Regeneration. Int. J. Mol. Sci. 2018, 19, 3604. [Google Scholar] [CrossRef]
- Rodrigues, A.F.M.; Torres, P.M.C.; Barros, M.J.S.; Presa, R.; Ribeiro, N.; Abrantes, J.C.C.; Belo, J.H.; Amaral, J.S.; Bañobre-López, M.; Bettencourt, A.; et al. Effective production of multifunctional magnetic-sensitive biomaterial by an extrusion-based additive manufacturing technique. Biomed. Mater. 2020, 16, 015011. [Google Scholar] [CrossRef]
- Goranov, V.; Shelyakova, T.; De Santis, R.; Haranava, Y.; Makhaniok, A.; Gloria, A.; Tampieri, A.; Russo, A.; Kon, E.; Marcacci, M.; et al. 3D Patterning of cells in Magnetic Scaffolds for Tissue Engineering. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Roh, S.; Okello, L.B.; Golbasi, N.; Hankwitz, J.P.; Liu, J.A.C.; Tracy, J.B.; Velev, O.D. 3D-Printed Silicone Soft Architectures with Programmed Magneto-Capillary Reconfiguration. Adv. Mater. Technol. 2019, 4, 1800528. [Google Scholar] [CrossRef]
- Nimeri, G.; Kau, C.H.; Abou-Kheir, N.S.; Corona, R. Acceleration of tooth movement during orthodontic treatment-a frontier in Orthodontics. Prog. Orthod. 2013, 14, 42. [Google Scholar] [CrossRef]
- Nishimura, M.; Chiba, M.; Ohashi, T.; Sato, M.; Shimizu, Y.; Igarashi, K.; Mitani, H. Periodontal tissue activation by vibration: Intermittent stimulation by resonance vibration accelerates experimental tooth movement in rats. Am. J. Orthod. Dentofac. Orthop. 2008, 133, 572–583. [Google Scholar] [CrossRef]
- Shimizu, Y. Movement of the lateral incisors in Macaca fuscata as loaded by a vibrating force. J. Jpn. Orthod. Soc. 1986, 45, 56–72. [Google Scholar]
- Kau, C.H. A radiographic analysis of tooth morphology following the use of a novel cyclical force device in orthodontics. Head Face Med. 2011, 7, 14. [Google Scholar] [CrossRef]
- Davidovitch, Z.; Finkelson, M.D.; Steigman, S.; Shanfeld, J.L.; Montgomery, P.C.; Korostoff, E. Electric currents, bone remodeling, and orthodontic tooth movement. Am. J. Orthod. 1980, 77, 33–47. [Google Scholar] [CrossRef]
- Saito, S.; Shimizu, N. Stimulatory effects of low-power laser irradiation on bone regeneration in midpalatal suture during expansion in the rat. Am. J. Orthod. Dentofac. Orthop. 1997, 111, 525–532. [Google Scholar] [CrossRef]
- Trelles, M.A.; Mayayo, E. Bone fracture consolidates faster with low-power laser. Lasers Surg. Med. 1987, 7, 36–45. [Google Scholar] [CrossRef]
- Takeda, Y. Irradiation effect of low-energy laser on alveolar bone after tooth extraction. Experimental study in rats. Int. J. Oral Maxillofac. Surg. 1988, 17, 388–391. [Google Scholar] [CrossRef]
- Fujita, S.; Yamaguchi, M.; Utsunomiya, T.; Yamamoto, H.; Kasai, K. Low-energy laser stimulates tooth movement velocity via expression of RANK and RANKL. Orthod. Craniofacial Res. 2008, 11, 143–155. [Google Scholar] [CrossRef]
- Karu, T.I. Mitochondrial Signaling in Mammalian Cells Activated by Red and Near-IR Radiation. Photochem. Photobiol. 2008, 84, 1091–1099. [Google Scholar] [CrossRef]
- Eells, J.T.; Henry, M.M.; Summerfelt, P.; Wong-Riley, M.T.T.; Buchmann, E.V.; Kane, M.; Whelan, N.T.; Whelan, H.T. Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proc. Natl. Acad. Sci. USA 2003, 100, 3439–3444. [Google Scholar] [CrossRef]
- Showkatbakhsh, R.; Jamilian, A.; Showkatbakhsh, M. The effect of pulsed electromagnetic fields on the acceleration of tooth movement. World J. Orthod. 2010, 11, e52–e56. [Google Scholar]
- Darendeliler, M.A.; Sinclair, P.M.; Kusy, R.P. The effects of samarium-cobalt magnets and pulsed electromagnetic fields on tooth movement. Am. J. Orthod. Dentofac. Orthop. 1995, 107, 578–588. [Google Scholar] [CrossRef]
- Darendeliler, M.A.; Zea, A.; Shen, G.; Zoellner, H. Effects of pulsed electromagnetic field vibration on tooth movement induced by magnetic and mechanical forces: A preliminary study. Aust. Dent. J. 2007, 52, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Chang, W.H.-S.; Huang, S.; Huang, S.; Shih, C. Pulsed electromagnetic fields stimulation affects osteoclast formation by modulation of osteoprotegerin, RANK ligand and macrophage colony-stimulating factor. J. Orthop. Res. 2005, 23, 1308–1314. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).