High Crystalline Quality Conductive Polypyrrole Film Prepared by Interface Chemical Oxidation Polymerization Method
Abstract
:1. Introduction
2. Materials and Methods
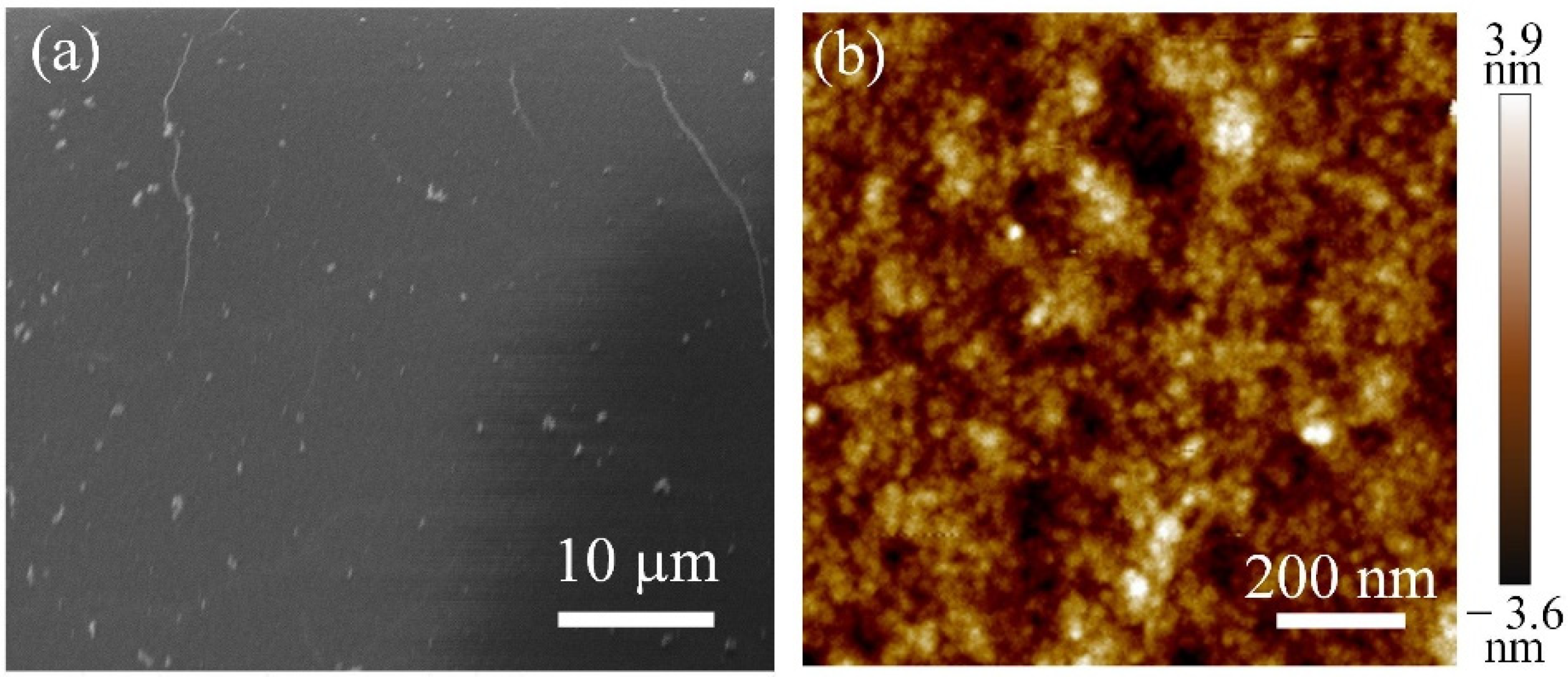
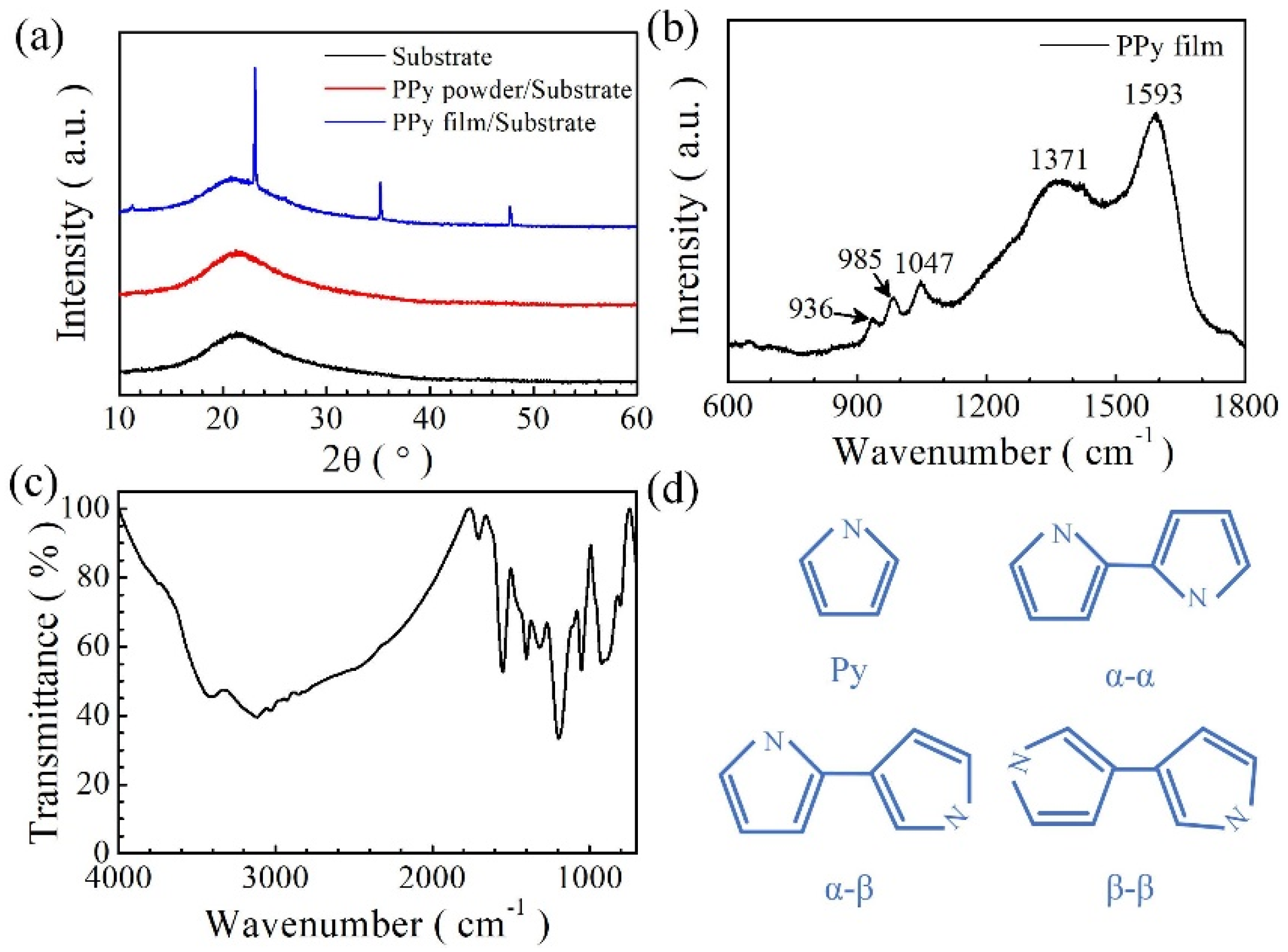
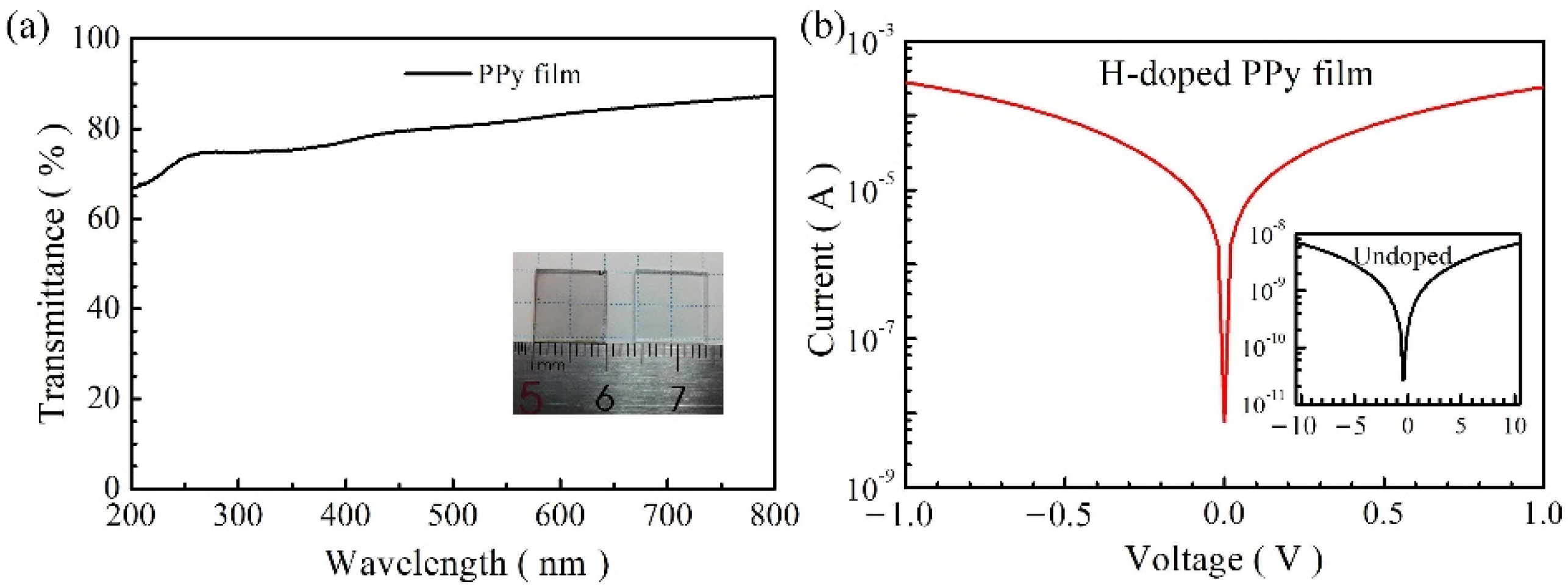
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shirakawa, H.; Ikeda, S. Infrared Spectra of Poly (acetylene). Polymer J. 1971, 2, 231–244. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.W. Investigation on the Low Luminous Efficiency in a Polymer Light-Emitting Diode with a High Work-function Cathode by Soft Contact Lamination. Adv. Funct. Mater. 2010, 17, 3128–3133. [Google Scholar] [CrossRef]
- Dhingra, M.; Shrivastava, S.; Senthil Kumar, P.; Annapoorni, S. ZnO/PPy Hybrid Heterojunction as an Ultraviolet Photosensor. J. Electron. Mater. 2013, 42, 1235–1241. [Google Scholar] [CrossRef]
- Zheng, W.J.; Li, X.C.; Dong, C.X.; Yan, X.M.; He, G.H. Fabrication of a visible light detector based on a coaxial polypyrrole/TiO2 nanorod heterojunction. RSC Adv. 2014, 4, 44868–44871. [Google Scholar] [CrossRef]
- Wen, P.; Tan, C.H.; Zhang, J.C.; Meng, F.B.; Jiang, L.; Sun, Y.H.; Chen, X.D. Chemically tunable photoresponse of ultrathin polypyrrole. Nanoscale 2017, 9, 7760–7764. [Google Scholar] [CrossRef] [PubMed]
- Kharat, H.J.; Kakde, K.P.; Savale, P.A.; Datta, K.; Ghosh, P.; Shirsat, M.D. Synthesis of polypyrrole films for the development of ammonia sensor. Polym. Adv. Technol. 2007, 18, 397–402. [Google Scholar] [CrossRef]
- Hua, B.; Shi, G. Gas Sensors Based on Conducting Polymers. Sensors 2007, 7, 267–307. [Google Scholar]
- Du, W.X.; Lee, H.J.; Byeon, J.H.; Kim, J.S.; Cho, K.S.; Kang, S.; Takada, M.; Kim, J.Y. Highly sensitive single-walled carbon nanotube/polypyrrole/phenylalanine core–shell nanorods for ammonia gas sensing. J. Mater. Chem. C 2020, 8, 15609–15615. [Google Scholar]
- Ahmad, S.; Khan, I.; Husain, A.; Khan, A.; Asiri, A.M. Electrical Conductivity Based Ammonia Sensing Properties of Polypyrrole/MoS2 Nanocomposite. Polymers 2020, 12, 3047. [Google Scholar] [CrossRef] [PubMed]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Zhang, D.C.; Zhang, X.; Chen, Y.; Yu, P.; Wang, C.H.; Ma, Y.W. Enhanced capacitance and rate capability of graphene/polypyrrole composite as electrode material for supercapacitors. J. Power Sources 2011, 196, 5990–5996. [Google Scholar] [CrossRef]
- Liu, T.Y.; Finn, L.; Yu, M.H.; Wang, H.Y.; Zhai, T.; Lu, X.H.; Tong, Y.X.; Li, Y. Polyaniline and Polypyrrole Pseudocapacitor Electrodes with Excellent Cycling Stability. Nano Lett. 2014, 14, 2522–2527. [Google Scholar] [CrossRef]
- Tang, H.J.; Wang, J.Y.; Yin, H.J.; Zhao, H.J.; Wang, D.; Tang, Z.Y. Growth of Polypyrrole Ultrathin Films on MoS2 Monolayers as High-Performance Supercapacitor Electrodes. Adv. Mater. 2014, 27, 1117–1123. [Google Scholar] [CrossRef]
- Xue, J.B.; Yang, Q.Q.; Guan, R.F.; Shen, Q.Q.; Liu, X.G.; Jia, H.S.; Li, Q. High-performance ordered porous Polypyrrole/ZnO films with improved specific capacitance for supercapacitors. Mater. Chem. Phys. 2020, 256, 123591. [Google Scholar] [CrossRef]
- Wen, J.; Ding, Y.; Zhong, J.; Chen, R.Y.; Gao, F.; Qiao, Y.L.; Fu, C.Q.; Wang, J.L.; Shen, L.; He, H.F. Ice-interface assisted large-scale preparation of polypyrrole/graphene oxide films for all-solid-state supercapacitors. RSC Adv. 2020, 10, 41503. [Google Scholar] [CrossRef]
- Xu, P.; Han, X.J.; Wang, C.; Zhou, D.H.; Lv, Z.S.; Wen, A.H.; Wang, X.H.; Zhang, B. Synthesis of electromagnetic functionalized nickel/polypyrrole core/shell composites. J. Phys. Chem. B 2008, 112, 10443–10448. [Google Scholar] [CrossRef]
- Yuan, L.Y.; Yao, B.; Hu, B.; Huo, K.F.; Chen, W.; Zhou, J. Polypyrrole-coated paper for flexible solid-state energy storage. Energy Environ. Sci. 2013, 6, 470–476. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q.C.; Wu, S.Y.; Xu, B.X.; Xu, H.X. Multilayer Polypyrrole Nanosheets with Self-Organized Surface Structures for Flexible and Efficient Solar-Thermal Energy Conversion. Adv. Mater. 2019, 31, 1807716. [Google Scholar] [CrossRef] [PubMed]
- Rikame, S.S.; Mungray, A.A.; Mungray, A.K. Electrochemical recovery of metal copper in microbial fuel cell using graphene oxide/polypyrrole cathode catalyst. Int. J. Energy Res. 2021, 45, 6863–6875. [Google Scholar] [CrossRef]
- Lu, Y.Q.; Shi, G.; Li, C.; Liang, Y.Q. Thin polypyrrole films prepared by chemical oxidative polymerization. J. Appl. Polym. Sci. 1998, 70, 2169–2172. [Google Scholar] [CrossRef]
- Sadki, S.; Schottland, P.; Brodie, N.; Sabouraud, G. The mechanisms of pyrrole electropolymerization. Chem. Soc. Rev. 2000, 29, 283–293. [Google Scholar]
- Kumar, D.; Sharma, R.C. Advances in conductive polymers. Eur. Polym. J. 1998, 34, 1053–1060. [Google Scholar] [CrossRef]
- Shi, G.Q.; Jin, S.; Xue, G.; Li, C. A Conducting Polymer Film Stronger Than Aluminum. Science 1995, 267, 994–996. [Google Scholar] [CrossRef]
- Huang, W.S.; MacDiarmin, A.G. Optical properties of polyaniline. Polymer 1993, 34, 1833–1845. [Google Scholar] [CrossRef]
- Stejskal, J.; Sapurina, I.; Prokes, J.; Zemek, J. In-situ polymerized polyaniline films. Synth. Met. 1999, 105, 195–202. [Google Scholar] [CrossRef]
- Malinauskas, A. Chemical deposition of conducting polymers. Polymer 2001, 42, 3957–3972. [Google Scholar] [CrossRef]
- Majumdar, S.; Mahanta, D. One-Step Preparation of Freestanding Polypyrrole Films at Air-Water Interface. Chem. Sel. 2017, 2, 9930–9933. [Google Scholar] [CrossRef]
- Majumdar, S.; Moral, R.; Mahanta, D. Rapid mixing polymerization: A simple method for preparation of free standing polypyrrole film and powder for the removal of anionic pollutants. Colloids Surf. A 2020, 595, 124643. [Google Scholar] [CrossRef]
- Li, M.; Yang, L.L. Intrinsic flexible polypyrrole film with excellent electrochemical performance. J. Mater. Sci. Mater. Electron. 2015, 26, 4875–4879. [Google Scholar] [CrossRef]
- Yurtsever, M.; Yurtsever, E. Structural studies of polypyrroles: I. An ab-initio evaluation of bonding through α and β carbons. Synth. Met. 1999, 98, 221–227. [Google Scholar] [CrossRef]

| Samples | Molar Ratio ((NH4)2S2O8/Py) | Conductivity (S/cm) |
|---|---|---|
| S1 | 5:1 | 3.8 × 10−4 |
| S2 | 2:1 | 2.8 × 10−4 |
| S3 | 1:1 | 7.0 × 10−3 |
| S4 | 1:2 | 1.45 |
| S5 | 1:5 | 2.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Song, R.; Li, L.; Fu, R.; Liu, Z.; Li, B. High Crystalline Quality Conductive Polypyrrole Film Prepared by Interface Chemical Oxidation Polymerization Method. Appl. Sci. 2022, 12, 58. https://doi.org/10.3390/app12010058
Wang Y, Song R, Li L, Fu R, Liu Z, Li B. High Crystalline Quality Conductive Polypyrrole Film Prepared by Interface Chemical Oxidation Polymerization Method. Applied Sciences. 2022; 12(1):58. https://doi.org/10.3390/app12010058
Chicago/Turabian StyleWang, Yuefei, Renjing Song, Li Li, Rongpeng Fu, Zhiguo Liu, and Bingsheng Li. 2022. "High Crystalline Quality Conductive Polypyrrole Film Prepared by Interface Chemical Oxidation Polymerization Method" Applied Sciences 12, no. 1: 58. https://doi.org/10.3390/app12010058
APA StyleWang, Y., Song, R., Li, L., Fu, R., Liu, Z., & Li, B. (2022). High Crystalline Quality Conductive Polypyrrole Film Prepared by Interface Chemical Oxidation Polymerization Method. Applied Sciences, 12(1), 58. https://doi.org/10.3390/app12010058






