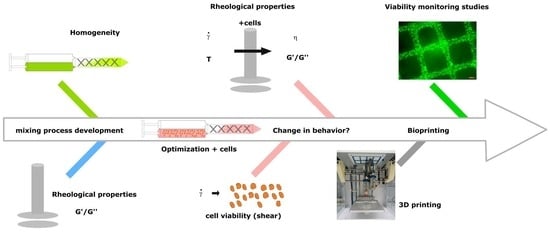
Optimized Cell Mixing Facilitates the Reproducible Bioprinting of Constructs with High Cell Viability
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Carrier Material
2.2. Mixing Process—Assessment and Optimization
2.2.1. Evaluation of Mixing Behavior
2.2.2. Optimization for Bioprinting
2.3. Analytical Methods for Mixing Evaluation
2.3.1. Mixing Homogeneity
2.3.2. Mixing Rheology
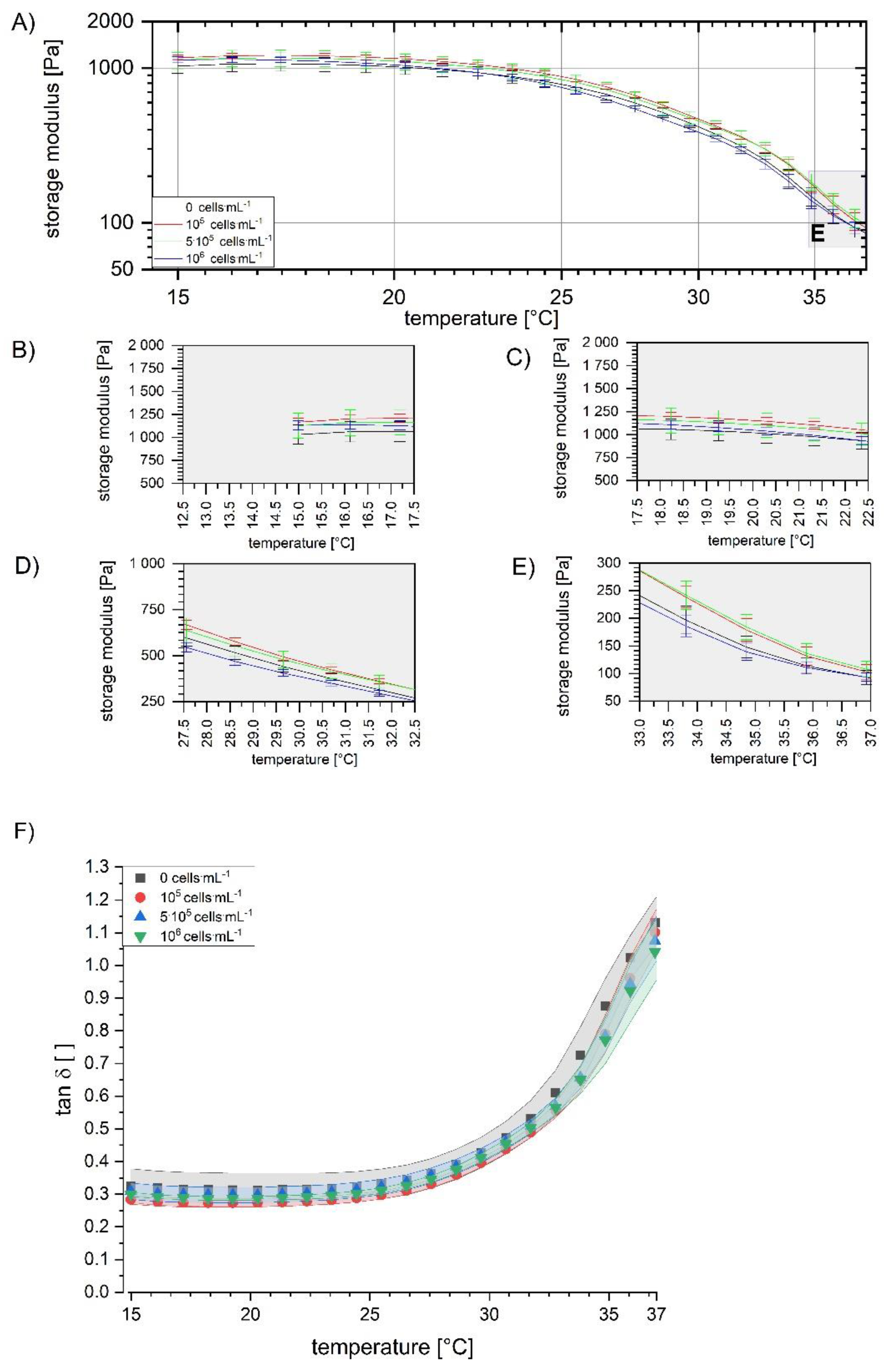
2.3.3. Temperature Sweeps
2.3.4. Cell Sedimentation
2.4. Estimation of Shear Stress
2.4.1. Cell culture and Bioink Preparation
2.4.2. Shear Stress during Printing
2.4.3. Rheological Assessment of Cells and Bioink
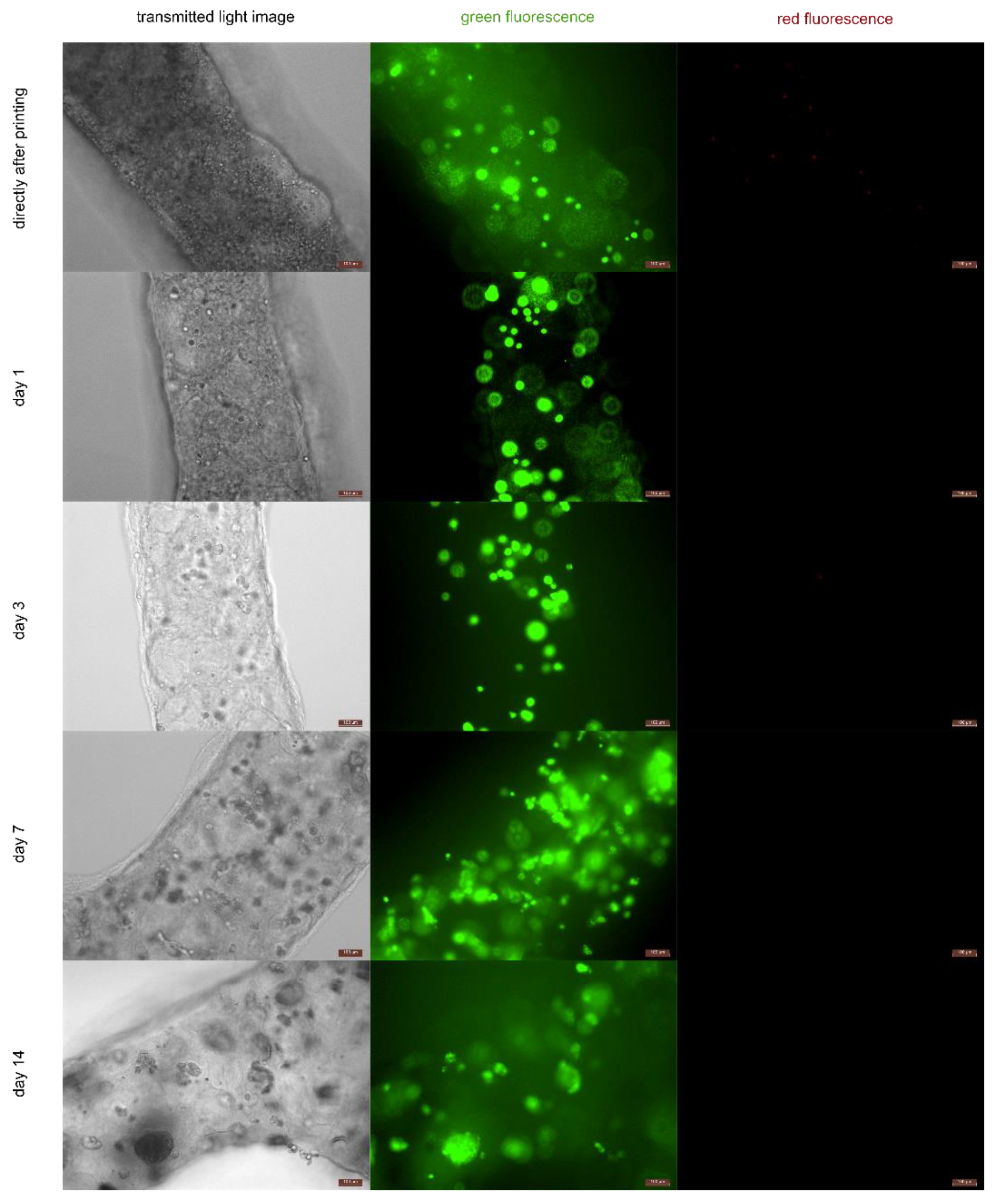
2.5. Cell Viability after Printing
2.6. Data Analysis
3. Results
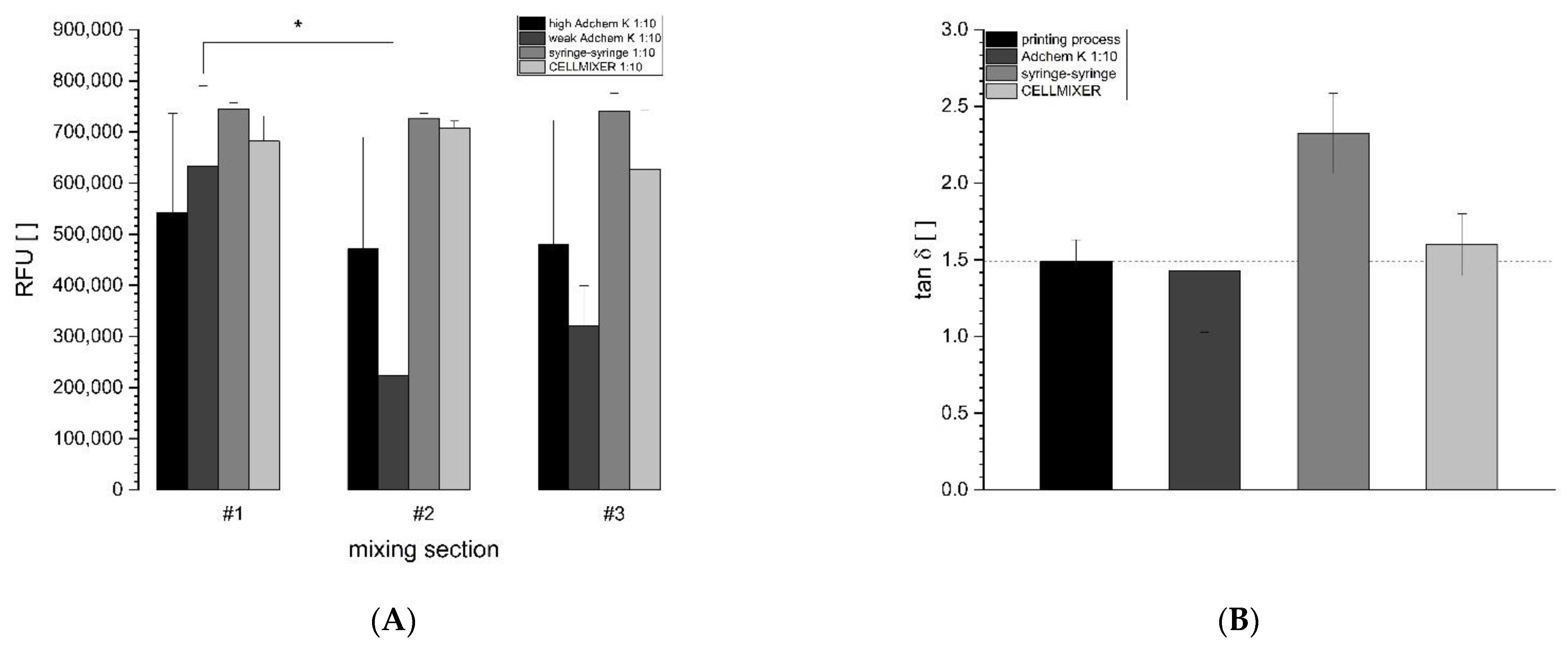
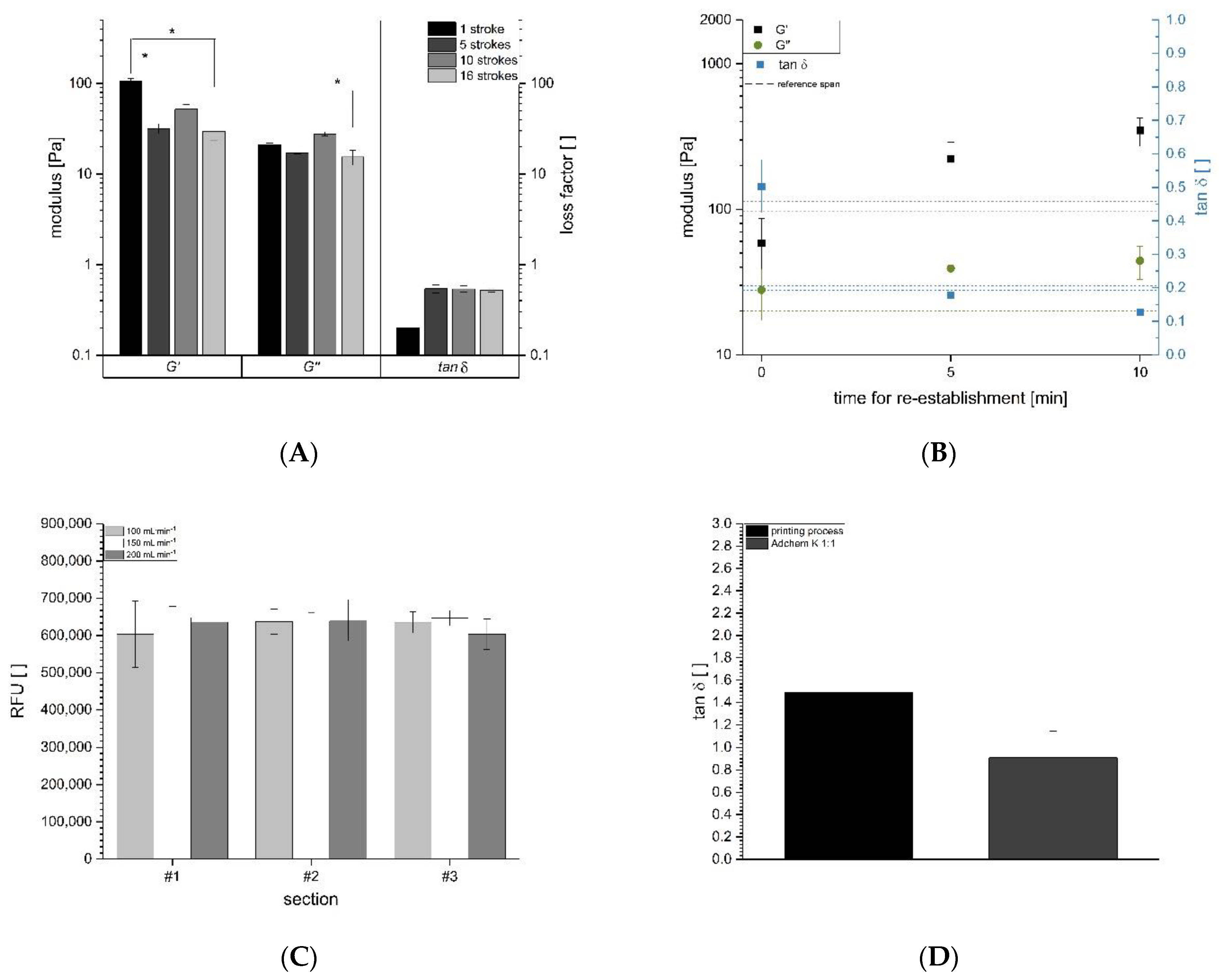
3.1. Assessment and Optimization of the Mixing Process
3.2. Optimization of the Mixing Process for Bioprinting
3.3. Estimation of Shear during the Printing Process and on Cells
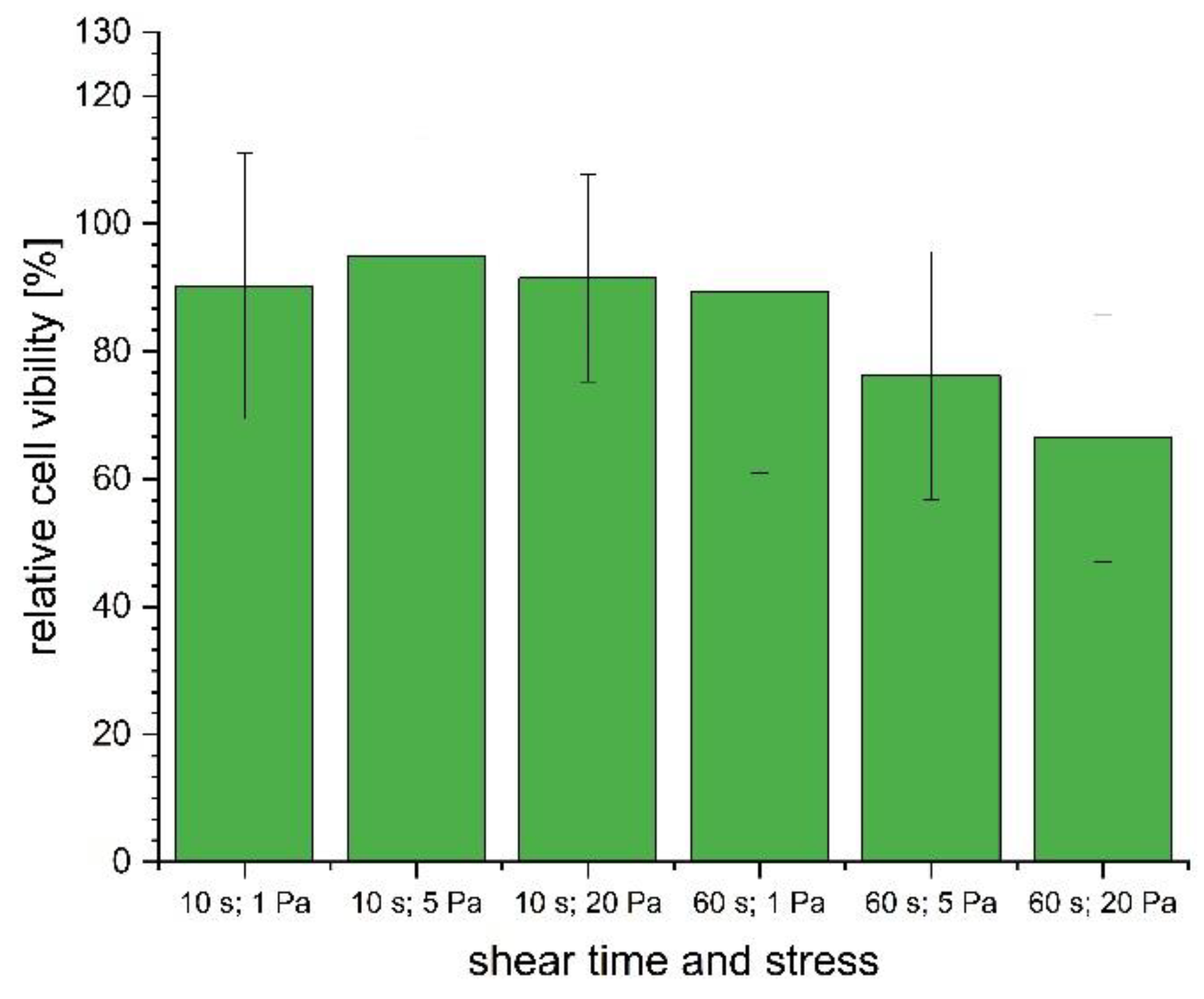
3.4. Cell Viability after Printing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Groll, J.; Boland, T.; Blunk, T.; Burdick, J.A.; Cho, D.-W.; Dalton, P.D.; Derby, B.; Forgacs, G.; Li, Q.; Mironov, V.A. Biofabrication: Reappraising the definition of an evolving field. Biofabrication 2016, 8, 13001. [Google Scholar] [CrossRef]
- Ramos, T.A.D.S.; Moroni, L. Tissue engineering and regenerative medicine 2019: The role of biofabrication—A year in review. Tissue Eng. Part C Methods 2020, 26, 91–106. [Google Scholar] [CrossRef]
- Hölzl, K.; Lin, S.; Tytgat, L.; van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016, 8, 32002. [Google Scholar] [CrossRef] [PubMed]
- Gillispie, G.; Prim, P.; Copus, J.; Fisher, J.; Mikos, A.G.; Yoo, J.J.; Atala, A.; Lee, S.J. Assessment methodologies for extrusion-based bioink printability. Biofabrication 2020, 12, 22003. [Google Scholar] [CrossRef]
- Markstedt, K.; Mantas, A.; Tournier, I.; Ávila, H.M.; Hägg, D.; Gatenholm, P. 3D bioprinting human chondrocytes with nanocellulose-alginate bioink for cartilage tissue engineering applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Gebeyehu, A.; Miranda, M.; Bahadur, D.; Patel, N.; Ramakrishnan, S.; Rishi, A.K.; Singh, M. Characterization and printability of sodium alginate-gelatin hydrogel for bioprinting NSCLC co-culture. Sci. Rep. 2019, 9, 19914. [Google Scholar] [CrossRef]
- Yin, J.; Yan, M.; Wang, Y.; Fu, J.; Suo, H. 3D bioprinting of low-concentration cell-laden gelatin methacrylate (GelMA) bioinks with a two-step cross-linking strategy. ACS Appl. Mater. Interfaces 2018, 10, 6849–6857. [Google Scholar] [CrossRef] [PubMed]
- White, C.R.; Frangos, J.A. The shear stress of it all: The cell membrane and mechanochemical transduction. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Chisti, Y. Hydrodynamic damage to animal cells. Crit. Rev. Biotechnol. 2001, 21, 67–110. [Google Scholar] [CrossRef]
- Billiet, T.; Gevaert, E.; de Schryver, T.; Cornelissen, M.; Dubruel, P. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials 2014, 35, 49–62. [Google Scholar] [CrossRef]
- Diamantides, N.; Dugopolski, C.; Blahut, E.; Kennedy, S.; Bonassar, L.J. High density cell seeding affects the rheology and printability of collagen bioinks. Biofabrication 2019, 11, 45016. [Google Scholar] [CrossRef] [PubMed]
- Pössl, A.; Hartzke, D.; Schmidts, T.M.; Runkel, F.E.; Schlupp, P. A targeted rheological bioink development guideline and its systematic correlation with printing behavior. Biofabrication 2021, 13, 035021. [Google Scholar] [CrossRef] [PubMed]
- Thayer, P.S.; Orrhult, L.S.; Martínez, H. Bioprinting of cartilage and skin tissue analogs utilizing a novel passive mixing unit technique for bioink precellularization. J. Vis. Exp. JoVE 2018, 131, e56372. [Google Scholar] [CrossRef]
- Park, K.; Jang, J.; Irimia, D.; Sturgis, J.; Lee, J.; Robinson, J.P.; Toner, M.; Bashir, R. ‘Living cantilever arrays’ for characterization of mass of single live cells in fluids. Lab A Chip 2008, 8, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Pössl, A.; Hartzke, D.; Schlupp, P.; Runkel, F.E. Calculation of Mass Transfer and Cell-Specific Consumption Rates to Improve Cell Viability in Bioink Tissue Constructs. Materials 2021, 14, 4387. [Google Scholar] [CrossRef]
- Castillo, E.; Codina, R. Stabilized stress-velocity-pressure finite element formulations of the Navier–Stokes problem for fluids with non-linear viscosity. Comput. Methods Appl. Mech. Eng. 2014, 279, 554–578. [Google Scholar] [CrossRef]
- Salzig, D.; Schmiermund, A.; Grace, P.P.; Elseberg, C.; Weber, C.; Czermak, P. Enzymatic detachment of therapeutic mesenchymal stromal cells grown on glass carriers in a bioreactor. Open Biomed. Eng. J. 2013, 7, 147. [Google Scholar] [CrossRef][Green Version]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef]
- Mouser, V.H.; Melchels, F.P.; Visser, J.; Dhert, W.J.; Gawlitta, D.; Malda, J. Yield stress determines bioprintability of hydrogels based on gelatin-methacryloyl and gellan gum for cartilage bioprinting. Biofabrication 2016, 8, 35003. [Google Scholar] [CrossRef]
- Kumar, V.; Shirke, V.; Nigam, K. Performance of Kenics static mixer over a wide range of Reynolds number. Chem. Eng. J. 2008, 139, 284–295. [Google Scholar] [CrossRef]
- Cohen, D.L.; Lo, W.; Tsavaris, A.; Peng, D.; Lipson, H.; Bonassar, L.J. Increased mixing improves hydrogel homogeneity and quality of three-dimensional printed constructs. Tissue Eng. Part C Methods 2011, 17, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, Z.; Xu, C. Sedimentation study of bioink containing living cells. J. Appl. Phys. 2019, 125, 114901. [Google Scholar] [CrossRef]
- Dubbin, K.; Tabet, A.; Heilshorn, S.C. Quantitative criteria to benchmark new and existing bio-inks for cell compatibility. Biofabrication 2017, 9, 44102. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.J.; Mirzahossein, E.; Iftekhar, E.N.; Bächer, C.; Schrüfer, S.; Schubert, D.W.; Fabry, B.; Gekle, S. Flow and hydrodynamic shear stress inside a printing needle during biofabrication. PLoS ONE 2020, 15, e0236371. [Google Scholar] [CrossRef]
- Yan, C.; Mackay, M.E.; Czymmek, K.; Nagarkar, R.P.; Schneider, J.; Pochan, D.J. Injectable solid peptide hydrogel as a cell carrier: Effects of shear flow on hydrogels and cell payload. Langmuir 2012, 28, 6076–6087. [Google Scholar] [CrossRef]
- Lucas, L.; Aravind, A.; Emma, P.; Christophe, M.; Edwin-Joffrey, C. Rheology, simulation and data analysis toward bioprinting cell viability awareness. Bioprinting 2021, 21, e00119. [Google Scholar]
- Li, Z.; Huang, S.; Liu, Y.; Yao, B.; Hu, T.; Shi, H.; Xie, J.; Fu, X. Tuning alginate-gelatin bioink properties by varying solvent and their impact on stem cell behavior. Sci. Rep. 2018, 8, 8020. [Google Scholar] [CrossRef]
- Panwar, A.; Tan, L.P. Current status of bioinks for micro-extrusion-based 3D bioprinting. Molecules 2016, 21, 685. [Google Scholar] [CrossRef]
- Blaeser, A.; Duarte Campos, D.F.; Puster, U.; Richtering, W.; Stevens, M.M.; Fischer, H. Controlling shear stress in 3D bioprinting is a key factor to balance printing resolution and stem cell integrity. Adv. Healthc. Mater. 2016, 5, 326–333. [Google Scholar] [CrossRef]
- Nair, K.; Gandhi, M.; Khalil, S.; Yan, K.C.; Marcolongo, M.; Barbee, K.; Sun, W. Characterization of cell viability during bioprinting processes. Biotechnol. J. Healthc. Nutr. Technol. 2009, 4, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, Y.; Mao, S.; Sun, W.; Yao, R. The influence of printing parameters on cell survival rate and printability in microextrusion-based 3D cell printing technology. Biofabrication 2015, 7, 45002. [Google Scholar] [CrossRef] [PubMed]
- Petry, F.; Weidner, T.; Czermak, P.; Salzig, D. Three-dimensional bioreactor technologies for the cocultivation of human mesenchymal stem/stromal cells and beta cells. Stem Cells Int. 2018, 2018, 2547098. [Google Scholar] [CrossRef] [PubMed]
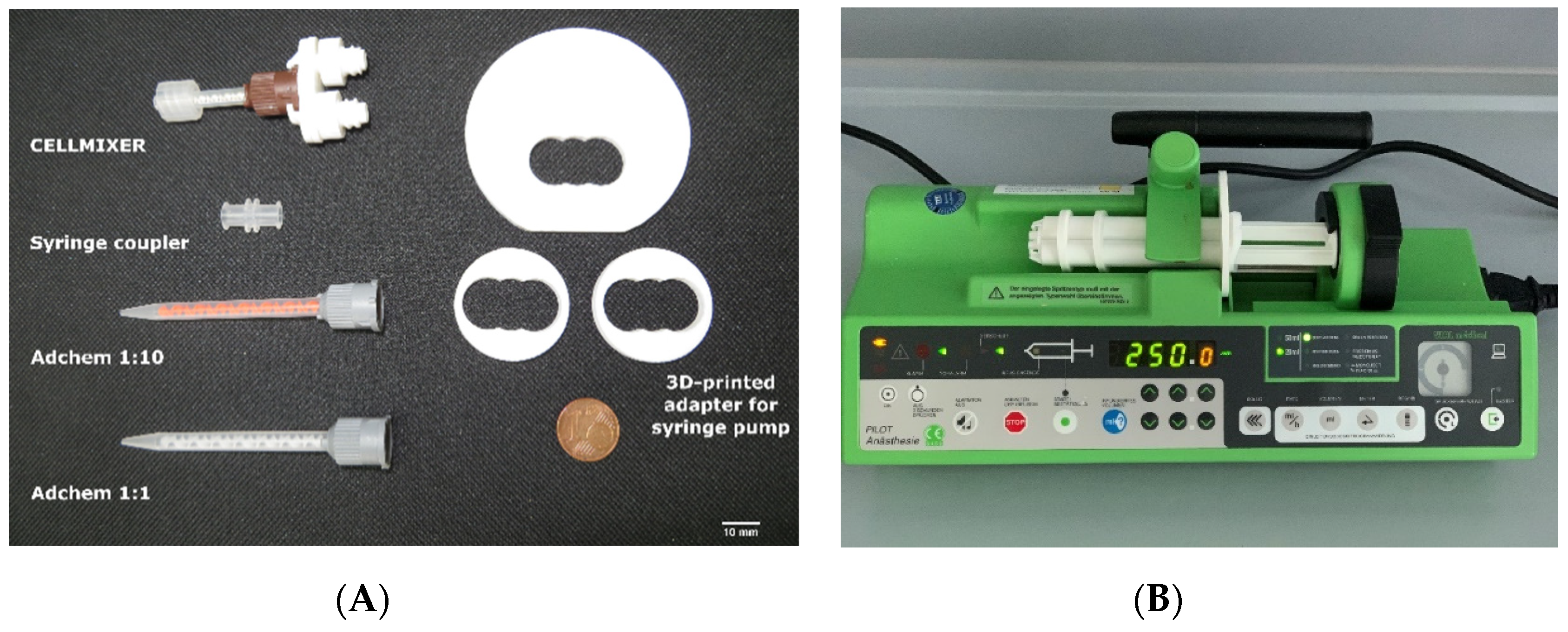



| System/ Mixing Ratio | Volume Chamber 1 (mL) | Volume Chamber 2 (mL) | Mixing Principle | Number of Mixing Steps | Mixer Length/Inner/Outlet Diameter (mm) |
|---|---|---|---|---|---|
| Adchem K/1:10 | 9.0 | 1.0 | Static mixer | 1 | 74.0/3.2/1.0 |
| CELLMIXER/1:10 | 2.7 | 0.3 | Static mixer | 1 | 25.7/1.8/1.8 |
| Syringe–syringe/1:10 | 2.7 | 0.3 | Tapering | 40 | 9.4/1.6/1.6 |
| Adchem K/1:1 | 6.5 | 6.5 | Static mixer | 1 | 74.0/3.2/1.0 |
| Challenge | Solution |
|---|---|
| Cell sedimentation in aqueous solution can occur during the process | Increase η and split the carrier material into two parts |
| Strongly differing viscosity causes unsteady flow inside the mixers and leads to heterogenous mixing results | Optimize rheological properties by using separate gelling agents in the bioink material, ensuring a steady flow from each chamber |
| The smaller cell suspension volume, the greater likelihood of cell damage | Select acceptable ratio from 1:10 to 1:1 |
| One mixing step reduces shear stress intensity and makes the process more scalable | Select a static mixer |
| Variability caused by manual mixing leads to poor reproducibility during printing | Mix with syringe pump instead to improve reproducibility |
| Bioink Material (Pössl et al. [12]) | Gelatin Solution | Water | ||||||
|---|---|---|---|---|---|---|---|---|
| Carreau–Yasuda 37 °C | Measured 37 °C | Measured 37 °C | Measured 37 °C | Measured 37 °C | Carreau–Yasuda 37 °C | Measured 28 °C | 37 °C | |
| Shear rate (s−1) | 0 | 1.0 | 10.0 | 100.0 | 1000.0 | Infinity | 0.1 | Not relevant |
| viscosity (Pa·s) | 280.05 | 21.00 | 8.00 | 2.50 | 0.50 | 0.0001 | 1127.14 | 0.001 |
| Resulting v (m·s−1) ·10−10 | 6.8 | 90.7 | 238.2 | 762.3 | 3811.4 | 19,057,237.9 | 1.7 | 1,905,723.8 |
| Sedimentation time (min) | 1,714,440.5 | 128,560.1 | 48,975.3 | 15,305.8 | 3061.0 | 0.6 | 6,900,247.9 | 6.1 |
| Volume Flow Rate (mL·min−1) | τ Wall (Pa) | Wall (s−1) | Reynolds Number Power Law | Reynolds Number Carreau–Yasuda | Residence Time z in Nozzle (s) |
|---|---|---|---|---|---|
| 0.025 | 112.2 | 19.6 | 0.000118 | 0.000014 | 54.30 |
| 0.05 | 159.3 | 39.3 | 0.000332 | 0.000027 | 27.14 |
| 0.10 (100 kPa) | 226.0 | 78.6 | 0.000935 | 0.000054 | 13.60 |
| 0.50 | 509.5 | 393.0 | 0.010375 | 0.000271 | 2.71 |
| 0.75 | 625.2 | 589.5 | 0.019022 | 0.000407 | 1.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pössl, A.; Hartzke, D.; Schlupp, P.; Runkel, F.E. Optimized Cell Mixing Facilitates the Reproducible Bioprinting of Constructs with High Cell Viability. Appl. Sci. 2022, 12, 326. https://doi.org/10.3390/app12010326
Pössl A, Hartzke D, Schlupp P, Runkel FE. Optimized Cell Mixing Facilitates the Reproducible Bioprinting of Constructs with High Cell Viability. Applied Sciences. 2022; 12(1):326. https://doi.org/10.3390/app12010326
Chicago/Turabian StylePössl, Axel, David Hartzke, Peggy Schlupp, and Frank E. Runkel. 2022. "Optimized Cell Mixing Facilitates the Reproducible Bioprinting of Constructs with High Cell Viability" Applied Sciences 12, no. 1: 326. https://doi.org/10.3390/app12010326
APA StylePössl, A., Hartzke, D., Schlupp, P., & Runkel, F. E. (2022). Optimized Cell Mixing Facilitates the Reproducible Bioprinting of Constructs with High Cell Viability. Applied Sciences, 12(1), 326. https://doi.org/10.3390/app12010326