Trapa japonica Flerov Extract Prevents Obesity by Regulating Adipogenesis and Lipolysis in Differentiated 3T3-L1 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.2. Cell Culture and Treatments
2.3. Water Soluble Tetrazolium Salt (WST) Assay
2.4. Western Blotting
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Oil Red O Staining
2.7. Glycerol Release Assay
2.8. Statistical Analysis
3. Results
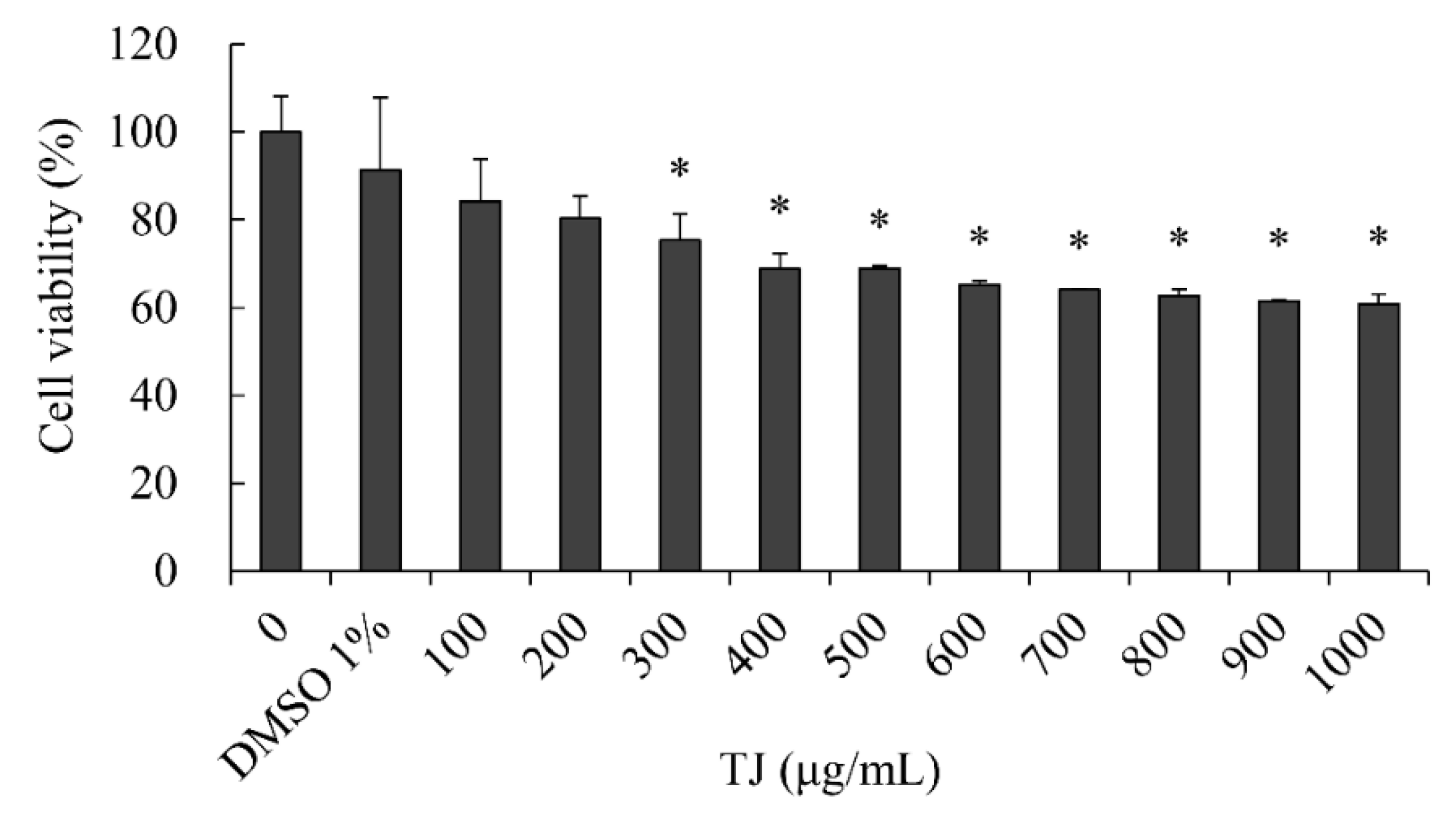
3.1. Effects of TJ on Cell Viability in 3T3-L1 Cells
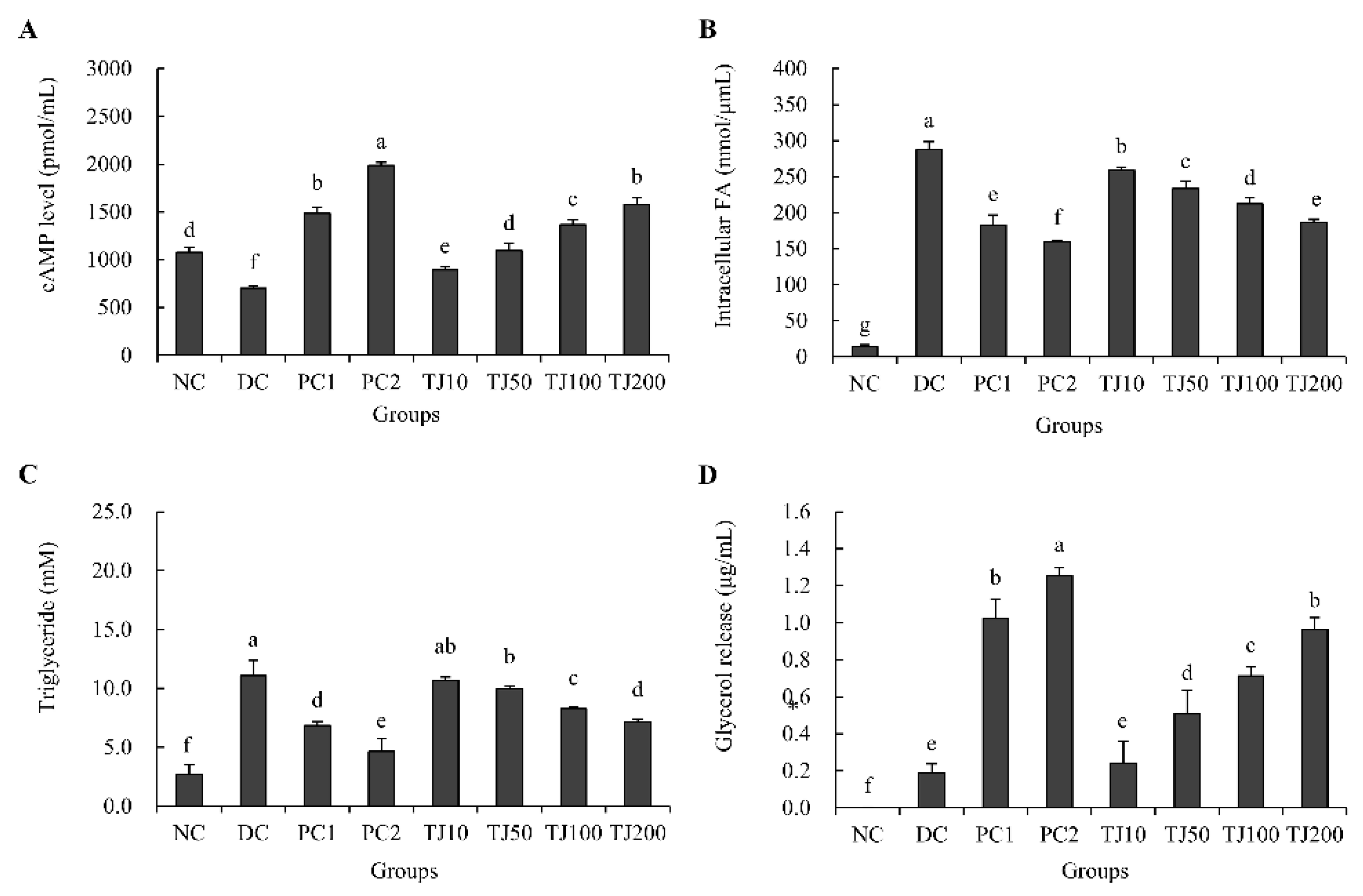
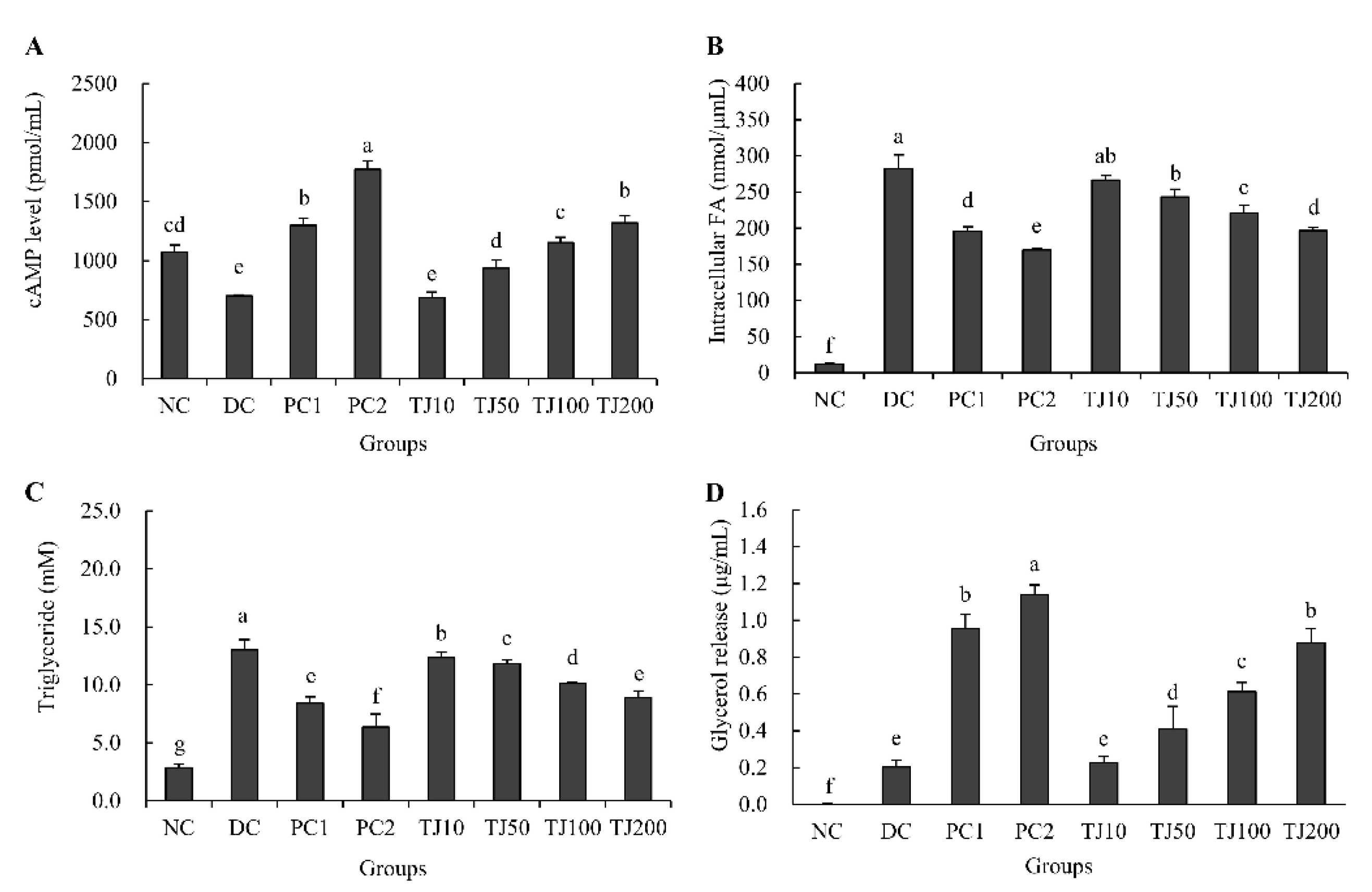
3.2. The Effects of the TJ in the cAMP Level, Intracellular FA, TG, and Glycerol Release Related to the Adipogenesis Mechanism in Differentiated 3T3-L1 Cells
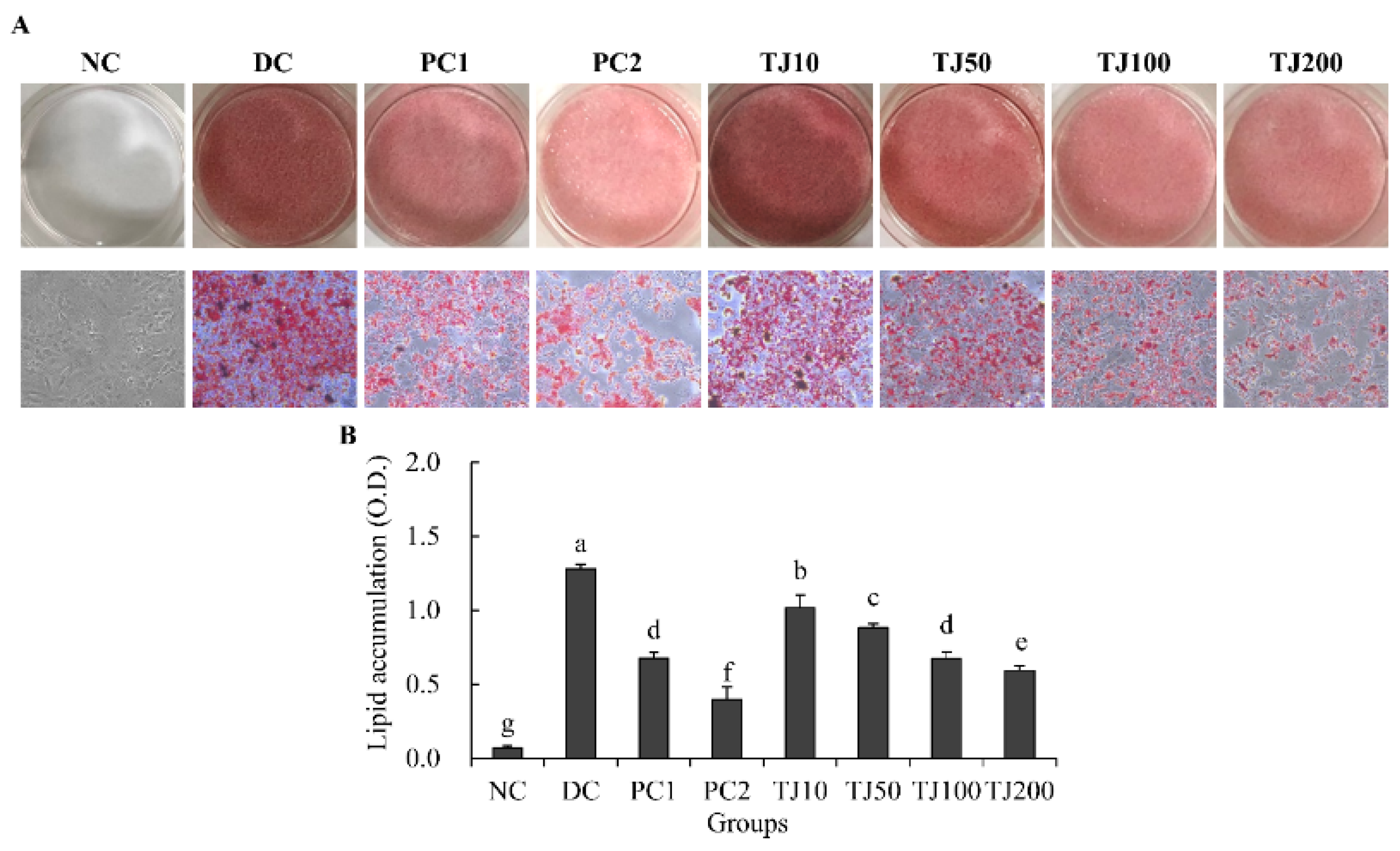
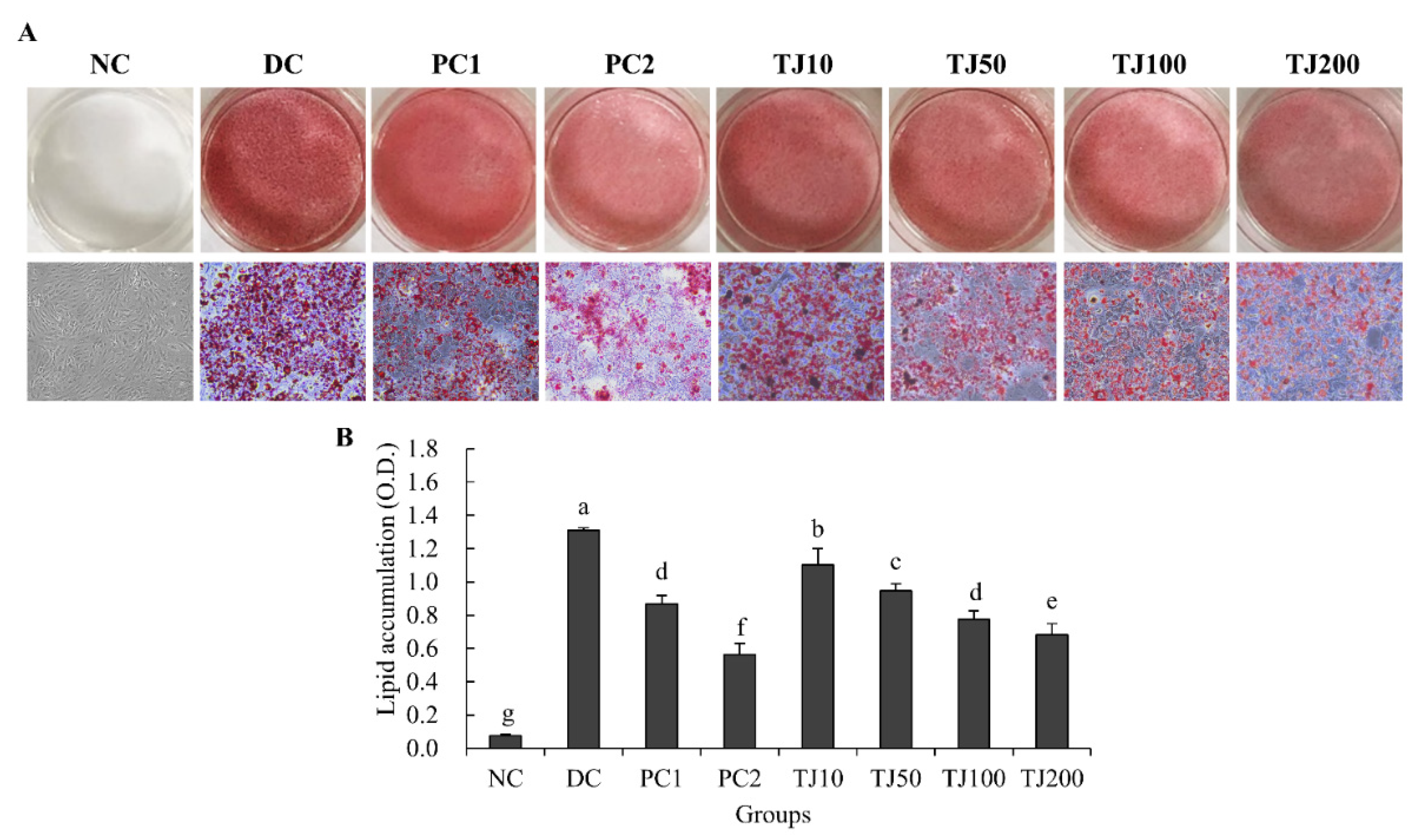
3.3. The TJ Inhibited the Lipid Accumulation Related to the Adipogenesis Mechanism in Differentiated 3T3-L1 Cells
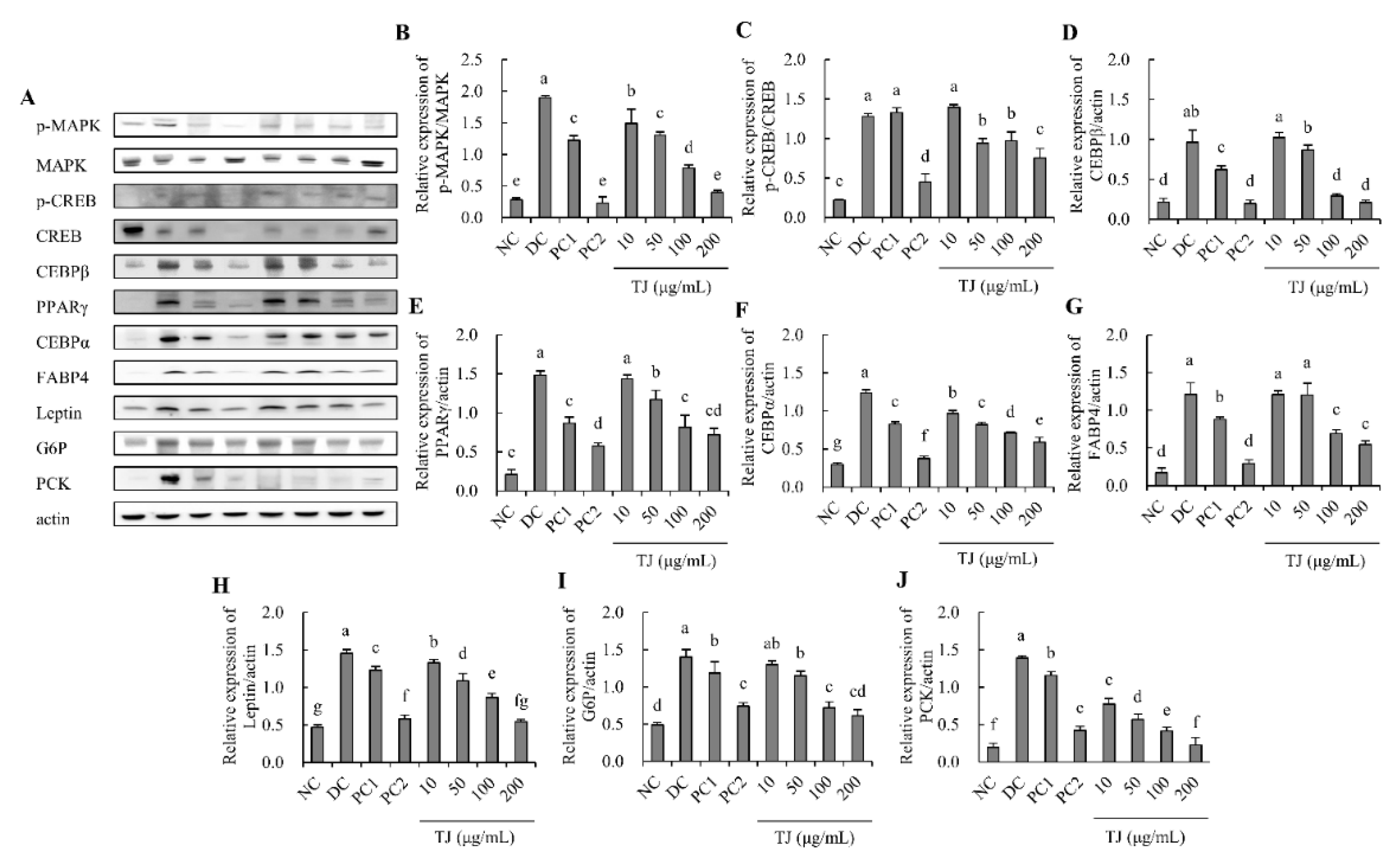
3.4. The TJ Regulated the Protein Expression Levels Related to the Adipogenesis Mechanism in Differentiated 3T3-L1 Cells
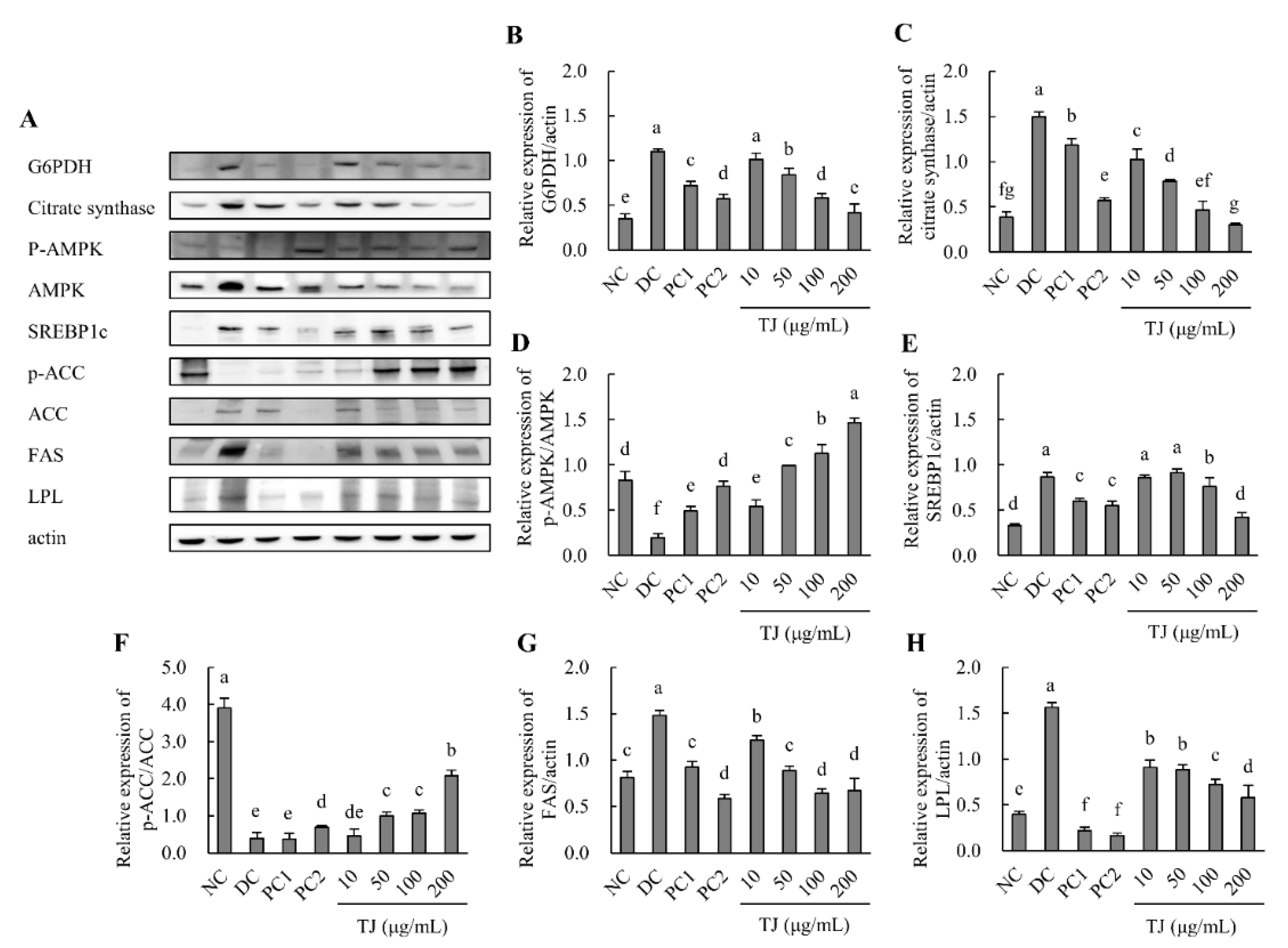
3.5. The TJ Regulated the Protein Expression Levels Related to the Lipogenesis Mechanism in Differentiated 3T3-L1 Cells
3.6. The Effects of the TJ in the cAMP Level, Intracellular FA, TG, and Glycerol Release Related to the Lipolysis Mechanism in Differentiated 3T3-L1 Cells
3.7. The TJ Inhibited the Lipid Accumulation Related to the Lipolysis Mechanism in Differentiated 3T3-L1 Cells
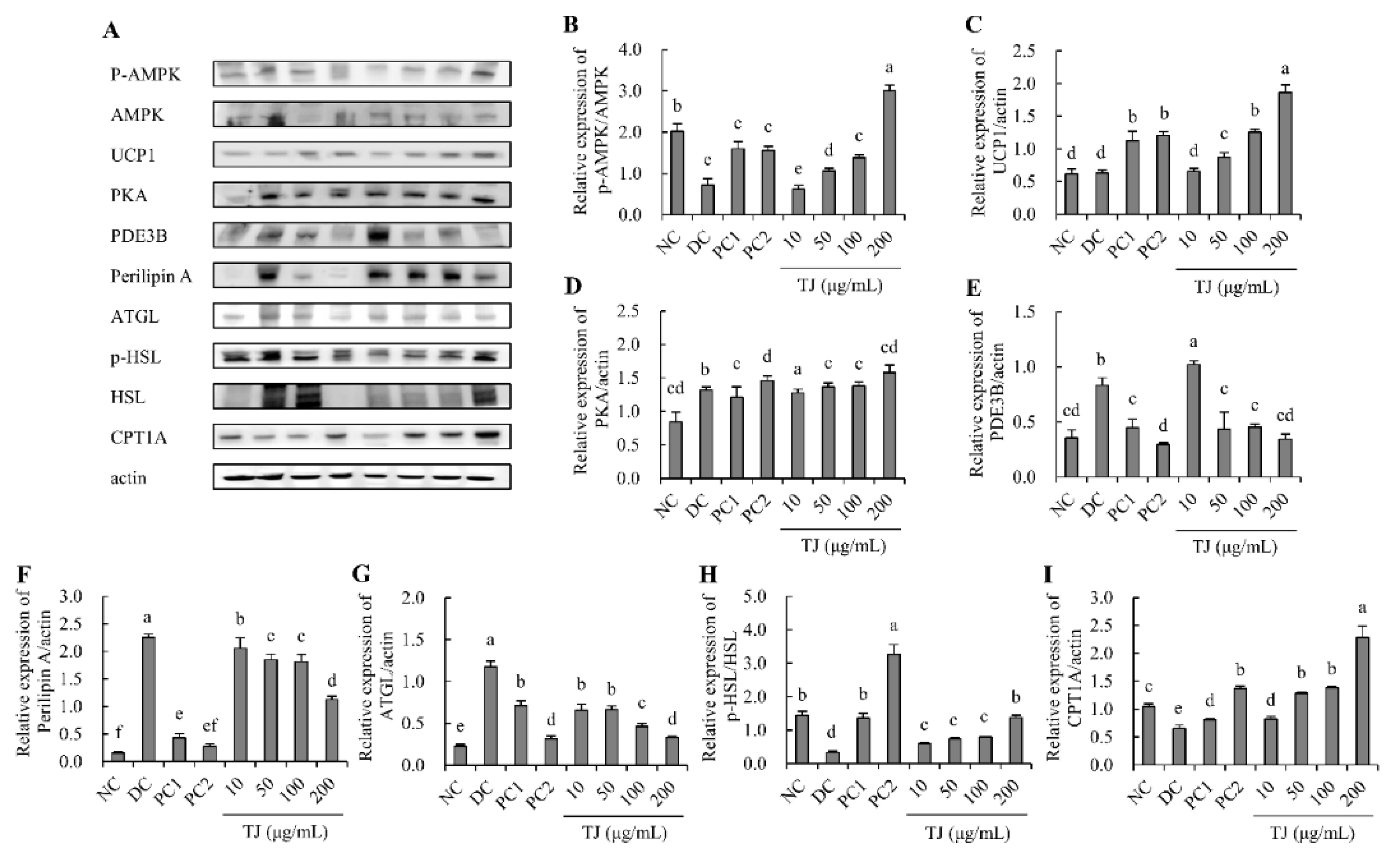
3.8. The TJ Regulated the Protein Expression Levels Related to the Lipolysis Mechanism in Differentiated 3T3-L1 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
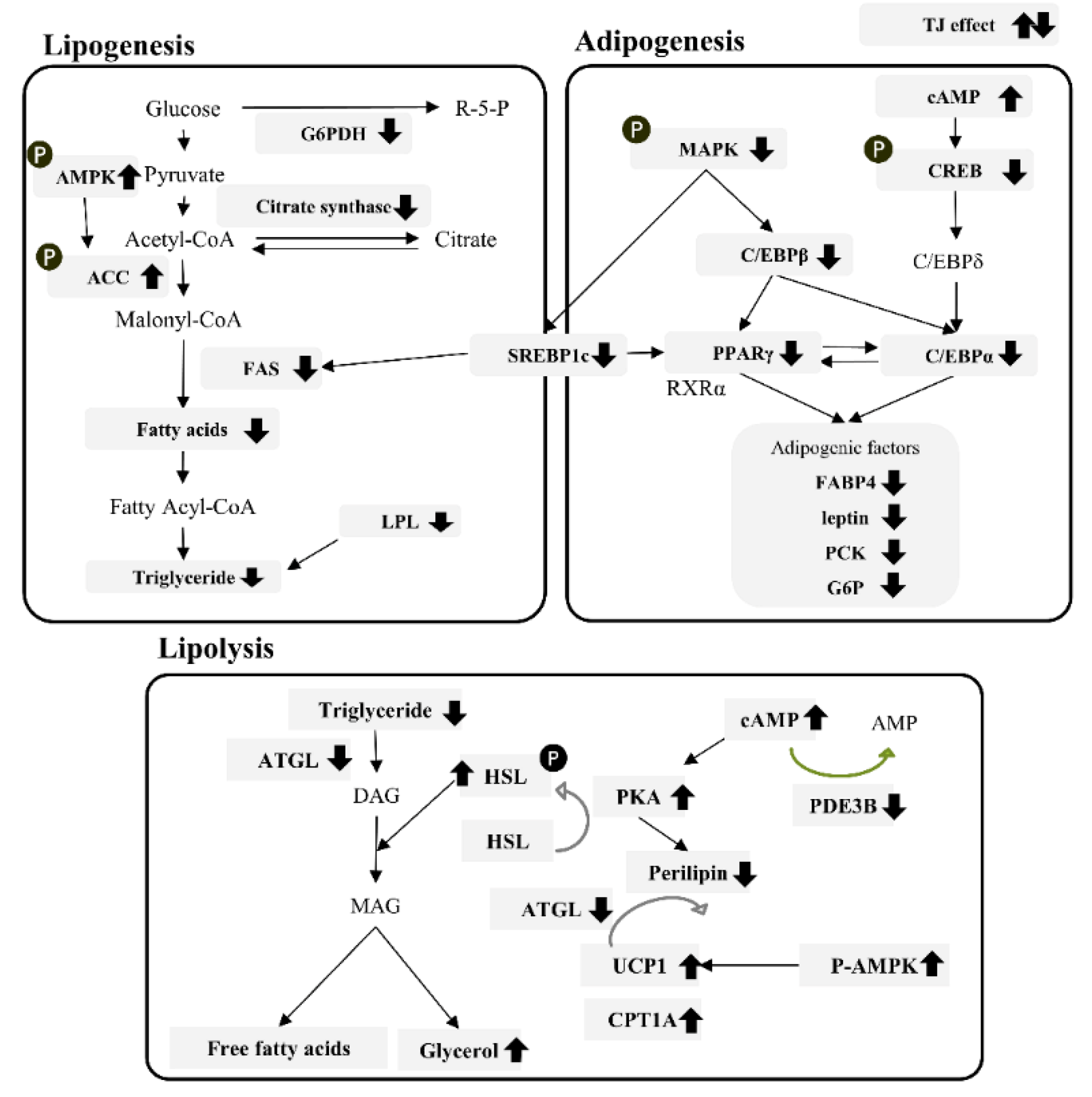
Appendix A
References
- Lavie, C.J.; Pandey, A.; Lau, D.H.; Alpert, M.A.; Sander, P. Obesity and atrial fibrillation prevalence, pathogenesis, and prognosis: Effects of weight loss and exercise. J. Am. Coll. Cardiol. 2017, 70, 2022–2035. [Google Scholar] [CrossRef] [PubMed]
- Taylor, V.H.; Forhan, M.; Vigod, S.N.; Mclntyre, R.S.; Morrison, K.M. The impact of obesity on quality of life. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Nepali, S.; Cha, J.-Y.; Ki, H.-H.; Lee, H.-Y.; Kim, Y.-H.; Kim, D.-K.; Song, B.-J.; Lee, Y.-M. Chrysanthemum indicum inhibits adipogenesis and activates the AMPK pathway in high-fat-diet-induced obese mice. Am. J. Chin. Med. 2018, 46, 119–136. [Google Scholar] [CrossRef]
- Kanneganti, T.-D.; Dixit, V.D. Immunological complications of obesity. Nut. Immunol. 2012, 13, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, A.; Feng, D.; Pessin, J.E.; Shinoda, K. The impact of single-cell genomics on adipose tissue research. Int. J. Mol. Sci. 2020, 21, 4773. [Google Scholar] [CrossRef] [PubMed]
- Wolins, N.E.; Quaynor, B.K.; Skinner, J.R.; Tzekov, A.; Park, C.; Choi, K.; Bickel, P.E. OP9 mouse stromal cells rapidly differentiate into adipocytes. Characterization of a useful new model of adipogenesis. J. Lipid Res. 2006, 47, 450–460. [Google Scholar] [CrossRef] [Green Version]
- Jemai, R.; Drira, R.; Makni, M.; Fetoui, H.; Sakamoto, K. Colocynth (Citrullus colocynthis) seed extracts attenuate adipogenesis by down-regulating PPARγ/SREBP-1c and C/EBPα in 3T3-L1 cells. Food. Biosci. 2020, 33, 100491. [Google Scholar] [CrossRef]
- White, U.A.; Stephens, J.M. Transcriptional factors that promote formation of white adipose tissue. Mol. Cell Endocrinol. 2010, 318, 10–14. [Google Scholar] [CrossRef] [Green Version]
- Menezes-Garcia, Z.; Oliveira, M.C.; Lima, R.L.; Soriani, F.M.; Cisalpino, D.; Botion, L.M.; Teixeira, M.M.; Souza, D.; Ferreira, A.V.M. Lack of platelet-activating factor receptor protects mice against diet-induced adipose inflammation and insulin-resistance despite fat pad expansion. Obesity 2014, 22, 663–672. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, N.; Yoo, G.; Kim, S.-N.; K, H.-J.; Jung, K.; Oh, D.-C.; Lee, Y.-H.; Kim, S.H. Phenolics and neolignans isolated from the fruits of Juglans mandshurica, Maxim. And their effects on lipolysis in adipocytes. Phytochemistry 2017, 137, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Koster, J.D.; Nelli, R.K.; Strieder-Barboza, C.; Souza, J.D.; Lock, A.L.; Contreras, G.A. The contribution of hormone sensitive lipase to adipose tissue lipolysis and its regulation by insulin in periparturient dairy cows. Sci. Rep. 2018, 8, 13378. [Google Scholar] [CrossRef]
- Rahn Landstrӧm, T.; Mei, J.; Karlsson, M.; Manganiello, V.; Degerman, E. Down-regulation of cyclic-nucleotide phosphodiesterase 3B in 3T3-L1 adipocytes induced by tumor necrosis factor alpha and cAMP. Biochem. J. 2000, 346, 337–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmen, G.Y.; Victor, S.M. Signaling mechanisms regulating lipolysis. Cell. Signal. 2006, 18, 401–408. [Google Scholar] [CrossRef]
- Suriyagoda, L.; Arima, S.; Suzuki, A.; Hoque, M.A. Variation in growth and yield performance of seventeen water chestnut accessions (Trapa spp.) collected from Asia and Europe. Plant. Prod. Sci. 2007, 10, 372–379. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.J.; Im, K.R.; Yoon, K.-S. Trapa japonica Flerov extract attenuates lipid accumulation through downregulation of adipogenic transcription factors in 3T3-L1 cells. Am. J. Mol. Biol. 2015, 5, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, M.; Yasutake, K.; Hino, M.; Ohwatari, H.; Ohmagari, N.; Takedomi, K.; Tanaka, T.; Nonaka, G. Inhibitory effects of polyphenols from water chestnut (Trapa japonica) husk on glycolytic enzymes and postprandial blood glucose elevation in mice. Food. Chem. 2014, 165, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Hwang, J.W.; Han, Y.K.; Kwon, H.J.; Hong, H.; Kim, E.H.; Moon, S.H.; Jeon, B.T.; Park, P.J. Antioxidant activity and protective effects of Trapa japonica pericarp extracts against tert-butylhydroperoxide-induced oxidative damage in chang cells. Food. Chem. Toxicol. 2014, 64, 49–56. [Google Scholar] [CrossRef]
- Zhan, G.; Pan, L.; Tu, K.; Jiao, S. Antitumor, antioxidant, and nitrite scavenging effects of chines water chestnut (Eleocharis dulcis) peel flavonoids. J. Food. Sci. 2016, 81, H2578–H2586. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Lee, M.; Oh, D.H.; Kim, J.L.; Park, M.-R.; Kim, T.G.; Kim, O.-K.; Lee, J. Emblica officinalis and Hordeum vulgare L. mixture regulates lipolytic activity in differentiatied 3T3-L1 cells. J. Med. Food. 2021, 24, 172–179. [Google Scholar] [CrossRef]
- Park, S.-J.; Kim, D.; Kim, J.-L.; Park, M.-R.; Kim, T.G.; Kim, O.-K.; Nam, D.-E. Indian gooseberry and Barley sprout mixture inhibits aipogenesis and lipogenesis activity in 3T3-L1 adipocytes. Appl. Sci. 2002, 10, 9078. [Google Scholar] [CrossRef]
- Speliotes, E.K.; Willer, C.J.; Berndt, S.I. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat. Genet. 2010, 42, 937–948. [Google Scholar] [CrossRef] [Green Version]
- Chu, D.-T.; Nguyet, N.T.M.; Dinh, T.C.; Lien, N.V.T.; Nguyen, K.-H.; Ngoc, V.T.N.; Tao, Y.; Son, L.H.; Le, D.-H.; Nga, V.B.; et al. An update on physical health and economic consequences of overweight and obesity. Diabetes Metab. Syndr. 2018, 12, 1095–1100. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.-S.; Kim, C.-T.; Kim, I.-H.; Kim, Y. Inhibitory effects of green tea catechin on the lipid accumulation in 3T3-L1 adipocytes. Phytother. Res. 2009, 23, 1088–1091. [Google Scholar] [CrossRef]
- Adesanya, O.A.; Senuga, T.O.; Oyenike, M.A.; Adam, M.A.; Adeoye, A.O.; Shittu, L.A.J. Effects of ethanolic extract of Irvingia gabonensis on the liver of progesterone induced obesity in female Swiss mice. Res. J. Health Sci. 2019, 7, 302–311. [Google Scholar] [CrossRef]
- Choi, J. Who cares for nutrition information at a restaurant? Foo-related lifestyles and their association to nutrition information conscious behaviors. Brit. Food J. 2016, 118, 1625–1640. [Google Scholar] [CrossRef]
- Bargale, M.; Smawarkar, N.J.; Sharma, Y.K. Mineralogical composition of water chestnut as compared to sweet potato. Indian J. Nutr. Diet. 1987, 24, 78–82. [Google Scholar]
- Kang, M.J.; Lee, S.K.; Song, J.H.; Kim, M.E.; Kim, M.J.; Jang, J.S.; Lee, J.H.; Kim, J.I. Water chestnut (Trapa japonica Flerov.) exerts inhibitory effect on postprandial glycemic response in rats and free radical scavenging activity in vitro. Food. Sci. Biotechnol. 2019, 18, 808–812. [Google Scholar]
- Talcott, S.T.; Lee, J.-H. Ellagic acid and flavonoid antioxidant content of muscadine wine and juice. J. Agric. Food Chem. 2002, 50, 3186–3192. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-M.; Deng, J.-Z.; Ma, J.; Chen, S.-N.; Marchall, R.; Jones, S.H.; Johnson, R.K.; Hecht, S.M. DNA damaging activity of ellagic acid derivatives. Bioorg. Med. Chem. 2003, 11, 1593–1596. [Google Scholar] [CrossRef]
- Zeng, J.-L.; Li, Z.-H.; Wang, Z.-C.; Zhang, H.-L. Green tea consumption and risk of pancreatic cancer: A meta-analysis. Nutrients 2014, 6, 4640–4650. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Yang, L.; Xu, F.; Lin, L.; Zheng, G. Combination therapy with catechins and caffeine inhibits fat accumulation in 3T3-L1 cells. Exp. Ther. Med. 2017, 13, 688–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.-R.; Lee, Y.-S.; Choi, B.-K.; Lee, H.J.; Park, S.-B.; Kim, T.-M.; Oh, H.J.; Yang, S.H.; Suh, J.-W. Root extracts of adenophora triphylla var. japonica improve obesity in 3T3-L1 adipocytes and high-fat diet-induced obese mice. Asian Pac. J. Trop. Med. 2015, 8, 898–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, K.E.; Colton, L.A.; Erickson, P.F.; Friedman, J.E.; Cha, H.C.; Keller, P.; MacDougald, O.A.; Klemm, D.J. Regulation of cyclin D1 and wnt10b gene expression by cAMP-responsive element-binding protein during early adipogenesis involves differential promotor methylation. J. Biol. Chem. 2008, 283, 35096–35105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidal-Puig, A.; Jimenez-Liñan, M.; Lowell, B.B.; Hamann, A.; Hu, E.; Spiegelman, B.; Flier, J.S.; Moller, D.E. Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. J. Clin. Investig. 1996, 97, 2553. [Google Scholar] [CrossRef] [Green Version]
- Yi, X.; Wu, P.; Liu, J.; Gong, Y.; Xu, X.; Li, W. Identification of the potential key genes for adipogenesis from human mesenchymal stem cells by RNA-Seq. J. Cell Physiol. 2019, 234, 20217–20227. [Google Scholar] [CrossRef]
- Li, W.; Rong, Y.; Bao, L.; Nie, B.; Ren, G.; Zheng, C.; Amin, R.; Arnold, R.D.; Jeganathan, R.B.; Huggins, K.W. Suppression of adipocyte differentiation and lipid accumulation by stearidonic acid (SDA) in 3T3-L1 cells. Lipid Health Dis. 2017, 16, 181. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-S.; Cha, B.-Y.; Choi, S.-S.; Choi, B.-K.; Yonezawa, T.; Teruya, T.; Nagai, K.; Woo, J.-T. Nobiletin improves obesity and insulin resistance in high-fat diet-induced obese mice. J. Nutr. Biochem. 2014, 24, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Browning, J.D.; Horton, J.D. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Investig. 2004, 114, 147. [Google Scholar] [CrossRef] [Green Version]
- Foretz, M.; Pacot, C.; Dugail, I.; Lemarchand, P.; Guichard, C.; Lièpvre, X.I.; Berthelier-Lubrana, C.; Spiegelman, B.; Kim, J.B.; Ferré, P.; et al. ADD1/SREBP-1c is required in the activation of hepatic lipogenic geneexpression by glucose. Mol. Cell Biol. 1999, 19, 3760–3768. [Google Scholar] [CrossRef] [Green Version]
- Molenear, A.; Mao, J.; Oden, K.; Seyfert, H.M. All three promoters of the acetyl-coenzyme A-carboxylase ALPA-encoding gene are expressed in mammary epithelial cells of ruminants. J. Histochem. Cytochem. 2003, 51, 1073–1081. [Google Scholar] [CrossRef] [Green Version]
- Smith, S. The animal fatty acid synthase: One gen, one polypeptide, seven enzymes. FASEB J. 1994, 8, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Jung, Y.; Seo, S.J.; Kim, S.-M.; Shim, Y.J.; Cho, S.H.; Chung, S.-I.; Yoon, Y. Berberine activates AMPK to suppress proteolytic processing, nuclear translocation and target DNA binding of SREBP-1c in 3T3-L1 adipocytes. Mol. Med. Rep. 2017, 15, 4139–4147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, B.-K.; Park, S.-B.; Lee, D.-R.; Lee, H.J.; Jin, Y.-Y.; Yang, S.H.; Suh, J.-W. Green coffee bean extract improves obesity by decreasing body fat in high-fat diet induced obese mice. Asian Pac. J. Trop. Med. 2016, 9, 635–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blomquist, C.; Chorell, E.; Ryberg, M.; Mellberg, C.; Worrsjö, E.; Makoveichuk, E.; Larsson, C.; Lindahl, B.; Olivecrona, G.; Olsson, T. Decreased lipogenesis-promoting factors in adiose tissue in postmenopausal women with overweight on a Paleolithic-type diet. Eur. J. Nutr. 2018, 57, 2877–2886. [Google Scholar] [CrossRef] [Green Version]
- Blancon-Rivero, J.; Xavier, F.E. Therapeutic potential of phosphodiesterase inhibitors for endothelial dysfunction-related diseases. Curr. Phar. Des. 2020, 26, 3633–3651. [Google Scholar] [CrossRef]
- Jiang, B.; Yang, Y.; Jin, H.; Shang, W.; Zhou, L. Qian, L.; Chen, M. Astragaloside IV attenuates lipolysis and improves insulin resistance induced by TNFalpha in 3T3-L1 adipocytes. Phytother. Res. 2008, 22, 1434–1439. [Google Scholar] [CrossRef] [PubMed]
- Sztalryd, C.; Brasaemle, D.L. The perilipin family of lipid droplet proteins: Gatekeepers of intracellular lipolysis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1221–1232. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-J.; Lee, M.; Kim, K.-Y.; Shin, S.; Choi, M.-W.; Hong, E.-J.; Lee, J. Trapa japonica Flerov Extract Prevents Obesity by Regulating Adipogenesis and Lipolysis in Differentiated 3T3-L1 Cells. Appl. Sci. 2022, 12, 290. https://doi.org/10.3390/app12010290
Park S-J, Lee M, Kim K-Y, Shin S, Choi M-W, Hong E-J, Lee J. Trapa japonica Flerov Extract Prevents Obesity by Regulating Adipogenesis and Lipolysis in Differentiated 3T3-L1 Cells. Applied Sciences. 2022; 12(1):290. https://doi.org/10.3390/app12010290
Chicago/Turabian StylePark, Soo-Jeung, Minhee Lee, Ki-Young Kim, Su Shin, Min-Woo Choi, Eun-Jin Hong, and Jeongmin Lee. 2022. "Trapa japonica Flerov Extract Prevents Obesity by Regulating Adipogenesis and Lipolysis in Differentiated 3T3-L1 Cells" Applied Sciences 12, no. 1: 290. https://doi.org/10.3390/app12010290
APA StylePark, S.-J., Lee, M., Kim, K.-Y., Shin, S., Choi, M.-W., Hong, E.-J., & Lee, J. (2022). Trapa japonica Flerov Extract Prevents Obesity by Regulating Adipogenesis and Lipolysis in Differentiated 3T3-L1 Cells. Applied Sciences, 12(1), 290. https://doi.org/10.3390/app12010290





